Abstract
The aim of our study was to analyze the presence of 5-methyl-cytosine (5-mC) and 5-hydroxymethyl-cytosine (5-hmC) in the genome of crustacean Daphnia pulex. First, the presence of 5-mC and 5-hmC in genomic DNA was demonstrated by using antibodies specific to either 5-mC or 5-hmC. Then, analysis of 5-mC and 5-hmC using pairs of restriction enzymes with different sensitivity to methylation and hydroxymethylation confirmed the presence of both modifications in selected regions of three genes (Cox4, Cand2 and Ephx1). To get a detailed picture of 5-hmC distribution over the D. pulex genome, we performed 5-hmC enrichment and sequenced the enriched fraction using next generation sequencing and non-enriched library (input) as a control. Comparison of input and enriched libraries showed that 5-hmC in exons is twice as frequent as in introns. Functional analysis indicated that 5-hmC abundance is associated with genes that are involved in the adenylate cyclase-activating G-protein-coupled receptor signaling pathway, molting cycles, morphogenesis and cell fate determination. Genes that lack 5-hmC tend to be involved in the regulation of the transforming growth factor beta receptor signaling pathway and in many mRNA-related processes. Our results suggest that epigenetic modifications are present in the genome of D. pulex and most likely are involved in the regulation of gene expression of this crustacean.
Keywords: Daphnia pulex, 5-methyl-cytosine, 5-hydroxymethyl-cytosine, whole genome sequencing, epigenetic modifications, Cox4, Cand2, Ephx1
1. Introduction
Daphnids are freshwater crustaceans that, dependent on environmental conditions, can reproduce either sexually or parthenogenetically. Water flea Daphnia pulex propagates by cyclical parthenogenesis producing subitaneous eggs. With deterioration of environmental quality, usually at the end of the growing season, these animals initiate sexual reproduction which results in two diapausing eggs encased in ephippium, a protective structure modified from carapace [1,2]. Emergence from diapausing eggs in daphnids takes place in the early season during a relatively short period [3,4,5], although these eggs, being resistant to external factors, can remain viable for extended time periods [6]. In addition, D. pulex exhibit different polyphenisms [7,8]. Since clonal lines are genetically identical but consist of phenotypically divergent individuals, this phenomenon could be attributed to the epigenetic changes [9,10].
Due to the relatively recently published genome sequence of D. pulex [11], the genome of this organism now is one of the most intensively studied among aquatic invertebrates. However, despite much encouragement [9,10], investigations of the epigenome of Daphnia are scarce. The presence of 5-methylcytosine was shown in the genome of D. magna [12,13]. Bioinformatic analysis indicated that D. pulex do have DNA methyltransferases as well [14], and potentially can methylate their genome; however, direct evidence is still lacking.
Until a few years back only one epigenetic DNA modification was well known—5-methylcytosine (5-mC). This modification has been extensively studied and a number of important epigenetic functions (e.g., gene regulation, X chromosome imprinting) are known. In 2009, 5-hydroxymethylcytosine (5-hmC) was rediscovered, resulting in a new age of epigenetics [15]. The modification of 5-hmC immediately became intensively studied and subsequent studies revealed the mechanism of producing this base in vivo via TET1-mediated oxidation [16]. While there are no TET oxygenases described in D. pulex, a recently published bioinformatic search suggested some candidates [17].
The aim of our study was to analyze the presence of 5-mC and 5-hmC in the genome of D. pulex using different methods, such as immuno-dot blot analysis, next generation sequencing (NGS) and digestion of genomic DNA with several pairs of restriction enzymes with different sensitivity to methylation and hydroxymethylation.
2. Experimental Section
2.1. Preparation of Biological Samples
A cyclically parthenogenetic population of D. pulex from a permanent pond in Vilnius was the source of animals for investigation [18]. The species was identified by analyzing both females and males. Clones of D. pulex were established from ephippia (winter eggs) that were collected in the pond after the ice cover had melted. In our laboratory, they were placed into trays filled with 0.45 μm membrane-filtered pond water and kept at 16 °C under permanent illumination. Exephippial hatchlings born during the two days were utilized for investigation and initiation of parthenogentic generation.
Exephippial hatchlings for the first three days of their life were raised in 200 mL volume vessels with ~40 individuals per vessel. Since the third day of their life, density of experimental animals was reduced to 20 individuals per vessel, and since maturation, the density was reduced to 10 specimens per vessel.
Parthenogenetic hatchlings were initiated from the second clutch of exephippial mothers. Before the clutch release, females were individually transferred into separate vessels. Each group of offspring of 40 specimens per vessel was composed by taking one hatchling from each 40 random clutches, thus these groups were mixtures of unique genotypes. Further, parthenogenetic offspring were raised in the same way as exephippial hatchlings.
Both morphs (exephippial and parthenogenetic) of D. pulex were raised at 20 °C under permanent illumination in membrane-filtered pond water under high food conditions, i.e., daily provision of Scenedesmus quadricauda ~2.0 mg/L. Before cropping, animals were kept in filtered water overnight to get their gut clear. Further, daphnids were transferred to distilled water and counted, then were filtered on 0.5 mm mesh size net, rinsed with distilled water, inspected under microscope, transferred into microcentrifuge tubes and immediately frozen in liquid nitrogen. Samples were preserved at −70 °C until analysis.
For the analysis, animals of both morphs were cropped at the four ontogenetic stages: three-day-old juvenile stage (juveniles, 40 specimens per sample), five-day-old preadult animal stage (preadults, 20 specimens per sample) which, in this species, corresponds to instar 4 [19], about 12-day-old female carrying the second clutch stage (adults I, 10 specimens per sample), and over 15-day-old female carrying the third-fourth clutch stage (adults II, 10 specimens per sample). All these samples for both exephippial and parthenogenetic D. pulex morphs were composed of multiple clones. For next generation sequencing, samples consisting of single clone parthenogentic females, mostly adults, were used.
2.2. Preparation of Genomic DNA
Genomic DNA was purified using GeneJETTM Plant Genomic DNA Purification Mini Kit (Thermo ScientificTM, Vilnius, Lithuania, protocol A) following manufacturer’s instructions. Concentration of purified DNA was measured using Nano Drop 2000 (Thermo Scientific) following manufacturer’s instructions.
