Abstract
Immunotherapy is emerging as the newest pillar of cancer treatment, with the potential to assume a place alongside surgical debulking, radiotherapy, and chemotherapy. Early experiences with antitumor vaccines demonstrated the feasibility and potential efficacy of this approach, and newer agents, such as immune checkpoint blocking antibodies and modern vaccine platforms, have ushered in a new era. These efforts are headlined by work in melanoma, prostate cancer, and renal cell carcinoma; however, substantial progress has been achieved in a variety of other cancers, including high-grade gliomas. A recurrent theme of this work is that immunotherapy is not a one-size-fits-all solution. Rather, dynamic, tumor-specific interactions within the tumor microenvironment continually shape the immunologic balance between tumor elimination and escape. High-grade gliomas are a particularly fascinating example. These aggressive, universally fatal tumors are highly resistant to radiotherapy and chemotherapy and inevitably recur after surgical resection. Located in the immune-privileged central nervous system, high-grade gliomas also use an array of defenses that serve as direct impediments to immune attack. Despite these challenges, vaccines have shown activity against high-grade gliomas, and anecdotal, preclinical, and early clinical data bolster the notion that durable remission is possible with immunotherapy. Realizing this potential, however, will require an approach tailored to the unique aspects of glioma biology.
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor in adults, with an incidence of 2 to 3 per 100,000 (1). Despite recent advances in chemotherapy, radiotherapy, and surgical resection, GBM remains a devastating diagnosis with a median survival duration of 14.6 months (2). Although GBM exploits many of the same molecular pathways that drive aggressive behavior in other solid tumors, several characteristics of GBM deserve special consideration. In most solid tumors, metastasis is a sentinel event in cancer progression and a frequent harbinger of incurable disease. Multifocal GBM, however, is atypical in that it remains unclear whether a multifocal disease pattern represents disease spread or recurrent de novo tumor development. Furthermore, metastasis outside the central nervous system (CNS) has been reported (3), but is infrequent and not a primary cause of morbidity and mortality.
Despite the ability to reliably achieve gross total resection with modern surgical techniques, neoplastic infiltration beyond the radiographically defined tumor margins leads to inevitable recurrence. Adjuvant therapy with radiation and alkylating chemotherapeutic agents, such as temozolomide and carmustine (2, 4), may delay disease progression, but outgrowth of resistant clones limits response durability. Mechanisms underlying resistance to radiochemotherapy are a topic of intense investigation with attention particularly focused on glioma stem cells (5), which are characteristically enriched for activity of the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT; ref. 6), and have demonstrated resistance to alkylating agents (7) and ionizing radiation (8). In addition, glioma stem cells are remarkably malleable, as illustrated by the finding that this cell population can differentiate into pericytes and vascular endothelium (9, 10). Such plasticity also potentiates the characteristic molecular heterogeneity reflected in the distinction “multiforme” (11). Accordingly, recent work has parsed high-grade gliomas into subclasses (12) and identified molecular profiles, such as isocitrate dehydrogenase mutations (IDH1/IDH2; ref. 13), MGMT methylation status (14), and EGFR amplification (12) with clear prognostic significance. Even these more discriminating classification schemes, however, are incomplete as they fail to account for intratumoral heterogeneity, which may present a more significant therapeutic challenge (15). One strategy for circumventing the lack of a clearly targetable molecular signature is to intervene in a process that is presumably critical to all cells comprising the tumor mass, such as angiogenesis. However, GBM cells have demonstrated a remarkable capacity to escape angiogenesis inhibitors through several mechanisms, including enhanced migratory behavior via upregulation of matrix metallo-proteinases (16).
Immunotherapy has recently emerged into the clinical mainstream with the approval of the first antigen-specific agent, sipuleucel-T, for castrate-resistant prostate cancer in 2010 (17), and the approval of an immune checkpoint inhibitor, ipilimumab, for metastatic melanoma in 2011 (18). Historically, immunotherapy for GBM has afforded valuable insights, but failed to generate comparable clinical results with melanoma, renal cell carcinoma, and prostate cancer. There are several fundamental reasons for this discrepancy. Unlike prostate cancer, which expresses reasonably well-characterized tumor-restricted antigens, and melanoma, which is clearly immunogenic, GBM expresses relatively few known tumor-restricted antigens and has been classically considered nonimmunogenic (19). These characteristics, coupled with location in the immunologically privileged brain—which is, in turn, confined within the edema-intolerant cranial vault—have likely tempered enthusiasm for the application of immunotherapy to GBM. Yet, recent successes indicate that immunotherapy for GBM may be effective and well tolerated, with several immunotherapy regimens currently in clinical trials.
