Abstract
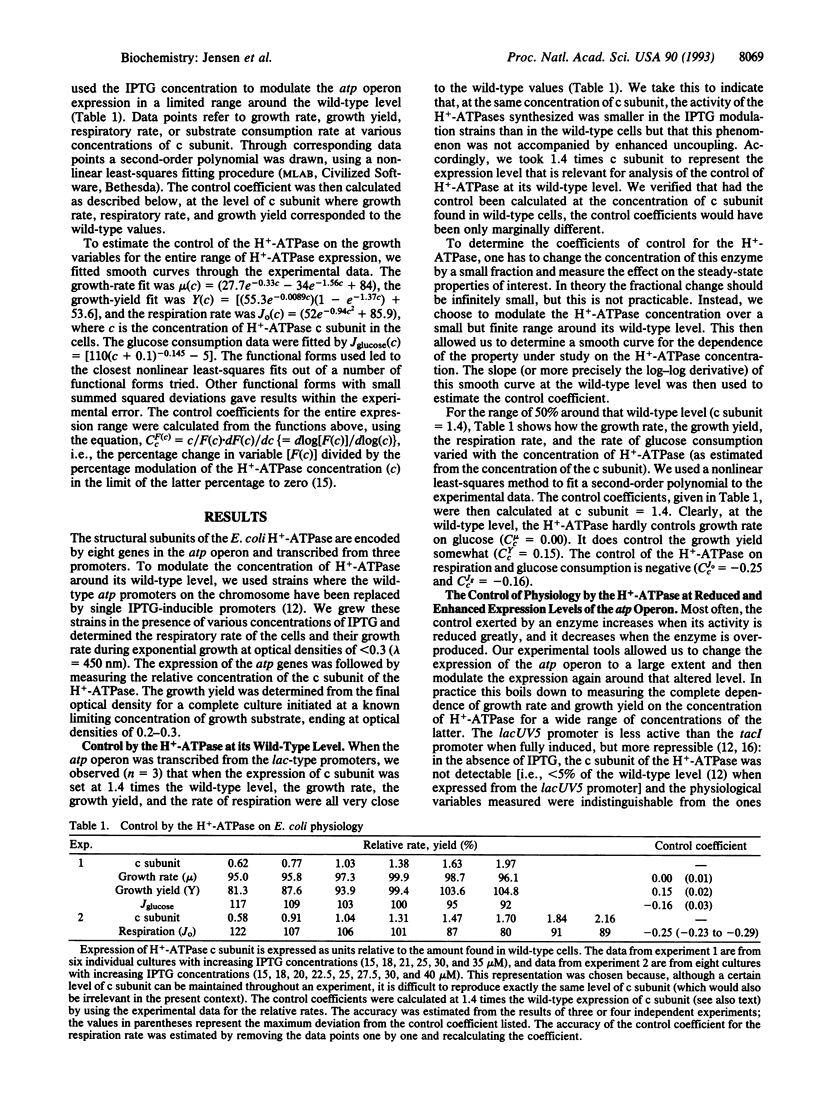
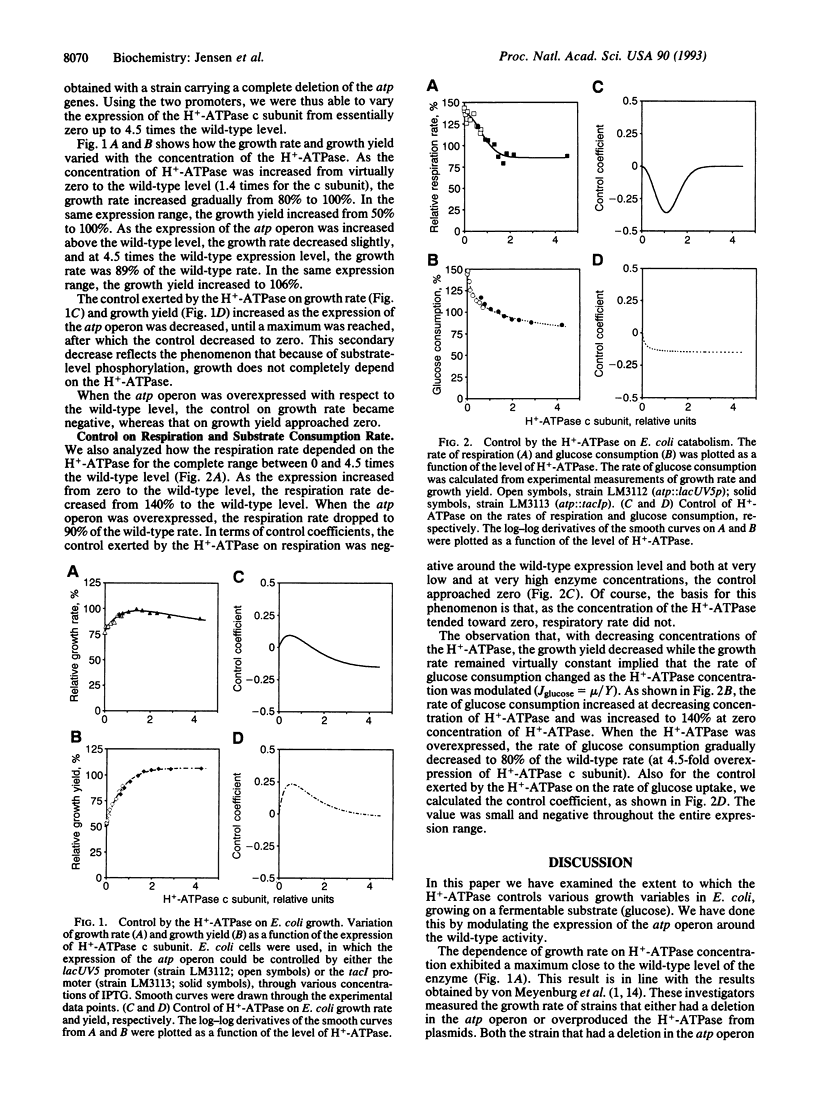
The H(+)-ATPase plays a central role in Escherichia coli free-energy transduction and hence in E. coli physiology. We here investigate the extent to which this enzyme also controls the growth rate, growth yield, and respiratory rate of E. coli. We modulate the expression of the atp operon and determine the effect on said properties. When quantified in terms of control coefficients, we find that, in the wild-type cell growing on glucose in minimal medium, this key enzyme (H(+)-ATPase) exerts virtually no control on growth rate (magnitude of C < 0.01), a minor positive control on growth yield (C = 0.15), and a small but negative control on respiration rate (C = -0.25). The control the enzyme exerts on the consumption rate of the carbon and free-energy substrate is negative (C = -0.15). We also studied how the control coefficients themselves vary with the expression of the atp operon. As the level of expression of the atp operon was reduced, the control exerted by the H(+)-ATPase on growth rate and growth yield increased slightly; the control on growth rate passed through a maximum (C = 0.1) and disappeared when the atp operon was not expressed at all, reflecting that with this substrate there are alternative routes for ATP synthesis. At elevated levels of the H(+)-ATPase compared to the wild type, the control exerted by the enzyme on growth rate became negative. The evolutionary context of the absence of control by the atp operon on growth rate is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstein C., Tiankova L., Kepes A. Respiratory control in Escherichia coli K 12. Eur J Biochem. 1979 Mar;94(2):387–392. doi: 10.1111/j.1432-1033.1979.tb12905.x. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Heinrich R., Rapoport S. M., Rapoport T. A. Metabolic regulation and mathematical models. Prog Biophys Mol Biol. 1977;32(1):1–82. [PubMed] [Google Scholar]
