Abstract
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome (MetS). Up to a third of NAFLD subjects are at risk for developing nonalcoholic steatohepatitis (NASH). Many rodent models fail to replicate both MetS and NASH. The purpose of this study was to develop a reliable mouse model of NASH and MetS using a diet containing cholesterol, saturated fat and carbohydrate that is reflective of Western diets of North Americans. Experimental design: We used adult male C57BL/6 J 4- to 5-week-old mice and administered a solid diet containing 0.2% cholesterol, 45% of its calories from fat, with 30% of the fat in the form of partially hydrogenated vegetable oil. We also provided carbohydrate largely as high-fructose corn syrup equivalent in water. In a separate cohort, we gave the identical diet in the absence of cholesterol. Glucose and insulin tolerance testing was conducted throughout the feeding period. The feeding was conducted for 16 weeks, and the mice were sacrificed for histological analysis, markers of MetS, liver inflammation, circulating lipids, as well as liver staining for fibrosis and alpha smooth muscle actin (α-SMA). Results: We found that cholesterol significantly increased serum leptin, interleukin-6, liver weight and liver weight/body weight ratio, fibrosis and liver α-SMA. Conclusions: Mice administered a diet accurately reflecting patterns associated with humans afflicted with MetS can reliably replicate features of MetS, NASH and significant liver fibrosis. The model we describe significantly reduces the time by several months for development of stage 3 hepatic fibrosis.
Keywords: Fibrosis, NASH, Adipocytokine, Cholesterol, Fructose
Nonalcoholic fatty liver disease (NAFLD) has increased in concordance with rates of obesity and is a key component of the metabolic syndrome (MetS). According to the Centers for Disease Control, the United States has seen a sharp increase in the prevalence of obesity from 14%–17% in 1994 to ≥26.0% in 2007 of the adult population. The data collected from the National Health and Nutrition Examination Survey reveal that the number of Americans classified as obese (body mass index ≥30) continues to increase, with the greatest increases occurring in adult men (30.4%) [1]. At present, targeted treatment for NAFLD-related liver disease or the fibrosis that leads to cirrhotic changes is not available. In part, our inability to target treatment for these significant clinical issues is related to our inability to replicate MetS and nonalcoholic steatohepatitis (NASH) with hepatic fibrosis in mice.
Nonalcoholic fatty liver disease is a spectrum of liver disorders classified on histological criteria. These include NASH as well as the more benign lesion, nonalcoholic fatty liver (NAFL), a bland steatosis, or fatty infiltration of hepatocytes. Roughly 20% of NAFLD patients may progress to have hepatic lesions including mixed lobular inflammation, hepatocyte degeneration or “ballooning,” and pericentral deposition of fibrillar collagen [2–4]. These features are hallmarks of NASH, along with Mallory bodies. The histological classification of NASH has been standardized by the National Institutes of Health NAFLD Clinical Research Network and is referenced [5]. Data from population-based studies have consistently demonstrated that most cases of NAFL in humans do not progress, although the ability to predict in which patients progression does occur is limited at present [6–8]. Together, these findings represent increased risk for cirrhosis, clinical end-stage liver disease and hepatocellular carcinoma [3,6,9]. In patients with the diagnosis of NASH, a significantly greater proportion (perhaps up to one third) will develop cirrhosis. NASH-related cirrhosis is currently the third leading indication for liver transplantation in the United States; however, NASH-related cirrhosis will likely surpass chronic alcoholism and chronic hepatitis C infection as the major indication for orthotopic liver transplantation.
There is a growing body of evidence linking a Western diet [high in saturated fat, trans-saturated fatty acids (trans-fat) and table sugar] with the increasing incidence of NASH [10]. In addition, diets containing trans-fat and high-fructose corn syrup (HFCS) have been shown independently to increase insulin resistance, total body fat mass, and liver lipid accumulation in rodent and human livers [10–14]. A major stumbling block for investigators, however, is to recapitulate a murine model which fulfills three major criteria. The first is that the rodent develops MetS. That is, the animal develops hypertension, dyslipidemia and, most importantly, insulin resistance. NAFLD is the hepatic manifestation of MetS. The second is a rodent model that precipitates hepatic fibrosis in the setting of MetS. Finally, the diet needs to mimic what Westerners, in this case Americans, eat. Many investigators have studied hepatic fibrosis using the methionine–choline deficiency diet, but in so doing fail to provide the most common phenotypic scenario in human NAFLD, which is insulin resistance. Nonetheless, creating a NASH phenotype in a mouse model which concomitantly develops MetS has been exceedingly challenging and required using supraphysiologic quantities of fat in rodent chow for 6 or more months, making studies to elucidate NASH-related mechanisms of hepatic fibrosis time consuming, inefficient and costly for investigators.