Redefining CNS Immune Privilege
Classification of the CNS as an immunologically privileged site originated with the observation that tissues engrafted into the brains of experimental animals were rejected more slowly than tissues transplanted to other sites (20). Subsequent work characterizing the blood–brain barrier (BBB), an absence of conventional lymphatic structures, a paucity of professional antigen-presenting cells (APC) within the brain parenchyma, low levels of major histo-compatibility complex (MHC) molecule expression, and the constitutive expression of immunosuppressive cytokines, such as IL10 and TGFβ, have established the CNS as immunologically distinct. The notion of immunologic privilege, however, is inconsistent with several clinical observations. For example, recent evidence suggests that downregulation of HLA class I expression corresponds with poor prognosis in GBM (21) and low CD4 counts in patients receiving standard therapy for high-grade gliomas portend shorter survival (22). Taken together, these observations suggest that a T-cell response to GBM could potentially modulate outcome.
Preclinical data further elucidate these inconsistencies by describing a highly dynamic immunologic compartment in the CNS. The BBB is relatively impenetrable to circulating immune cells and antibodies in the quiescent state; however, transcription of inflammatory chemokines is upregulated in the presence of danger signals, such as pathogen-associated molecular patterns. Transcription of the IFNγ-inducible chemokines CXCL9, CXCL10, and CXCL11 subsequently potentiates immune cell incursion (23, 24) via interactions with CXCR3, resulting in lymphocyte arrest and binding of cell adhesion molecules (CAM) to α4 and β1 integrins (25). Taken together, these data describe a well-orchestrated mechanism for potential lymphocyte egress into an evolving CNS tumor. Thus, the BBB, which was once thought to be a major impediment to immune cells accessing the CNS, may play little or no role in limiting active T-cell responses.
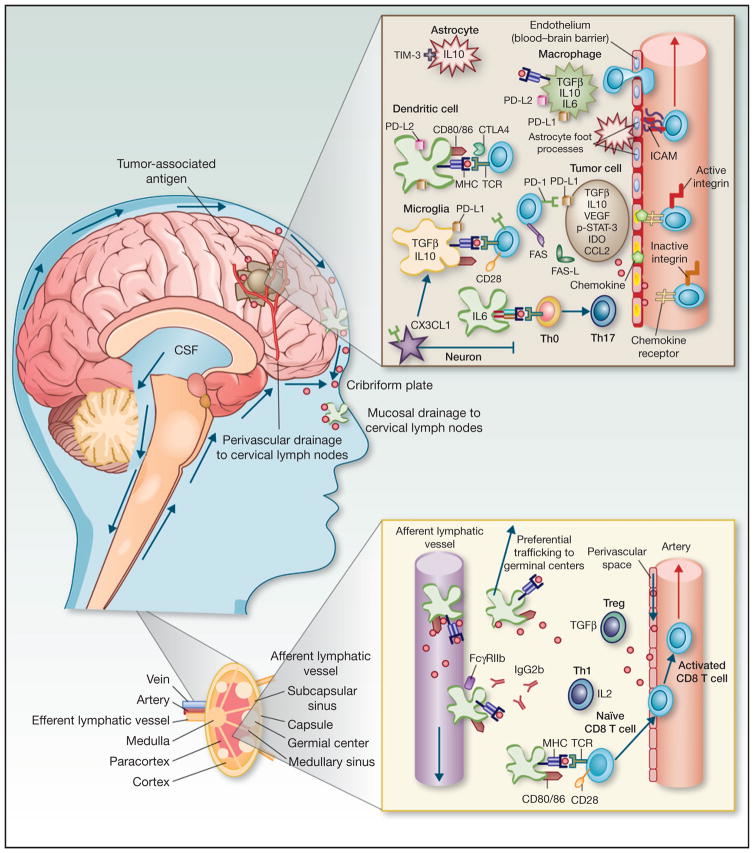
Adaptive immune responses are driven by professional APCs presenting antigen to T and B lymphocytes in a lymph node. Two distinct pathways for antigen egress from the CNS have been described: (i) antigens and antigen-loaded APCs are conveyed in the cerebrospinal fluid, pass through the cribriform plate, and drain to cervical lymph nodes via the nasal mucosa (26); (ii) interstitial fluid drains in a parallel pathway along capillary wall basement membranes and between smooth muscle cells in the tunica media of muscular arteries to reach the cervical lymph nodes (27). The relative role of each of these routes in tumor antigen presentation is unknown and deserves further study. Once an antigen reaches the cervical lymph nodes, however, it is likely that immune responses to CNS antigens may be constrained by the same mechanisms associated with mucosal tolerance induction, including TGFβ production by T regulatory cells (Treg; ref. 28), production of antibodies that signal through inhibitory Fc receptors like CD32 (FcRIIB; ref. 29), and preferential presentation in germinal centers, resulting in skewing toward B-cell predominant responses (30).
