Abstract
Transgenic expression of antigen-specific T-cell receptor (TCR) genes is a promising approach for immunotherapy against infectious diseases and cancers. A key to the efficient application of this approach is the rapid and specific isolation and cloning of TCRs. Current methods are often labor-intensive, nonspecific, and/or relatively slow. Here, we describe an efficient system for antigen-specific αβTCR cloning and CDR3 substitution. We demonstrate the capability of cloning influenza-specific TCRs within 10 days using single-cell polymerase chain reaction (PCR) and Gibson Assembly techniques. This process can be accelerated to 5 days by generating receptor libraries, requiring only the exchange of the antigen-specific CDR3 region into an existing backbone. We describe the construction of this library for human γδ TCRs and report the cloning and expression of a TRGV9/TRDV2 receptor that is activated by zoledronic acid. The functional activity of these αβ and γδ TCRs can be characterized in a novel reporter cell line (Nur77-GFP Jurkat 76 TCRα–β–) for screening of TCR specificity and avidity. In summary, we provide a rapid method for the cloning, expression, and functional characterization of human and mouse TCRs that can assist in the development of TCR-mediated therapeutics.
Introduction
T cells play a vital role in the control of viral infections and tumors. T cells are activated by antigen-presenting cells via interactions between peptide-major histocompatibility complex and T-cell receptors (TCRs). This interaction can induce proliferation and the development of effector functions, including cytokine production and cytotoxic activity. T cells can also infiltrate infected or transformed tissues, e.g., as tumor-infiltrating lymphocytes, to perform these effector functions.1,2 However, in some chronic viral infections and tumors, responding effector T cells progressively get exhausted and become dysfunctional.3–5 In addition, control of tumors and/or infection may require large numbers of highly reactive lymphocytes that cannot be achieved due to normal tolerance mechanisms. One effective method to overcome this barrier is the use of therapeutic adoptive transfer of lymphocytes.2,6,7
Adoptive transfer of lymphocytes such as in vitro-expanded or TCR-engineered antigen-specific T cells has been successfully used to control viruses and tumors in patients.8–12 In vitro expansion of viral or tumor-specific T cells require significant time to prepare and the targets are not usually fully characterized. Lymphocytes expressing engineered TCRs and chimeric antigen receptors target specific antigens, with chimeric antigen receptors recognizing surface antigens through immunoglobulin-type interactions10,13 and TCRs recognizing tumor-associated peptide-major histocompatibility complexes. Chimeric antigen receptor therapy directed against surface antigens requires a tumor-associated antigen that can be universally targeted (even on healthy, nontumor tissue) without significant toxicity. Tumor-specific antigens that are targeted by TCRs represent an attractive alternative that can provide greater specificity and reduce nontumor-associated toxicities.14–16 Additionally, engineered T cells expressing high-affinity antigen receptors can be conditioned to overcome immune tolerance, which has been a major limitation for immunotherapy.14,15,17 Apart from the clinical applications, a robust system for the cloning and expression of TCRs is a valuable tool for the investigation of TCR structure and functions.18–20
Techniques to rapidly profile and clone antigen-specific TCRs have improved and shortened the process of TCR-engineered immunotherapy.21,22 These approaches are useful contributions to the field and are able to handle large cell inputs very effectively. However, for certain applications, the reported methods still have some limitations. First, approaches that rely on deep sequencing and cloning of bulk sorted cells can still be limited by target cell numbers. In contrast, single-cell approaches can utilize input sizes starting with a single cell but are less efficient at dealing with high cell number inputs (greater than 10,000 cells). As a result, single-cell methods are best directed at defined samples such as antigen-specific responses or tissue-associated infiltrating cells. Second, for bulk sorting, pairing of TCR chains requires algorithmic imputation, which can have difficulty dealing with cells expressing two distinct TCR chains of one type (e.g., two TCRα chains), which are quite common. A recently reported algorithm has addressed this concern and efficiently pairs bulk processed TCRs, using barcoded pools of cells.23 This method, though, requires relatively large inputs to successfully pair and would likely not be appropriate for very small sample sizes as might be obtained from tissue biopsies or tetramer sorting of small populations.
Third, while currently described methods are able to generate full-length receptors either by synthesis or by 5’ rapid amplification of cDNA ends (RACE)-associated approaches at the single cell level, these methods often are reported to require expansion of the isolated cells prior to TCR isolation. Lastly, the majority of antiviral and antitumor adoptive therapy has focused on αβ T-cell clones due to their exquisite antigen specificity. However, γδ T cells have also been shown to mediate antiviral and antitumor effects and are novel candidates for therapeutic development.24,25 To date, there is little research about profiling and utilizing the TCRγδ repertoire for therapeutic purposes, and as such applying γδ T cells for immunotherapeutic applications may be a promising future approach in conjunction with traditional TCRαβ techniques. Therefore, it is important to establish a system to define the repertoire and functional activity for γδ T cells. Additionally, improving efficiencies for cloning αβ TCRs from single cells may have complementary uses in the lab and in the clinic.
To overcome these limitations, we developed a rapid cloning and expression system for specific TCRs. In conjunction with single-cell multiplex polymerase chain reaction (PCR) techniques for TCRαβ or TCRγδ profiling,26,27 and Gibson Assembly cloning of synthesized DNA, we were able to rapidly sequence and clone specific TCRs in a retroviral expression vector. Moreover, by generating V region-specific libraries, this protocol can be significantly accelerated by only requiring the substitution of the CDR3 region,28 resulting in cloned TCRs in appropriate expression vectors in as little as 5 days after cell isolation with highly robust, inexpensive methods. Thus, this protocol provides an efficient and relatively high-throughput means for TCR engineering for therapeutic or research applications.
Results
Paired TCRγδ analysis of human PBMC samples at the single cell level
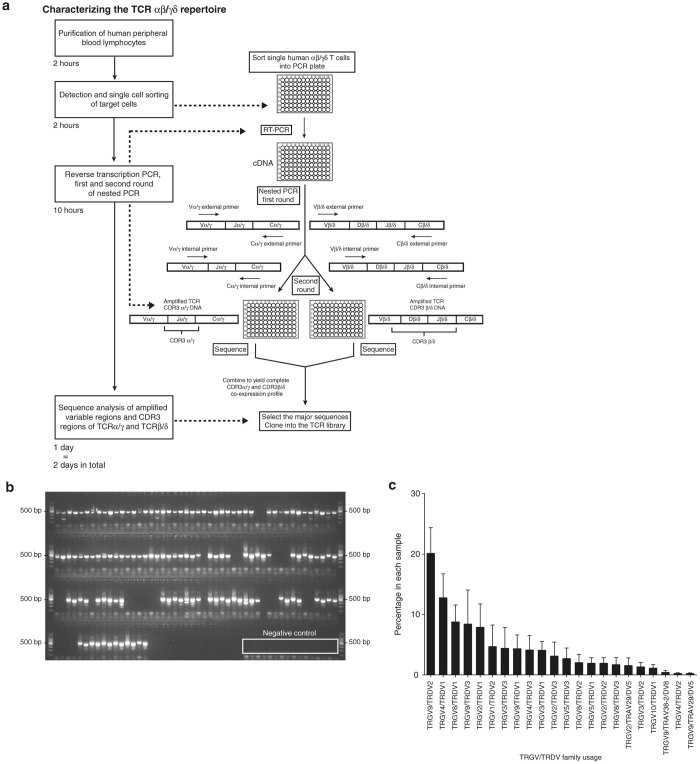
Following on our previously reported single-cell PCR protocols for TCRαβ,26,27 we developed a similar strategy to characterize the paired TCRγδ repertoire in humans (Figure 1a). The primers were designed for all nonpseudogene TRGV and TRDV regions along with antisense primers for their respective constant regions. Two sets of primers (external and internal) were designed in order to perform a nested PCR (Table 1). The PCR products were examined by agarose gel electrophoresis before sequencing (Figure 1b). The average success rate for obtaining paired CDR3γ and CDR3δ sequences at the single cell level from human peripheral blood mononuclear cell (PBMC) samples is 71.25 ± 18.75%. The TRGV/TRDV family usage was determined from the multiplex PCR products (Figure 1c). On average, 20% of the sequences from our analysis of 14 human PBMCs were TRGV9/TRDV2. This technique along with the established mouse and human αβ single-cell multiplex PCR offers a rapid method (turnaround time ~3 days per 160 cells) for characterizing paired TCRγδ chains at the single cell level. The data of paired TRGV/TRDV family usage percentage in each human sample are shown in Supplementary Table S1.