We recently reported data in a mouse model on a diet high in saturated fat and trans-fat: the American Lifestyle-Induced Obesity Syndrome feeding model, introduced by Tetri et al., which mimicked the Western-diet-induced pathophysiology of MetS observed in humans [15–17]. In our previous studies, we demonstrated that administering this diet to 4- to 5-week-old male mice induced hepatic insulin resistance within 6 weeks and systemic insulin resistance within 8 weeks, and recapitulated the features of MetS including dyslipidemia, significant weight gain, hepatic steatosis and high blood pressure [16,17]. However, we failed to induce hepatic fibrosis in studies as long as 16 weeks. Recent studies by Min et al. showed that increased hepatic synthesis and dysregulation of cholesterol are associated with the severity of NASH [18]. Additional studies by Savard et al. utilized a 30-week feeding model with 1% cholesterol to examine the effect cholesterol in developing NASH; their results showed a significant increase in inflammation and perisinusoidal fibrosis [19]. Consequently, the aim of this study was to develop a mouse model that included all of the components of a diet typical of Americans, and induced MetS and NASH with induced fibrosis by 16 weeks while maintaining a diet that was in proportion (in terms of fat and cholesterol) to what average Americans consume on a daily basis.
1. Research design and methods
1.1. Animal studies
Seventy-two male C57BL/6 J mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Four- to 5-week-old male mice were cared for in accordance with protocols approved by the Animal Care and Use Committee of Emory University. Animals were housed in laboratory cages at 23°C under a 12-h light/dark cycle. Mice were fed either standard chow, the high-fat, trans-fat (HFTF) diet as described by Tetri et al. or the HFTF diet plus 0.2% cholesterol (HFTFX) (Harlan Teklad custom diet TD.120330) [15–17]. The HFTF and HFTFX diets derive 45% of their calories from fat, with 30% of the fat in the form of partially hydrogenated vegetable oil (28% saturated, 57% monounsaturated fatty acids, 13% polyunsaturated fatty acids; HFTF custom diet TD06303, Harlan Teklad) [15]. The HFTF and HFTFX mice were also given HFCS equivalents in rodent drinking water at 42 g/L (55% fructose, 45% glucose w/w). The HFTFX diet also contained 2.0 g/kg of cholesterol. Food and water consumption was measured by weighing new and remaining food and water three times weekly. At the onset and throughout the study, fasting blood samples were obtained. At necropsy, liver and fat samples were snap-frozen in liquid nitrogen and stored at −80°C.
1.2. Pathology
Tissues were prepared as described previously [9,16,17]. Briefly, liver was removed, weighed and divided into three samples for cryosection, formalin fixation and frozen samples. Hematoxylin and eosin stain (formalin fixed embedded in paraffin), Sirius red (Sigma-Aldrich, St. Louis, MO, USA), Masson Trichrome (Thermo Scientific) and immunohistochemistry were performed on the cryosections [9,16,17,20]. Frozen samples were used for oil red O staining and performed as described Mehlem et al. [21]. Hydroxyproline colorimetric assay (BioVision, Milpitas, CA, USA) was performed as described by the manufacturer on frozen samples. Visceral fat was removed, weighed and stored at −80°C.
1.3. Glucose and insulin tolerance testing
For the glucose tolerance test, mice were fasted for 8 h. Glucose (2 g/kg) was then administered intraperitoneally using a 31-gauge insulin syringe. Glucose levels were measured at 0, 15, 30, 60, 90 and 120 min by tail vein sampling with portable glucometer. Insulin tolerance was measured as described previously [15]. Briefly, mice were fasted for 6 h and injected intraperitoneally with 0.6 U/kg human regular insulin at a concentration of 0.2 U/ml with a 31-gauge insulin syringe. Glucose levels were measured by tail vein sampling with a portable glucometer at 0, 15, 30, 45 and 60 min.
1.4. Blood chemistry
Blood drawn from the submandibular vein was used to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol and serum triglycerides. Lipids were measured on the CX7 chemistry autoanalyzer (Beckman Coulter Diagnostics, Miami, FL, USA). Adipokines were measured using the Milliplex Map Mouse Serum Adipokine panel (EMD Millipore, Billerica, MA, USA).
1.5. Statistical analysis
The data are presented as means±standard error of the mean (S.E.M.). Statistical analysis was performed using JMP v.8.01 (SAS Institute, Cary, NC, USA). All data were initially analyzed using one-way analysis of variance. The Student’s t test was also used to determine difference between groups.
2. Results
2.1. Both diets result in obesity and insulin resistance in mice
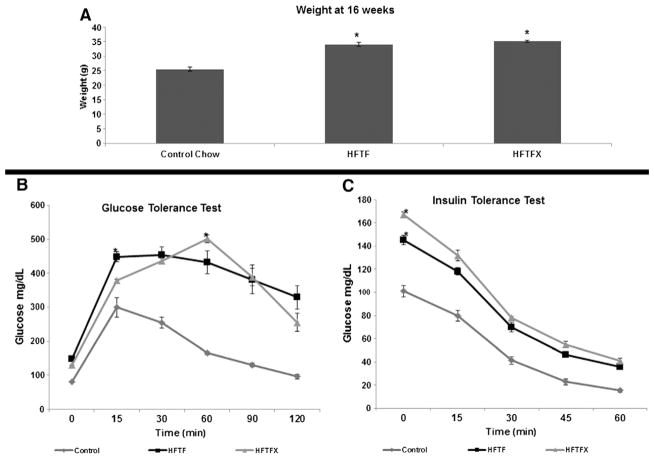
C57BL6 mice fed for 16 weeks on either the HFTF or HFTFX diet were significantly heavier (33%–38%; P<.05) than mice fed the standard laboratory chow (Fig. 1A and Table 1). Both cohorts also had significantly higher fasting blood glucose than mice fed standard chow (Fig. 1B–C). Additionally, both glucose tolerance and insulin tolerance tests demonstrated that both the HFTF and HFTFX mice were insulin resistant; both groups had significantly larger area under the curve values (AUC) than the control group (Fig. 1B–C). There was no significant difference in AUC values between the high-fat-fed groups. The addition of cholesterol was shown to have no appreciable effect on body weight, insulin sensitivity or glucose disposal.