Microglia, the resident tissue macrophages of the CNS, are a particularly important component of this system. Microglia are of myeloid origin and migrate to the brain during development, but are phenotypically distinct from macrophages in peripheral tissues (31). The interplay between peripheral and local myeloid cell populations is illustrated by the finding that circulating monocytes readily repopulate the microglia niche in CD11b-herpes simplex virus thymidine kinase (CD11b-HSVTK) transgenic mice receiving intraventricular injection of ganciclovir (32). However, resident microglia are confined to the CNS, and whether cells that express dendritic cell (DC) markers and originate in the CNS have access to peripheral tissues remains a focus of investigation (33). In addition to their prominent role in innate immunity, which is reviewed in detail elsewhere (34), microglia play a crucial, albeit more nebulous role in adaptive immunity by representing antigen to activated lymphocytes, a critical process for preventing deletion of lymphocytes that enter the brain parenchyma (31). Microglia may work in concert with local DCs, which migrate in from the peripheral circulation and are also found in the meninges and choroid plexus (35), although the relative contributions of resident and migrating APCs in shaping the adaptive immune response is another aspect of CNS immunology that is poorly understood. In summary, CNS antigens may be presented in draining lymph nodes, by local microglia, and/or by immigrating DCs. A number of tolerogenic mechanisms have been identified in each of these pathways, which are depicted in Fig. 1.
Figure 1.
Summary of adaptiveimmune responseactivation and tolerance mechanisms against CNS tumor antigens. Antigens drain to cervical lymph nodes along two distinct pathways: (i) soluble antigens and activated APCs pass through the cribriform plate and are conveyed to cervical lymph nodes via lymphatic pathways draining the nasal mucosa, (ii) soluble antigens in interstitial fluid drain along capillary wall basement membranes and between smooth muscle cells in the tunica media of muscular arteries to reach the cervical lymph nodes. In the cervical lymph nodes, CNS APCs preferentially migrate to germinal centers, stimulating B-cell predominant responses. The presence of CD32 (FcγRIIB) and Tregs producing TGFβ, further blunt the immune response. Activated T cells acquire CNS tropism via expression of α4 and β1 integrins. In the CNS vasculature, these integrins bind CAMs expressed on the surface of vascular endothelial cells, mediating T-cell egress. Once in the tumor microenvironment, expression of immune checkpoint ligands on a variety of cell types indicates that immune checkpoint signaling may play a central role in suppression of cytotoxic effector function. Inhibitory cytokines, including TGFβ, IL10 are also produced bya variety ofcelltypes,including microglia, astrocytes, tumor cells, and immigrating macrophages. CX3CL1(fractalkine) expressed byneurons inhibits activation of microglia. Furthermore, skewing toward Th17 differentiation limits maturation of CD8 T cells from Th0 cells entering the inflamed CNS. This combination of immunosuppressive mechanisms both intrinsic and extrinsic tothe tumor cells themselves is likely to subserveblunted immune responses against glioma antigens. CSF, cerebrospinal fluid.
Glioma Vaccines: Initiating an Antitumor Immune Response
Antitumor vaccines are the most established and well-studied immunotherapeutic modality. A target-specific antigen is administered in the context of danger signals (adjuvant), resulting in antigen uptake by APCs, expression of proinflammatory costimulatory molecules, such as CD80/CD86, and presentation of peptide fragments in the context of MHC molecules. Recognition of the antigen-MHC complex by specific CD4 or CD8 T cells in this milieu triggers T-cell activation, proliferation, tracking to the periphery, homing to sites of antigen expression, and effector function—in particular, lysis of antigen-expressing targets by CD8 T cells. This framework highlights two fundamental challenges in developing effective anti-tumor vaccines. First, unlike pathogenic microorganisms, which are molecularly distinct from host cells, tumor cells are derived from host tissues and, therefore, have similar molecular expression patterns. Second, many tumors have commandeered mechanisms of tolerance induction that have evolved in healthy tissues to protect against autoimmunity. Thus, a proinflammatory stimulus that might be sufficient to initiate a response against an invading organism is frequently inadequate to eliminate tumor cells.
Several known tumor-associated antigens are now being targeted in GBM, including HER-2, TRP-2, gp100, MAGE-1, IL13α2, and AIM-2 (36); however, the most extensively studied vaccine strategies have targeted the tumor-restricted neoantigen EGFR variant III (EGFRvIII; ref. 37). Mutated EGFRs are molecular drivers of malignant cell proliferation, differentiation, survival, invasion, and angiogenesis in many solid tumors, including GBM. EGFRvIII is a truncated, constitutively active EGFR variant expressed in 15% to 60% of GBMs, but only rarely expressed in healthy tissues (38–40). The first clinical trial targeting EGFRvIII in GBM involved administration of a 14-amino acid peptide (PEPvIII) conjugated to the foreign antigen keyhole limpet hemocyanin (KLH; ref. 41). This agent is also known as rindopepimut or CDX-110 (Cell-dex). Eighteen patients harboring tumors with confirmed EGFRvIII expression received the vaccine in combination with standard radiotherapy and chemotherapy. No significant adverse events were reported, and median survival among this cohort was 26 months. Interestingly, 82% of the tumors that eventually recurred in these patients did not express EGFRvIII. This finding is potentially encouraging in light of the immunoediting hypothesis, which postulates that tumors may lose proteins targeted by the immune system in an effort to “escape” immunologic pressure (42). However, caution is warranted, because the effects of radiotherapy and chemotherapy on EGFRvIII have not yet been fully characterized (43). Nevertheless, subsequent trials of peptide vaccines targeting EGFRvIII have been initiated and interim results generally support that the vaccine is immunologically active and has an acceptable safety profile.