Figure 1.
Unbiased single-cell amplification of paired T-cell receptor (TCR) CDR3 regions. (a) Overview of the multiplex polymerase chain reaction (PCR) protocol to amplify and sequence paired TCR CDR3 α/γ and CDR3 β/δ. After sorting single human αβ or γδ T cells into a 96-well plate, reverse transcription is performed to obtain single-cell cDNA. Taking human γδ T cells as an example, a first round of PCR is performed by using an external primer mixture of nine TRGV and eight TRDV sense and single TRGC and TRDC antisense primers following reverse transcription (RT) PCR. The first-round PCR products are subjected to two separate second-round PCRs using a corresponding internal primers mix (nine sense TRGV, single antisense TRGC, and eight sense TRDV, single antisense TRDC, respectively). The timeline of this process is shown on the left. (b) An agarose gel electrophoresis image of TCR segments containing CDR3γ and CDR3δ is shown. Paired CDR3γ and CDR3δ products from the same cell were loaded in adjacent lanes. Negative control PCR reactions are shown in the boxed region and in the ladder lane, a 500 bp label is shown. (c) Paired TRGV/TRDV usage is determined by multiplex PCR and sequencing (n=14 human apheresis rings). The percentage of different TRGV/TRDV usage in each sample was assessed (mean ± standard error of the mean).
Table 1. Primers targeting human T-cell receptor-γ (TRGV) and δ (TRDV) genes.
| TRGV gene(s) targeted by primer | External primer sequence | Internal primer sequence |
|---|---|---|
| HuTRGV3.5 | 5′TCTTCCAACTTGGAAGGG3’ | 5′GGTCATCTGCTGAAATCAC3′ |
| HuTRGV7 | 5′TCTTCCAACTTGCAAGGG3’ | 5′GGTCATCTGCTGTAATCACTTG3′ |
| HuTRGVA | 5′GGGTCATCCTGTTTCCAG3′ | 5′TACCTAAGGACCTGTGTAGAGG3′ |
| HuTRGVB | 5′TGGCCTCCCAAAGTACTG3′ | 5′TCCTCTTTCTATGTCCCAGG3′ |
| HuTRGV8 | 5′CCAACTTGGAAGGGAGAAC3′ | 5′AAAATGCCGTCTACACCC3′ |
| HuTRGV9 | 5′CCAGGTCACCTAGAGCAAC3′ | 5′TGTCCATTTCATATGACGG3′ |
| HuTRGV10 | 5′TTATCAAAAGTGGAGCAGTTC3′ | 5′CAGCTATCCATTTCCACGG3′ |
| HuTRGV11 | 5′GAACAACCTGAAATATCTATTTCC3′ | 5′CATATCTTGGAAGGCATCC3′ |
| HuTRGV1.2.4.6 | 5′GGGTCATCTGCTGAAATCAC3′ | 5′CCAGGAGGGGAAGGC3′ |
| HuTRGC | 5′GGTGTTCCCCTCCTGG3′ | 5′CCCAGAATCGTGTTGCT3′ |
| TRDV gene(s) targeted by primer | External primer sequence | Internal primer sequence |
| HuTRDV1 | 5′GCCCAGAAGGTTACTCAAG3′ | 5′AGCAAAGAGATGATTTTCCTTA3′ |
| HuTRDV2 | 5′ATTGAGTTGGTGCCTGAAC3′ | 5′TATATCAACTGGTACAGGAAGACC3′ |
| HuTRDV3 | 5′TGTGACAAAGTAACCCAGAGTTC3′ | 5′GGTACTGCTCTGCACTTACGAC3′ |
| HuTRDV4/TRAV14 | 5′CAAACCCAACCAGGAATG3′ | 5′AGGAAAAGGAGGCTGTGAC3′ |
| HuTRDV5/TRAV29 | 5′GCAAGTTAAGCAAAATTCACC3′ | 5′CTGCTGAAGGTCCTACATTC3′ |
| HuTRDV6/TRAV23 | 5′TTGATAGTCCAGAAAGGAGG3′ | 5′CGTTTGACTACTTTCCATGG3′ |
| HuTRDV7/TRAV36 | 5′GACAAGGTGGTACAAAGCC3′ | 5′ATCTCTGGTTGTCCACGAG3′ |
| HuTRDV8/TRAV38-2 | 5′CAGTCACTCAGTCTCAACCAG3′ | 5′TCTGGTACAAGCAGCCTC3′ |
| HuTRDC | 5′CTTCATATTTACCAAGCTTGACAG3′ | 5′GATGACAATAGCAGGATCAAAC3′ |
Establishment of human TCRαβ and TCRγδ retroviral expression clones
Many of the downstream applications of paired TCRαβ or TCRγδ sequence analysis require cloning and expression of the antigen-specific receptors for immunological studies such as structural and functional characterization, biochemical characterization, epitope identification, and gene therapy.18,29,30 Thus, we sought to develop a rapid cloning method to improve on conventional restriction enzyme-mediated ligation techniques, which can be cumbersome and time consuming. In addition, use of restriction enzymes for cloning becomes problematic because of the potential for restriction sites to appear in some variable regions and the nongermline CDR3 sequences of the TCR chains. The vector that we chose for TCR expression is pMICherry, which has been successfully used to construct TCR clones for the generation of retrogenic mice.31,32 A schematic diagram of the cloned TCR chains in the pMICherry vector is shown in Figure 2a.