Fig. 1.
Body weight, glucose and insulin tolerance tests after 16 weeks of feeding. (A) Weights at 16 weeks. Mice were fed control chow, HFTF or HFTFX. (B–C) Glucose and insulin tolerance tests were performed at 16 weeks. Both the HFTF and HFTFX groups have significantly larger AUCs than control. “*” denotes significant difference (P<.05, n=8 per group).
Table 1.
Body weight, visceral adiposity and liver weight in mice
| Body weight at 16 weeks | Visceral fat weight (g) | Liver weight (g) | Liver as % of body weight | Fat weight as % of body weight | |
|---|---|---|---|---|---|
| Control | a25.56±0.63g | a0.34±0.07 | a1.04±0.09 | a4.06±0.20 | a1.35±0.15 |
| HFTF | b34.03±0.67g | b1.61±0.07 | b1.60±0.08 | b4.6±0.21 | b4.74±0.16 |
| HFTFX | b35.17±0.27g | b1.58±0.09 | c2.54±0.11 | c7.2±0.23 | b4.47±0.22 |
Values are means±S.E.M. (n=8 per group). Superscript letters indicate significant differences between groups (P<.05). Groups with the same letter indicate no significant difference. Student’s t test was used in analysis.
2.2. Cholesterol — independent of visceral adiposity — significantly impacts liver weight and morphology
Mice fed either the HFTF or HFTFX diet had significantly more visceral fat than mice fed standard chow (P<.0001), and this trend persisted even when adjusted for body weight (Table 1).
Mice fed the HFTF diet had significantly heavier livers than the control group (Table 1; approximately 50%, P<.05); however, the livers from mice fed the HFTFX diet had liver weights approximately 144% heavier than mice fed the control chow and 58% heavier than mice fed the HFTF alone (P<.0001). This is an important distinction and suggests that livers exposed to cholesterol may have advanced hepatic NASH-related histopathology that cannot be accounted for by simply increased steatosis.
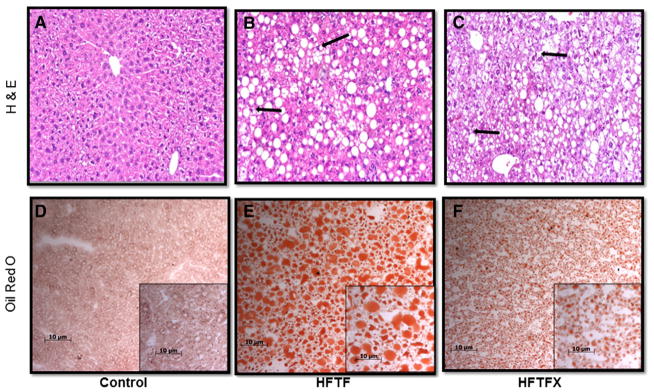
The characteristic chicken wiring pattern, hepatocyte ballooning and Mallory bodies seen in human NASH are present in the livers of high-fat-fed mice with and without cholesterol (Fig. 2B–C). Oil red O staining reveals significant lipid accumulation in the livers of both the HFTF and HFTFX groups (Fig. 2D–F). The HFTFX group’s lipid accumulation appears to be primarily microvesicular in comparison to that of the HFTF group, which contains large macrovesicular droplets.
Fig. 2.
(A–C) Hematoxylin and eosin staining of liver sections (magnification, 100×). Black arrows denote Mallory body formation and hepatocyte ballooning. (D–F) Oil red O staining of liver sections. Red staining indicates lipid accumulation (magnification, 100×; insert, 200×).