Recently, a phase II clinical trial of rindopepimut plus granulocyte-macrophage colony-stimulating factor (GM-CSF; ACT III) in newly diagnosed GBM was completed and a phase II trial of rindopepimut plus GM-CSF in combination with bevacizumab in recurrent GBM (ReACT) reported interval results. ACT III included 65 patients with newly diagnosed GBM who received three biweekly intradermal injections followed by monthly injections until radiographic tumor progression, at which time patients received standard temozolomide. Reported median overall survival (OS; 21.3 months) compared favorably with historic controls (44). The ReACT trial is ongoing and involves randomization to bevacizumab, rindopepimut, or combination therapy. Interval results from ReACT reported a 12-month OS and 3.7-month progression-free survival with rindopepimut plus bevacizumab compared with 7.9 and 2.0 months, respectively, with bevacizumab alone (45). Several other studies are ongoing, notably, including a phase III study of rindopepimut plus GM-CSF in patients with newly diagnosed GBM (ACT IV).
Administration of tumor antigen-loaded DCs has the theoretical advantage of activating host lymphocytes while bypassing the requirement for antigen processing by host APCs, which are often dysfunctional in patients with cancer (46). Trials of DC vaccines targeting EGFRvIII have generated comparable data with peptide vaccine trials. An early feasibility study by Sampson and colleagues involving administration of PEPvIII-KLH–pulsed autologous DCs to 12 patients reported only mild toxicity and a median OS of 22.8 months (47). Polyvalent DC vaccines, including ICT-107 (Immunocellular Therapeutics) and the autologous antigen-based DC-Vax(R)-L Brain (Northwest Biotherapeutics), have also generated encouraging results, with phase I data indicating limited toxicity and median survival in the range of 30 to 40 months (36, 48). A phase II trial of ICT-107 is ongoing (NCT01280552) and a phase III trial of DC-Vax-L is currently recruiting (NCT00045968).
The seminal work in glioma vaccine development took place in an era of cancer immunotherapy dominated by peptide and DC vaccines. More recently, a new generation of vaccine platforms is demonstrating encouraging efficacy for targeted immunotherapy. One particularly promising vaccine platform is recombinant Listeria monocytogenes, a facultative intracellular pathogen that efficiently delivers antigens to the class I processing pathway (49). Live-attenuated listeria vaccines have demonstrated an impressive capacity to protect against tumor challenge and mediate regression of established tumors (50). Early trials in pancreatic cancer have confirmed that recombinant listeria vaccines can be administered safely and stimulate robust antitumor immunity (51). Clinical trials of CRS-207 (Aduro Biotech) are currently ongoing in pancreatic cancer (NCT1417000) and recruiting for mesothelioma (NCT01675765), as well as HPV-associated cancers (ADXS11-001; Advaxis, NCT01671488, NCT 01116245, NCT01598792, NCT01266460). A recombinant live-attenuated listeria vaccine expressing EGFRvIII and NY-ESO-1 has been constructed, and a phase I trial of this agent in patients with GBM (NCT01967758) is currently enrolling (Table 1).
Table 1.
Selected trials of immunotherapy in GBM
| Reference or NCCT identifier | Design | No. of Subjects | Phase | Results/endpoints |
|---|---|---|---|---|
| Completed | ||||
| (41) | PEPvIII-KLH was administered 2 weeks after surgery and once per month thereafter until radiographic progression. | 18 | II | PFS: 14.2 Months MS: 26 Months |
| (36) | 21 patients (17 newly diagnosed, 3 recurrent, and 1 brainstem glioma) underwent leukapheresis; DCs were isolated and pulsed with tumor-associated antigen peptides administered at 3-week intervals. | 21 | I | PFS 16.9 months MS: 38.4 months |
| (48) | Glioma lysate–pulsed DCs and imiquimod or poly-ICLC adjuvant were administered every 3 months until tumor progression. | 23 | I | PFS: 15.9 months MS: 31.4 months |
| (44) | CDX-110 was administered in three biweekly intradermal injections followed by monthly injections until radiographic tumor progression; patients then received temozolomide. | 65 | II | MS: 21.3 months |
| Ongoing | ||||
| NCT01480479 | A randomized trial of patients receiving standard-of-care surgery, radiation, and temozolomide with half of the patients randomized to receive CDX-110. | 440 | III | MS |
| NCT00045968 | Randomized, controlled trial with participants in the placebo arm receiving autologous PBMC, while participants in the experimental arm will receive DCVax-L; treatment will be given at days 0, 10, and 20 and weeks 8, 16, 32, 72, 96, and 120. | 300 | III | PFS MS |
| NCT01280552 | All patients receive standard of care surgery, radiation and temozolomide; after a 6-week washout period, patients undergo apheresis and DCs are purified, cultured, and pulsed with synthetic tumor antigens; pulsed DCs are then administered to the experimental group, while the placebo group receives unpulsed autologous DCs. | 200 | II | MS |
| NCT00458601 | A multicenter trial of CDX-110 in combination with standard-of-care radiation, surgery, and temolzolomide. | 82 | II | PFS |
| NCT01967758 | Dose escalation study of live-attenuated L. monocytogenes expressing EGFRvIII peptide and full-length NY-ESO-1 will be administered to patients with recurrent EGFRvIII-positive high-grade gliomas; patients will be vaccinated up to four times separated by 21 days. | TBD | I | Safety Immunogenicity |
| NCT01952769 | Patients with diffuse pontine gliomas or recurrent high-grade gliomas will receive CT-011 at 6 mg/kg every other week until disease progression or a serious adverse event occurs. | 30 | I–II | PFS at 6 months Toxicity |
| (85) | In development: a randomized two-arm study of ipilimumab. | TBD | II–III | TBD |
Abbreviations: MS, median survival; PBMC, peripheral blood mononuclear cells; PFS, progression-free survival; TBD, to be decided.
