Abstract
Background
The dopamine D2 receptor (D2R) has received much attention in obesity studies. Data indicate that D2R is reduced in obesity and that the TaqA1 D2R variant may be more prevalent among obese persons. It is often suggested that reduced D2R generates a “reward deficiency” and altered appetitive motivation that induces compulsive eating and contributes to obesity. Although dopamine is known to regulate physical activity, it is often neglected in these studies, leaving open the question of whether reduced D2R contributes to obesity through alterations in energy expenditure and activity.
Methods
We generated a D2R knockdown (KD) mouse line and assessed both energy expenditure and appetitive motivation under conditions of diet-induced obesity.
Results
The KD mice did not gain more weight or show increased appetitive motivation compared to wild-type (WT) in a standard environment; however, in an enriched environment with voluntary exercise opportunities, KD mice exhibited dramatically lower activity and became more obese than WT, obtaining no protective benefit from exercise opportunities.
Conclusions
These data suggest the primary contribution of altered D2R signaling to obesity lies in altered energy expenditure rather than the induction of compulsive overeating.
Keywords: D2R, behavioral thrift, dietary induced obesity, running wheels, voluntary exercise, reward deficiency
INTRODUCTION
Hypodopaminergic function, particularly reduced dopamine D2 receptor (D2R) signaling, has been implicated in obesity in both human (1–5) and animal studies (6–9), giving rise to the reward deficiency hypothesis that suggests individuals increase reward seeking-- compulsive eating-- in order to release dopamine to compensate for diminished dopamine activity (10–12). However, despite the prominence of the hypothesis in the field, it remains controversial and empirical support has not been consistent.
Although initial studies supported the link between decreased D2R and obesity, contradictory data have begun to emerge. For example, earlier imaging studies reported reduced D2R availability in obese subjects (2; 5), but more recently other studies have called this into question (13–17). Several studies have reported an association between obesity and the TaqA1 genetic variant of D2R (18–22), but an extensive, prospective study with thousands of subjects did not support that linkage (23). Consequently, the precise contribution of altered D2R to obesity remains uncertain, though presumably important.
We have recently developed an alternative hypothesis of dopamine function and propose that its primary role is to regulate behavioral energy expenditure to adapt energy allocation to the environmental energy economy, the behavioral thrift hypothesis of dopamine (24). For decades it has been well established that dopamine can regulate activity (for review, 24), including locomotor, exploratory and voluntary activity, which is why dopamine activating drugs are termed psychostimulants. The hypodopaminergia associated with obesity (5; 8; 9; 25–28), in contrast, would be expected to decrease activity, shifting energy balance toward greater energy conservation and storage, facilitating obesity (24). However, none of the studies of D2R and obesity in either humans or animals assess activity levels using any measure. While the role of dopamine in regulating energy expenditure in obesity or under conditions conducive to obesity has received little investigation, the contribution of sedentary lifestyle to obesity is increasingly gaining attention (29–37).
Most evidence in support of the D2R reward deficiency hypothesis of obesity is correlational, with limited direct experimental testing. Therefore, it is unclear whether altered D2R and reward processing is cause or consequence of obesity (38–40) or, alternatively a process that co-occurs with obesity where both are mediated by another mechanism, such as altered insulin or leptin signaling (14; 41–43). Research prior to the D2R reward deficiency hypothesis of obesity generally demonstrated that D2R antagonism did not alter feeding, though it altered willingness to work for food and physical activity (e.g., 44; 45; for review, 46). More recently, two studies directly testing a causal link between D2R and obesity obtained contradictory results. In the first, Johnson and Kenny (7) used RNA interference to knockdown D2R in striatal neurons and observed acceleration in the development of dietary induced obesity. However, the authors did not assess the potential contribution of reduced activity that may have been caused by the D2R knockdown nor consider the possibility that alterations in physical activity might account for accelerated obesity, an explanation more consistent with earlier literature. In contrast, Kim et al (42) used D2R knockouts and found the mice exhibited a lean phenotype, which the authors suggest may be mediated via D2R interactions with leptin, resulting in increased leptin signaling. Like Johnson and Kenny (2010), Kim et al (2010) did not assess alterations in activity. Notably, Kim et al (2010) used D2R knockouts. Complete knockouts often induce more severe abnormalities than knockdowns, provoking the question of whether a knockdown, more consistent with reduced rather than ablated D2R signaling, would yield different results.
In the present study, we used a D2R knockdown (KD) mouse line generated via gene targeting of the D2R locus. While the complete D2R knockout mice exhibit dwarfism (47) and impaired glucose regulation (48), the KD mice are healthy and do not display dwarfism or glucose dysregulation (reported below). We set out to directly test the following causal effects (1) if reduced D2R signaling contributes to dietary induced obesity (DIO) and (2) the relative contribution of increased consumption and decreased energy expenditure.
METHODS AND MATERIALS
Subjects and dietary induced obesity paradigm
Mice were group housed except during the home cage operant and indirect calorimetry tests where they were singly housed. Mice were provided either standard chow or high-fat diet (Bio-Serv, F3282, 60% fat calories) and were weighed 1x per week. Half the mice had running wheels in their home cagess throughout the experiment. For the primary dietary induced obesity experiment, homozygous KD mice on C57BL6/J background (originally on 129/SvJ background; backcrossed to C57BL6/J for > 10 generations) were used and compared with wild-type C57BL6/J (WT) from Jackson Laboratories. Both male and female mice were used. The WT mice were 10 weeks of age and the KD mice from 8 to 29 weeks (mean, 15 weeks) at the start of the experiment. However, there were no age effects in our measures. For the open-field, both male and female KD homozygotes and littermate WT controls all between the ages of 8–12 weeks (mean 11 weeks) were tested. For the indirect calorimetry, wheel acquisition, and re-feeding experiments we used littermate WT, heterozygotes and homozygotes to examine gene-dose effects using a range of ages (12–28 weeks, means: WT 18.5 ± 1; KD homozygotes, 19.4 ± 1.7; KD heterozygotes 20.6 ± 1.5; no significant difference, F (2,21) = .432, p = .65). All Animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Home cage concurrent choice
Mice were singly housed in home cagess equipped with operant levers and pellet dispenser and provided freely available diet. Mice could earn 20mg sucrose pellets on a resetting progressive ratio schedule (PR2, i.e., after 30 min inactivity, the incrementing ratio reset to the beginning of the sequence). Running wheel activity was recorded in 1-minute bins. Consumption of free food was measured daily.