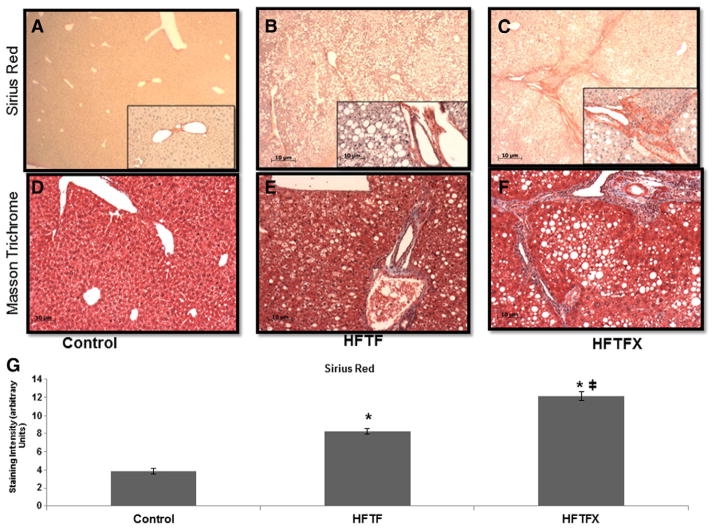
Histological staining is an important component to establish the extent of hepatic fibrosis. Sirius red staining and trichrome staining were both used to measure the amount of hepatic fibrosis. The impact of cholesterol is clearly demonstrated in liver stained for fibrosis progression (Fig. 3A–F). The HFTFX diet in comparison to the control diet and HFTF has significant increases in Sirius staining as well as trichrome stain — both markers of dense collagen deposition. The addition of cholesterol appeared to have a profibrogenic effect within the model (Fig. 3G). Both Sirius red and trichrome staining intensity revealed significant fibrosis development in comparison to the HFTF diet group alone, indicating that cholesterol increases the profibrogenic potential of this diet. We quantified these data using histomorphometric analysis to ascertain relative density units of Sirius red staining from various sections of liver tissue sampled of all mice from the three feeding cohorts (Fig. 3G).
Fig. 3.
(A–C) Siruis red staining of liver sections (magnification, 40×; inset, 100×). (D–F) Masson Trichome staining (magnification, 100×). (G) Quantification of Sirius red staining. “*” indicates significant difference from control (P<.05); “╪” indicates a significant difference between HFTF and HFTFX (P<.05).
2.3. The addition of cholesterol to the diet increases profibrotic cytokines leptin and interleukin (IL)-6 in an additive manner
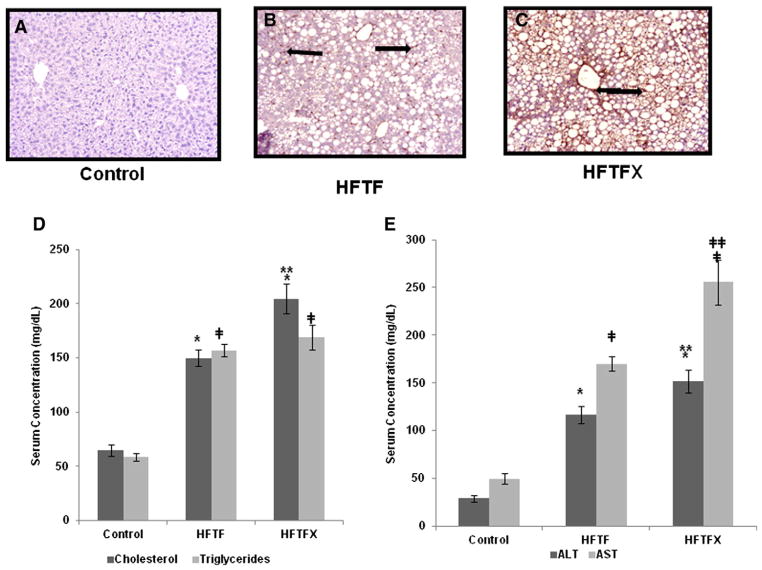
To provide a potential explanation as to why choleseterol can be profibrogenic in the liver, we analyzed key adipocytokines known to be associated with injury and fibrosis. Mice fed either the HFTF or HFTFX diet had extremely high circulating levels of insulin and significantly higher tumor necrosis factor (TNF)-α levels (Table 2). These data were anticipated since the mice became hyperinsulinemic which results in low-grade inflammation consistent with chronic obesity. Interestingly, the addition of cholesterol significantly increased circulating leptin and IL-6 levels. Hence, the HFTFX-fed mice had significantly higher levels of leptin and IL-6 than did the mice fed the HFTF alone (Table 2). These data strongly suggest that the addition of cholesterol creates a synergistic effect on adipocytokines associated with development of NAFLD as well as hepatic fibrosis. Adiponectin has been shown to be a potent antifibrogenic cytokine, and while the levels were markedly reduced in the HFTFX mouse cohort when compared to the HFTF group, the difference was not statistically significant. Surprisingly, there was no difference in monochemoattractant protein-1 (MCP-1) between the groups, indicating that there may be other factors increasing the infiltration of immune cells. Two other adipocytokines — resistin and plasminogen activator inhibitor (PAI)-1 — revealed mixed results in either feeding cohort. The exact role of these adipocytokine in the progression or severity of NASH is still under debate. Both adipocytokines have been shown to be elevated in NASH patients [22–24]. However, neither adipocytokine at present is known to play a crucial role in the progression or resolution of NASH. It is clear that cholesterol resulted in increased hepatic inflammation since the ALT and AST levels were significantly higher in the HFTFX group when compared to either the control or HFTF-fed mice (P<.005) (Fig. 4D–E).
Table 2.