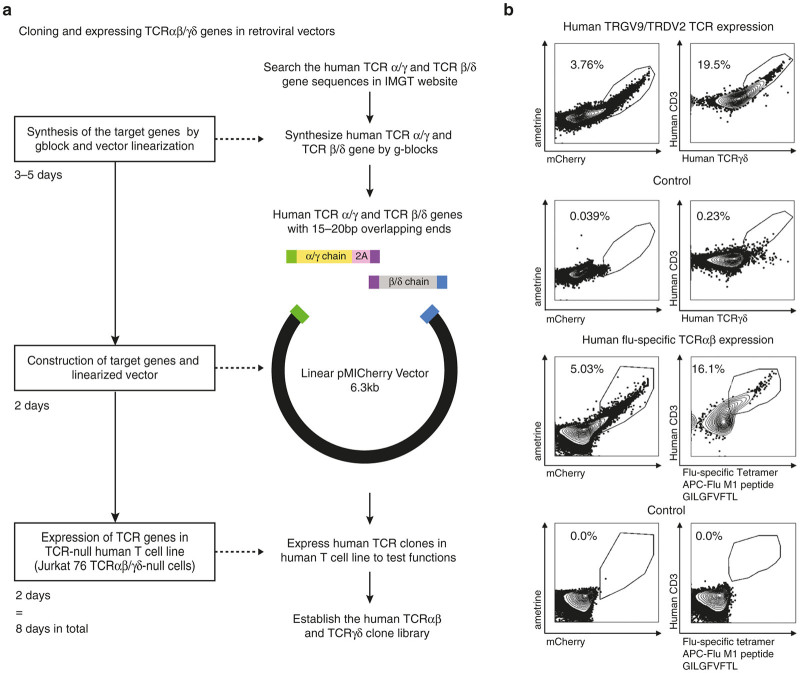
Figure 2.
Rapid cloning and expression of human TCRαβ or TCRγδ in a retroviral vector. (a) A schematic diagram of T-cell receptor (TCR) cloning using gBlock synthesized DNA fragments and a linearized retroviral vector (pMICherry) is shown. Family-specific TRGV and TRDV full-length TCR chains were synthesized with a 15–20 bp overlap sequence (purple) in the 2A region (pink). Together with a linearized pMICherry expression vector, a three-way ligation is performed by using Gibson Assembly Cloning. The timeline of this process is presented on the left. (b) Expression of TCR constructs in the Jurkat 76 TCRα–β– cell line. The vectors with human TRGV9/TRDV2 TCR genes (top panel) and human influenza-specific TCRαβ genes (bottom panel) were cotransfected with the human CD3 construct into the Jurkat 76 TCRα–β– cell line. The flow cytometry results of transfected cells are shown
To clone full-length TCR chains, as a proof of principle, we chose a TRGV9/TRDV2 clone to demonstrate the feasibility of our cloning system, since the TRGV9/TRDV2 clonotype is dominant in the TCRγδ repertoire analysis from human PBMCs (Figure 1c). Similarly, a human TCRαβ pair was chosen derived from an influenza-specific CD8 T cell from an infected individual (unpublished data). Using the IMGT-reported human TRGV, TRDV, TRGC, and TRDC sequences for TCRγδ or human TRAV, TRBV, TRAC and TRBC sequences for TCRαβ and our single-cell CDR3γ and δ or CDR3α and β sequence data we constructed full-length TCRγ and δ chains and TCRα and β chains joined by the 2A “self-cleaving” site in silico. 2A oligopeptides can interact with the ribosomal exit tunnel to terminate sequence translation at the final codon (Pro) of the 2A sequence, and reinitiate translation of the following sequence.33 Recently, multi-cistronic 2A-based retroviral vectors have been widely used for TCR:CD3 structural and functional studies.31,32,34–37 The entire sequence of TCRγ-2A-TCRδ along with an 25 bp overhang complementary to the ends of the linearized pMICherry vector were synthesized in two fragments of approximately 1 kb each as gBlock DNA fragments (Integrated DNA Technologies) with an internal 25 bp overlap in the 2A segment. By using Gibson Assembly Master Mix, we ligated the two gBlocks spanning the TCRγ-2A-TCRδ with the linearized vector in a three-way ligation. The process of cloning is shown in Figure 2a. After this cloning procedure, an average of 70.9% of the colonies picked after transformation were entirely matched with target sequences. The others contained either point mutations resulting from the cloning process or no inserts. To date, we have cloned more than 30 different TCR constructs by using this system, including, mouse and human TCR αβ and TCRγδ. The cloning system is highly reproducible and we have succeeded in generating clones in all attempts.
To test the functionality of the TCR clones that were made following the method described in Figure 2a, we transfected the human TRGV9/TRDV2 construct into the Jurkat 76 TCRα–β– cell line and checked for the cell surface expression by anti-TCRγδ and anti-CD3 antibody staining and flow cytometry. Although Jurkat cells have endogenous CD3, the expression of TCRγδ was not robust. Since γδ T cells do not develop in CD3-deficient mice and patients,38 we cloned the human CD3 complex into an MSCV vector (pMIAmetrine) and cotransfected it with our human TCR constructs. mCherry and ametrine are the reporter genes in the pMICherry vector with human TCR genes and the pMIAmetrine vector with human CD3 genes, respectively. We demonstrated that cotransfection of the human CD3 construct with the human TCRγδ and αβ chains can improve the surface expression level of TCR in a Jurkat cell line (Supplementary Figure S1). 3.76% of the cells were double positive for mCherry and ametrine, and 19.5% of the double-positive cells were TCRγδ and CD3 positive, which proved the functionality of our cloning and expression platform (top panel, Figure 2b). We analyzed the expression of the influenza virus-specific TCRαβ by staining the transfected cells with allophycocyanin (APC) conjugated influenza-M1 tetramer (HLA-A*0201, GILGFVFTL) and CD3 antibody. The FACS plot shows that 5.03% of the transfected cells were double positive for mCherry and ametrine, 16.1% of which were positive for tetramer staining (bottom panel, Figure 2b).
Effective TCR activation reporting by Nur77-GFP Jurkat 76 TCRα–β– cells
An important application of TCR cloning and expression is to screen molecules that can activate or inhibit TCR signaling. Thus far, the common methods to detect TCR activation are using ELISA to detect cytokines (e.g., IFNγ) in the cell culture medium,21,22 intracellular staining to report cytokine production by flow cytometry, or qRT-PCR to quantify the mRNA expression of cytokines, which are time-consuming, labor-intensive, and expensive. Hence, we established a TCR activation reporter cell line, Nur77-GFP Jurkat 76 TCRα–β–(NJ76 cells). The Nur77-GFP reporting system has been demonstrated to reflect specific TCR signal strength by GFP expression.39–41 Here, we established the NJ76 cell line by stably transducing Nur77-GFP BAC DNA into Jurkat 76 TCRα–β– cells.
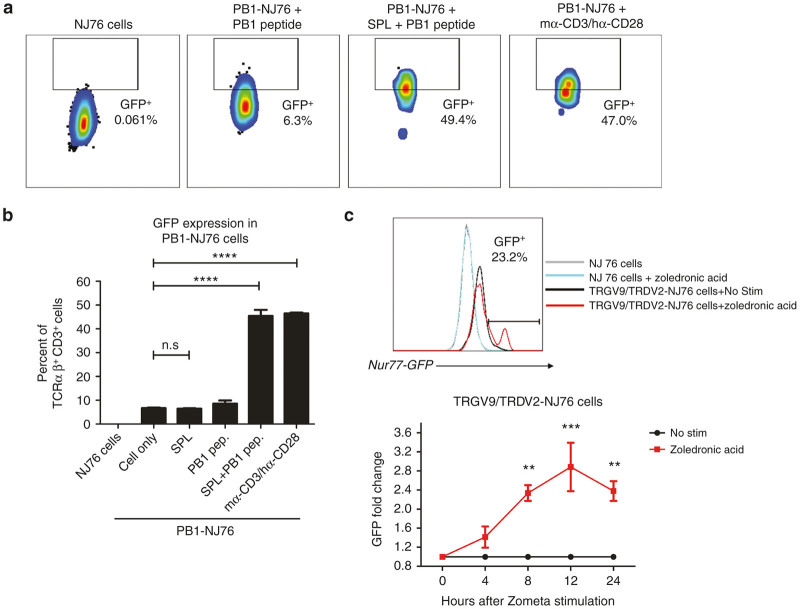
To test the functionality of NJ76 cells in reporting TCR activation, we transfected NJ76 cells with a murine KbPB1703-specific TCR αβ derived from influenza-infected mice along with mouse CD3. The KbPB1703+TCRαβ+ NJ76 cells (PB1-NJ76) were incubated with mouse splenocytes, the influenza PB1703 peptide, splenotytes and peptide, or mouse α-CD3/human α-CD28 as a positive control for 4 hours and GFP expression in transfected NJ76 cells was detected by flow cytometry (Figure 3a). The quantification of GFP levels is shown in Figure 3b. The results show that PB1-NJ76 cells can robustly express GFP after specific peptide stimulation (PB1) with antigen-presenting cells. The gating strategy for GFP detection is shown in Supplementary Figure S2.
