Abstract
Fusion of intracellular transport vesicles requires soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) and Sec1/Munc18-family (SM) proteins. Membrane-bridging SNARE complexes are critical for fusion, but their spontaneous assembly is inefficient and may require SM proteins in vivo. We report x-ray structures of Vps33, the SM subunit of the yeast vacuolar homotypic fusion and vacuole protein sorting (HOPS) complex, bound to two individual SNAREs. The two SNAREs, one from each membrane, are held in the correct orientation and register for subsequent complex assembly. Vps33 and potentially other SM proteins could thus act as templates for generating partially zipped SNARE assembly intermediates. HOPS was essential to mediate SNARE complex assembly at physiological SNARE concentrations. Thus, Vps33 appears to catalyze SNARE complex assembly through specific SNARE motif recognition.
SNARE proteins are essential for intracellular membrane fusion (1). They contain a 60- to 70- residue SNARE motif capable of assembling with three other SNARE motifs to form a parallel four-helical bundle (fig. S1). Most SNAREs also contain a C-terminal transmembrane anchor adjacent to their SNARE motif. The assembly of “trans” SNARE complexes – those that include SNAREs anchored in two apposed membranes – brings the C-terminal anchors into close proximity and promotes membrane fusion. In addition to their C-terminal anchors and adjacent SNARE motifs, many SNAREs contain N-terminal domains that regulate SNARE assembly and/or recruit additional proteins to the sites of membrane fusion (Fig. 1A).
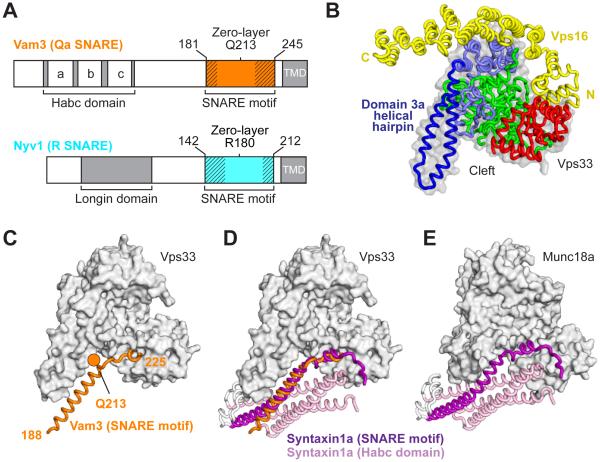
Fig. 1. Structure of the Qa-SNARE Vam3 bound to the SM protein Vps33.
(A) Shown are the C. thermophilum SNAREs whose SNARE motifs were co-crystallized with Vps33–Vps16. Cross-hatching indicates regions that were disordered in the resulting crystal structures (see also Fig. 2). TMD, transmembrane domain. (B) Vps33–Vps16 crystal structure, highlighting domains 1 (red), 2 (green), and 3 (blue). (C) Complex containing the Vam3 SNARE motif (see fig. S2). The zero-layer Q is highlighted. Vps16 is present in this structure but is omitted for clarity. (D and E) Comparison between Vps33–Vam3 and Munc18a–syntaxin1a (PDB code 3C98) complexes (see fig. S4).
SNAREs are classified by their position within the four-helix bundle and the residue that they contribute to the so-called “zero layer” at the bundle’s midpoint (fig. S1) (2). R-SNAREs contribute an arginine to the zero layer, while Qa-, Qb-, and Qc-SNAREs each contribute glutamines. Polar interactions among the four zero-layer residues within the otherwise hydrophobic core of the four-helix bundle ensure that the SNAREs assemble in the proper register (fig. S1).
Membrane fusion also requires Rab GTPases, tethering factors, and Sec1-Munc18 (SM) proteins. SM proteins interact with individual SNARE motifs as well as with partial (Qabc) and complete (RQabc) SNARE complexes, suggesting that they participate in SNARE complex assembly (3-6). Yet paradoxically, the first structure of an SM-SNARE complex revealed the SM protein bound to an auto-inhibited conformation of a Qa-SNARE (see Fig. 1E) (7, 8). Without other structures containing an SM protein and SNARE motifs, the question of how – or even whether – SM proteins promote SNARE assembly remains unresolved.