Adipocytokines and insulin
| Control | HFTF | HFTFX | |
|---|---|---|---|
| MCP-1 (pg/ml) | a 11.60±2.87 | a 17.39±2.94 | a 21.81±3.0 |
| Insulin (pg/ml) | a 270.26±477.0 | b 1978.24±550.80 | b 2140.82±509.94 |
| Leptin (pg/ml) | a 288.78±87.11 | b 3385.65±905.74 | c 5093.26±452.42 |
| Adiponectin (μg/ml) | a 110,307.4±7179.7 | b 71,420.0±1658.9 | b 55,820.0±1682.1 |
| IL-6 (pg/ml) | a 7.15±1.19 | b 15.11±1.74 | c 20.91±1.45 |
| TNF-α (pg/ml) | a 1.22±0.32 | b 2.90±0.30 | b 3.51±0.26 |
| Resistin (pg/ml) | a 1957.87±179.29 | ab 3436.19±406.91 | b 4949.27±712.31 |
| PAI-1 (pg/ml) | a 1747.10±124.80 | ab 2347.91±94.86 | b 2962.09±385.37 |
Values are means±S.E.M. (n=8 per group). Superscript letters with the same letter indicate no significant difference between the groups. Groups with the same superscript letter indicate that there is a significant differences between groups (P<.05). Student’s t test was used in analysis.
Fig. 4.
(A–C) Liver sections stained for α-SMA activation, a marker for stellatecell activation. Black arrows indicateactivated stellatecells. (D) Serum levels of cholesterol and triglycerides. (F) Serum levels of ALT and AST. “*” or “╪” indicates significant difference from control (P<.0001); “**” or “╪╪” indicates a significant difference between HFTF and HFTFX (P<.001).
Activated stellate cells (HSCs) are principal players in the development of liver fibrosis, regardless of liver injury. Previous work from our laboratory has shown that high concentrations of leptin and inflammation can activate HSCs. To examine whether stellate cell activation was significant in the HFTFX-fed mice, we stained liver sections with tagged anti-alpha smooth muscle actin (α-SMA) antibodies, a marker of HSC “activation” (Fig. 4A–C). Our results indicate that the HFTFX-fed mouse had significantly more activated HSCs in respective liver tissue examined.
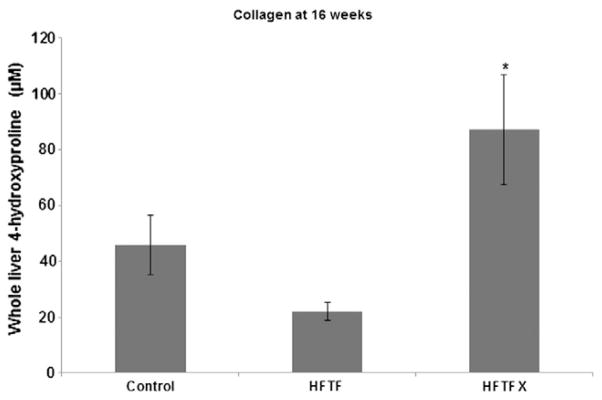
The immunohistochemical data and liver weight/body weight ratios indicate that increased collagen was present in the livers of mice fed the HFTFX diet. As an indirect measure of collagen content, hydroxyproline assays demonstrated that, indeed, the mice on the HFTFX diet had significantly higher hydroxyproline levels when compared to all other mouse cohorts (Fig. 5).
Fig. 5.
Hydroxyproline content of livers from mouse cohorts; “*” indicates significant difference from control (P<.05).
3. Discussion
We have provided significant evidence for the use of the modified HFTFX diet administered over 16 weeks to study NASH in murine models. Importantly, the nutrients of this model also result in key features of MetS as well as NASH. In comparison to other mouse models, early exposure to this diet (feeding that begins at 4–5 weeks of age) appears to disrupt the compensatory mechanism used to dispose of excessive free fatty acids (FFA), whereby the liver serves as a “sink” for toxic FFA [25]. There is a growing evidence emerging from pediatric studies demonstrating that advanced fibrosis can occur even with a relatively short exposure to a Western diet [26,27]. There are data that mice which are weaned and immediately initiated on Western diets may be more likely to develop a phenotype akin to human MetS along with NASH and pericellular fibrosis. Our data would appear to support this assertion, and this may explain the robust hepatic NASH lesions we have observed in this model, in contrast to outcomes of others who have used a similar model in recent publications. We are currently examining a mechanistic role for innate immune cells to account for these early fibrotic findings in the liver.