Glucose and insulin challenges
Mice were fasted for 6 hours prior to the procedure. A fasting glucose reading was taken prior to the challenge (time 0) and then either 1 g/kg dextrose or .75 U/kg of human insulin were administered (i.p.) for the glucose and insulin challenges, respectively. Subsequent glucose blood levels were determined at 15, 60, 90 and 120 minutes. Glucose was measured using Accu-check with blood from the tail vein.
Further information on methods available in supplementary material.
RESULTS
Validation of D2R knock down mouse line
In mice heterozygous and homozygous for the knockdown, D2R mRNA was reduced to 55% and 3%, respectively, of WT littermates (Fig S1 in supplement for details). The KD mice exhibited a normal range body weight (below) and exhibit no baseline impairment in glucose regulation (below). Homozygote KD mice were used throughout unless otherwise noted.
KD mice do not exhibit greater weight gain in DIO but fail to benefit from voluntary exercise opportunities
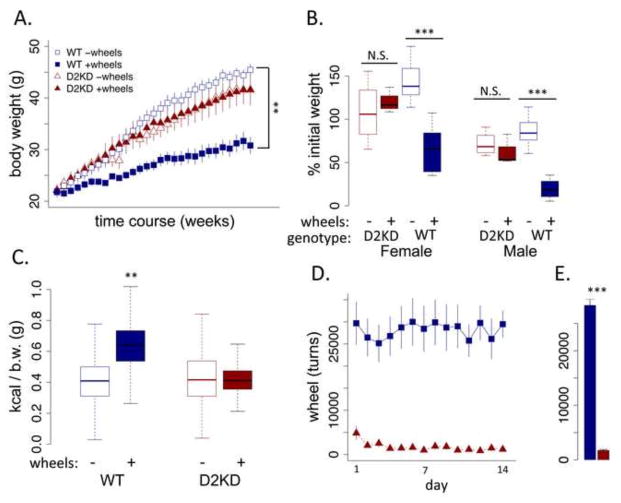
There was no significant genotype difference between WT and KD mice in initial weight. Both WT and KD mice exhibited substantial weight gain across 29 weeks on a high-fat diet (Fig 1A; genotype main effect, F (1,33) = 2.614, p = .11), showing an approximately 100% increase in body weight from initial to final weights (Fig 1B). However, provision of running opportunities dramatically decreased weight gain and was protective against obesity in WT mice (Fig 1A and B; wheel main effect, F (1,31) = 40.95, p < .001), but not KD mice where no difference was observed between those with and without running wheels (genotype x wheel interaction, F (1,31) = 27.19, p < .001). In this and other experiments, we used both male and female mice. Throughout we observed few sex differences, reviewed in supplement. Measures and analysis described below were all conducted with these mice unless otherwise noted.
Figure 1. Effect of running wheels on WT and KD mice in dietary induced obesity.
(A) Body weights across experiment (weights taken 1x/week). (B) Percent change from initial to final weight, sexes shown separately. (C) total caloric consumption during a 14-day home cage concurrent choice with freely available high fat diet and sucrose via operant responding (b.w., body weight). (D) total daily wheel running across 14-day period. (D) Average daily running across 14-day period. ** p < .01, *** p < .001, N.S., not significant. WT: N=16/group; KD: N=8/group. (E) Average daily running across 14-day period. **p < .01, ***p < .001. WT: n = 16/group; D2KD: n = 8/group. N.S., not significant.
KD mice show decreased voluntary activity not increased consumption
Following 29 weeks exposure to the high-fat diet, the group-housed mice were individually housed during a 2-week test period in home cage equipped for concurrent choice task with free access to the same high-fat diet and continued access to running wheels in the wheel groups. WT mice with access to wheels consumed significantly more food compared to WT mice without access to wheels (Fig 1C, genotype x wheels, F (1,29) = 10.02, p < .01), suggesting the availability of an exercise wheel increases both expenditure and intake but maintaining a lower body weight with less storage. In contrast, the availability of a running wheel had no effect on consumption in the KD mice. Regardless of wheel status, the KD mice consumed the same amount as WT mice without wheel access. To assess whether the KD may have difficulty feeding in an energy-depleted state, we fasted the mice and measured re-feeding. After an initial 60 minutes of re-feeding there was no difference between genotypes (see supplement).
KD mice provided running wheels showed dramatically lower activity compared to WT (Fig 1D and E, F (1,12) = 16.3, p < .01). These data indicate that the KD mice expended dramatically less energy on running, suggesting that the primary vulnerability to obesity associated with a reduction in D2R signaling is decreased physical activity rather than increased appetitive motivation.