DNA integrity was assessed in 1% agarose gel (TopVision™ agarose (Thermo Scientific), 50× TAE buffer (Thermo Scientific), 0.05 mg/mL ethidium bromide (Thermo Scientific). DNA samples were mixed with 6× DNA Loading Dye (Thermo Scientific).
2.3. Detection of Global Genome Methylation and Hydroxymethylation Using Immuno-Dot Blot Analysis
Immuno-dot blot was conducted using Biotin Chromogenic Detection Kit (Thermo Scientific) following manufacturer’s instructions. Primary 5-mC antibody (Active Motif, cat. no. 61255) was diluted 1000 times and primary 5-hmC antibody (Active Motif, cat. No. 39770) was diluted 10,000 times. Secondary rabbit antibody (Active Motif) in 5-hmC sample was diluted 2000 times and secondary mouse antibody (Active Motif) in 5-mC sample was diluted 5000 times. Total genomic methylation and hydroxymethylation levels were assesed by using TotalLab software, signal intensity was normalized according to amount of DNA used (genomic DNA of D. Pulex—200 ng, methylated and unmethylated DNA of the plasmid pUC—100 ng, human genomic DNA—625 ng, 5-hydroxymethylated DNA of Staphylococcus aureus—250 ng).
2.4. Local Methylation and Hydroxymethylation Analysis in Selected Genes of D. pulex
Local DNA modifications were analyzed in genomic regions located in three genes (Table 1): cullin-associated NEDD8-dissociated protein 2 (Cand2), cytochrome c oxidase, subunit IV (Cox4), and juvenile hormone epoxide hydrolase 1 (Ephx1). Primers for qPCR analysis (Table 2) were designed using NCBI PRIMER-BLAST [20] in such way that amplicons are covering both 5'-CCGG-3' and 5'-TCGA-3' sites in exonic regions. Primers with ΔG bigger than −2 kcal/mol were chosen
Table 1.
Target genes and their parameters.
| Gene | Gene Symbol | Genomic Location | Length, bp | Number of Exons | Length of Exons, bp |
|---|---|---|---|---|---|
| Juvenile hormone epoxide hydrolase 1 | Ephx1 | scaffold_121: 159299–164657 | 5358 | 11 | 1598 |
| Cullin-associated Nedd8-dissociated 2 | Cand2 | scaffold_4446: 1087–2177 | 1090 | 4 | 882 |
| Cytochrome c oxidase, subunit IV | Cox4 | scaffold_23: 1334549–1335729 | 1180 | 4 | 780 |
Table 2.
qPCR primer sequences for D. pulex genes of interest.
| Gene | Primer | Primer Sequence |
|---|---|---|
| Cox4 | Forward | AGTTGGAGACCCAGTTAAAGC |
| Reverse | AGGTTTGGCAGAAAGATGCTC | |
| Cand2 | Forward | GAAATACTTGCACCGCCAGAG |
| Reverse | TACTCCTGCAGCATTTCCGTG | |
| Ephx1 | Forward | CTCAAAACCCAGTGGGGAGG |
| Reverse | TTGTCGGATTCTTGAGTCAGC |
In order to evaluate the methylation level of target genes, 100 ng purified genomic DNA was treated by EpiJETTM DNA Methylation Analysis Kit (MspI/HpaII) and (TaqI/HpyF30I) (Thermo Scientific) following manufacturer’s instructions. In order to assess target-gene hydroxymethylation level 100 ng purified genomic DNA was treated by EpiJET 5-hmC Analysis Kit (Thermo Scientific) following manufacturer’s instructions. qPCR was performed using 2× Maxima™ SYBR Green qPCR Master Mix (Thermo Scientific) following manufacturer’s instructions. Percent methylation/hydroxymethylation level at the target-gene site was evaluated according to formula: 100/(1 + E%)^(CT average of restriction endonuclease treated DNA-CT average of non-treated DNA), where E% is qPCR efficiency, calculated by StepOne Plus™ (Applied Biosystems, Carlsbad, CA, USA) from a standard curve, consisting of three DNA dilutions. All results were obtained from at least four replicates.
2.5. Next Generation Sequencing
For next generation sequencing, DNA from parthenogenetic D. pulex adults was used. Prior to DNA extraction, individuals from single clone were treated with Sephadex beads to clean the gut and 500 mg/l of tetracycline to reduce bacterial contamination, exactly as described by Colbourne et al. [11]. Then 100 ng of extracted DNA was fragmented using MuSeekTM Library Preparation Kit, Illumina compatible (Thermo Scientific) and purified using GeneJET NGS Cleanup Kit (Thermo Scientific). Libraries for 5-hmC enrichment analysis were end-repaired, purified and 5-hmC was enriched using EpiJET 5-hmC Enrichment Kit (Thermo Scientific) according to manufacturer’s protocol. The enriched and non-enriched control (input) were PCR-amplified for 14 cycles and gel size selected for next generation sequencing. The libraries were analyzed using Agilent Bioanalyzer (Agilent) and KAPA library quantification kit (KAPA biosystems). The next generation sequencing for de novo assembly was performed using Illumina MiSeq platform with MiSeq reagent kit v3 600-cycle (Illumina). Libraries for 5-hmC analysis were sequenced using v3 150-cycle kit. The sequencing data is deposited in NCBI’s Sequence Read Archive. Reads for the reference genome assembly are deposited as SRR2968969 entry, reads of the two runs of the 5-hmC enriched library are deposited as SRR2970595 and SRR2970600.
2.6. NGS Data Analysis
2.6.1. Initial Processing of Reads
Initially, the reads were processed using Cutadapt 1.8.1 [21]. Shorter-than-70-bp reads were discarded and the 3ʹ end of reads were trimmed to have quality value higher or equal to 20.
2.6.2. Assembly of Genome
Genome assembly was done in two steps:
Reads that matched to draft D. pulex genome [11] were collected using Kraken 0.10.5 [22]. The overlapping 3' ends of reads were merged using FLASH v1.2.11 [23]. The assembly was performed using Spades 3.5.0 [24]. Bacterial contigs were filtered out using Kraken 0.10.5 and the remaining contigs were used for the subsequent step.