Immune Checkpoints: Objective Antitumor Responses in Multiple Cancers
Multiple mechanisms exist to protect healthy tissues against autoimmunity. In the earliest stages of lymphocyte development, T-cell receptors (TCR) that bind autoantigens are deleted during negative selection in the thymus. This process is imperfect, however, necessitating the evolution of mechanisms that inactivate autoreactive lymphocytes in the periphery. Immune checkpoints, a class of membrane-bound, inhibitory molecules expressed by activated and exhausted lymphocytes, play a critical role, as clearly evidenced in preclinical knockout models of one of the most extensively studied immune checkpoints, Cytotoxic T lymphocyte antigen-4 (CTLA-4). Mice lacking CTLA-4 develop lethal autoimmunity in multiple organ systems at an early age (52). Immune checkpoint molecules are often relatively overexpressed in the setting of advanced cancer, as tumors use immune checkpoint signaling to protect against immunologic elimination. The discovery of immune checkpoints and the development of agents targeting these pathways represent a major breakthrough in the field of cancer immunotherapy.
Immune checkpoint pathways seem to be nonredundant, with distinct signaling mechanisms (53) and locations of activity (54, 55). CTLA-4, for example, is expressed exclusively by T cells and serves as a major checkpoint at the level of initial T-cell activation in secondary lymphoid organs. Mechanistically, CTLA-4 and the activating T-cell molecule CD28 share the ligands CD80 and CD86 (56); however, CTLA-4 binds these ligands with higher affinity, sequestering CD80/CD86 and inhibiting T-cell activation (57). CTLA-4 binding further inhibits T-cell activation by dephosphorylating the CD3ζ chain via activity of SHP2 and PP2A phosphatases (58). One of the important challenges to applying CTLA4 blockade for cancer therapy, however, is the lack of specificity associated with inhibiting a checkpoint that functions early in the process of lymphocyte activation. This prediction is clinically manifest in the development of immune-related adverse events, including colitis, hepatitis, dermatitis, and hypophysitis (59). Nevertheless, CTLA-4 blocking antibodies have demonstrated clinically meaningful activity against advanced cancers and the monoclonal antibody ipilimumab (Yervoy; Bristol-Myers Squibb) was the first immune checkpoint–targeted agent to receive the FDA approval based on a survival benefit noted in phase III trials in melanoma (60, 61).
Several recent studies have evaluated the activity of ipilimumab against brain metastases. Most notably, Margolin and colleagues (62) enrolled 72 patients with advanced melanoma and brain metastases. These patients were divided into two cohorts. Cohort A (51 patients) included asymptomatic patients not on steroids, while cohort B (21 patients) included symptomatic patients on a stable steroid regimen. Patients who had previously received immunomodulatory antibodies were excluded, and patients that had received previous radiotherapy were included only if they exhibited subsequent disease progression (63). Patients received one dose of ipilimumab every 3 weeks for four doses and patients with stable disease at 24 weeks received one dose every 12 weeks. Twenty-four percent of patients in cohort A and 10% of patients in cohort B achieved stable or improved CNS disease burden, which was consistent with control of systemic lesions (27% in cohort A and 5% in cohort B). In contrast, recent retrospective data from Mathew and colleagues are less encouraging as they indicated no difference in local disease control in patients undergoing stereotactic radiosurgery alone versus stereotactic radiosurgery in combination with ipilimumab (64). These data should be interpreted with caution, however, as the retrospective nature of the study precluded precise timing and sequencing of radio-therapy and ipilimumab. The relative sequence of radiotherapy and immunotherapy has yet to be formally evaluated in patients with intracranial tumors, but is a fundamental component of combining radiotherapy with CTLA-4 blockade in other tumor models (65).