KD mice have reduced bouts, duration, and running speed
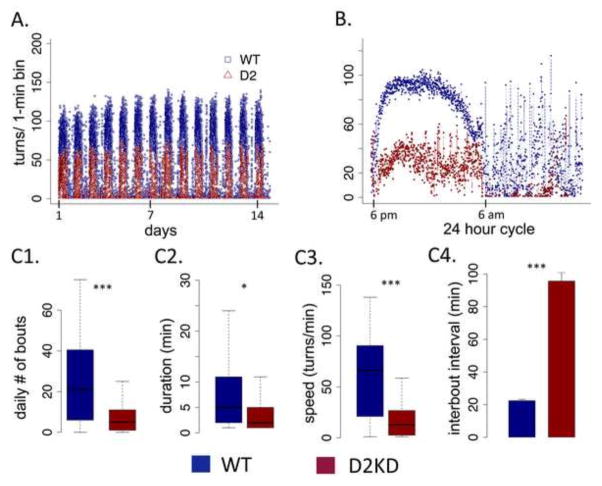
Figure 2A shows average running activity in the WT (blue) and KD (red) mice in one-minute bins across the entire experiment. Though the KD mice ran considerably less, they did run. The circadian pattern was similar between genotypes and not disturbed in the KD mice. Plotting the data across a 24-hour period averaged across the 14-day experiment (Fig 2B), both genotypes showed a similar onset of running at lights out (6 pm) with scattered episodes of running during the inactive period (6 am). We see greatly reduced wheel running by KD mice in both the active and inactive periods. To characterize activity patterns in greater detail, we performed a bout analysis with a bout defined as consecutive minute bins with activity (Table 1). A single 1-minute bin without activity terminated a bout. The greatest difference between genotypes appeared in the total number of bouts (Fig 2C1), with the KD mice showing dramatically fewer total bouts of running (F (1,13) = 190.49, p < .001), consistent with much greater inter-bout intervals (Fig 2C4; F (1,13) = 90.21, p < .001). KD mice also showed significantly reduced bout duration (F (1,13) = 5.45, p < .05), on average running about half as long as WT mice, though this was less pronounced. The number of turns in a minute of running reflects both the average distance and the speed of running. The KD mice ran significantly slower than WT (Fig 2C3 F (1,13) = 33.54, p < .001). Overall, the KD mice showed a consistent pattern of reduced running wheel activity on all measures, having fewer bouts of running that were, on average, shorter and slower. In a separate experiment, we tested initial acquisition of running behavior. All genotypes show similar acquisition of running behavior with a trend toward a gene-dose response where reduced D2R reduces running; however this did not reach significance, suggesting the marked difference observed here emerges over time and exposure to the running wheel (see supplement Fig S2).
Figure 2. Wheel activity patterns in WT and KD mice.
(A) Total number of wheel turns in one-minute bins across the entire 14-day experiment. (B) Wheel turns in one-minute bins across circadian cycles of a 24 hour period, averaged across experiment. (C) Bout analysis, showing (C1) average daily number of running bouts, (C2) average duration of bouts, (C3) average speed of running (turns/min) and (C4) the average inter-bout interval. * p < .05, ** p < .01, *** p < .001, N.S., not significant difference. N=11, WT; N=6, KD.
Table 1. Bout analysis by genotype, sex and circadian phase.
Duration in minutes, speed in revolutions/min.
| ACTIVE PHASE | WT | D2KD | ||
|---|---|---|---|---|
| male | female | male | female | |
|
| ||||
| # bouts | 42.5 ±1.18 | 39.8±.69 | 13.28±.93 | 9.53±1.11 |
|
| ||||
| duration | 7.12±.12 | 9.41±.14 | 4.52±.17 | 4.64±.47 |
|
| ||||
| turns | 563.55±11.6 | 837.49±16.1 | 134.48±7.77 | 142.53±22.07 |
|
| ||||
| speed | 64.38±.68 | 66.25±.61 | 19.01±.55 | 14.98±.9 |
|
| ||||
| INACTIVE PHASE | ||||
|
| ||||
| # bouts | 6.33±.52 | 6.81±.43 | 2.23±.32 | 1.42±.31 |
|
| ||||
| duration | 2.27±.11 | 9.41±.14 | 2.26±.27 | 1.5±.13 |
|
| ||||
| turns | 66.2±7.4 | 113.9±9.9 | 42.8±11.8 | 11.35±5.24 |
|
| ||||
| speed | 17.14±1.05 | 22.43±.95 | 8.2±.96 | 4.1±1.13 |
KD mice show reduced exploratory activity in the open field
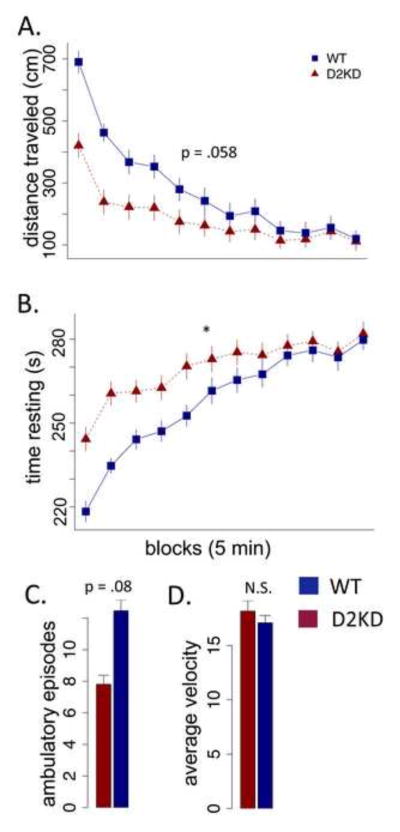
It is possible that the observed reduction in wheel running in KD mice is caused by impaired running rather than decreased energy expenditure; the KD mice perform poorly on the accelerating rotarod (data not shown). Reduction of D2R may affect multiple functions, including both motor learning and motivational regulation of both energy intake-- appetitive motivation-- and energy expenditure, voluntary exercise. The question is the degree to which reduced wheel running arises as a consequence of motor learning deficits versus altered regulation of energy expenditure. The bout analysis (Fig 2) suggests a primary motivation/expenditure deficit. During both active and inactive cycles the KD mice run much slower than WT mice; however, during the active cycle the KD mice increase their speed and run at speeds comparable to the WT during the inactive period, suggesting the KD mice were capable of running WT speeds during the inactive cycle but did not. To assess activity in a non-skilled paradigm, we tested the genotypes in the open field using naive mice not exposed to wheel-running or high fat diet. Consistent with decreased energy expenditure, the KD mice showed reduced open field activity (Fig 3A F (1,16) = 4.15, p = .0584; genotype x block, F (1,628) = 4.15, p < .001) and significantly increased resting time (Fig 3B F (1,16) = 4.552, p < .05). Moreover, they exhibited a trend toward fewer ambulatory episodes (Fig 3C F (1,16) = 3.42, p = .08); however, there was no difference in average velocity during ambulation (Fig 3D F (1,16) = .164, p = .69). Thus, the KD mice showed decreased energy expenditure in the unskilled open field test with no evidence of motor slowing (i.e., their movement velocity was the same).
Figure 3. Open field activity in WT and KD mice.