- Heinrich R., Schuster S., Holzhütter H. G. Mathematical analysis of enzymic reaction systems using optimization principles. Eur J Biochem. 1991 Oct 1;201(1):1–21. doi: 10.1111/j.1432-1033.1991.tb16251.x. [DOI] [PubMed] [Google Scholar]
- Jensen P. R., Michelsen O. Carbon and energy metabolism of atp mutants of Escherichia coli. J Bacteriol. 1992 Dec;174(23):7635–7641. doi: 10.1128/jb.174.23.7635-7641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. R., Westerhoff H. V., Michelsen O. Excess capacity of H(+)-ATPase and inverse respiratory control in Escherichia coli. EMBO J. 1993 Apr;12(4):1277–1282. doi: 10.1002/j.1460-2075.1993.tb05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. R., Westerhoff H. V., Michelsen O. The use of lac-type promoters in control analysis. Eur J Biochem. 1993 Jan 15;211(1-2):181–191. doi: 10.1111/j.1432-1033.1993.tb19885.x. [DOI] [PubMed] [Google Scholar]
- Kahn D., Westerhoff H. V. Control theory of regulatory cascades. J Theor Biol. 1991 Nov 21;153(2):255–285. doi: 10.1016/s0022-5193(05)80426-6. [DOI] [PubMed] [Google Scholar]
- Moreno-Sánchez R. Contribution of the translocator of adenine nucleotides and the ATP synthase to the control of oxidative phosphorylation and arsenylation in liver mitochondria. J Biol Chem. 1985 Oct 15;260(23):12554–12560. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo F., Serrano R. Growth control strength and active site of yeast plasma membrane ATPase studied by site-directed mutagenesis. Eur J Biochem. 1989 Dec 22;186(3):501–507. doi: 10.1111/j.1432-1033.1989.tb15235.x. [DOI] [PubMed] [Google Scholar]