Figure 3.
Nur77-GFP Jurkat 76 TCRα–β– cells can report the T-cell receptor (TCR) signaling activation. (a) Following cotransfection of a murine KbPB1703-specific TCR αβ derived from influenza-infected mice and a mouse CD3 construct, the KbPB1703+TCRαβ+ NJ76 cells (PB1-NJ76) were stimulated either with influenza PB1703 peptide alone or PB1703 peptide-pulsed splenotytes for 4 hours. Anti-mouse CD3/α-human CD28 stimulation was also done as a positive control. The GFP expression was assessed by flow cytometry. (b) The quantification of GFP expression in PB1-NJ76 cells is shown. Statistical differences were determined by one-way analysis of variance (ANOVA). (c) The transfected human TRGV9/TRDV2-NJ76 cells were pulsed with zoledronic acid (50 μg/ml) for 3 hours at 37 °C, and washed and incubated at 37 °C for 12 hours. The GFP expression of stimulated NJ76 cells (gray line), nonstimulated TRGV9/TRDV2-NJ76 (black line) and stimulated TRGV9/TRDV2-NJ76 cells (red line) is shown (top panel). Fold change of GFP expression in stimulated TRGV9/TRDV2-NJ76 cells (red line) compared to nonstimulated TRGV9/TRDV2-NJ76 (black line) is shown as a time course (bottom panel). Statistical differences were determined by two-way ANOVA; P < 0.05 was considered statistically significant. Data are mean ± standard error of the mean of two independent experiments. **P < 0.01, ***P < 0.001, ****P < 0.0001. n.s., nonsignificant.
This TCR-activation reporting system has also been tested for TCRγδ signaling. Zoledronic acid (Zometa, Novartis, Basel, Switzerland) is an aminobisphosphonate that has demonstrated antitumor effects via inhibition of tumor growth and angiogenesis and induction of malignant cell apoptosis in humans.42–44 In addition, zoledronic acid can specifically stimulate and expand human TRGV9/TRDV2 cells.45–48 Since it can result in the accumulation of upstream metabolites in the mevalonate pathway, e.g., IPP, which induce the expansion of γδ T cells in vitro and in vivo, zoledronic acid pretreatment can increase the cytolysis of some cancer cell lines by γδ T cells.49 After transfection of the human TRGV9/TRDV2 vector and human CD3 vector into NJ76 cells, we pulsed the transfected TRGV9/TRDV2-NJ76 cells with 50 μg/ml of zoledronic acid for 3 hours, and washed and incubated the cells at 37 °C for 12 hours. We quantified the GFP expression level in transfected TRGV9/TRDV2-NJ76 cells and control cells by flow cytometry. The Nur77-GFP expression level is shown in Figure 3c (top panel). The transfected TRGV9/TRDV2-NJ76 cells showed a significantly higher level of GFP expression, which demonstrates that zoledronic acid can trigger γδ TCR signaling for the stimulation and expansion of γδ T cells. The fold change of GFP expression over time in stimulated TRGV9/TRDV2-NJ76 cells (red line) and nonstimulated TRGV9/TRDV2-NJ76 (black line) is shown in Figure 3c (bottom panel).
Rapid TCR cloning by CDR3 substitution using overlap extension PCR and TCR library
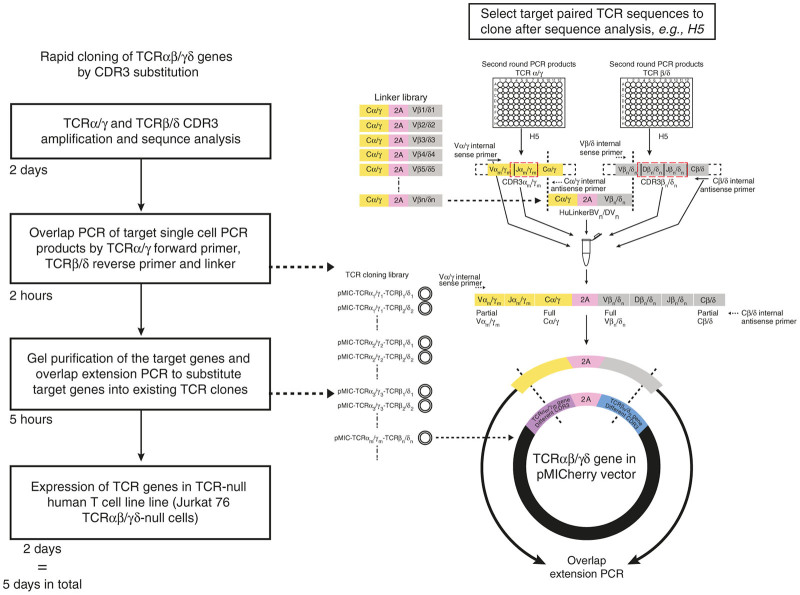
For TCRs, the only hypervariable regions are the CDR3 regions. Thus, cloning full-length TCRs de novo for each application may expend unnecessary resources. To improve on this, we have generated a library of potential TRGV and TRDV “backbone” combinations that only require the swapping of individual CDR3 regions directly from PCR products. For example, in TRGV9/TRDV2 cells from PBMCs of healthy donors, the CDR3s of both γ and δ chains were found to be highly diverse (Table 2). To rapidly generate a library of diverse TRGV/TRDV clones, we designed DNA linkers, whose ends overlap with the TRGC and TRDV in our single-cell PCR products. These DNA linkers contain the TRGC region, 2A and one of the TRDV regions, as is shown in Figure 4. By overlapping PCR with the single-cell PCR products, DNA linkers, TRGV sense primers, and TRDC antisense primers, we can connect any pair of TCRγδ together. Next, we use these first-step PCR products as a mega primer with the appropriate clone from our library as a template for the second-step overlap extension PCR. By using this substitution method, we have successfully cloned different γδ TCRs with matched CDR3s from our human single-cell PCR products. In principle, the same approach could be used with αβ TCRs, although the clone library would be larger. As an estimate, this CDR3 substitution approach can shorten the cloning process to within 5 days (Figure 4).