The SM protein Vps33 is a stable subunit of the hetero-hexameric HOPS complex, a tethering factor required for the fusion of yeast vacuoles (9). Vps33 binds to the SNARE motifs of two vacuolar SNAREs, the R-SNARE Nyv1 and the Qa-SNARE Vam3, as well as to the complete vacuolar SNARE complex (4, 5, 10). Here, we generated x-ray structures of Vps33 bound to the SNARE motifs of Nyv1 and Vam3 (figs. S2 and S3, table S1). Both structures also contained a second HOPS subunit, Vps16 (Fig. 1B) (11-13). All proteins were from the thermophilic fungus Chaetomium thermophilum.
The SNARE motif of the Qa-SNARE Vam3 binds within the cleft formed by the arch-shaped Vps33 (Fig. 1C and fig. S2). The well-ordered portion of Vam3 closely resembles the corresponding region of the auto-inhibited Qa-SNARE syntaxin1a bound to the SM protein Munc18a (Fig. 1, D and E, and figs. S2 and S4) (7, 8, 14). The SNARE motif of the R-SNARE Nyv1 binds in a non-overlapping site, outside the cleft, on Vps33 (Fig. 2A and fig. S3). It fills the entire length of the groove formed between the antiparallel α-helices of the domain 3a helical hairpin (Fig. 1B). The Nyv1-binding groove represents the largest conserved region on the surface of Vps33; moreover, an analogous highly-conserved groove is observed on the surface of Munc18 (Fig. 2B). The two ordered regions of Nyv1 are separated by a short region for which well-defined electron density is lacking; notably, this region flanks the zero-layer arginine, R180. Nyv1 appears to be anchored in position by the layer +6 core residue, F201 (fig. S1), whose side chain inserts into a conserved hydrophobic pocket (Fig. 2A, inset). F201 itself is also widely conserved (as either phenylalanine or tyrosine) among R-SNAREs.
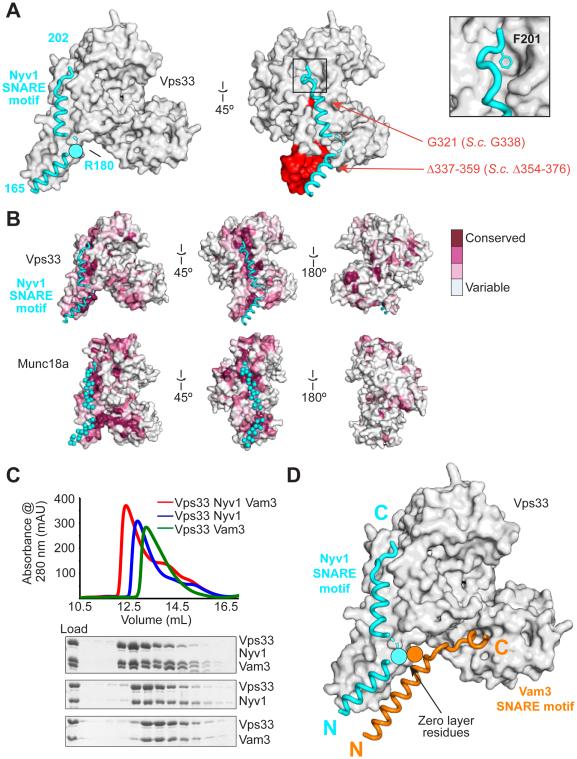
Fig. 2. Binding to a conserved surface groove on Vps33 orients and aligns the R-SNARE Nyv1 for assembly.
(A) Vps33–Nyv1 crystal structure (Vps16 omitted for clarity; see fig. S3). Red indicates mutations employed in Fig. 3. (B) The Nyv1 binding site is conserved (as calculated by ConSurf (30)) among Vps33 homologs. The same site, indicated with cyan spheres, is conserved among Munc18 homologs (shown is 3PUJ). (C) Vps33, Nyv1, and Vam3 form a ternary complex as judged by size exclusion chromatography. Nyv1 and Vam3 SNARE motifs contain an N-terminal maltose binding protein tag. (D) Model showing both SNARE domains binding simultaneously to Vps33.