The overlapping 3ʹ ends of reads were merged using FLASH v1.2.11. Then they were assembled using Spades 3.5.0 together with the contigs from the first step. Contigs that matched to draft D. pulex genome [11] were collected using Kraken 0.10.5. Out of these contigs the bacterial ones were discarded using second run of filtering with Kraken 0.10.5.
In this way, prepared assembly was used to evaluate sequencing of some control libraries after 5-hmC enrichment. However, the distribution of 5-hmC across gene body and its association with potential gene function were analyzed after discarding reads with exceptionally high coverage (higher than 50× coverage). The statistics about assemblies were collected using QUAST 3.1 [25].
2.6.3. Detection of Genes and Their Functional Annotation
Genes were detected using GlimmerHMM-3.0.4 [26], which was trained on already published D. pulex genome [11]. Functions of the detected genes were inferred (GO terms assigned) using PANNZER (Protein ANNotation with Z-scoRE) and its database (v.1.0) [27].
2.6.4. The 5-hmC Peak Calling and Analysis
Before alignment reads were processed using Cutadapt 1.8.1. Shorter-than-35-bp reads were discarded and the 3ʹ end of reads were trimmed to have quality value higher or equal to 20. Reads were aligned to contigs using Bowtie2 v2.1.0 [28]. Peaks were detected using MACS2 v2.0.10 [29] allowing at most three duplicate reads. The distribution of peaks across the genome was analyzed using RSeQC v2.5 [30], BEDTools [31] and custom Python scripts. For functional analysis we selected two groups of genes: (1) genes having exons completely void of peaks; (2) genes having a density of peaks across gene body equal or higher than 10 PPKM (peaks per kilobase of transcript per million of peaks). Coordinates of protein coding exons for each gene were predicted with GlimmerHMM-3.0.4 [26]. PPKM is analogous to RPKM (reads per kilobase per million) measure that is used in RNA sequencing. PPKM measure normalizes counts of peaks to the length of protein-coding DNA and allows us to compare exons’ hydroxymethylation in genes of different lengths. The PPKM cut off was arbitrarily adjusted to have approximately same number of genes in both groups. The enrichment of all GO terms of these genes was evaluated between these two groups. Only those GO terms were considered for analysis which were detected in both groups. For further analysis we selected only the GO terms of which frequency was significantly different. The p-value 0.01 was used in cases of genes that had 5-hmC and for genes that lacked the peaks the p-value cut-off was 0.05. The selected GO terms were analyzed and visualized using REViGO [32].
3. Results and Discussion
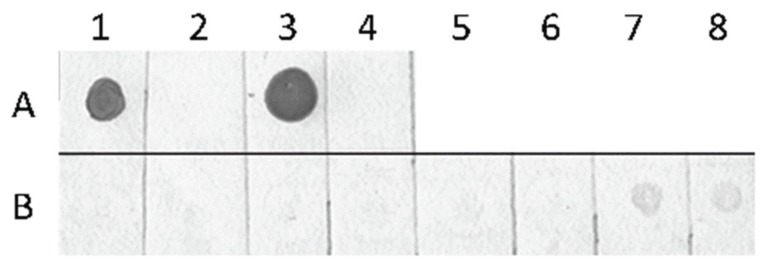
The presence of cytosine methylation and hydroxymethylation in D. pulex genomic DNA was first assessed by immunological reaction, using antibodies specific for either 5-mC or 5-hmC. Immuno-dot blot using antibody specific for 5-mC showed a very weak signal only at a dot corresponding to the DNA of the oldest individuals (Figure 1). In younger individuals no signal was detectable, which could be due to a very low amount of 5-mC that is below the sensitivity of the immuno-dot blot assay.
Figure 1.
Immuno-dot blot using antibody specific for 5-methylcytosine. Lane A represents 100 ng of methylated DNA from the plasmid pUC (A1), 100 ng of unmethylated DNA from pUC (A2), 625 ng of human genomic DNA (A3), 250 ng of 5-hydroxymethylated DNA from Staphylococcus aureus (A4). Lane B—200 ng of genomic DNAs from D. pulex juveniles (B1, B2), preadults (B3, B4), adults I (B5, B6), and adults II (B7, B8); even numbers—individuals hatched form parthenogenetic eggs; odd numbers—individuals hatched from ephippial eggs.
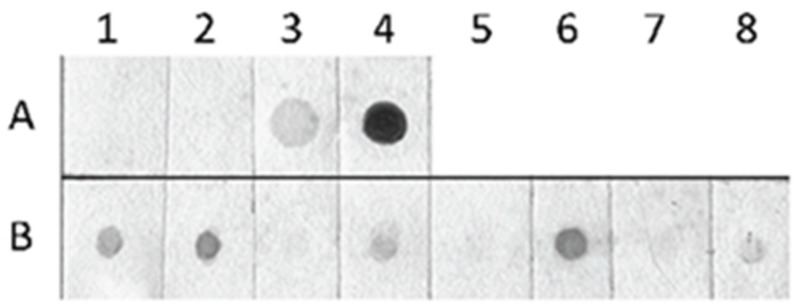
A different picture was seen in the case of immuno-dot blot using antibody specific for 5-hmC (Figure 2). A relatively strong signal was seen in dots with DNA from juveniles, preadults and adults I, and a only weak signal in dots with DNA from adults II. In all cases, variation between two morphs (hatched from winter and parthenogenetic eggs) of each ontogenetic stage could be noticed (in dots B3, B5 and B7 no signal was detectable).
Figure 2.
Immuno-dot blot using antibody specific for 5-hydroxymethylcytosine. Lane A represents 100 ng of methylated DNA from the plasmid pUC (A1), 100 ng of unmethylated DNA from pUC (A2), 625 ng of human genomic DNA (A3), 250 ng of 5-hydroxymethylated DNA from Staphylococcus aureus (A4). Lane B—200 ng of genomic DNAs from D. pulex juveniles (B1, B2), preadults (B3, B4), adults I (B5, B6), and adults II (B7, B8); even numbers—individuals hatched form parthenogenetic eggs; odd numbers—individuals hatched from ephippial eggs.