Table 2. CDR3 amino acid sequences of paired human TRGV9/TRDV2 cells isolated from peripheral blood mononuclear cells (n = 14).
| Paired amino acid sequence in TRGV9-CDR3 region | Paired amino acid sequence in TRDV2-CDR3 region | Frequency |
|---|---|---|
| ALFIQELGKKIKV | ACDVLGDTEGRLI | 2 |
| ALWDGPYYKKL | ACDTVFTGGYSSWDTRQMF | 2 |
| ALWDIPPGQELGKKIKV | ACDTLGETSSWDTRQMF | 2 |
| ALWEAQELGKKIKV | ACDSGGYSSWDTRQMF | 2 |
| ALWEARQELGKKIKV | ACDTLFPGGSATDKLI | 2 |
| ALWEGTRGQELGKKIKV | ACDTVGAHTDKLI | 2 |
| ALWEVGDQELGKKIKV | ACDPLNTGGSFSLYTDKLI | 2 |
| ALWEVHSELGKKIKV | ACDTGGFRSSWDTRQMF | 2 |
| ALWEVHSELGKKIKV | ACDTGGFRSSWDTRQMF | 2 |
| ALWEVLELGKKIKV | ACDTVGMGIRLGDKLI | 2 |
| ALWEVLVGELGKKIKV | ACDILGINTDKLI | 2 |
| ALWEVPELGKKIKV | ACERLGDYVPDKLI | 2 |
| ALWEVQELGKKIKV | ACDRLLGDTDKLI | 2 |
| ALWEVQELGKKIKV | ACDTVAPRIGGLKYTDKLI | 2 |
| ALWEVQELGKKIKV | ACDTVGGPYTDKLI | 2 |
| ALWEVQELGKKIKV | ACDTVGGTAQ | 2 |
| ALWEVQELGKKIKV | ACDTVSGGSTPTWYTDKLI | 2 |
| ALWEVQELGKKIKV | ACDTVSIFTGDTTDKLI | 2 |
| ALWEVRELGKKIKV | ACDTILIFSPTGGDTDKLI | 2 |
| ALWEVRELGKKIKV | ACVPLGDWTDKLI | 2 |
| ALWEVRKQELGKKIKV | ACDTLGDDFDKLI | 2 |
| ALWEVTHNRQELGKKIKV | ACDTLLGTEAWDTRQMF | 2 |
| ALWGGAAGAYYKKL | ACDGKTTDTDKLI | 2 |
| ALWGGELGKKIKV | ACDLLGDTRYTDKLI | 2 |
| ALWVQELGKKIKV | ACVGITGDTDKLI | 2 |
| ALWEAHQELGKKIKV | ACDSLGDSVDKLI | 1 |
| ALWEANKKL | ACDLLRGAGGQIDKLI | 1 |
| ALWEAQELGKKIKV | ACDTVGGAFDTDKLI | 1 |
| ALWEATGLGKKIKV | ACDMGDTRSWDTRQMF | 1 |
| ALWEDLELGKKIKV | ACDTVSWGKNTDKLI | 1 |
| ALWEKEELGKKIKV | ACDTGDWGSSWDTRQMF | 1 |
| ALWEKELGKKIKV | ACDILDSTGGTDLTAQLF | 1 |
| ALWEMTQELGKKIKV | ACDTVRNTGGYAFAGIDKLI | 1 |
| ALWEPQELGKKIKV | ACDKVLGDSSWDTRQMF | 1 |
| ALWESKELGKKIKV | ACEGLGATQSSWDTRQMF | 1 |
| ALWEVGELGKKIKV | ACDKLLGDNELI | 1 |
| ALWEVHKLGKKIKV | ACDSLLGKGTDKLI | 1 |
| ALWEVKELGKKIKV | ACDTLRGSADKLI | 1 |
| ALWEVLQQELGKKIKV | ACDTVPARHTDKLI | 1 |
| ALWEVPVLGKKIKV | ACDTADRSSYTDKLI | 1 |
| ALWEVQELGKKIKV | ACDTLLGDPSSSWDTRQMF | 1 |
| ALWEVQELGKKIKV | ACDTLSGGYARTDKLI | 1 |
| ALWEVQELGKKIKV | ACDTVGILGDTGLGLI | 1 |
| ALWEVRELGKKIKV | ACDTIVSGYDGYDKLI | 1 |
| ALWEVRELGKKIKV | ACSILGDKTSDKLI | 1 |
| ALWEVRQELGKKIKV | ACDTVSQRGGYSDKLI | 1 |
| ALWEVRVQELGKKIKV | ACDPLERVGGPANTDKLI | 1 |
| ALWEVTELGKKIKV | ACDVLGDTGDDKLI | 1 |
| ALWGRELGKKIKV | ACDTVGSNTDKLI | 1 |
| ALWVQELGKKIKV | ACDVLGDTEADKLI | 1 |
| ALYGSPSGEELGKKNQG | ACDPLEGAGGHNTDKLI | 1 |
Figure 4.
Schematic strategy of CDR3 substitution by overlap extension polymerase chain reaction (PCR). Based on the library of TCRαβ and TCRγδ established by the described cloning platform, the strategy for CDR3 substitution using multiplex single cell PCR products and linker DNA is shown. After the sequence analysis of single-cell PCR (shown in Figure 1a), the target pairs of T-cell receptors (TCRs) are chosen from the respective second round paired PCR plates, which include TCRα/γm and TCRβ/δn (m represents a particular TRAV or TRGV subfamily; n represents a particular TRBV or TRDV subfamily). Beforehand, we generated a library of linker DNA by gBlock synthesis (Integrated DNA Technologies) (Figure 5). The linker DNA consists of TRAC/TRGC-2A-TRBVn/TRDVn (n represents the TRB/DV subfamily) sequence. Using the single cell PCR products of α/γ and β/δ chains of the desired clonotypes and the relevant linker gBlock DNA, we carried out an overlap PCR with TRAm/GVm internal sense primer and TRB/DC internal antisense primer. The PCR products were visualized on an agarose gel, and subsequently purified to use as “mega-primer” for cloning into the existing construct from our TCR cloning library (pMIC-TCRα/γm-TCR β/δn) with the same TRGV and TRDV family usage but different CDR3s by overlap extension PCR. The timeline of the whole process is on the left.
Discussion
Here, we report several useful techniques for the analysis of TCR biology, including a single-cell–based protocol for γδ TCR amplification, a rapid protocol for TCR cloning and expression, and a novel platform for functional characterization of TCR clones. Our system provides an accurate and efficient method to approach rapid cloning at the single cell level, which can improve the development of multiple applications, including TCR-mediated immunotherapy.
The most prominent recent immunotherapy approaches involve T-cell checkpoint blockade inhibitors.50 However, these therapies depend on the presence of significant numbers of antitumor T-cell responses. The ex vivo expansion of tumor-infiltrating lymphocytes has also been successful, but is time consuming.51 Our protocol could significantly accelerate the amount of time needed to generate large numbers of antitumor T cells, by allowing the efficient transduction of identified antitumor receptors. The key to the application of this form of directed T-cell immunotherapy is the rapid and accurate isolation and cloning of paired TCRs. Thus far, various methods have been developed for the cloning of TCR genes by traditional PCR, but the acquisition and expression of TCRs is often labor-intensive, time-consuming, expensive, and nonspecific. The system we have described provides efficient acquisition of TCR gene products for cloning based on single-cell isolation, with an amplification success rate of isolated paired single-cell TCRγ and TCRδ CDR3 products of 71.25 ± 18.75% based on total sorted single cells in each sample. We have also developed a platform for screening TCR activation after cloning. By inserting the Nur77 reporter into a Jurkat 76 TCRα-β-cell line, we have generated a useful system for monitoring specific TCR activation, as demonstrated by stimulation of PB1-NJ76 by its cognate influenza-derived peptide and stimulation of TRGV9/TRDV2 T cells with zoledronic acid (Zometa).
