Abstract
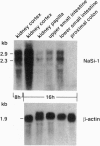
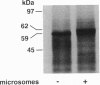
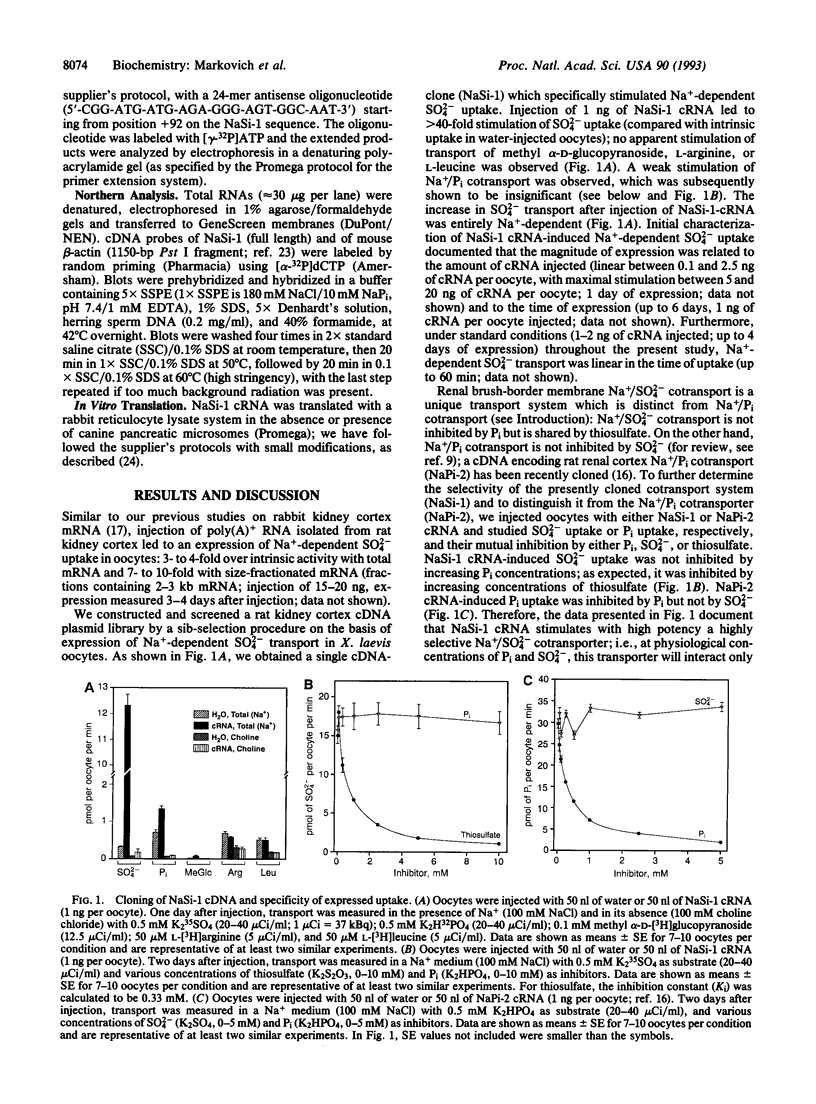
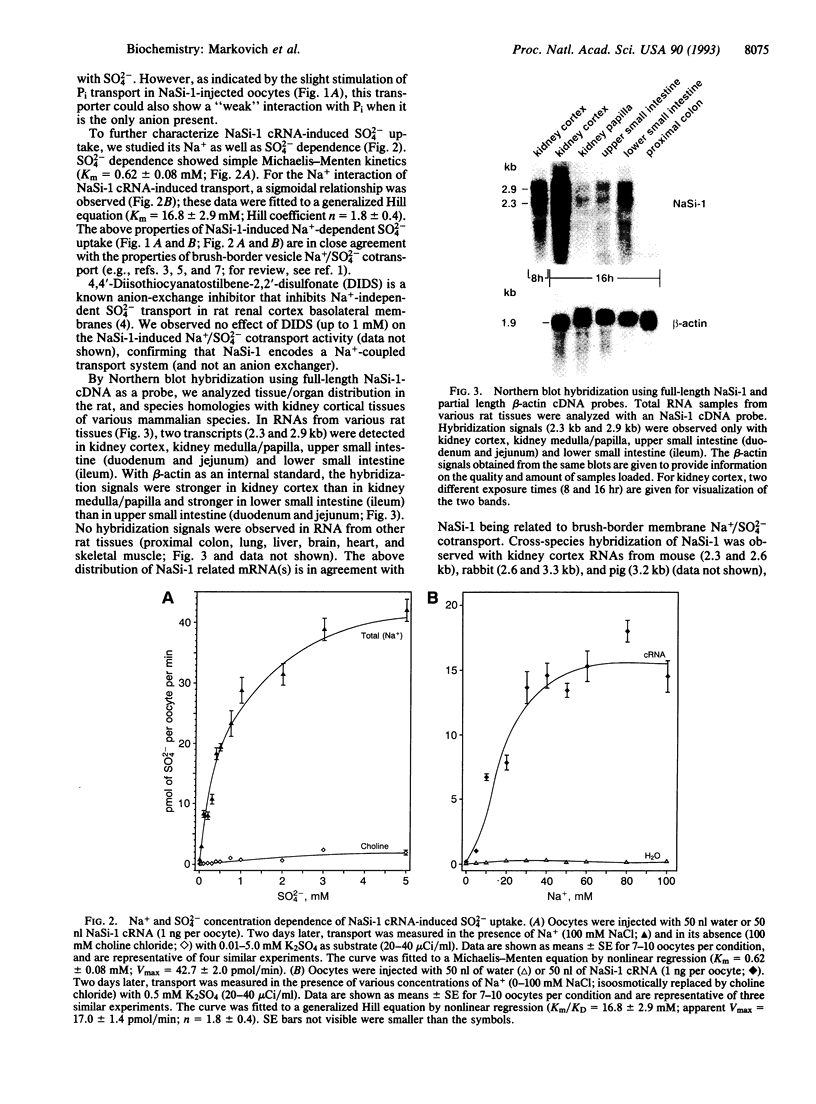
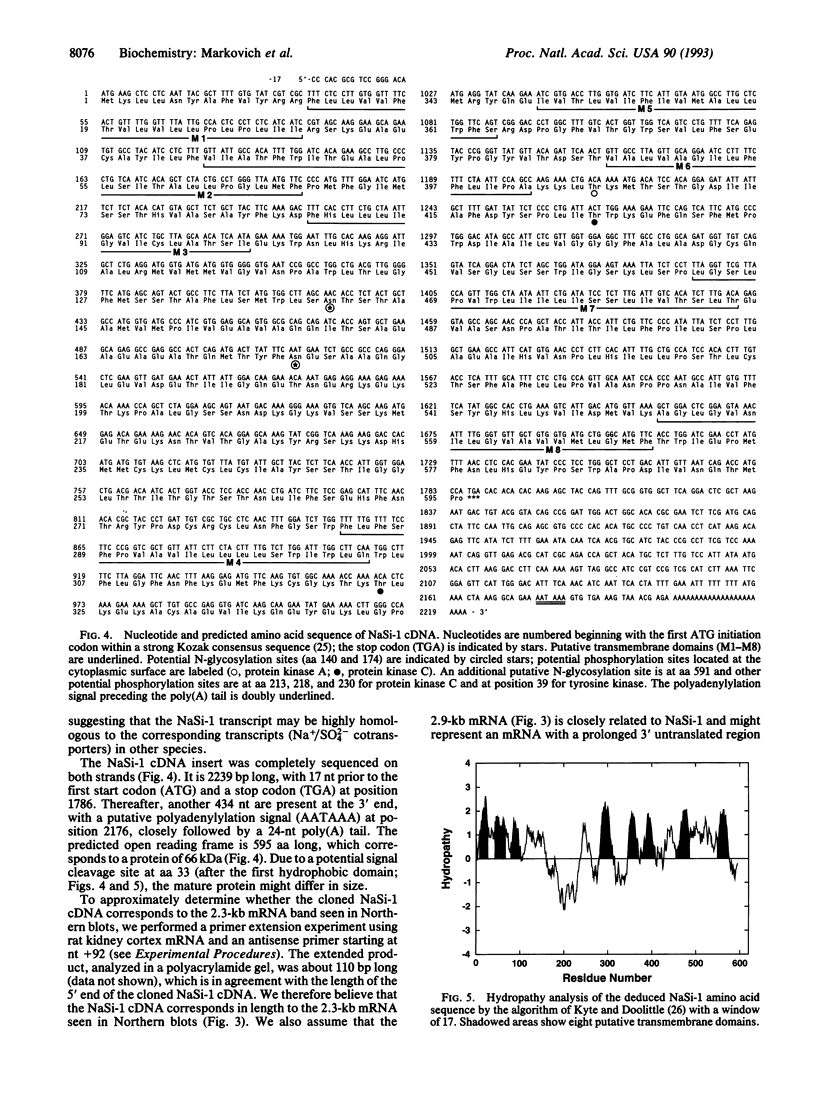
Injection of rat kidney cortex mRNA into Xenopus laevis oocytes leads to a stimulation of Na(+)-dependent SO4(2-) uptake. Based on this information, we have isolated from a corresponding library a cDNA (NaSi-1) that is most likely related to a Na+/SO4(2-) cotransport system. NaSi-1 cRNA leads in a time- and dose-dependent manner to specific stimulation of Na(+)-dependent SO4(2-) uptake in oocytes. The apparent affinity constants of the NaSi-1 cRNA-expressed transport resemble those of Na+/SO4(2-) cotransport in brush-border membrane. The NaSi-1 cDNA contains 2239 bp [including a poly(A) tail] and encodes a protein of 595 amino acids (66.05 kDa); the hydropathy profile suggests at least eight membrane-spanning regions. In vitro translation of NaSi-1 cRNA results in a protein of the expected size and suggests glycosylation. Northern blot analysis shows signals of 2.3 and 2.9 kb in kidney (more abundant in cortex than in papilla/medulla) and in mucosa of small intestine of rats. The above data indicate that we have structurally identified a membrane protein involved in renal and small-intestinal brush-border membrane Na+/SO4(2-) cotransport.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn G. A., Murer H. Functional roles of Na+ and H+ in SO2-4 transport by rabbit ileal brush border membrane vesicles. J Membr Biol. 1984;78(3):177–186. doi: 10.1007/BF01925966. [DOI] [PubMed] [Google Scholar]