(A) Ambulatory distance (cm) in 5-minute blocks across a 60 minute testing session, averaged across 3 consecutive sessions. (B) Time resting (no ambulation) in 5-minute blocks. (C) Average number of ambulatory blocks per 5-minute block. (D) Average ambulatory velocity across entire session. * p < .05, N=8.
KD mice show increased metabolism and decreased activity in indirect calorimetry
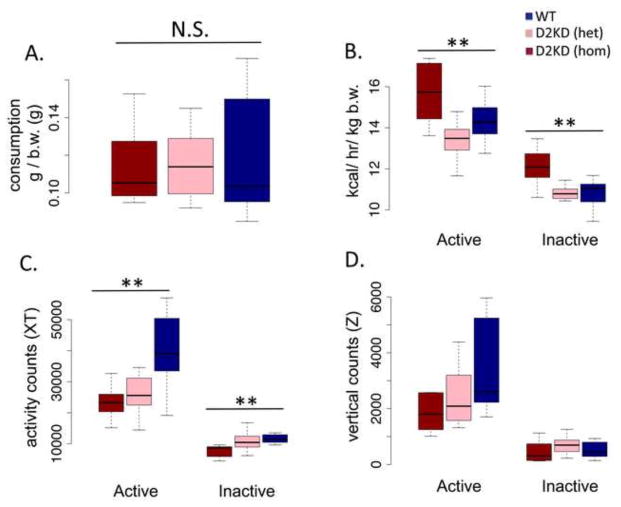
To assess basal metabolism (in the absence of exercise wheels and on standard chow), we tested a naive group of male mice, including heterozygotes for the KD, using indirect calorimetry, measuring metabolic rate, consumption and activity. All mice were approximately 24 weeks of age (no age difference between genotypes), weighing 26–29 grams, with a trend (F (2,21) = 3.069, p = .06) toward the homozygote D2KD weighing less (homozygote mean, 26.7 ± .86 vs. 28.9 ± .97 for WT). Percent of body mass composed of fat, as assessed by DEXA (see supplement), was not significantly different between genotypes (F (2,21) = 2.17, p = .13; homozygotes, 16.05 ± .85; heterozygotes, 18.48 ± .90; WT, 16.45 ± .91). Consistent with findings reported above, there was no difference in food consumption normalized to body weight between the genotypes (Fig 4A, F (2,21) = .157, p = .85). Surprisingly, however, the KD mice showed increased basal metabolism (Fig 4B, F (2,21) = 7.64, p < .01). Although the mechanism by which this might arise is not clear (see discussion), it indicates that the observed vulnerability to weight gain due to reduced voluntary activity does not arise from lower basal metabolic rate. Consistent with reduced voluntary (wheel running) and exploratory (open field) activity, the KD mice showed a significant reduction in basal activity (Fig 4C, F (2,21) = 6.872, p < .01). Interestingly, the heterozygotes were similar to WT in terms of basal metabolic rate (Fig 4B); however, in terms of basal activity, measured in the calorimetry chambers, they were closer to the KD homozygotes, suggesting that regulation of basal locomotor activity might be particularly sensitive to D2R signaling. There were no significant differences in vertical activity counts (Fig 4D, F (2,21) = 1.73, p = .20).
Figure 4. Indirect calorimetry.
(A) average daily consumption normalized to body weight, (B) metabolic rate (kcal/hr/kg bodyweigh, b.w.), (C) horizontal activity counts and (D) vertical activity counts for active and inactive periods for KD homozygotes (red), heterozygotes (pink) and WT littermates (blue). ** p < .01, N = 7, WT; 9, hom; 8, het.
KD mice do not show enhanced appetitive motivation in concurrent choice
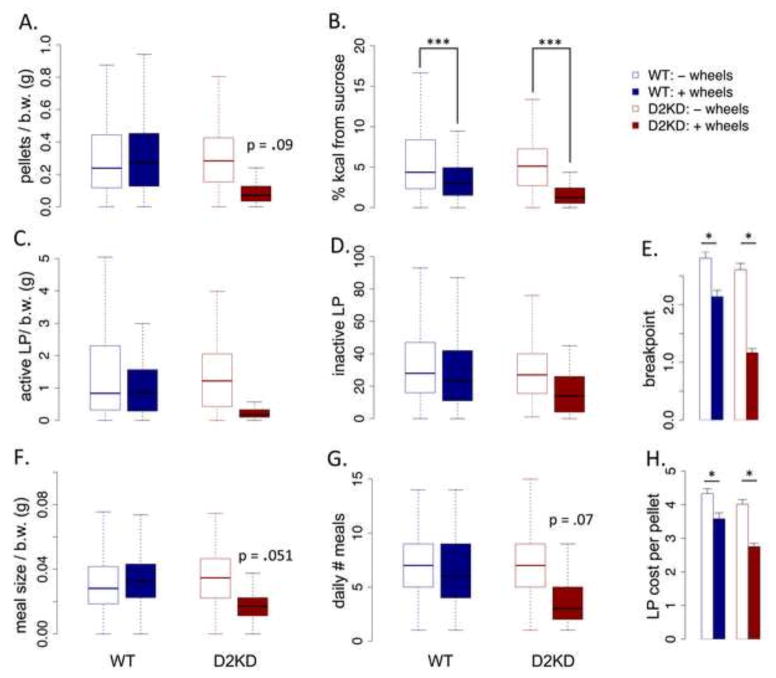
Mice were housed in home cage operant chambers with high-fat diet freely available in the cage and sucrose pellets available through operant responding on a resetting progressive ratio (PR2) where the ratio reset after 30 minutes of inactivity on the levers (Beeler et al 2012c). Because the mean body weight between groups differed, results (where appropriate) are normalized to body weight. No differences were observed in average daily sucrose pellets earned between groups (F (1,35) = 1.82, p = .18), though the KD mice with running wheel access exhibited a trend toward decreased sucrose consumption (Fig 5A; genotype x wheels, F (1,29) = 2.9, p = .09). The percentage of total caloric intake derived from sucrose was not significantly different between genotypes (F (1,29) = 2.74, p = .11), but provision of a running wheel decreased sucrose as a percentage of intake across both genotypes (Fig 5B; F (1,29) = 21.6, p < .001). There were no significant genotype differences in the overall amount of active and inactive lever pressing (Fig 5C and D; active lever, F (1,29) = 1.59, p = .21). Average breakpoint was not different between genotypes (Fig 5E; F (1,29) = 2.22, p = .14); however, wheel access significantly reduced breakpoint in both genotypes (F (1,29) = 7.81, p < .01). There was no genotype effect on meal size (effectively, normalized breakpoint; Fig 5F, F (1,29) = 1.51, p = .22), but a trend toward the KD mice with wheel access eating smaller meals (genotype x wheels, F (1,29) = 4.12, p = .051). Similarly, there was no genotype effect on the number of bouts of pressing for sucrose (Fig 5G, F (1,29) = 3.0, p = .09), although again the KD mice with wheel access show a trend toward reduced meal number (Fig 5G, F (1,29) = 3.41, p = .075). In the home cage progressive ratio, mice can modulate the average cost of sucrose pellets by shifting between longer, more costly episodes of pressing and shorter, less costly (but more frequent) episodes (Fig 5F, meal size, Fig 5G, number of daily meals). Consistent with breakpoint, provision of a running wheel decreased cost per pellet in both genotypes (Fig 5H, F (1,29) = 4.81, p < .05).