Considering the variability of CDR3 sequences and TCR variable regions (approximately 1018 combinations in human TCRγδ cells and 1016 combinations for TCRαβ cells52) and the complexity of cloning all the different clones de novo, we established a method using overlap extension PCR of a linker molecule with amplified single-cell CDR3 products and a constructed γδ TCR library to rapidly (less than 5 days) generate diverse TCR clones. The applications of this rapid approach for immunotherapy are obvious. Tumor-specific T cells have been characterized by broad nonspecific surface phenotypes that can be used to isolate, clone, and express potential tumor-targeted clones.53 The recent advancement of tumor sequencing has allowed for identification of tumor neoantigens and overexpressed self-antigens.54–57 Combining these technologies will allow for promptly characterized and tailored antitumor therapy.
T-cell transfers have also been used for the treatment of opportunistic infections in immunosuppressed patients, particularly after hematopoietic stem cell transplant. The reactivation of herpes viruses like human cytomegalovirus and Epstein-Barr virus is a clinical dilemma that cannot always be addressed with antivirals.24,58,59 Analogous to tumor-infiltrating lymphocyte therapies, ex vivo expansion of antiviral T-cell specificities can be clinically useful, but suffers from similar workflow limitations. By generating a library of specific TCR constructs reactive against a range of viruses and HLA types, TCR-directed therapies could be used prophylactically or immediately at the earliest signs of reactivation.
In addition to these therapeutic applications, our protocol significantly improves the workflow for cloning and expressing TCRs for study in vitro. This can include the characterization of biochemical features of the TCR-peptide-major histocompatibility complex interaction, or, in the case where ligands have not been identified, transduced cell lines can be used for the screening of novel antigens. This is particularly useful in the context of γδ T cells, where very few ligands have been identified and confirmed.18,60 The GFP reporter line we have engineered can be used directly in high-throughput screening platforms; alternative reporters (such as luciferase) can be easily substituted as well.
In conclusion, we introduce a novel method to rapidly clone, express, and characterize the function of paired αβ and γδ TCR chains from single cells. Our platform addresses the nonspecific, labor-intensive, and time-consuming issues of traditional PCR-based cloning and it provides a relatively high-throughput, accurate, and efficient method of TCR engineering for therapeutic or research applications.
Materials and Methods
Subjects and PBMC samples
Samples were obtained on research protocols approved by St. Jude Children’s Research Hospital’s institutional review boards (Memphis, TN). Peripheral whole blood was collected from heparinized apheresis rings from healthy immunocompetent individuals not taking immunomodulatory pharmaceutical agents. PBMCs were isolated via density-gradient centrifugation (GE Healthcare Ficoll-Paque PLUS, Marlborough, MA), and red blood cells (RBCs) were removed using RBC lysis buffer (8.3 g NH4Cl, 1 g KHCO3, and 1 ml 0.1% Phenol Red in 1 l distilled water). Isolated PBMCs were frozen in −80 °C for future use. All PBMCs used in the paper were stored frozen. Compared to fresh PBMC data from healthy apheresis rings, our frozen PBMCs did not have a significantly lower success rate for single-cell amplification (data not shown).
Single-cell sorting and staining
PBMCs were treated with human FcR blocking reagent (Miltenyi Biotec, Auburn, CA) on ice for 20 minutes. Human TCRγδ cells were isolated by staining with PE-conjugated anti-human TCRγ/δ (Biolegend, San Diego, CA, clone: B1), fluorescein isothiocyanate (FITC) conjugated anti-human CD3 (Biolegend clone: OKT3), a dump gate consisting of APC-conjugated anti-human CD11b/CD14/CD19 (Biolegend, CD11b clone: ICRF44; Biolegend, CD14 clone: HCD14; Biolegend, CD19 clone: HIB19) and Live/Dead Violet exclusion dye (Invitrogen, Carlsbad, CA, L34955) on ice for 30 minutes. After staining, TCRγ/δ+ CD3+ cells were sorted directly into a 96-well PCR plate (Bio-Rad, Hercules, CA) with a sorter (Model sy3200, Sony Biotech Synergy sorter, Sony Biotech, San Jose, CA) by the following gating strategy: autofluorescence gate—lymphocytes gate—single cell gate—live/dead gate—dump gate (CD11b/14/19)—TCRγδ/CD3 gate (Supplementary Figure S3). The last two columns of the plate were left empty for use as PCR negative controls. After sorting, plates were stored at −80 °C until downstream processing. Human αβ T cells were also isolated using a similar method by using the staining and gating strategy described in this paper.27
Reverse transcription, multiplex, nested single-cell PCR and sequencing
Complementary DNA (cDNA) from TCRγδ and TCRαβ mRNA was reverse transcribed directly from the sorted and stored single cells in the PCR plate without any RNA extraction step using the iScript cDNA Synthesis Kit (Bio-Rad) in a 2.5 μl reaction mix as per the method described previously.26 The cDNA synthesis was carried out by incubating at 25 °C for 5 minutes, 42 °C for 30 minutes, and 80 °C for 5 minutes. Alternatively, we used the SuperScript VILO cDNA synthesis kit (Invitrogen), which produces a higher success rate for single-cell PCR by incubating the reaction mixture at 25 °C for 10 minutes, 42 °C for 60 minutes, and 80 °C for 5 minutes. The TCRαβ transcripts from each cell were amplified by a multiplex nested PCR strategy as described previously.26,27 For amplification of TCRγδ transcripts, the overall strategy was similar to the published TCRαβ amplification, except for the primers described in Table 1. We designed nine TRGV external sense primers, nine TRGV internal sense primers, eight TRDV external sense primers, and eight TRDV internal sense primers targeted for individual TRGV and TRDV families based on the sequences derived from the IMGT database (http://www.imgt.org/genedb/; ref. 61). For the antisense primer, we designed single TRGC external, TRGC internal, TRDC external, and TRDC internal primers complementary to the published TRGC and TRDC sequences in IMGT. Human TRAV14/DV4, TRAV23/DV6, TRAV29/DV5, TRAV36/DV7, and TRAV38-2/DV8 are shared primers in TRAV and TRDV primer sets. The primers were synthesized by Integrated DNA Technologies and stored at −20 °C at a stock concentration of 100 μmol/l in TE with low EDTA (pH 8.0). The primers for each category (sense external, sense internal of TRGV and TRDV) were combined so that the final concentration of each primer in the mixture was 10 μmol/l. The antisense primers were diluted to 10 μmol/l. The PCR conditions for the TCRγδ nested PCR were 95 °C for 2 minutes, followed by 35 cycles of 95 °C for 20 seconds, 53 °C for 20 seconds, and 72 °C for 45 seconds, followed by final extension of 72 °C for 7 minutes. The PCR products were run on a 2% agarose gel to check for the success rate of the PCR as well as contamination following which the products were purified by a modified Exonuclease I - rShrimp alkaline phosphatase (ExoSAP-IT, Bio-Rad) method62 to eliminate unincorporated primers and dNTPs for high-quality DNA sequencing. One microliter of the single-cell PCR product was added into the mixture of 4.6 μl of Tris-Cl (50 mmol/l, pH 8.0), 0.2 μl of Exonuclease I and 0.2 μl of rShimp alkaline phosphatase and was incubated at 37 °C for 15 minutes and 80 °C for 15 minutes. The purified PCR products were sequenced using the relevant TRAC, TRBC, TRGC, or TRDC primer. A schematic of the PCR strategy is shown in Figure 1a.