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Bertran J., Magagnin S., Werner A., Markovich D., Biber J., Testar X., Zorzano A., Kühn L. C., Palacin M., Murer H. Stimulation of system y(+)-like amino acid transport by the heavy chain of human 4F2 surface antigen in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5606–5610. doi: 10.1073/pnas.89.12.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran J., Werner A., Moore M. L., Stange G., Markovich D., Biber J., Testar X., Zorzano A., Palacin M., Murer H. Expression cloning of a cDNA from rabbit kidney cortex that induces a single transport system for cystine and dibasic and neutral amino acids. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5601–5605. doi: 10.1073/pnas.89.12.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran J., Werner A., Stange G., Markovich D., Biber J., Testar X., Zorzano A., Palacin M., Murer H. Expression of Na(+)-independent amino acid transport in Xenopus laevis oocytes by injection of rabbit kidney cortex mRNA. Biochem J. 1992 Feb 1;281(Pt 3):717–723. doi: 10.1042/bj2810717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bästlein C., Burckhardt G. Sensitivity of rat renal luminal and contraluminal sulfate transport systems to DIDS. Am J Physiol. 1986 Feb;250(2 Pt 2):F226–F234. doi: 10.1152/ajprenal.1986.250.2.F226. [DOI] [PubMed] [Google Scholar]
- David C., Ullrich K. J. Substrate specificity of the luminal Na(+)-dependent sulphate transport system in the proximal renal tubule as compared to the contraluminal sulphate exchange system. Pflugers Arch. 1992 Aug;421(5):455–465. doi: 10.1007/BF00370256. [DOI] [PubMed] [Google Scholar]