Figure 5. Home cage concurrent choice with freely available high fat diet.
(A) average daily sucrose pellets earned per gram of body weight. (B) Percentage of total daily kilocalorie (kcal) intake derived from sucrose. (C) average daily active lever presses (LP) for sucrose normalized to body weight (b.w.). (D) average daily inactive lever presses (not normalized). (E) Average breakpoint for bouts of sucrose seeking. (F) Average size of bouts of sucrose consumption normalized to body weight. (G) Average daily number of bouts of sucrose seeking. (H) Average cost per pellet in lever presses. * p < .05, ** p < .01, *** p < .001, N.S., not significant. WT: N=16/group; KD: N=8/group.
In summary, the KD mice did not exhibit increased appetitive motivation in a concurrent choice task. The provision of a running wheel decreased effort toward sucrose in both genotypes, an effect more pronounced in the KD mice. In the KD mice, this cannot be attributed to a motor impairment as the KD animals without wheel access press the same amount overall as WT mice. In the WT mice with wheel access, the reduced breakpoint might be attributed to reduced bodyweight; however, these mice consumed more free chow, suggesting that the reduction in sucrose seeking is motivational rather than reflecting overall decreased consumption. These data suggest that wheel running can decrease effort expended toward sucrose, possibly suggesting a competition in the allocation of energy between two sources of reward. This competition may be more pronounced in the KD mice that, overall, exhibit less behavioral energy expenditure. Importantly, these data also indicate that although the KD mice run dramatically less than WT, this minimal running can impact their appetitive energy expenditure.
KD mice glucose regulation unaltered by provision of running wheels
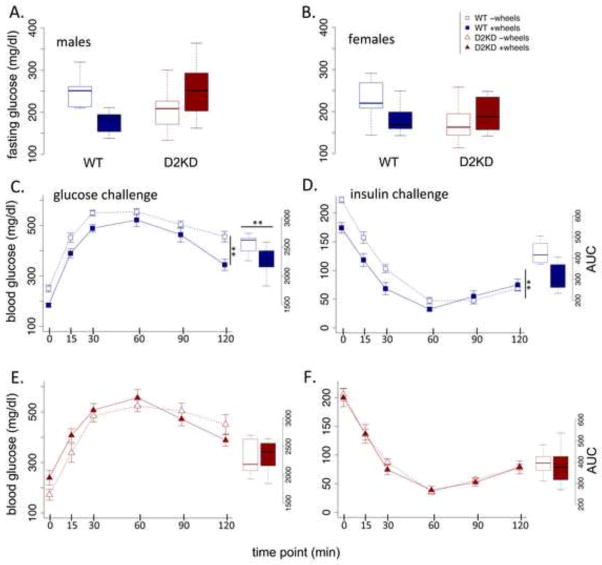
It is well known that exercise can reduce risk of type 2 diabetes, including in mice on high fat diets. We administered both glucose and insulin challenges to the mice to assess whether different patterns of voluntary activity could be observed in glucose regulation. There was no main effect of genotype on fasting glucose levels (Fig 6A and B; F (1,34) = .71, p = .40). The opportunity for voluntary exercise significantly reduced fasting glucose levels (wheel main effect, F (1,34) = 8.49, p < .01); an effect more pronounced in the WT mice (genotype x wheel, F (1,34) = 22.26, p < .001). In both the glucose and insulin challenge, a difference was observed between WT mice with and without wheel access (Fig 6C and D). WT mice with wheel access showed significantly improved glucose clearance (Fig 6C; WT AUC, F (1,25) = 8.59, p < .01) and a trend toward greater sensitivity to insulin (Fig 6D; WT AUC, F (1,25) = 2.66, p = .11). In contrast, no difference was observed in the KD mice as a result of having access to running wheels (Fig 6E and F; KD AUC, glucose, F (1,13) = .04, p = .84; insulin, F (1,13) = .12, p = .72).
Figure 6. Effect of wheels on glucose regulation in WT and KD mice.
Fasting (6 hr) blood glucose levels for (A) male and (B) female mice. (C and E) Glucose challenge (dextrose, 1 g/kg total body mass, i.p.) for WT (blue) and KD (red). Offset: area under the curve (AUC). (D and F) Insulin challenge (0.5 U/kg total body mass, i.p.), WT (blue), KD (red). Offset: AUC. ** p < .01, *** p < .001, N.S., not significant difference. WT: N=16/group; KD: N=8/group.
DISCUSSION
Reduction of D2R in the KD mice did not increase obesity in a standard DIO paradigm. The lack of an observed increase in consumption is inconsistent with the reward deficiency hypothesis, suggesting that reduced D2 receptor expression does not drive compulsive appetitive behavior. This is further supported in the home cage concurrent choice task where we did not observe greater effort exerted for sucrose pellets, a ‘preferred food’, in the KD mice; in fact, both genotypes exhibited reduced effort for sucrose with the provision of wheel access, an effect more pronounced in the KD mice, despite their low levels of running.