- Ruyter G. J., Postma P. W., van Dam K. Control of glucose metabolism by enzyme IIGlc of the phosphoenolpyruvate-dependent phosphotransferase system in Escherichia coli. J Bacteriol. 1991 Oct;173(19):6184–6191. doi: 10.1128/jb.173.19.6184-6191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Respiratory control in Escherichia coli. FEBS Lett. 1980 Oct 20;120(1):128–130. doi: 10.1016/0014-5793(80)81062-3. [DOI] [PubMed] [Google Scholar]
- Walsh K., Koshland D. E., Jr Characterization of rate-controlling steps in vivo by use of an adjustable expression vector. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3577–3581. doi: 10.1073/pnas.82.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., Groen A. K., Van Roermund C. W., Tager J. M. Factors determining the relative contribution of the adenine-nucleotide translocator and the ADP-regenerating system to the control of oxidative phosphorylation in isolated rat-liver mitochondria. Eur J Biochem. 1984 Jul 16;142(2):417–424. doi: 10.1111/j.1432-1033.1984.tb08303.x. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V. Control, regulation and thermodynamics of free-energy transduction. Biochimie. 1989 Aug;71(8):877–886. doi: 10.1016/0300-9084(89)90071-0. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Plomp P. J., Groen A. K., Wanders R. J., Bode J. A., van Dam K. On the origin of the limited control of mitochondrial respiration by the adenine nucleotide translocator. Arch Biochem Biophys. 1987 Aug 15;257(1):154–169. doi: 10.1016/0003-9861(87)90554-6. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., van Heeswijk W., Kahn D., Kell D. B. Quantitative approaches to the analysis of the control and regulation of microbial metabolism. Antonie Van Leeuwenhoek. 1991 Oct-Nov;60(3-4):193–207. doi: 10.1007/BF00430365. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., Nielsen J., Hansen F. G. Promoters of the atp operon coding for the membrane-bound ATP synthase of Escherichia coli mapped by Tn10 insertion mutations. Mol Gen Genet. 1982;188(2):240–248. doi: 10.1007/BF00332682. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