- Deguchi Y., Yamato I., Anraku Y. Nucleotide sequence of gltS, the Na+/glutamate symport carrier gene of Escherichia coli B. J Biol Chem. 1990 Dec 15;265(35):21704–21708. [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Kong C. T., Yet S. F., Lever J. E. Cloning and expression of a mammalian Na+/amino acid cotransporter with sequence similarity to Na+/glucose cotransporters. J Biol Chem. 1993 Jan 25;268(3):1509–1512. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lücke H., Stange G., Murer H. Sulphate-ion/sodium-ion co-transport by brush-border membrane vesicles isolated from rat kidney cortex. Biochem J. 1979 Jul 15;182(1):223–229. doi: 10.1042/bj1820223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magagnin S., Bertran J., Werner A., Markovich D., Biber J., Palacín M., Murer H. Poly(A)+ RNA from rabbit intestinal mucosa induces b0,+ and y+ amino acid transport activities in Xenopus laevis oocytes. J Biol Chem. 1992 Aug 5;267(22):15384–15390. [PubMed] [Google Scholar]
- Magagnin S., Werner A., Markovich D., Sorribas V., Stange G., Biber J., Murer H. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovich D., Stange G., Bertran J., Palacin M., Werner A., Biber J., Murer H. Two mRNA transcripts (rBAT-1 and rBAT-2) are involved in system b0,(+)-related amino acid transport. J Biol Chem. 1993 Jan 15;268(2):1362–1367. [PubMed] [Google Scholar]
- Murer H. Homer Smith Award. Cellular mechanisms in proximal tubular Pi reabsorption: some answers and more questions. J Am Soc Nephrol. 1992 Jun;2(12):1649–1665. doi: 10.1681/ASN.V2121649. [DOI] [PubMed] [Google Scholar]
- Pajor A. M., Hirayama B. A., Wright E. M. Molecular evidence for two renal Na+/glucose cotransporters. Biochim Biophys Acta. 1992 Apr 29;1106(1):216–220. doi: 10.1016/0005-2736(92)90241-d. [DOI] [PubMed] [Google Scholar]