As it was mentioned above, no direct measurements of methylation and hydroxymethylation levels in D. pulex have been published until now. However, DNA methylation was assessed in close species—D. magna, where by analyzing two genomic fragments the authors [12] determined relatively low methylation level—from 0.22% to 0.44% methylated CpG sites. Our data demonstrate, for the first time, the presence of both 5-mC and 5-hmC in the genome of D. pulex. A weak signal of 5-mC is in a good agreement with bioinformatics findings of other authors [14], where the profile of evolutionary CpG depletion in protein-coding genes of D. pulex suggests existence of DNA methylation in this crustacean. However, we feel that the immune-dot blot assay is not sensitive enough for such kinds of studies. It also does not allow any reliable quantitative analysis, since in invertebrates DNA modifications could occur in non-CpG context [33] and crude DNA preparations may contain significant amounts of DNA from other species, including various endosymbionts [34]. Unfortunately, more precise methods such as HPLC or MS analysis of total DNA do not discriminate the origins of DNA modifications, making the results of such analysis unreliable as well, and while the bisulphite sequencing will overcome the contaminating DNA issue, it will not discriminate between 5-mC and 5-hmC, thus providing no additional information about the distribution of 5-hmC over the genome of D. pulex. Keeping these reasons in mind, we decided to perform additional analysis, including digestion of genomic DNA with several pairs of restriction enzymes with different sensitivity to methylation and hydroxymethylation followed by locus-specific qPCR and total 5-hmC enrichment/NGS analysis.
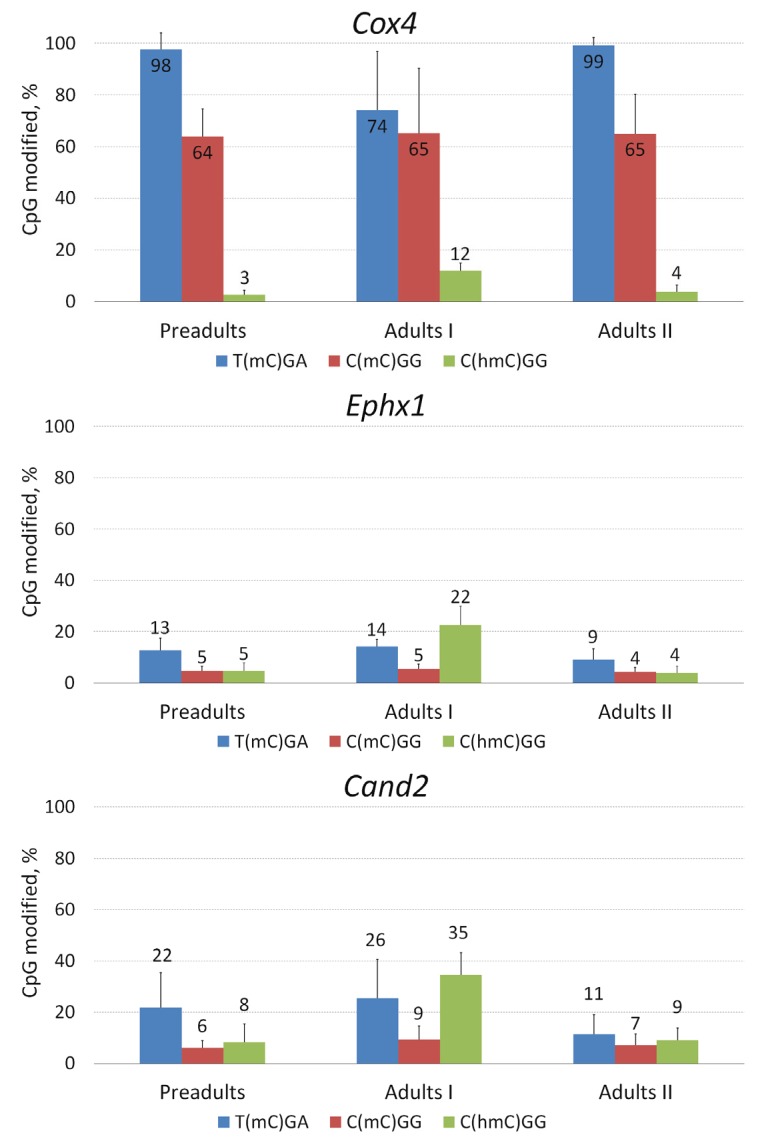
Thus, in our next set of experiments, we analyzed methylation and hydroxymethylation in regions of selected genes of D. pulex (Figure 3). For this analysis we used pairs of restriction endonucleases MspI/HpaII, which could detect methylation of the internal CpG in the CCGG tetranucleotide, and HpyF30I/TaqI, which could detect methylated cytosine in the TCGA tetranucleotide. First, 5-hmC was assessed in CCGG sites using T4 phage β-glucosyltransferase, which is capable of specifically modifying 5-hmC residues by adding a glucose moiety to 5-hmC, and restriction enzyme Epi MspI, the activity of which is completely blocked when 5-hmC is glucosylated.
Figure 3.
Percentage of CpG-modified sites in selected regions of three D. pulex genes—Cox4, Ephx1 and Cand2. Blue columns represent percentage of methylated CpG in TCGA sequences, red columns—percentage of methylated CpG in CCGG sequences, green columns—percentage of hydroxymethyated CpG in CCGG sequences. Error bars represent standard deviation.
Three genes that could be associated with the development of different morphs in D. pulex were chosen for the analysis. These were the cullin-associated NEDD8-dissociated 2 (Cand2) gene, cytochrome C oxidase subunit IV (Cox4) gene and juvenile hormone epoxide hydrolase 1 (Ephx1) gene. The Cand2 gene has not been annotated in the genome of D. pulex; however, a few orthologs are known in other species, Drosophila melanogaster in particular. It has been demonstrated that the product of this gene interacts with trancription factor TBP and promotes myogenesis [35]. Cox4 is a cytochrome c oxidase subunit responsible for the assembly of the whole cytochrome c oxydase [36]. It has been previously shown that Cox4 is differently expressed in post-diapause individuals of Artemia franciscana [37] and in different morphs of Acyrthosiphon pisum [38]. Juvenile hormone epoxide hydrolase 1 (Ephx1) participates in the catabolism of the juvenile hormone and seems to be a key enzyme in juvenile hormone degradation [39].