In WT mice, provision of running wheels-- an opportunity for voluntary exercise-- dramatically reduced weight gain under a high-fat diet that normally induces obesity, as observed previously (34; 49–55). In contrast, the KD mice obtained no protective benefit from running wheels. Though the KD mice did run in the wheels and demonstrated similar patterns of circadian activity, they ran many fewer bouts, of shorter duration and slower speed, consistent with decreased energy expenditure. Though we cannot conclusively rule out the possibility of motor impairment in KD mice that diminishes their activity on the running wheel, the problem appears to lie in motivation to run rather than ability (see also 56). The KD mice show reduced wheel running speed, but the circadian patterns of activity remain similar. For example, during the inactive phase the KD mice run slower than WT (Table 1). However, during the active cycle, while the KD mice again run much slower than WT, they achieve speeds approximating those observed in the WT during the inactive phase, suggesting their reduced speed, at least during the inactive cycle, did not arise from inability to run. Critically, we also observe a reduction in activity in both the open field and indirect calorimetry (basal activity), both of which measure unskilled activity. Overall, our data suggest that the KD mice exhibit reduced physical activity.
Our finding of increased metabolic rate in the KD mice was unexpected, but consistent with a prior observation of increased metabolic rate (also measured using indirect calorimetry) in D2R knockout mice (42). In that study, D2R knockout resulted in a lean phenotype, including reduced consumption, which the authors attributed to altered leptin signaling in the hypothalamus. Though KD mice may be expected to show a less severe phenotype than knockout mice, the increased metabolic rate we observe may be attributable to a similar mechanism. Though we cannot speculate on the relationship between increased metabolic rate and decreased physical activity in the KD mice, what is clear is that reduced D2R in both these lines does not increase appetitive motivation, but rather induces complex effects on energy regulation, apparently increasing basal metabolism while reducing physical activity, though Kim et al did not report locomotor activity.
Finally, the glucose and insulin challenge data indicate that such differences in activity can affect glucose regulation. The provision of running opportunities to WT mice increases their glucose clearance and insulin sensitivity, while the reduced voluntary activity in the KD mice precludes this potentially protective effect. Though in one sense this seems obvious as the KD mice exhibit dramatically reduced activity, the amount of activity necessary to yield an ameliorative effect on glucose metabolism is not well established. Thus, it could have been that even minimal voluntary exercise, as exhibited by the KD mice, might have altered glucose regulation, as it apparently altered appetitive motivation in the concurrent choice task (Fig 5). However, these data suggest that activity is reduced in the KD mice sufficiently to preclude potential protective effects against metabolic disorder. Additionally, the glucose and insulin challenge data indicate that the KD mice, unlike the D2R knockouts (48), do not exhibit impaired glucose homeostasis compared to WT mice.
Together, these data suggest that the role of reduced D2 receptor in obesity lies not in increasing appetitive motivation and generating compulsive eating, but rather in altering activity and energy expenditure, favoring reduced behavioral expenditure of energy. More broadly, these data are consistent with a fundamental role for dopamine in regulating behavioral energy expenditure, as long suggested by Salamone and colleagues (for review, 46). Over decades, they have demonstrated that reduced dopamine diminishes the willingness to work for reward as well as locomotor activity without altering free-feeding food preferences (46). The thrift hypothesis builds upon Salamone’s insight, suggesting dopamine not only regulates behavioral energy expenditure along an conserve-expend axis, but additionally regulates the degree to which prior reward biases behavioral choice with decreased dopamine inducing greater exploitation of prior reward information (for review, 24). In the present study we observe no change in appetitive behavior and choice in the home cage concurrent choice as a consequence of reduced D2R signaling alone; however, in the context of an enriched environment with access to a running wheel, we observe a decrease in pursuit of sucrose among both genotypes, more pronounced in the KD mice. We speculate that in an enriched environment with more rewarding options for energy expenditure, decreased D2R induces a regime of energy conservation in which energy expenditure has to be divided rather than increased, though testing this hypothesis remains for future studies.
Several cautions are in order. First, the D2R knockdown is global and constitutive. Thus, we cannot isolate its effects to specific neural substrates, nor can we rule out compensations. However, there are two primary views of reduced D2R function in obesity. In one, the reduction precedes and causes obesity, perhaps as a consequence of genetic variance (e.g., 4; 13; 20; 57; 58). This potential mechanism is analogous to our genetic knock down as one might expect that similar compensations might occur in individuals with genetically reduced D2R function. The other proposed mechanism is that obesity causes a reduction in D2R, which then further contributes to and maintains obesity. The development of obesity, however, is a gradual process and, presumably so is the reduction in D2R. Consequently, it is likely that even with this second proposed mechanism, compensations would be induced by reduced D2R expression.
Perhaps more importantly, a recent human imaging study by Guo et al (16) has suggested that obesity induced changes in D2R binding potential are not uniform, demonstrating a positive correlation between D2R binding in the dorsal striatal regions and both BMI and opportunistic eating, while the ventral striatum was negatively correlated, though this latter finding did not reach significance. These data suggest that obesity may induce region-specific alterations in D2R, which may differentially contribute to behavior. Thus, our global knockdown may obscure subtleties in obesity-induced regulation of D2R and its behavioral effects. Importantly, in the Guo study, the strongest finding was that lateral striatal D2R binding increased with BMI. The present findings highlight the importance of assessing energy expenditure, almost without exception neglected, when investigating dopamine-related contributions to obesity.
Finally, we cannot assess the exact degree to which potential motor impairments induced by D2R knock down contributed to decreased wheel running behavior. The expenditure of calories does not require proficient but rather persistent running, which the KD mice do not exhibit. Crucially, we observe decreased activity in measures of non-skilled voluntary activity as well, including the open field and basal activity as measured in the calorimetry experiments.
Over the last two decades, there has been much focus on diet as the root cause of the increase in obesity rates, the so-called obesogenic ‘Western diet.’ However, people have also become considerably more sedentary over recent decades, which is believed to contribute to obesity and risk of metabolic and cardiovascular disease (59–66). Despite evidence for central control of physical activity level by multiple substrates (e.g., 67–69), the neural systems regulating voluntary physical activity remain poorly understood (68). Here we show that reduced D2R expression, frequently believed to contribute to obesity, significantly alters patterns of energy expenditure with little effect on appetitive behavior, suggesting a key role of dopamine dysregulation in obesity might lie in altered behavioral energy regulation.