- Renfro J. L., Clark N. B., Metts R. E., Lynch M. A. Glucocorticoid inhibition of Na-SO4 transport by chick renal brush-border membranes. Am J Physiol. 1989 Jun;256(6 Pt 2):R1176–R1183. doi: 10.1152/ajpregu.1989.256.6.R1176. [DOI] [PubMed] [Google Scholar]
- Schneider E. G., Durham J. C., Sacktor B. Sodium-dependent transport of inorganic sulfate by rabbit renal brush-border membrane vesicles. Effects of other ions. J Biol Chem. 1984 Dec 10;259(23):14591–14599. [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallgren L. G. Inorganic sulphates in relation to the serum thyroxine level and in renal failure. Acta Med Scand Suppl. 1980;640:1–100. [PubMed] [Google Scholar]
- Tenenhouse H. S., Lee J., Harvey N. Renal brush-border membrane Na(+)-sulfate cotransport: stimulation by thyroid hormone. Am J Physiol. 1991 Sep;261(3 Pt 2):F420–F426. doi: 10.1152/ajprenal.1991.261.3.F420. [DOI] [PubMed] [Google Scholar]
- Turner R. J. Sodium-dependent sulfate transport in renal outer cortical brush border membrane vesicles. Am J Physiol. 1984 Nov;247(5 Pt 2):F793–F798. doi: 10.1152/ajprenal.1984.247.5.F793. [DOI] [PubMed] [Google Scholar]
- Werner A., Biber J., Forgo J., Palacin M., Murer H. Expression of renal transport systems for inorganic phosphate and sulfate in Xenopus laevis oocytes. J Biol Chem. 1990 Jul 25;265(21):12331–12336. [PubMed] [Google Scholar]
- Werner A., Moore M. L., Mantei N., Biber J., Semenza G., Murer H. Cloning and expression of cDNA for a Na/Pi cotransport system of kidney cortex. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9608–9612. doi: 10.1073/pnas.88.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Hager K. M., Turk E. Sodium cotransport proteins. Curr Opin Cell Biol. 1992 Aug;4(4):696–702. doi: 10.1016/0955-0674(92)90091-p. [DOI] [PubMed] [Google Scholar]