In selected regions of the Cox4 gene, high levels of methylation were observed in both CCGG and TCGA sites. Higher methylation levels were observed at the TCGA site when compared to the CCGG site (p < 0.0001, Mann-Whitney U-test), except in the adults I stage (p = 0.48). Statistically significant variations in methylation levels at the TCGA site between different ontogenetic stages were found (p < 0.01, Kruskal-Wallis test). We also detected 5-hydroxymethylation of CCGG sites, the highest levels being in the adults I stage. Variation in 5-hmC levels between different ontogenetic stages was significant as well (p < 0.01).
In selected regions of Ephx1 and Cand2 genes, methylation levels were much lower than in the Cox4 gene, especially at CCGG sites, and levels of 5-hydroxymethylation were much higher. However, other tendencies were exactly the same: higher methylation was observed in TCGA sites when compared to CCGG sites (p < 0.01 for both genes and all developmental stages, Mann-Whitney U-test) and significant variation between developmental stages was found for methylation at the TCGA site (p < 0.01 for both genes, Kruskal-Wallis test). Mehylation at CCGG sites was low (actually at the lower limit of detection) and did not differ between different developmental stages. Again, variation in 5-hmC levels between different ontogenetic stages was significant for both Ephx1 and Cand2 genes (p < 0.01).
Thus, our analysis showed that both 5-mC and 5-hmC are present in gene bodies of three analyzed genes, and higher methylation levels are consistently found at TCGA sites when compared to CCGG sites. Since levels of both 5-mC and 5-hmC are changing during ontogeny of D. pulex, it may indicate that these epigenetic modifications are actively involved in gene expression of this crustacean.
The currently published D. pulex genome was sequenced using the Sanger sequencing method [11]. Here we describe the first attempt to assemble the genome using new generation sequencing data. For this we sequenced the genome using the Illumina MiSeq platform. The sequencing resulted in 1.4 M pair-ended reads and 1.7 G sequenced bases. This was assembled to 17,338 individual contigs with N50 of 10,601. All these contigs presented ~54% of the currently published D. pulex reference genome. The data on the quality of the assembly is given in Table 3. Detailed analysis showed that the D. pulex used in this study exhibited a lot of difference at the nucleotide level when compared with a published reference genome. The total identity for the sequenced part was only 93.6% between the two clones suggesting large differences in D. pulex of different populations.
Table 3.
Comparison of basic quality measures of assembled and reference genomes of Daphnia pulex.
| Assembled Genome | Reference Genome [11] | |||
|---|---|---|---|---|
| Assembly | Contigs | Contigs used for peak calling and annotation | Contigs | Scaffolds |
| # contigs | 17338 | 5572 | 19008 | 5191 |
| Total length | 85410271 | 66080228 | 158604366 | 197261574 |
| GC (%) | 40.74 | 41.08 | 40.76 | 40.77 |
| N50 | 10601 | 13511 | 49250 | 642089 |
| # N's per 100 kbp | 0 | 0 | 1.33 | 19582.65 |
It is well known that, morphologically, D. pulex is almost indistinguishable from the relative species D. pulicaria when comparing females [40]. However, in our samples only 6% of the reads mapped to the D. pulicaria genome, and most of the remaining reads mapped to the genome of D. pulex. Thus, it is obvious that mismatches between the reference genome and our sequenced Daphnia genome could not be attributed to incorrect identification of the species.
One explanation could be that different populations of D. pulex exhibit different genomes. The reference genome is sequenced using D. pulex clone “The chosen one” which is established in the USA. It is known that different clones of D. pulex from different continents have quite significant differences in their DNA sequences and that mutation rates of the mitochondria genome are one of the highest among different species [41].
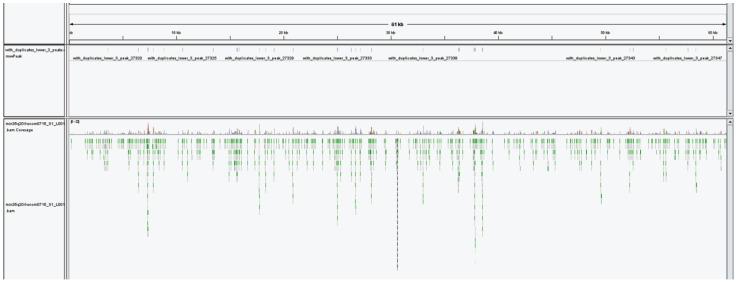
To further investigate 5-hmC distribution over the D. pulex genome, we performed 5-hmC enrichment and sequenced the enriched fraction using next generation sequencing and non-enriched library (input) as a control. Two technical repeats were analyzed for enriched libraries. The sequencing resulted in ~17 M pair-end 75 bp reads for input and 40 M reads for enriched DNA (two runs). After alignment of the input library to the assembled genome, we did not manage to have even coverage; thus, for peak calling we used only the enriched library and detected more than 27,000 peaks (Figure 4). Although this is quite a risky approach, we managed to get valuable information as the detected peaks were not distributed randomly. Furthermore, we investigated the distribution of the peaks on the genomic elements to find if the 5-hmC were concentrated in biologically significant genomic regions. Firstly, we identified functional genomic elements on the newly assembled genome using annotation data from the reference D. pulex genome. Secondly, the peaks were assigned to different elements and density per kilobase of the sequence was calculated (Table 4).
Figure 4.
A representative region of the of assembled D. pulex genome contigs with mapped reads from one 5-hmC-enriched library (snapshot from IGV [42]). The upper lane shows detected peaks. The lower lanes shows individual reads along with coverage map.
Table 4.
Analysis of 5-hmC enrichment over D. pulex functional elements. Tags denote number of locations where 5-hmC was found to be enriched. Tags/Kb—density of peaks per kilobase of analyzed sequence in a given genomic element. CDS: coding sequence site; TES: transcription end site; TSS: transcription start site.
| Group | Total_Bases | Tag_Count | Tags/Kb |
|---|---|---|---|
| CDS_Exons | 17597397 | 10651 | 0.61 |
| Introns | 17376020 | 4652 | 0.27 |
| TSS_up_1 kb | 12615965 | 1012 | 0.08 |
| TSS_up_5 kb | 35083848 | 2304 | 0.07 |
| TSS_up_10 kb | 57615402 | 2585 | 0.04 |
| TES_down_1 kb | 11356574 | 1191 | 0.10 |
| TES_down_5 kb | 31789833 | 2351 | 0.07 |
| TES_down_10 kb | 53166077 | 2506 | 0.05 |
This analysis clearly showed that 5-hmC was located not randomly over the D. pulex genome but had a strong preference to gene body sequences with exons having highest level of enrichment (Table 4). Moreover, such 5-hmC distribution is already described in other eukaryotic organisms such as mammals where 5-hmC is preferentially enriched over exonic sequences [43]. This finding suggests the idea that 5-hmC acts as a conservative regulatory element on gene bodies over the different organisms.