gBlock gene fragments, Gibson Assembly, and transformation
The gBlock gene fragments encoding the library of TRGVs and TRDVs were obtained from Integrated DNA Technologies, Coralville, IA. The expression vector pMICherry (10 μg), which was modified from the parental pMIGII63 by changing GFP to an mCherry reporter, was double digested by EcoR I (20 units) and Xho I (20 units) restriction enzymes (New England Biolabs) at 37 °C for 3 hours as per manufacturer’s instruction. Agarose gel purified-linearized pMICherry vector (100 ng) and 2× TCR gBlock inserts were ligated in a three-way ligation, including the TCRγ gene, TCRδ gene, and linearized vector by using the Gibson Assembly Cloning kit (New England Biolabs) per manufacturer’s instructions. Two microliters of the ligation mixture were transformed into DH5α Competent Escherichia coli (New England Biolabs) per manufacturer’s instructions.
Generation of human CD3 construct
Human CD3 δ, γ, ϵ, and ζ genes were amplified from human PBMC cDNA using the primers in Table 3. All the genes were linked together by overlap PCR with species-specific 2A regions inserted.34 The types and amino acid sequences of the 2As used are shown in Table 4. The CD3 gene complex was then cloned into an MSCV-based retroviral vector that contains an IRES31,32,64 and ametrine as a reporter gene.
Table 3. 2A primers targeting human CD3δ, γ, ϵ, and ζ genes.
| TRGV gene(s) targeted by primer | Primer sequence |
|---|---|
| CD3δ sense | 5′CCCTCACTCCTTCTCTAGGCGCCGGAATTCGCCAGGATGGAACATAGCACG3′ |
| CD3δ antisense | 5′CCACGTCTCCCGCCAACTTGAGAAGGTCAAAATTCAAAGTCTGTTTCACCGGTCCCTTGTTCCGAGCC3′ |
| CD3γ sense | 5′GAATTTTGACCTTCTCAAGTTGGCGGGAGACGTGGAGTCCAACCCAGGGCCCATGGAACAGGGGAAG3′ |
| CD3γ antisense | 5′CCTCGACGTCACCGCATGTTAGCAGACTTCCTCTGCCCTCAGATCTTCTATTCCTCCTCAAC3′ |
| CD3ϵ sense | 5’CAGAGGAAGTCTGCTAACATGCGGTGACGTCGAGGAGAATCCTGGCCCAATGCAGTCGGGCACTC3′ |
| CD3ϵ antisense | 5′GTTTTCTTCCACGTCTCCTGCTTGCTTTAACAGAGAGAAGTTCGTGGCGGATCCTCCGATGCGTCTCTG3′ |
| CD3ζ sense | 5′CTCTCTGTTAAAGCAAGCAGGAGACGTGGAAGAAAACCCCGGTCCCATGAAGTGGAAAGTG3′ |
| CD3ζ antisense | 5′GAGGGAGAGGGGCGGAATTGATCCTCGAGCAATTGTTAGCGAGGGGCCAG3′ |
Table 4. Types and sequences of 2A regions.
| 2A type | 2A amino acid sequence | Separation |
|---|---|---|
| F2A (foot-and-mouth disease virus) | VKQTLNFDLLKLAGDVESNPGP | CD3δ and CD3γ |
| T2A (Thosea asigna virus) | EGRGSLLTCGDVEENPGP | CD3γ and CD3ϵ |
| P2A (porcine teschovirus-1) | ATNFSLLKQAGDVEENPGP | CD3ϵ and CD3ζ |
DNA isolation, cell culture, and transfection
Recombinant pMICherry plasmids with full-length TCRαβ or TCRγδ inserts were isolated in small scale by using a NucleoSpin Plasmid kit (Clontech, Mountain View, CA) and in large scale for transfection using a Plasmid Midi kit (Qiagen, Hilden, Germany) per the manufacturer’s instructions. The Neon Transfection System was used to transfect 10 μg TCRαβ or γδ DNA in the pMICherry vector into the human Jurkat 76 TCRα–β– cell line (2 × 107 cells/ml, 100 μl),65 followed by three pulses with a voltage of 1,350V and a width of 10 ms. The transfected cells were cultured for 48 hours before being assayed for TCRαβ or TCRγδ expression on the surface by FACS analysis. The human Jurkat 76 cells TCRα–β– cell line was cultured in complete-RPMI 1640 medium, which is RPMI 1640 with 10% of fetal bovine serum, 1% Penicillin Streptomycin, and 1% L-glutamine at 37 °C and 5% CO2.
Immunofluorescent and flow cytometric analysis
For surface staining, cells (1–5 × 105) were harvested from culture and washed with FACS buffer (PBS with 1% of BSA and 0.1% sodium azide) prior to staining. The cells were treated with human FcR blocking reagent (Miltenyi Biotec) on ice for 20 minutes, and cells were then treated with various fluorescent conjugated antibodies against cell surface markers in FACS buffer. Human γδ T cells were stained with APC-conjugated anti-human TCRγ/δ (Biolegend, clone: B1) or APC-conjugated anti-human TCRα/β (Biolegend, clone: IP26) and Pacific Blue-conjugated anti-human CD3 (Biolegend, clone: OKT3). For influenza-specific tetramer staining, cells (1–5 × 105) were stained with APC-conjugated Influenza-M1 tetramer (Beckman Coulter, Brea, CA, HLA-A*0201, GILGFVFTL) in FACS buffer at room temperature for 1 hour prior to surface staining with the same staining antibodies described.
Modification of the CDR3 region by two-step overlap extension PCR cloning
The substitution of the CDR3 was carried out by an overlap extension PCR cloning protocol.28 A schematic diagram of the procedure is shown in Figure 4. Briefly, we generated a library of linker DNA by gBlock synthesis at Integrated DNA Technologies (Figure 5). The linker DNA consists of TRGC-2A-TRDVx (X represents the TRDV family) sequence. Using the single-cell PCR products of γ and δ chains of the desired clonotype and the relevant linker gBlock DNA, we carried out an overlap PCR. The PCR reaction was set up and carried out as follows: 12.5 μl 2× Phusion high-fidelity DNA polymerase (New England Biolabs), 0.25 μl of 100× DMSO, 1 μl of 10 μmol/l TRGV internal sense primer, 1 μl of TRDV internal antisense primer (Table 1), 1 ng of linker DNA, and deionized H2O up to 25 μl. The PCR program was 98 °C for 30 seconds; 34 cycles of each at 98 °C for 10 seconds, 58 °C for 30 seconds, 72 °C for 1 minute; then finally 72 °C for 10 minutes. The PCR products were visualized on a 1% agarose gel, and purified from the gel to use for cloning into the existing construct with the same TRGV and TRDV family usage. The reaction conditions used were as follows: 20 ng of a TCR construct in pMICherry vector with identical TRGV and TRDV but an irrelevant CDR3γ and δ, with 50 ng of the first-step PCR products, 12.5 μl of 2× Phusion high-fidelity DNA polymerase, 0.25 μl of 100× DMSO, and deionized H2O up to 25 μl. The PCR conditions used were 98 °C for 30 seconds; 17 cycles of each at 98 °C for 10 seconds, 65 °C for 30 seconds, 72 °C for 4 minutes; then finally 72 °C for 10 minutes. The PCR products were incubated with 1 μl DpnI enzyme (New England Biolabs, Ipswich, MA) at 37 °C for 1 hour, and 2–3 μl of the digested products transformed into NovaBlue Singles competent cells (EMD Millipore, Darmstadt, Germany).