Indeed, even in our diets without cholesterol, there was still modest fibrosis after 16 weeks. We hypothesize that one of the reasons models that utilize older mice require longer periods of exposure or higher fat and cholesterol amounts is because older mice are able to metabolize fat with higher efficiency. Consequently, investigators using adult mice, particularly using a diet with only a single nutrient (i.e., saturated fat) to induce hepatic steatosis, typically would require abnormally high levels of fat — 60% to 65% of total calories consumed over prolonged extended periods of time (6 months) as opposed to 45%, a value more typical of the American diet. Six-month feeding studies to facilitate hepatic fibrosis are not just an inefficient method to conduct animal experiments but are also quite costly. Importantly, the degree of fibrosis varies. While the best model to induce hepatic fibrosis by diet heretofore is using a diet with methionine–choline deficiency, this diet does not induce insulin resistance or MetS and becomes a major concern in reporting data related to the study of human NASH. By contrast, we have observed that cholesterol as a nutrient additive can induce significant fibrosis by 16 weeks. The increased collagen deposition, assessed in this report by Sirius red, trichrome stains and histomorphometric analysis, would explain why the liver weights observed in the HFTFX mice were considerably heavier compared to the liver weight and liver weight/body weight ratios observed in the mice fed the HFTF diet alone. We would also point out that we used a 0.2% cholesterol diet. While others have reported a clear association that cholesterol is fibrogenic, we did not use daily quantities of cholesterol significantly higher than a typical atherogenic diet or a diet consumed by average Americans [28–30].
The cytokine profile reported here corroborates previously published data but merits further study [31–33]. However, two adipocytokines that were significantly increased in the cholesterol feeding model were IL-6 and leptin. We, and others, have published extensive data regarding the role of leptin in the pathogenesis of fibrosis, regardless of the etiology of the liver injury sustained [32,34]. Machado et al. showed in morbidly obese patients that leptin levels increased significantly in proportion to increased fibrosis [32]. IL-6 is thought to be a major player in the development of NASH; numerous studies have demonstrated that IL-6 is significantly elevated in NASH patients [35,36].
The increased incidence of NAFLD and cardiovascular disease (CVD) in patients closely parallels the increased consumption of HFCS, cholesterol, saturated fat and trans-fat in the United States [10]. The sharp rise in the prevalence of T2DM and NASH is associated with changes in the food supply, particularly increased use of trans-fat, HFCS and cholesterol. In Denmark, the ban on trans-fats in food has resulted in a 60% decrease in CVD incidence [37]. Nonetheless, naturally occurring saturated fatty acids, as well as cholesterol, as discussed here, still pose significant risks in patients afflicted with MetS.
While single-nutrient studies have been extremely valuable in expanding our understanding of the fibrosis mechanisms related to cirrhosis, additional models which mirror human dietary consumption are clearly still needed for improved research in the spectrum of NAFLD as well as for early-phase pharmacologic testing. Our model offers a timely advance and parallels recent developments of genomic, proteomic and metabolomic analyses of human and mouse tissue. Taken together, such advances offer the possibility of biomarker development which would offer predictive value to clinicians as to which patients are at risk for significant morbidity and cardiovascular mortality.
The effect of fructose is different from other carbohydrates because fructose does not stimulate insulin secretion from pancreatic β-cells. Despite claims that “sugar is sugar,” studies using fructose or HFCS have demonstrated that high fructose consumption causes inflammation, leptin resistance, steatosis and decreased catabolism of fatty acids [38–41]. In this study, we did incorporate fructose as part of the water supply in experimental treatment arms. In prior studies, we did not find a significant difference in pair-feeding studies using this method of carbohydrate delivery. Similar to the Tetri study, our model incorporated HFCS into the rodent drinking water. The use of “sugar water” is similar to the beverages Americans drink periodically between meals, which result in frequent spikes in circulating insulin. These insulin spikes in blood glucose and subsequent increases in circulating insulin are likely to increase the inflammatory cytokine profile in mice, with concomitant activation of innate and adaptive immune responses. Separately, no single nutrient can recapitulate every metabolic derangement associated with MetS. HFCS studies by Collision et al. in mice revealed that, although mice fed this diet developed significant hepatic steatosis and did result in up-regulation of key lipogenic genes, the mice did not exhibit dyslipidemia and had normal fasting serum triglyceride levels [42].
In humans, trans-fat ingestion has been shown to decrease the high-density to low-density lipoprotein (LDL) cholesterol ratio [43]. Studies by Bassett et al. in low-density lipoprotein receptor-deficient mice (LDL−/−) demonstrated that mice fed a diet enriched with trans-fat developed atherosclerotic lesions even in the absence of dietary cholesterol [44]. In contrast, studies by Dorfman et al. demonstrated that rats fed a diet low in fat but enriched with trans-fat had significant increases in visceral fat and liver steatosis; however, they found that the diet had no impact on fasting glucose, insulin and triglyceride levels [45]. Studies by Wouters et al. implicated cholesterol as the primary determinant of NASH. Using both LDL-R−/− mice and ApoE2 knock-in mice, they were able to show that omitting cholesterol prevented hepatic inflammation [46,47]. Additionally, they showed that, in mice which developed NASH, there was an increase in serum free cholesterol without significant changes in serum cholesterol ester concentrations. Savard et al. showed that omitting dietary cholesterol reduced plasma very low density lipoprotein cholesterol and also protected the liver against developing hepatic inflammation. Furthermore, they also demonstrated that steatosis did not appear to be necessary for hepatic inflammation [48].