This result was the first demonstration of 5-hmC presence in D. pulex and it also confirms the dot-blot and qPCR data where we found that D. pulex DNA have 5-hmC in their genome.
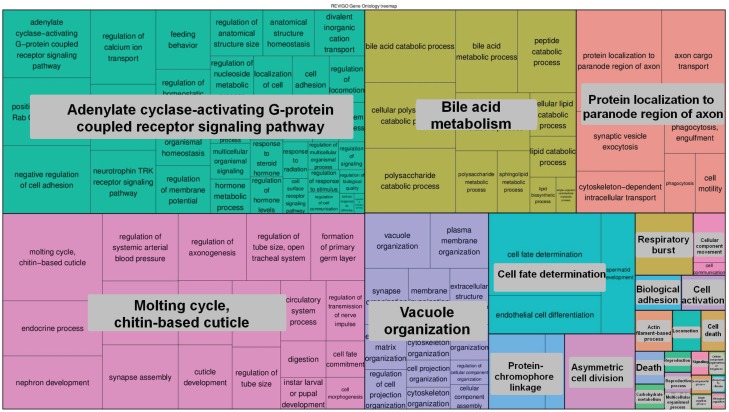
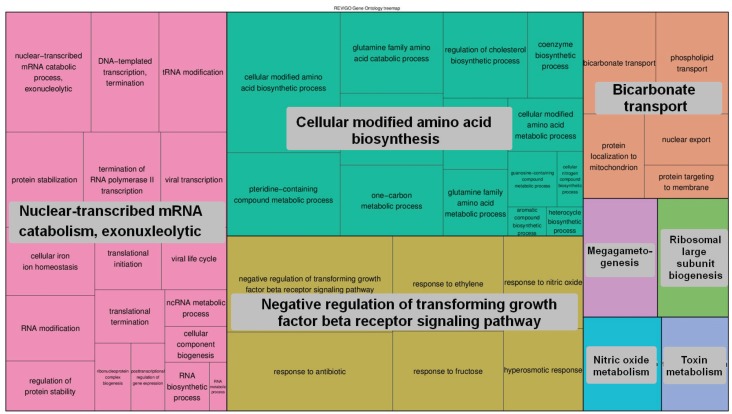
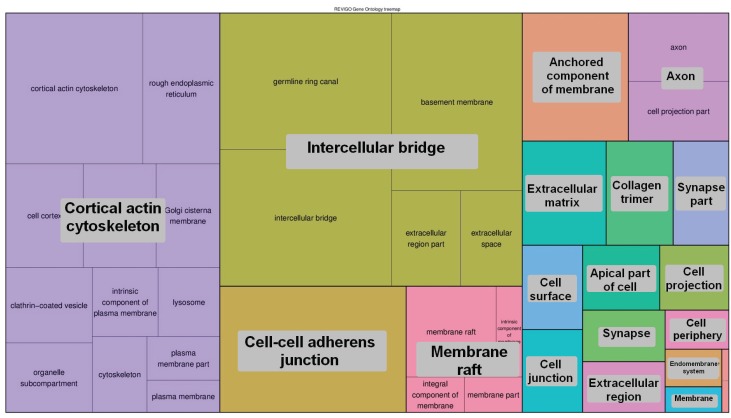
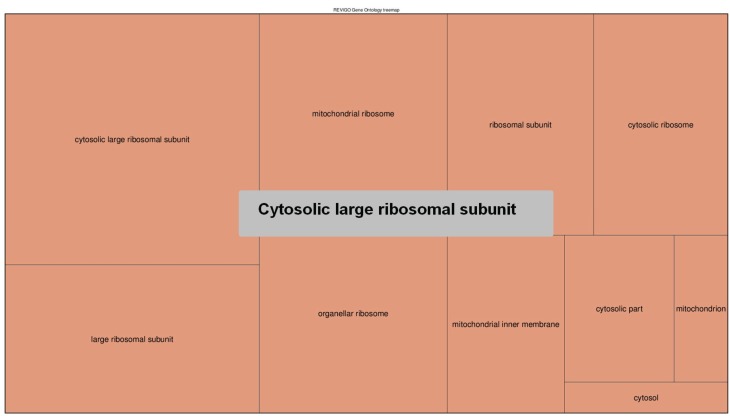
For functional analysis we selected two groups of genes: (a) 1663 genes having exons completely void of peaks; (b) 2121 genes having a density of peaks across the gene body equal or higher than 10 PPKM. The detected enrichment of GO terms was clusterized and presented in Figure 5, Figure 6, Figure 7 and Figure 8. Quantitative data and a full list of GO terms can be found in Supplementary Files S1–S4. Our analysis suggests that 5-hmC abundance is associated with genes that are involved in many biological processes and notably in the adenylate cyclase-activating G-protein-coupled receptor signaling pathway and molting cycles. In addition, many genes with 5-hmC are involved in morphogenesis and, interestingly, in cell fate determination. On the other hand, the genes that lack 5-hmC tend to be involved in the regulation of the transforming growth factor beta (TGF-β) receptor signaling pathway and in many mRNA-related processes.
Figure 5.
The GO terms (biological process) that were enriched for genes prone to 5-hmC modification. The areas of rectangles are proportional to the relative increase in frequency of GO terms comparing genes that had 5-hmC related peaks to genes that completely lacked them. The corresponding quantitative data and a full list of GO terms can be found in Supplementary File S1.
Figure 6.
The GO terms (biological process) that were enriched for genes void of 5-hmC. The areas of rectangles are proportional to the relative increase in frequency of GO terms comparing genes that completely lacked 5-hmC-related peaks to genes that had them. The corresponding quantitative data and a full list of GO terms can be found in Supplementary File S2.