Vam3 and Nyv1 SNARE motifs can bind to Vps33 simultaneously (Fig. 2C and fig. S5). Overlaying the corresponding structures revealed a striking resemblance to a half-zippered SNARE complex (Fig. 2D). Notably, Vam3 and Nyv1 bind to Vps33 in the same orientation (N- to-C) and with approximately the same register – that is, with their zero-layer residues in close proximity. This complex may represent an intermediate that links apposed membranes via the templated folding of two complementary SNAREs on the surface of the SM protein Vps33.
Single-residue substitutions along the length of the Nyv1-binding groove (Fig. 2A) reduced binding of Nyv1 (fig. S6). Conversely, deleting the C-terminal region of Nyv1, or substituting the conserved residue F201 with alanine, reduced binding to Vps33 (fig S7). We also prepared an internal deletion mutant, Vps33(Δ337-359), which removed the region that interacts with the N-terminal portions of both the Nyv1 and Vam3 SNARE motifs (Fig. 2A and fig. S6B). This mutant was stably folded, bound Vps16, and was incorporated into HOPS complexes, but it did not bind to either Nyv1 or Vam3 (fig. S6, K and L).
Next, we used Saccharomyces cerevisiae to examine the functional relevance of interactions between Vps33 and the Nyv1/Vam3 SNARE motifs in vivo. Wild-type yeast has 1-3 large vacuoles per cell (15). We examined two Vps33 mutants (Fig. 2A), one that selectively destabilized binding to Nyv1 (G338E) and one that displayed little or no binding to either Nyv1 or Vam3 (Δ354-376) (fig. S6). Vps33(G338E) cells often displayed 5-20 vacuoles per cell (Fig. 3A and fig. S8), while Vps33(Δ354-376) cells resembled Δvps33 cells in having no discernable vacuolar structures. Thus, both the Vps33–R-SNARE interaction and the helical hairpin of domain 3a are important for vacuolar fusion.
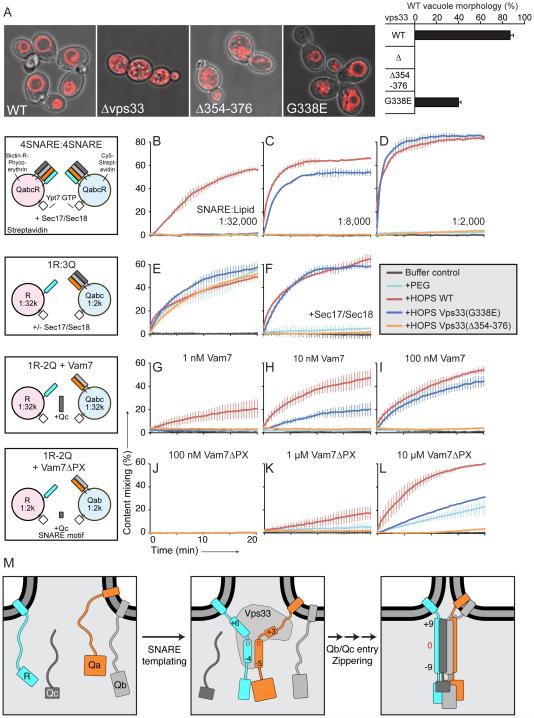
Fig. 3. Functional consequences of disrupting R-SNARE binding.
(A) Yeast vacuolar morphology as visualized by FM4-64 staining (see fig. S8). Cells containing 1-3 vacuoles were classified as having wild-type morphology. All error bars in this figure represent mean ± SD (N = 3). (B-L) In vitro fusion assay using purified HOPS containing wild-type or mutant Vps33. Proteoliposomes show an increase in FRET signal upon fusion and mixing of their luminal contents. Control experiments employ PEG as an artificial tethering factor. (B-D) Vps33(G338E) HOPS is fusion deficient at low SNARE concentrations. (E and F) Wild-type and mutant HOPS support membrane tethering. (G-L) Vps33(G338E) HOPS is compromised in recruiting (G-I) full-length Vam7 and (J-L) Vam7ΔPX into fusogenic trans-SNARE complexes. (M) Model showing the Vps33-templated initiation of trans-SNARE complex assembly. Numbers refer to SNARE complex core layers (see fig. S1). The remaining subunits of the HOPS complex, as well as Ypt7, are omitted for clarity. Further experiments are needed to clarify whether the fully zippered SNAREs remain associated with HOPS.