The thrift hypothesis of dopamine suggests that altered dopamine function will shift voluntary energy expenditure, with the reduced dopamine function associated with obesity paradoxically favoring behavioral energy conservation, ie., reduced physical activity. Such maladaptive regulation of behavioral energy expenditure may contribute not only to promoting obesity, but also help explain the motivational resistance often incurred by exercise programs aimed at reducing weight in obese and overweight individuals.
Supplementary Material
Acknowledgments
This work was supported by NIDA, R01DA25875 (JB), NIDDK, R56DK088515 (XZ) and NIH DK20595 (HY). We thank Jared T. Hinkle for technical assistance. We also thank Claus Kemkemer from the Long lab and Wei Zhang from the Kronfrost lab.
Footnotes
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van de Giessen E, Celik F, Schweitzer DH, van den Brink W, Booij J. Dopamine D2/3 receptor availability and amphetamine-induced dopamine release in obesity. J Psychopharmacol (Oxford) 2014;28:866–873. doi: 10.1177/0269881114531664. [DOI] [PubMed] [Google Scholar]
- 2.de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, et al. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011;1:37. doi: 10.1186/2191-219X-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Wang G-J, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 6.Huang X-F, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, et al. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res. 2006;175:415–419. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum K, Liu Y, Shriner R, Gold MS. Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr Pharm Des. 2011;17:1158–1167. doi: 10.2174/138161211795656819. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 12.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2010;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenstein SA, Antenor-Dorsey JAV, Gredysa DM, Koller JM, Bihun EC, Ranck SA, et al. A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[(11)C]methyl)benperidol. Synapse. 2013;67:748–756. doi: 10.1002/syn.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn JP, Kessler RM, Feurer ID, Volkow ND, Patterson BW, Ansari MS, et al. Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care. 2012;35:1105–1111. doi: 10.2337/dc11-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Giessen E, de Bruin K, la Fleur SE, van den Brink W, Booij J. Triple monoamine inhibitor tesofensine decreases food intake, body weight, and striatal dopamine D2/D3 receptor availability in diet-induced obese rats. European Neuropsychopharmacology. 2012;22:290–299. doi: 10.1016/j.euroneuro.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry. 2014;19:1078–1084. doi: 10.1038/mp.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35:3959–3965. doi: 10.1523/JNEUROSCI.4744-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpenter CL, Wong AM, Li Z, Noble EP, Heber D. Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. Obesity (Silver Spring) 2013;21:E467–73. doi: 10.1002/oby.20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ALC, Blum K, Chen TJH, Giordano J, Downs BW, Han D, et al. Correlation of the Taq1 dopamine D2 receptor gene and percent body fat in obese and screened control subjects: a preliminary report. Food Funct. 2012;3:40–48. doi: 10.1039/c1fo10089k. [DOI] [PubMed] [Google Scholar]
- 20.Barnard ND, Noble EP, Ritchie T, Cohen J, Jenkins DJA, Turner-McGrievy G, et al. D2 dopamine receptor Taq1A polymorphism, body weight, and dietary intake in type 2 diabetes. Nutrition. 2009;25:58–65. doi: 10.1016/j.nut.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comings DE, Gade R, MacMurray JP, Muhleman D, Peters WR. Genetic variants of the human obesity (OB) gene: association with body mass index in young women, psychiatric symptoms, and interaction with the dopamine D2 receptor (DRD2) gene. Mol Psychiatry. 1996;1:325–335. [PubMed] [Google Scholar]
- 22.Noble EP, Noble RE, Ritchie T, Syndulko K, Bohlman MC, Noble LA, et al. D2 dopamine receptor gene and obesity. Int J Eat Disord. 1994;15:205–217. doi: 10.1002/1098-108x(199404)15:3<205::aid-eat2260150303>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 23.Hardman CA, Rogers PJ, Timpson NJ, Munafò MR. Lack of association between DRD2 and OPRM1 genotypes and adiposity. Int J Obes (Lond) 2014;38:730–736. doi: 10.1038/ijo.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beeler JA, Frazier CRM, Zhuang X. Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources. Front Integr Neurosci. 2012;6:49. doi: 10.3389/fnint.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Chiara G, Tanda G, Cadoni C, Acquas E, Bassareo V, Carboni E. Homologies and differences in the action of drugs of abuse and a conventional reinforcer (food) on dopamine transmission: an interpretative framework of the mechanism of drug dependence. Adv Pharmacol. 1998;42:983–987. doi: 10.1016/s1054-3589(08)60911-4. [DOI] [PubMed] [Google Scholar]
- 26.Davis JF, Tracy AL, Schurdak JD, Tschöp MH, Lipton JW, Clegg DJ, Benoit SC. Behav Neurosci. Vol. 122. American Psychological Association; 2008. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat; pp. 1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, South T, Han M, Chen J, Wang R, Huang X-F. High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 2009;1268:181–189. doi: 10.1016/j.brainres.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 28.Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- 29.Chaput J-P, Klingenberg L, Rosenkilde M, Gilbert J-A, Tremblay A, Sjödin A. Physical activity plays an important role in body weight regulation. J Obes. 2011;2011 doi: 10.1155/2011/360257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elder SJ, Roberts SB. The effects of exercise on food intake and body fatness: a summary of published studies. Nutr Rev. 2007;65:1–19. doi: 10.1111/j.1753-4887.2007.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 32.Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics. 2007;28:146–157. doi: 10.1152/physiolgenomics.00174.2006. [DOI] [PubMed] [Google Scholar]
- 33.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 34.Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R771–8. doi: 10.1152/ajpregu.00650.2003. [DOI] [PubMed] [Google Scholar]