Figure 7.
The GO terms (cellular components) that were enriched for genes prone to 5-hmC modification. The areas of rectangles are proportional to the relative increase in frequency of GO terms comparing genes that had 5-hmC-related peaks to genes that completely lacked them. The corresponding quantitative data and a full list of GO terms can be found in Supplementary File S3.
Figure 8.
The GO terms (cellular components) that were enriched for genes void of 5-hmC. The areas of rectangles are proportional to the relative increase in frequency of GO terms comparing genes that completely lacked 5-hmC-related peaks to genes that had them. The corresponding quantitative data and a full list of GO terms can be found in Supplementary File S4.
Quite an interesting discovery is the association between the low 5-hmC level and the TGF-β receptor signaling pathway, since it has been previously shown that TGF-β regulates DNA methyltransferase expression [44] in prostate cancer and TGF-β induces global changes in DNA methylation in ovarian cancer cells [45]. Therefore, it is very likely that TGF-β plays an important role in DNA epigenetic modifications in invertebrates as well. Additionally, it is evident from Figure 8 that genes lacking 5-hmC modifications mainly encode ribosomal proteins. It is quite interesting to note that other studies on mouse brain [46] indicated that neuron-specific 5-hmC-marked genes were enriched among genes coding ribosomal proteins. Thus, again, a lack of 5-hmC in genes of ribosomal proteins may be a general feature of eukaryotic organisms.
4. Conclusions
For the first time, we were able to demonstrate the presence of both 5-mC and 5-hmC in the genome of Daphnia pulex. Our analysis showed that both 5-mC and 5-hmC are present in gene bodies of three analyzed genes. It was also discovered that 5-hmC distribution across the genome is not random: in exons it is twice as frequent as in introns. Together with the results of the functional analysis of genes with no 5-hmC and a high number of peaks with 5-hmC, it may indicate that this epigenetic modification could act as a conservative regulatory element on gene bodies over the different eukaryotic organisms.
Acknowledgments
This study was supported by the Research Council of Lithuania, Project No. MIP-031/2012.
Supplementary Files
Author Contributions
Kęstutis Arbačiauskas, Juozas Lazutka and Arūnas Lagunavičius designed the study. Kęstutis Arbačiauskas collected biological samples. Dovilė Strepetkaitė and Rasa Sabaliauskaitė conducted immuno-dot blot, DNA methylation and hydroxymethylation experiments and prepared genomic library for NGS. Eimantas Astromskas conducted NGS, Gediminas Alzbutas performed bioinformatic analysis of the data. Juozas Lazutka analyzed data and wrote the first draft of the manuscript. All authors contributed substantially to revisions and approved the final manuscript. Dovilė Strepetkaitė and Gediminas Alzbutas equally contributed to this research.
Conflicts of Interest
This work was partially supported by Thermo Fisher Scientific Baltics. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Stross R.G., Hill R.G. Diapause induction in Daphnia requires two stimuli. Science. 1965;150:1462–1464. doi: 10.1126/science.150.3702.1462. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho G.R., Hughes R.N. The effect of food availability, female-density and photoperiod on ephippia production in Daphnia magna Straus (Crustacea, Cladocera) Freshw. Biol. 1983;13:37–46. doi: 10.1111/j.1365-2427.1983.tb00655.x. [DOI] [Google Scholar]
- 3.Caceres C.E. Interspecific variation in the abundance, production, and emergence of Daphnia diapausing eggs. Ecology. 1998;79:1699–1710. doi: 10.1890/0012-9658(1998)079[1699:IVITAP]2.0.CO;2. [DOI] [Google Scholar]
- 4.Wolf H.G., Carvalho G.R. Testing eggs of lake-Daphnia II. In situ observations on the hatching of eggs and their contribution to population and community structure. Freshw. Biol. 1989;22:471–478. doi: 10.1111/j.1365-2427.1989.tb01119.x. [DOI] [Google Scholar]
- 5.Hairston N.G., Jr., Hansen A.M., Schaffner W.R. The effect of diapause emergence on the seasonal dynamics of zooplankton assemblage. Freshw. Biol. 2000;22:471–478. doi: 10.1046/j.1365-2427.2000.00386.x. [DOI] [Google Scholar]
- 6.Hairston N.G., Jr., van Brunt R.A., Kearns C.M., Engstrom D.R. Age and survivorship of diapausing eggs in a sediment egg bank. Ecology. 1995;76:1706–1711. doi: 10.2307/1940704. [DOI] [Google Scholar]
- 7.Spanier K., Leese F., Mayer C., Colbourne J.K., Gilbert D., Pfrender M.E., Tollrian R. Predator induced defences in Daphnia pulex: Selection and evaluation of internal reference genes for gene expression studies with real-time PCR. BMC Mol. Biol. 2010 doi: 10.1186/1471-2199-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbačiauskas K. Seasonal phenotypes of Daphnia: Post-diapause and directly developing offspring. J. Limnol. 2004;63:S7–S15. doi: 10.4081/jlimnol.2004.s1.7. [DOI] [Google Scholar]
- 9.Harris K.M.D., Bartlett N.J., Lloyd V.K. Daphnia as an emerging epigenetic model organism. Genet. Res. Intern. 2012 doi: 10.1155/2012/147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirbahai L., Chipman J.K. Epigenetic memory of environmental organisms: A reflection oflifetime stressor exposures. Mutat. Res. 2014;764:10–17. doi: 10.1016/j.mrgentox.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Colbourne J.K., Pfrender M.E., Gilbert D., Thomas W.K., Tucker A., Oakley T.H., Tokishita S., Aerts A., Arnold G.J., Basu M.K., et al. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandegehuchte M.B., Kyndt T., Vanholme B., Haegeman A., Gheysen G., Janssen C.R. Occurrence of DNA methylation in Daphnia magna and influence of multigeneration Cd exposure. Environ. Intern. 2009;35:700–706. doi: 10.1016/j.envint.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Vandegehuchte M.B., Lemiere F., Janssen C.R. Quantitative DNA-methylation in Daphnia magna and effects of multigeneration Zn exposure. Comp. Biochem. Physiol. 2009;150:343–348. doi: 10.1016/j.cbpc.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Glastad K.M., Hunt B.G., Yi S.V., Goodisman M.A.D. DNA methylation in insects: On the brink of the epigenomic era. Insect Mol. Biol. 2011;20:553–565. doi: 10.1111/j.1365-2583.2011.01092.x. [DOI] [PubMed] [Google Scholar]