We recognize that a 4-month dietary model still represents a significant investment with regard to time and cost; however, we are now testing this dietary model in mice for 12 weeks to determine the degree of fibrosis we detect. Finally, the model significantly reduced time and expense in our laboratory but has resulted in significant fibrosis along with MetS. Future work from our laboratory and our collaborators at Emory will demonstrate in publications based on preliminary data that this formula is highly useful for study purposes. In addition, while little is currently known as to exactly how cholesterol may be profibrogenic, one possibility may be that cholesterol is engulfed by hepatic stellate cells and in turn results in their activation. Tontonoz and colleagues reported several years ago [49] and, more recently, Tomita and colleagues together provide a molecular explanation for cholesterol as a profibrogenic substance [28].
In summary, the murine model and feeding strategy appear to be an advance in our laboratory toolbox in mimicking human MetS and NASH in mice. Three possible reasons for our findings may include the age of the mice when feeding commenced, the use of “sugar” water which could be likened to consumption of beverages with HFCS and a diet high (but not out of average daily consumption) in cholesterol. We are now testing other diets to reduce cost and time to achieve realistic endpoints for MetS and NASH.
Acknowledgments
J.E.M. developed and designed the study, conducted the experiments, analyzed the data, wrote the manuscript and edited the manuscript for intellectual content. P.P.F maintained the animals used in the study and performed experiments. P.K. analyzed data and edited the manuscript for intellectual content. T.S. performed immunohistochemistry staining and performed additional experiments. S.K. reviewed and analyzed data, and edited the manuscript for intellectual content. F.A.A. developed and designed the study, edited the manuscript for intellectual content, obtained the majority of the funding for the study and had primary responsibility for final content.
Footnotes
This work was supported by Public Health Service Grants DK062092 and I01X001746 to F.A.A. Jamie Eugene Mells is supported by the K12 GM000680NIGMS Fellowship in Research and Science Teaching (FIRST) Institutional Research and Academic Career Development Award.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–80. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 3.Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474–86. doi: 10.3748/wjg.14.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–74. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 5.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 7.Haas JT, Biddinger SB. Dissecting the role of insulin resistance in the metabolic syndrome. Curr Opin Lipidol. 2009;20:206–10. doi: 10.1097/MOL.0b013e32832b2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellentani S, Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD) Ann Hepatol. 2009;8(Suppl 1):S4–8. [PubMed] [Google Scholar]
- 9.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–81. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remig V, Franklin B, Margolis S, Kostas G, Nece T, Street JC. Trans fats in America: a review of their use, consumption, health implications, and regulation. J Am Diet Assoc. 2010;110:585–92. doi: 10.1016/j.jada.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004;53:454–7. doi: 10.1016/j.metabol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Parekh PI, Petro AE, Tiller JM, Feinglos MN, Surwit RS. Reversal of diet-induced obesity and diabetes in C57BL/6J mice. Metabolism. 1998;47:1089–96. doi: 10.1016/s0026-0495(98)90283-9. [DOI] [PubMed] [Google Scholar]
- 13.Surwit RS, Wang S, Petro AE, Sanchis D, Raimbault S, Ricquier D, et al. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc Natl Acad Sci U S A. 1998;95:4061–5. doi: 10.1073/pnas.95.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem. 2008;19:567–76. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295:G987–95. doi: 10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mells JE, Fu PP, Sharma S, Olson D, Cheng L, Handy JA, et al. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol. 2012;302:G225–35. doi: 10.1152/ajpgi.00274.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One. 2011;6:e25269. doi: 10.1371/journal.pone.0025269. http://dx.doi.org/10.1371/journal.pone.0025269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15:665–74. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savard C, Tartaglione EV, Kuver R, Haigh WG, Farrell GC, Subramanian S, et al. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57:81–92. doi: 10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–92. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc. 2013;8:1149–54. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick E, Dew TK, Quaglia A, Sherwood RA, Mitry RR, Dhawan A. Analysis of adipokine concentrations in paediatric non-alcoholic fatty liver disease. Pediatr Obes. 2012;7:471–9. doi: 10.1111/j.2047-6310.2012.00082.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsochatzis E, Papatheodoridis GV, Hadziyannis E, Georgiou A, Kafiri G, Tiniakos DG, et al. Serum adipokine levels in chronic liver diseases: association of resistin levels with fibrosis severity. Scand J Gastroenterol. 2008;43:1128–36. doi: 10.1080/00365520802085387. [DOI] [PubMed] [Google Scholar]
- 24.Edwards CR, Hindle AK, Latham PS, Fu SW, Brody FJ. Resistin expression correlates with steatohepatitis in morbidly obese patients. Surg Endosc. 2013;27:1310–4. doi: 10.1007/s00464-012-2603-y. [DOI] [PubMed] [Google Scholar]