The precise location at which another major immune checkpoint, programmed cell death-1 (PD-1) encounters its ligand(s) PD-L1 and/or PD-L2 remains unclear; but, there is general agreement that the PD-1 pathway is involved in governing the activity of T cells in peripheral tissues (66). The PD-1 ligands, PD-L1 and PD-L2, are expressed on the surface of tumor cells (67–69) and tumor-associated macrophages (54), as well as supportive structures in tumor environment, such as vascular endothelial cells (70) and presumed bystanders, including neurons (71). Studies have suggested that expression of PD-L1 on the tumor cell surface is associated with a higher likelihood of response to PD-1 blockade (67). Several pharmaceutical entities are actively developing PD-1 (Merck; Bristol-Myers Squibb; Curetech), and PD-L1 (Medimmune; Roche) blocking antibodies. Two of these antibodies have achieved FDA designations that facilitate accelerated approval. Bristol-Myers Squibb’s nivolumab has been granted fast-track status (72) and Merck’s lambrolizumab was recently tabbed as a breakthrough therapy (73). Additionally, a phase I–II trial evaluating the effectiveness of PD-1 blockade with CT-011 (pidilizumab) in patients with recurrent high-grade gliomas opened in November 2013 and is currently recruiting participants (NCT01952769).
Other immune checkpoints that are being investigated for clinical translation include lymphocyte activation gene-3 (LAG-3) and T-cell immunoglobulin mucin-3 (TIM-3). LAG-3 is a CD4 homolog that binds MHC class II (74) and seems to play a role in controlling expansion of previously activated T cells rather than independent modulation of T-cell activity (75). This hypothesis has been borne out in preclinical models (76). TIM-3 is a glycoprotein with extra-cellular immunoglobulin and mucin domains that binds Galectin-9. Like LAG-3, TIM-3 seems to be principally involved in fine-tuning ongoing immune responses (77).
Combination Immunotherapy
Studies in checkpoint signaling illustrate a general tenet of immune tolerance: It is generated, maintained, and refined by multiple overlapping, yet nonredundant pathways, which operate in distinct anatomic and physiologic compartments. A striking clinical example comes from a recent clinical trial combining nivolumab and ipilimumab in patients with metastatic melanoma (78). The authors reported greater than 80% reduction in tumor burden in 53% of patients, albeit with a toxicity profile somewhat more pronounced than that observed using ipilimumab monotherapy. Furthermore, recent work by our group suggests that synergistic responses may be achieved by combing immunotherapy with radiotherapy in a glioma model (79). Several other candidate combinatorial strategies are being evaluated, and accumulating data suggest that intervention at multiple levels of the immune response will be required to achieve maximum antitumor activity.
We suspect that a combination immunotherapy regimen will be required to achieve meaningful responses in GBM. Specifically, such a regimen would involve activation of a systemic tumor antigen-directed response in addition to combinatorial checkpoint blockade and manipulation of the tumor microenvironment. Our focus on vaccines and checkpoint inhibitors in this review reflects an illustrative, though by no means exclusive, means of translating this strategy. This notion is supported by work in other tumors. GVAX has been safely administered in combination with CTLA-4 blockade (80), a combination that has demonstrated efficacy in preclinical models (65, 81) and combining GVAX with PD-1 blockade has been similarly promising (82). Although listeria vaccines are only beginning to be applied to GBM, this vaccine platform may prove particularly advantageous for combinatorial therapy as listeria vaccines can drive T-cell proliferation without PD-1 upregulation (83) and increase T effector to Treg ratios in the brain tumor microenvironment (Jackson, Lim, and Drake; unpublished data). In the process of translating these findings to a combinatorial clinical regimen, it should be noted that intracranial tumors might respond differently to listeria vaccination than peripheral tumors (84). Additional data from our group indicate that myeloid cells in the brain tumor microenvironment may modulate systemic immunologic responses to brain tumor–associated antigens differently than myeloid cells associated with otherwise equivalent non-CNS tumors (Jackson, Lim, and Drake; unpublished data). Taken together, these concepts reinforce the status of the CNS as a unique immunologic compartment, which mandates special considerations for GBM immunotherapy.
Conclusions
GBM remains a devastating clinical diagnosis and significant scientific challenge. Effectively developing immunotherapy against GBM will require combinatorial strategies that target multiple levels of immune tolerance. These efforts will likely be guided by successes in other tumors, but the unique aspects of GBM pathology must remain at the forefront. Many aspects of GBM tumor antigen presentation described in this review are extrapolated from studies conducted in autoimmune and infectious models. Although this framework is useful in considering general principles of CNS immunity, further investigation in neuro-oncologic models is clearly needed. The role of local myeloid cells in GBM pathogenesis and progression is also unclear, but may be critical to understanding the balance between tumor-mediated tolerance and persistent adaptive immune responses. Immune checkpoint blockade is dramatically changing the field of cancer immunotherapy, but effective implementation of checkpoint blockade in GBM will likely require a more detailed understanding of the role of these pathways both within the CNS and peripherally in this patient population. In summary, the unique and poorly understood interplay between peripheral and CNS immune responses in the setting of intracranial tumors makes the challenge of GBM immunotherapy singularly intriguing. Unlocking these connections will be critical to developing efficacious combination immunotherapy regimens for patients with high-grade gliomas.