- 35.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawley JA, Holloszy JO. Exercise: it’s the real thing! Nutr Rev. 2009;67:172–178. doi: 10.1111/j.1753-4887.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 37.Powell KE, Blair SN. The public health burdens of sedentary living habits: theoretical but realistic estimates. Med Sci Sports Exerc. 1994;26:851–856. [PubMed] [Google Scholar]
- 38.Berridge KC, Ho C-Y, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int J Obes (Lond) 2013;37:1095–1103. doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kok P, Roelfsema F, Frölich M, van Pelt J, Meinders AE, Pijl H. Activation of dopamine D2 receptors lowers circadian leptin concentrations in obese women. J Clin Endocrinol Metab. 2006;91:3236–3240. doi: 10.1210/jc.2005-2529. [DOI] [PubMed] [Google Scholar]
- 42.Kim KS, Yoon YR, Lee HJ, Yoon S, Kim S-Y, Shin SW, et al. Enhanced hypothalamic leptin signaling in mice lacking dopamine D2 receptors. J Biol Chem. 2010;285:8905–8917. doi: 10.1074/jbc.M109.079590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaffly J, Michaelides M, Wang G-J, Pessin JE, Volkow ND, Thanos PK. Leptin increases striatal dopamine D2 receptor binding in leptin-deficient obese (ob/ob) mice. Synapse. 2010;64:503–510. doi: 10.1002/syn.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baldo B, Sadeghian K, Basso A. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 45.Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) (1991) 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- 46.Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Current opinion in pharmacology. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 47.García-Tornadú I, Rubinstein M, Gaylinn BD, Hill D, Arany E, Low MJ, et al. GH in the dwarf dopaminergic D2 receptor knockout mouse: somatotrope population, GH release, and responsiveness to GH-releasing factors and somatostatin. J Endocrinol. 2006;190:611–619. doi: 10.1677/joe.1.06902. [DOI] [PubMed] [Google Scholar]
- 48.García-Tornadú I, Ornstein AM, Chamson-Reig A, Wheeler MB, Hill DJ, Arany E, et al. Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology. 2010;151:1441–1450. doi: 10.1210/en.2009-0996. [DOI] [PubMed] [Google Scholar]
- 49.Zachwieja JJ, Hendry SL, Smith SR, Harris RB. Voluntary wheel running decreases adipose tissue mass and expression of leptin mRNA in Osborne-Mendel rats. Diabetes. 1997;46:1159–1166. doi: 10.2337/diab.46.7.1159. [DOI] [PubMed] [Google Scholar]
- 50.Rhodes JS, Gammie SC, Garland T. Neurobiology of Mice Selected for High Voluntary Wheel-running Activity. Integr Comp Biol. 2005;45:438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- 51.Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond, B, Biol Sci. 2006;361:1211–1218. doi: 10.1098/rstb.2006.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patterson CM, Levin BE. Role of exercise in the central regulation of energy homeostasis and in the prevention of obesity. Neuroendocrinology. 2008;87:65–70. doi: 10.1159/000100982. [DOI] [PubMed] [Google Scholar]
- 53.Patterson CM, Bouret SG, Dunn-Meynell AA, Levin BE. Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am J Physiol Regul Integr Comp Physiol. 2009;296:R537–48. doi: 10.1152/ajpregu.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meek TH, Eisenmann JC, Garland T. Western diet increases wheel running in mice selectively bred for high voluntary wheel running. Int J Obes (Lond) 2010;34:960–969. doi: 10.1038/ijo.2010.25. [DOI] [PubMed] [Google Scholar]
- 55.Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, et al. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58:355–367. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klinker F, Hasan K, Paulus W, Nitsche MA, Liebetanz D. Pharmacological blockade and genetic absence of the dopamine D2 receptor specifically modulate voluntary locomotor activity in mice. Behav Brain Res. 2013;242:117–124. doi: 10.1016/j.bbr.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 57.Heber D, Carpenter CL. Addictive genes and the relationship to obesity and inflammation. Mol Neurobiol. 2011;44:160–165. doi: 10.1007/s12035-011-8180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stice E, Yokum S, Zald D, Dagher A. Dopamine-based reward circuitry responsivity, genetics, and overeating. Curr Top Behav Neurosci. 2011;6:81–93. doi: 10.1007/7854_2010_89. [DOI] [PubMed] [Google Scholar]
- 59.Martínez-González MA, Martínez JA, Hu FB, Gibney MJ, Kearney J. Physical inactivity, sedentary lifestyle and obesity in the European Union. Int J Obes Relat Metab Disord. 1999;23:1192–1201. doi: 10.1038/sj.ijo.0801049. [DOI] [PubMed] [Google Scholar]
- 60.Martínez JA, Kearney JM, Kafatos A, Paquet S, Martínez-González MA. Variables independently associated with self-reported obesity in the European Union. Public Health Nutr. 1999;2:125–133. doi: 10.1017/s1368980099000178. [DOI] [PubMed] [Google Scholar]
- 61.Ladabaum U, Mannalithara A, Myer PA, Singh G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am J Med. 2014;127:717–727 e12. doi: 10.1016/j.amjmed.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: a focus on physical activity and lifestyle changes. Curr Mol Med. 2008;8:519–532. doi: 10.2174/156652408785747915. [DOI] [PubMed] [Google Scholar]
- 63.Hu FB. Diet and exercise for new-onset type 2 diabetes? Lancet. 2011;378:101–102. doi: 10.1016/S0140-6736(11)60692-2. [DOI] [PubMed] [Google Scholar]
- 64.Héroux M, Janssen I, Lam M, Lee D-C, Hebert JR, Sui X, Blair SN. Dietary patterns and the risk of mortality: impact of cardiorespiratory fitness. Int J Epidemiol. 2010;39:197–209. doi: 10.1093/ije/dyp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31:369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 66.Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32:590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noble EE, Billington CJ, Kotz CM, Wang C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am J Physiol Regul Integr Comp Physiol. 2014;307(6):R737–45. doi: 10.1152/ajpregu.00118.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zink AN, Perez-Leighton CE, Kotz CM. The orexin neuropeptide system: physical activity and hypothalamic function throughout the aging process. Front Syst Neurosci. 2014;8:211. doi: 10.3389/fnsys.2014.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, Levine JA. Orexin A mediation of time spent moving in rats: Neural mechanisms. Neuroscience. 2006;142(1):29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.