To substantiate that Vps33 guides SNARE complex assembly, we purified S. cerevisiae Vps33(G338E)- and Vps33(Δ354-376)-containing HOPS for use in in vitro fusion reactions (16). Briefly, this assay employs proteoliposomes of defined lipid and protein composition, with a lumenally deposited FRET pair separated in different liposome populations; upon fusion, lumenal contents are mixed and an increase in FRET signal is observed. To recapitulate the conditions present on vacuoles, we first analyzed proteoliposomes bearing the Rab protein Ypt7 and all four vacuolar SNAREs (R, Qa, Qb, and Qc). Fusion of these proteoliposomes requires (in addition to HOPS) that the SNAREs be liberated from “cis” complexes by the disassembly activity of Sec17/Sec18. At low SNARE:lipid ratios (1:32,000), wild-type HOPS supported membrane fusion while neither of the mutant HOPS complexes did (Fig. 3B). The defect manifested by Vps33(G338E) HOPS was fully rescued at unphysiologically high (1:2000) SNARE:lipid ratios (Fig. 3D). The increased importance of Vps33–R-SNARE binding when SNARE concentrations are limiting supports a key role for this interaction in promoting SNARE assembly.
Prior to trans-SNARE complex assembly, membranes are tethered by large complexes such as HOPS (17). To test whether the deficiency of mutant HOPS in supporting membrane fusion stems from a tethering defect, we used proteoliposomes bearing either the R-SNARE or the preassembled Qabc-SNARE complex. Under this specific condition, with high Qabc-SNARE concentrations to drive spontaneous SNARE complex formation, fusion can be achieved even with a non-specific tethering agent such as PEG (18). Mutant HOPS complexes were as effective as wild-type HOPS in stimulating this reaction, establishing that they support membrane tethering (Fig. 3E). Addition of Sec17/Sec18 under these conditions did not disrupt reactions containing wild-type or Vps33(G338E) HOPS, but rendered Vps33(Δ354-376) HOPS unable to support fusion (Fig. 3F). This suggests that the distal portion of Vps33 domain 3a may help protect trans-SNARE complexes from premature disassembly by Sec17/Sec18 (19).
HOPS recruits the Qc-SNARE Vam7, which naturally lacks a transmembrane anchor, into fusogenic trans-SNARE complexes (18). The direct affinity of HOPS for Vam7’s PX domain (4, 5, 20) likely contributes to this recruitment, but Vps33 might also assist by presenting a partially assembled SNARE complex. To test this idea, we performed fusion reactions in which Vam7 recruitment is rate-limiting – that is, in which the Qabc-SNARE complex is not pre-assembled (18). At low nanomolar concentrations of full-length Vam7, the fusion activity of mutant HOPS was greatly reduced relative to wild-type (Fig. 3G), although higher Vam7 concentrations rescued the defect caused by Vps33(G338E) (Fig. 3, H and I). When Vam7 lacking an alternative recruitment mechanism (Vam7ΔPX) was used in place of the full-length protein, the mutant HOPS complexes had little activity beyond membrane tethering (21) (Fig. 3, J to L). Vam7ΔPX recruitment thus appears to depend on the partially-zipped R-SNARE–Qa-SNARE complex presented by Vps33. Taken together, our findings support a direct role for Vps33 binding to Nyv1 (and Vam3) in promoting the assembly of fusogenic SNARE complexes.
Binding to R-SNAREs, although less studied than binding to Qa-SNAREs, is widely conserved among SM proteins (4, 22, 23). Our work highlights the role of an R-SNARE binding groove that lies outside the SM cleft and is lined by conserved residues in the domain 3a helical hairpin. Analogous conserved grooves are found in other SM proteins including Munc18 (Fig. 2B) and Sly1 (fig. S9A) and may likewise be used for R-SNARE binding. Consistent with this idea, a mutation (L348R) in the proposed R-SNARE binding groove of Munc18a disrupted binding to the R-SNARE VAMP2 (24). Conversely, mutations directly flanking the VAMP2 phenylalanine residue equivalent to Nyv1 F201 (Fig. 2A) compromised Munc18-stimulated proteoliposome fusion (25).