Footnotes
Disclosure of Potential Conflicts of Interest
M. Lim reports receiving commercial research grants from Accuray and Arbor Pharmaceuticals; speakers bureau honoraria from Accuray and Stryker CMF; and is a consultant/advisory board member for Stryker CMF. C.G. Drake reports receiving commercial research grants from Aduro Biotech, Bristol-Myers Squibb, and Janssen; has ownership interest (including patents) in Compugen; and is a consultant/advisory board member for Bristol-Myers Squibb, Compugen, Dendreon, and Roche/Genentech. No potential conflicts of interest were disclosed by the other author.
References
- 1.Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104:2798–806. doi: 10.1002/cncr.21539. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Romero-Rojas AE, Diaz-Perez JA, Amaro D, Lozano-Castillo A, Chinchilla-Olaya SI. Glioblastoma metastasis to parotid gland and neck lymph nodes: fine-needle aspiration cytology with histopathologic correlation. Head Neck Pathol. 2013;7:409–15. doi: 10.1007/s12105-013-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleinberg LR, Weingart J, Burger P, Carson K, Grossman SA, Li K, et al. Clinical course and pathologic findings after Gliadel and radiotherapy for newly diagnosed malignant glioma: implications for patient management. Cancer Invest. 2004;22:1–9. doi: 10.1081/cnv-120027575. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melguizo C, Prados J, Gonzalez B, Ortiz R, Concha A, Alvarez PJ, et al. MGMT promoter methylation status and MGMT and CD133 immuno-histochemical expression as prognostic markers in glioblastoma patients treated with temozolomide plus radiotherapy. J Transl Med. 2012;10:250. doi: 10.1186/1479-5876-10-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 9.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–52. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin CR, Laterra J. Neuro-oncology: unmasking the multiforme in glioblastoma. Nat Rev Neurol. 2010;6:304–5. doi: 10.1038/nrneurol.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandes AA, Tosoni A, Franceschi E, Sotti G, Frezza G, Amista P, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27:1275–9. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton MG, Roldan G, Magliocco A, McIntyre JB, Parney I, Easaw JC. Determination of the methylation status of MGMT in different regions within glioblastoma multiforme. J Neurooncol. 2011;102:255–60. doi: 10.1007/s11060-010-0307-5. [DOI] [PubMed] [Google Scholar]
- 16.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–99. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 17.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–93. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958–62. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136:1631–47. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- 21.Yeung JT, Hamilton RL, Ohnishi K, Ikeura M, Potter DM, Nikiforova MN, et al. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin Cancer Res. 2013;19:1816–26. doi: 10.1158/1078-0432.CCR-12-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–80. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorbachev AV, Kobayashi H, Kudo D, Tannenbaum CS, Finke JH, Shu S, et al. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T-cell–mediated suppression of cutaneous tumors. J Immunol. 2007;178:2278–86. doi: 10.4049/jimmunol.178.4.2278. [DOI] [PubMed] [Google Scholar]
- 24.Klein RS, Izikson L, Means T, Gibson HD, Lin E, Sobel RA, et al. IFN-inducible protein 10/CXC chemokine ligand 10-independent induction of experimental autoimmune encephalomyelitis. J Immunol. 2004;172:550–9. doi: 10.4049/jimmunol.172.1.550. [DOI] [PubMed] [Google Scholar]
- 25.Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, et al. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–84. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2:269–76. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 27.Laman JD, Weller RO. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol. 2013;8:840–56. doi: 10.1007/s11481-013-9470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong Y, Wang X, Ji Q, Mao X, Tang H, Yi G, et al. CD4+LAP+ and CD4+CD25+Foxp3+ regulatory T cells induced by nasal oxidized low-density lipoprotein suppress effector T cells response and attenuate atherosclerosis in ApoE+/+ mice. J Clin Immunol. 2012;32:1104–17. doi: 10.1007/s10875-012-9699-7. [DOI] [PubMed] [Google Scholar]
- 29.Samsom JN, van Berkel LA, van Helvoort JM, Unger WW, Jansen W, Thepen T, et al. Fc gamma RIIB regulates nasal and oral tolerance: a role for dendritic cells. J Immunol. 2005;174:5279–87. doi: 10.4049/jimmunol.174.9.5279. [DOI] [PubMed] [Google Scholar]
- 30.Harling-Berg CJ, Park TJ, Knopf PM. Role of the cervical lymphatics in the Th2-type hierarchy of CNS immune regulation. J Neuroimmunol. 1999;101:111–27. doi: 10.1016/s0165-5728(99)00130-7. [DOI] [PubMed] [Google Scholar]
- 31.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–62. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 32.Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci U S A. 2012;109:18150–5. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hochmeister S, Zeitelhofer M, Bauer J, Nicolussi EM, Fischer MT, Heinke B, et al. After injection into the striatum, in vitro-differentiated microglia- and bone marrow-derived dendritic cells can leave the central nervous system via the blood stream. Am J Pathol. 2008;173:1669–81. doi: 10.2353/ajpath.2008.080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–71. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuno MA, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immun-other. 2013;62:125–35. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–33. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphrey PA, Wong AJ, Vogelstein B, Friedman HS, Werner MH, Bigner DD, et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res. 1988;48:2231–8. [PubMed] [Google Scholar]