- 15.Kriaucionis S., Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahiliani M., Koh K.P., Shen Y., Pastor W.A., Bandukwala H., Brudno Y., Agarwal S., Iyer L.M., Liu D.R., Aravind L., et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer L.M., Zhang D., de Souza R.F., Pukkila P.J., Rao A., Aravind L. Lineage-specific expansions of TET/JBP genes and a new class of DNA transposons shape fungal genomic and epigenetic landscapes. PNAS. 2014;111:1676–1683. doi: 10.1073/pnas.1321818111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbačiauskas K, Gasiūnaitė Z.R. Growth and fecundity of Daphnia after diapause and their impact on the development of a population. Hydrobiologia. 1996;320:209–222. [Google Scholar]
- 19.Arbačiauskas K. Life-history traits of exephippial and parthenogenetically derived daphnids: Indicators of different life-history strategies. Arch. Hydrobiol. Spec. Issues Adv. Limnol. 1998;52:339–358. [Google Scholar]
- 20.Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012 doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMB J. 2011 doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 22.Wood D.E., Salzberg S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014 doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magoč T., Salzberg S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurevich A., Saveliev V., Vyahhi N., Tesler G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majoros W.H., Pertea M., Salzberg S.L. TigrScan and GlimmerHMM: Two open source ab initio eukaryotic gene-finders. Bioinformatics. 2004;20:2878–2879. doi: 10.1093/bioinformatics/bth315. [DOI] [PubMed] [Google Scholar]
- 27.Koskinen P., Törönen P., Nokso-Koivisto J., Holm L. PANNZER: High-throughput functional annotation of uncharacterized proteins in an error-prone environment. Bioinformatics. 2015;31:1544–1552. doi: 10.1093/bioinformatics/btu851. [DOI] [PubMed] [Google Scholar]
- 28.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nussbaum C., Myers R.M., Brown M., Li W., et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008 doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Wang S., Li W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 31.Quinlan A.R., Hall I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Supek F., Bošnjak M., Škunca N., Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6:1. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Field L.M., Lyko F., Mandrioli M., Prantera G. DNA methylation in insects. Insect Mol. Biol. 2004;13:109–115. doi: 10.1111/j.0962-1075.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- 34.Qi W., Nong G., Preston J.F., Ben-Ami F., Ebert D. Comparative metagenomics of Daphnia symbionts. BMC Genomics. 2009 doi: 10.1186/1471-2164-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiraishi S., Zhou C., Aoki T., Sato N., Chiba T., Tanaka K., Yoshida S., Nabeshima Y., Nabeshima Y., Tamura T.A. TBP-interacting protein 120B (TIP120B)/cullin-associated and neddylation-dissociated 2 (CAND2) inhibits SCF-dependent ubiquitination of myogenin and accelerates myogenic differentiation. J. Biol. Chem. 2007;282:9017–9028. doi: 10.1074/jbc.M611513200. [DOI] [PubMed] [Google Scholar]
- 36.Rufini A., Niklison-Chirou M.V., Inoue S., Tomasini R., Harris I.S., Marino A., Federici M., Dinsdale D., Knight R.A., Melino G., et al. TAp73 depletion accelerates aging through metabolic dysregulation. Genes Dev. 2012;26:2009–2014. doi: 10.1101/gad.197640.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W.H., Ge X., Wang W., Yu J., Hu S. A gene catalogue for post-diapause development of an anhydrobiotic arthropod Artemia franciscana. BMC Genomics. 2009 doi: 10.1186/1471-2164-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brisson J. Aphid wing dimorphisms: Linking environmental and genetic control of trait variation. Phil. Transact. R. Soc. Ser. B. 2010;365:605–616. doi: 10.1098/rstb.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nijhout H.F., Riddiford L.M., Mirth C., Shingleton A.W., Suzuki Y., Callier V. The developmental control of size in insects. WIREs Dev. Biol. 2014;3:113–134. doi: 10.1002/wdev.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benzie J.A.H. Cladocera: The Genus Daphnia (Including Daphniopsis) Backhuys Publishers; Leiden, The Netherland: 2005. [Google Scholar]
- 41.Duggan I.C., Robinson K.V., Burns C.W., Banks J.C., Hogg I.D. Identifying incertebrate invasionsusing morphological and molecular analyse: North American Daphnia “pulex” in New Zealand fresh water. Aquat. Invasions. 2012;7:585–590. doi: 10.3391/ai.2012.7.4.015. [DOI] [Google Scholar]
- 42.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen L., Li X., Yan L., Tan Y., Li R., Zhao Y., Wang Y., Xie J., Zhang Y., Song C., et al. Whole-genome analysis of 5-hydroxymethylcytosine and 5-methylcytosine at base resolution in the human brain. Genome Biol. 2014 doi: 10.1186/gb-2014-15-3-r49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q., Chen L., Helfand B.T., Jang T.L., Sharma V., Kozlowski J., Kuzel T.M., Zhu L.J., Yang X.J., Javonovic B., et al. TGF-β regulates DNA methyltransferase expression in prostate cancer, correlates with aggressive capabilities, and predicts disease recurrence. PLoS ONE. 2011;6:1–13. doi: 10.1371/journal.pone.0025168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cardenas H., Vieth E., Lee J., Segar M., Liu Y., Nephew K.P., Matei D. TGF-beta induces global changes in DNA methylation during the epithelial-to-mesenchymal transition in ovarian cancer cells. Epigenetics. 2014;9:1461–1472. doi: 10.4161/15592294.2014.971608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo J.U., Szulwach K.E., Su Y., Li Y., Yao B., Xu Z., Shin J.H., Xie B., Gao Y., Ming G.L., et al. Genome-wide antagonism between 5-hydroxymethylcytosine and DNA methylation in the adult mouse brain. Front. Biol. 2014;9:66–74. doi: 10.1007/s11515-014-1295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.