Comparisons with other SM proteins also suggest that access to the R-SNARE binding site may be regulated. The first SM protein structure revealed an auto-inhibited syntaxin and a bent hairpin conformation for Munc18a domain 3a (Fig. 1E and fig. S4) (8). The bent hairpin is incompatible with the R-SNARE binding mode observed for Vps33. Thus “opening” of syntaxin, perhaps promoted by Munc13 (26), would relieve the steric blockade on R-SNARE binding. A second example is yeast Sly1, which contains an insertion that forms a “lid” over the presumptive R-SNARE binding site (fig. S9A) (27). A dominant gain-of-function allele (SLY1-20) contains a single residue substitution (E532K) (28) predicted to open the lid (27), exposing the R-SNARE binding site beneath. Other lid mutations, including the deletion of an entire α-helix, display the same dominant phenotype, bypassing otherwise-essential components of the ER–Golgi transport machinery (29). These components may therefore regulate fusion by controlling access to the R-SNARE binding site on the SM protein.
Our work reveals a likely early intermediate in Vps33-mediated SNARE complex assembly, with the SNARE motifs of Vam3 and Nyv1 oriented and in register for further assembly (Fig. 3M). Although the N-terminal helices of Nyv1 and Vam3 appear poised for four-helix bundle formation, they would need to move somewhat closer together, and rotate around their long axes, to complete the process. These adjustments may be facilitated by the flexibility of the distal portion of the domain 3a helical hairpin. Finally, full zippering of the four-helix bundle, and the attendant close membrane apposition, will require dislodging the C-terminal portions of the R- and Qa-SNAREs from the SM template.
Supplementary Material
One-sentence summary.
Structures of an SM protein engaging two SNARE motifs reveal a mechanism of coordinated SNARE complex formation.
ACKNOWLEDGMENTS
We thank H. Robinson and the staff of NSLS beamline X29 for assistance with data collection and A. Merz, M. Rose, and members of our labs for reagents, discussion, and critical comments on the manuscript. This work was funded by NIH (GM071574 to FMH and GM23377 to WTW) and a pre-doctoral fellowship from NSF (RWB). The atomic coordinates and structure factors are deposited in the Protein Data Bank with accession codes 5BV1 (Vps33/Vps16), 5BUZ (Vps33/Vps16/Vam3), and 5BV0 (Vps33/Vps16/Nyv1).
Footnotes
SUPPLEMENTARY MATERIALS
Materials and Methods Figs. S1 to S9
Table S1 References (31-44)
REFERENCES
- 1.Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Ann. Rev. Cell Dev. Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- 4.Lobingier BT, Merz AJ. Sec1/Munc18 protein Vps33 binds to SNARE domains and the quaternary SNARE complex. Mol. Biol. Cell. 2012;23:4611–4622. doi: 10.1091/mbc.E12-05-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krämer L, Ungermann C. HOPS drives vacuole fusion by binding the vacuolar SNARE complex and the Vam7 PX domain via two distinct sites. Mol. Biol. Cell. 2011;22:2601–2611. doi: 10.1091/mbc.E11-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulubova I, et al. Munc18-1 binds directly to the neuronal SNARE complex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 9.Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu. Rev. Cell Dev. Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- 10.Dulubova I, Yamaguchi T, Wang Y, Sudhof TC, Rizo J. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat. Struct. Biol. 2001;8:258–264. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- 11.Baker RW, Jeffrey PD, Hughson FM. Crystal structures of the Sec1/Munc18 (SM) protein Vps33, alone and bound to the homotypic fusion and vacuolar protein sorting (HOPS) subunit Vps16. PLoS One. doi: 10.1371/journal.pone.0067409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham SC, et al. Structural basis of Vps33A recruitment to the human HOPS complex by Vps16. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13345–13350. doi: 10.1073/pnas.1307074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bröcker C, et al. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1991–1996. doi: 10.1073/pnas.1117797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu SH, et al. Possible roles for Munc18-1 domain 3a and Syntaxin1 N-peptide and C-terminal anchor in SNARE complex formation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1040–1045. doi: 10.1073/pnas.0914906108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banta LM, Robinson JS, Klionsky DJ, Emr SD. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol. 1988;107:1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zucchi PC, Zick M. Membrane fusion catalyzed by a Rab, SNAREs, and SNARE chaperones is accompanied by enhanced permeability to small molecules and by lysis. Mol. Biol. Cell. 2011;22:4635–4646. doi: 10.1091/mbc.E11-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu. Rev. Cell Dev. Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- 18.Zick M, Wickner W. The tethering complex HOPS catalyzes assembly of the soluble SNARE Vam7 into fusogenic trans-SNARE complexes. Mol. Biol. Cell. 2013;24:3746–3753. doi: 10.1091/mbc.E13-07-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Jun Y, Thompson J, Yates J, Wickner W. HOPS prevents the disassembly of trans-SNARE complexes by Sec17p/Sec18p during membrane fusion. EMBO J. 2010;29:1948–1960. doi: 10.1038/emboj.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennison SM, Bowen ME, Brunger AT, Lentz BR. Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys. J. 2006;90:1661–1675. doi: 10.1529/biophysj.105.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Su L, Rizo J. Binding of Munc18-1 to synaptobrevin and to the SNARE four-helix bundle. Biochemistry. 2010;49:1568–1576. doi: 10.1021/bi9021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpp LN, Ciufo LF, Shanks SG, Boyd A, Bryant NJ. The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes. J. Cell Biol. 2006;173:927–936. doi: 10.1083/jcb.200512024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parisotto D, et al. An extended helical conformation in domain 3a of Munc18-1 provides a template for SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex assembly. J. Biol. Chem. 2014;289:9639–9650. doi: 10.1074/jbc.M113.514273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Ma C, Su L, Seven AB, Xu Y, Rizo J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 2013;339:421–425. doi: 10.1126/science.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bracher A, Weissenhorn W. Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. EMBO J. 2002;21:6114–6124. doi: 10.1093/emboj/cdf608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dascher C, Ossig R, Gallwitz D, Schmitt HD. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol. Cell. Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Schmitt HD, Gallwitz D, Peng RW. Mutations of the SM protein Sly1 resulting in bypass of GTPase requirement in vesicular transport are confined to a short helical region. FEBS Lett. 2007;581:5698–5702. doi: 10.1016/j.febslet.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38:W529–533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheich C, Kümmel D, Soumailakakis D, Heinemann U, Büssow K. Vectors for co-expression of an unrestricted number of proteins. Nucleic Acids Res. 2007;35:e43. doi: 10.1093/nar/gkm067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz ML, Merz AJ. Capture and release of partially zipped trans-SNARE complexes on intact organelles. J. Cell Biol. 2009;185:535–549. doi: 10.1083/jcb.200811082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF) EMBO J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- 34.Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 2008;27:2031–2042. doi: 10.1038/emboj.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fratti RA, Wickner W. Distinct targeting and fusion functions of the PX and SNARE domains of yeast vacuolar Vam7p. J. Biol. Chem. 2007;282:13133–13138. doi: 10.1074/jbc.M700584200. [DOI] [PubMed] [Google Scholar]
- 36.Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. Meth. Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 37.Vonrhein C, et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D. 2011;67:293–302. doi: 10.1107/S0907444911007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 39.Emsley P, Cowtan K. Coot. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 40.Adams PD, et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 41.Gietz RD, Schiestl RH. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007;2:31–34. doi: 10.1038/nprot.2007.13. [DOI] [PubMed] [Google Scholar]
- 42.Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol. Biol. Cell. 2008;19:2500–2508. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 44.Burkhardt P, et al. Primordial neurosecretory apparatus identified in the choanoflagellate Monosiga brevicollis. Proc. Natl. Acad. Sci. U. S. A. 2011;108:15264–15269. doi: 10.1073/pnas.1106189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.