- 39.Yamazaki H, Fukui Y, Ueyama Y, Tamaoki N, Kawamoto T, Taniguchi S, et al. Amplification of the structurally and functionally altered epidermal growth factor receptor gene (c-erbB) in human brain tumors. Mol Cell Biol. 1988;8:1816–20. doi: 10.1128/mcb.8.4.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humphrey PA, Wong AJ, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A. 1990;87:4207–11. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 43.Lesniak MS. Immunotherapy for glioblastoma: the devil is in the details. J Clin Oncol. 2011;29:3105–6. doi: 10.1200/JCO.2011.34.9019. [DOI] [PubMed] [Google Scholar]
- 44.Lai R, Recht L, Reardon DA, Paleologos N, Groves M, Rosenfeld MR, et al. Long-term follow-up of ACT III: a phase II trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma [abstract]. Proceedings of the 16th Annual Scientific Meeting of the Society for Neuro-Oncology; 2011; Orange County, CA. 2011. Abstract nr #IM-03. [Google Scholar]
- 45.Celldex CS. Therapeutics’ rindopepimut demonstrates promising clinical activity in patients with EGFRvIII-positive recurrent glioblastoma at SNO. 2013 [cited 2014 March 9, 2014]. Available from: http://ir.celldex.com/releasedetail.cfm?ReleaseID=809242.
- 46.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, 2nd, Lally-Goss D, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8:2773–9. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–15. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikonomidis G, Paterson Y, Kos FJ, Portnoy DA. Delivery of a viral antigen to the class I processing and presentation pathway by Listeria monocytogenes. J Exp Med. 1994;180:2209–18. doi: 10.1084/jem.180.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brockstedt DG, Bahjat KS, Giedlin MA, Liu W, Leong M, Luckett W, et al. Killed but metabolically active microbes: a new vaccine paradigm for eliciting effector T-cell responses and protective immunity. Nat Med. 2005;11:853–60. doi: 10.1038/nm1276. [DOI] [PubMed] [Google Scholar]
- 51.Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–68. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 53.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19:3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 57.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 58.Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19:4917–24. doi: 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37:499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 62.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–65. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 63.Nieder C. Ipilimumab in patients with melanoma and brain metastases. Lancet Oncol. 2012;13:e277–8. doi: 10.1016/S1470-2045(12)70303-0. [DOI] [PubMed] [Google Scholar]
- 64.Mathew M, Tam M, Ott PA, Pavlick AC, Rush SC, Donahue BR, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013;23:191–5. doi: 10.1097/CMR.0b013e32835f3d90. [DOI] [PubMed] [Google Scholar]
- 65.Wada S, Jackson CM, Yoshimura K, Yen HR, Getnet D, Harris TJ, et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med. 2013;11:89. doi: 10.1186/1479-5876-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatam LJ, Devoti JA, Rosenthal DW, Lam F, Abramson AL, Steinberg BM, et al. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/ L2 expression. Clin Cancer Res. 2012;18:1925–35. doi: 10.1158/1078-0432.CCR-11-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 70.Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2002;9:133–45. doi: 10.1038/sj/mn/7800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Carlsson R, Ambjorn M, Hasan M, Badn W, Darabi A, et al. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J Neurosci. 2013;33:14231–45. doi: 10.1523/JNEUROSCI.5812-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bristol-Myers Squibb Reports First Quarter 2013 Financial Results. Thursday, April 25, 2013. 2013 Q1 Financial Report. cited 2014 March 1. Available from: http://news.bms.com/press-release/financial-news/bristol-myers-squibb-reports-first-quarter-2013-financial-results.
- 73.PD-1 inhibitor becomes ‘breakthrough therapy.’. Cancer Discov. 2013;3:OF14. doi: 10.1158/2159-8290.CD-NB2013-074. [DOI] [PubMed] [Google Scholar]
- 74.Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327–37. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–78. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–41. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 78.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–9. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–12. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olino K, Wada S, Edil BH, Pan X, Meckel K, Weber W, et al. Tumor-associated antigen expressing Listeria monocytogenes induces effective primary and memory T-cell responses against hepatic colorectal cancer metastases. Ann Surg Oncol. 2012;19(Suppl 3):S597–607. doi: 10.1245/s10434-011-2037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liau LM, Jensen ER, Kremen TJ, Odesa SK, Sykes SN, Soung MC, et al. Tumor immunity within the central nervous system stimulated by recombinant Listeria monocytogenes vaccination. Cancer Res. 2002;62:2287–93. [PubMed] [Google Scholar]
- 85.Tanaka S, Louis DN, Curry WT, Batchelor TT, Dietrich J. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat Rev Clin Oncol. 2013;10:14–26. doi: 10.1038/nrclinonc.2012.204. [DOI] [PubMed] [Google Scholar]