- 25.McClain CJ, Barve S, Deaciuc I. Good fat/bad fat. Hepatology. 2007;45:1343–6. doi: 10.1002/hep.21788. [DOI] [PubMed] [Google Scholar]
- 26.Swiderska-Syn M, Suzuki A, Guy CD, Schwimmer JB, Abdelmalek MF, Lavine JE, et al. Hedgehog pathway and pediatric nonalcoholic fatty liver disease. Hepatology. 2013;57:1814–25. doi: 10.1002/hep.26230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepa-titis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97:2460–2. doi: 10.1111/j.1572-0241.2002.06003.x. [DOI] [PubMed] [Google Scholar]
- 28.Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, Narimatsu K, et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2014;59:154–69. doi: 10.1002/hep.26604. [DOI] [PubMed] [Google Scholar]
- 29.Chow EC, Magomedova L, Quach HP, Patel R, Durk MR, Fan J, et al. Vitamin D Receptor Activation Down-regulates the Small Heterodimer Partner and Increases CYP7A1 to Lower Cholesterol. Gastroenterology. 2014;146:1048–59. e1047. doi: 10.1053/j.gastro.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 30.Hintze KJ, Benninghoff AD, Ward RE. Formulation of the Total Western Diet (TWD) as a basal diet for rodent cancer studies. J Agric Food Chem. 2012;60:6736–42. doi: 10.1021/jf204509a. [DOI] [PubMed] [Google Scholar]
- 31.Felipo V, Urios A, Garcia-Torres ML, El Mlili N, del Olmo JA, Civera M, et al. Alterations in adipocytokines and cGMP homeostasis in morbid obesity patients reverse after bariatric surgery. Obesity (Silver Spring) 2013;21:229–37. doi: 10.1002/oby.20008. [DOI] [PubMed] [Google Scholar]
- 32.Machado MV, Coutinho J, Carepa F, Costa A, Proenca H, Cortez-Pinto H. How adiponectin, leptin, and ghrelin orchestrate together and correlate with the severity of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2012;24:1166–72. doi: 10.1097/MEG.0b013e32835609b0. [DOI] [PubMed] [Google Scholar]
- 33.Asano T, Watanabe K, Kubota N, Gunji T, Omata M, Kadowaki T, et al. Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J Gastroenterol Hepatol. 2009;24:1669–76. doi: 10.1111/j.1440-1746.2009.06039.x. [DOI] [PubMed] [Google Scholar]
- 34.Tsochatzis EA, Papatheodoridis GV, Archimandritis AJ. Adipokines in nonalcoholic steatohepatitis: from pathogenesis to implications in diagnosis and therapy. Mediators Inflamm. 2009;2009:831670. doi: 10.1155/2009/831670. http://dx.doi.org/10.1155/2009/831670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemoine M, Ratziu V, Kim M, Maachi M, Wendum D, Paye F, et al. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int. 2009;29:1431–8. doi: 10.1111/j.1478-3231.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- 36.Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, Stivaktas PI, et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol. 2013;58:1125–32. doi: 10.1016/j.jhep.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 37.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–44. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roglans N, Vila L, Farre M, Alegret M, Sanchez RM, Vazquez-Carrera M, et al. Impairment of hepatic Stat-3 activation and reduction of PPARalpha activity in fructose-fed rats. Hepatology. 2007;45:778–88. doi: 10.1002/hep.21499. [DOI] [PubMed] [Google Scholar]
- 39.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 40.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983–92. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–9. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collison KS, Saleh SM, Bakheet RH, Al-Rabiah RK, Inglis AL, Makhoul NJ, et al. Diabetes of the liver: the link between nonalcoholic fatty liver disease and HFCS-55. Obesity (Silver Spring) 2009;17:2003–13. doi: 10.1038/oby.2009.58. [DOI] [PubMed] [Google Scholar]
- 43.Huang Z, Wang B, Pace RD, Yoon S. Trans fat intake lowers total cholesterol and high-density lipoprotein cholesterol levels without changing insulin sensitivity index in Wistar rats. Nutr Res. 2009;29:206–12. doi: 10.1016/j.nutres.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Bassett CM, McCullough RS, Edel AL, Maddaford TG, Dibrov E, Blackwood DP, et al. Trans-fatty acids in the diet stimulate atherosclerosis. Metabolism. 2009;58:1802–8. doi: 10.1016/j.metabol.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Dorfman SE, Laurent D, Gounarides JS, Li X, Mullarkey TL, Rocheford EC, et al. Metabolic implications of dietary trans-fatty acids. Obesity (Silver Spring) 2009;17:1200–7. doi: 10.1038/oby.2008.662. [DOI] [PubMed] [Google Scholar]
- 46.Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lutjohann D, et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–86. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 47.Wouters K, van Bilsen M, van Gorp PJ, Bieghs V, Lutjohann D, Kerksiek A, et al. Intrahepatic cholesterol influences progression, inhibition and reversal of non-alcoholic steatohepatitis in hyperlipidemic mice. FEBS Lett. 2010;584:1001–5. doi: 10.1016/j.febslet.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 48.Savard C, Tartaglione EV, Kuver R, Geoffrey Haigh W, Farrell GC, Subramanian S, et al. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57(1):81–92. doi: 10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaven SW, Wroblewski K, Wang J, Hong C, Bensinger S, Tsukamoto H, et al. Liver X receptor signaling is a determinant of stellate cell activation and susceptibility to fibrotic liver disease. Gastroenterology. 2011;140:1052–62. doi: 10.1053/j.gastro.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]