Figure 5.
Human TCRγδ linker DNA library. Yellow color indicates TC sequence; pink color indicates 2A sequence; and gray color indicates TRDVx sequence.
Nur77-GFP Jurkat 76 TCRα–β– cell line
To characterize the functionality of TCRαβ or γδ clones, we established the Nur77-GFP Jurkat 76 TCRα–β– cell line (NJ76 cells). After linearization of a Nur77-GFP BAC clone (constructed based on pTARBAC)39 by mixing 10 μg BAC DNA, 2 μl 10× reaction buffer, 10 units of PI-SceI restriction enzyme (New England Biolabs), and nuclease-free water to make the volume up to 20 μl with incubation at 37 °C for 3 hours and inactivation at 65 °C for 20 minutes, we added 80 μl of nuclease-free water, 15 μl of sterile sodium acetate (3M, pH 7.0), and 300 μl of ethanol to the reaction mixture, and centrifuged at 12,000g for 30 minutes at 4 °C. The resulting DNA pellet was washed with 75% ethanol, dried in the air, and resuspended by Tris-EDTA buffer (pH 8.0). We used the Neon Transfection System (Invitrogen) following the manufacturer’s instruction to transfect the linearized BAC DNA into the human Jurkat 76 TCRα–β– cell line (2 × 107 cells/ml, 100 μl), with three pulses with a voltage of 1,350V and a width of 10 ms. Cells were then cultured in complete-RPMI 1640 medium containing 500 μg/ml Geneticin (Invitrogen) for selection.
Stimulation of KbPB1703+TCRαβ+ NJ76 cells (PB1-NJ76) by flu peptide PB1
NJ76 cell transfected with a murine KbPB1703-specific TCRαβ derived from influenza-infected mice and transfected cells were incubated with mouse splenocytes (cell number ratio of PB1-NJ76/splenocytes is 2:1), the influenza PB1703-711 peptide (1 μmol/ml), mouse splenocytes and peptide, and mouse α-CD3 (Biolegend, 2C11; 10 μg/ml) and human α-CD28 (Biolegend, CD28.2; 10 μg/ml) in c-RPMI 1640 medium at 37 °C for 4 hours. The GFP expression in the mouse TCRαβ + CD3+ cell population was quantified by flow cytometry.
Stimulation of TRGV9/TRDV2-NJ76 cells by Zoledronic acid
NJ76 cells transfected with a TRGV9/TRDV2 clone were incubated with 50 μg/ml zoledronic acid (Zometa) in c-RPMI 1640 medium at 37 °C for 3 hours, washed three times and incubated for 12 hours. The GFP expression in the TCRγδ + CD3+ cell population was quantified by flow cytometry.
Acknowledgments
This work was supported by the St. Baldrick’s and Assisi Foundations (MD), 5 P30CA02176536 (Development Funds to MD), The Hartwell Foundation Individual Biomedical Research Award (PT), AI107625 (PT), and ALSAC. The authors would like to acknowledge Nicole R. Cunningham, Haverford College, for the Nur77-GFP BAC DNA and Michael Nishimura, Loyola University, for Jurkat 76 TCRα–β– cell line. The authors also would like to thank Jin He and Scarlett Evans for technical support.
The authors declare no conflict of interest.
References
- Restifo, NP, Dudley, ME and Rosenberg, SA (2012). Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, SA and Restifo, NP (2015). Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry, EJ, Ha, SJ, Kaech, SM, Haining, WN, Sarkar, S, Kalia, V et al. (2007). Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27: 670–684. [DOI] [PubMed] [Google Scholar]
- Baitsch, L, Baumgaertner, P, Devêvre, E, Raghav, SK, Legat, A, Barba, L et al. (2011). Exhaustion of tumor-specific CD8⁺ T cells in metastases from melanoma patients. J Clin Invest 121: 2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry, EJ and Kurachi, M (2015). Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15: 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser, MJ, Shapira-Frommer, R, Itzhaki, O, Treves, AJ, Zippel, DB, Levy, D et al. (2013). Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res 19: 4792–4800. [DOI] [PubMed] [Google Scholar]
- Klaver, Y, Kunert, A, Sleijfer, S, Debets, R and Lamers, CH (2015). Adoptive T-cell therapy: a need for standard immune monitoring. Immunotherapy 7: 513–533. [DOI] [PubMed] [Google Scholar]
- Lamers, CH, Willemsen, R, van Elzakker, P, van Steenbergen-Langeveld, S, Broertjes, M, Oosterwijk-Wakka, J et al. (2011). Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 117: 72–82. [DOI] [PubMed] [Google Scholar]
- Chapuis, AG, Thompson, JA, Margolin, KA, Rodmyre, R, Lai, IP, Dowdy, K et al. (2012). Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci U S A 109: 4592–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, EQ, Li, XL, Wang, CR, Li, TF and Han, SY (2013). Chimeric antigen receptor-engineered T cells for cancer immunotherapy: progress and challenges. J Hematol Oncol 6: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs, CS, Borman, ZA, Cassard, L, Gattinoni, L, Spolski, R, Yu, Z et al. (2009). Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA 106: 17469–17474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw, MH, Westwood, JA, Parker, LL, Wang, G, Eshhar, Z, Mavroukakis, SA et al. (2006). A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 12(20 Pt 1): 6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, CA and Dotti, G (2011). Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther 11: 855–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Rohena, A, Carpenito, C, Perez, EE, Richardson, M, Parry, RV, Milone, M et al. (2008). Genetic engineering of T cells for adoptive immunotherapy. Immunol Res 42: 166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt, TM, Ragnarsson, GB and Greenberg, PD (2009). T cell receptor gene therapy for cancer. Hum Gene Ther 20: 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauss, HJ, Morris, EC and Abken, H (2015). Cancer gene therapy with T cell receptors and chimeric antigen receptors. Curr Opin Pharmacol 24: 113–118. [DOI] [PubMed] [Google Scholar]
- Schmitt, TM, Stromnes, IM, Chapuis, AG and Greenberg, PD (2015). New strategies in engineering T-cell receptor gene-modified T cells to more effectively target malignancies. Clin Cancer Res 21: 5191–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavassori, S, Kumar, A, Wan, GS, Ramanjaneyulu, GS, Cavallari, M, El Daker, S et al. (2013). Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol 14: 908–916. [DOI] [PubMed] [Google Scholar]
- Birnbaum, ME, Mendoza, JL, Sethi, DK, Dong, S, Glanville, J, Dobbins, J et al. (2014). Deconstructing the peptide-MHC specificity of T cell recognition. Cell 157: 1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma, AM, Castro, CD, Mayassi, T, Bembinster, LA, Bai, L, Picard, D et al. (2013). Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity 39: 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann, C, Heemskerk, B, Kvistborg, P, Kluin, RJ, Bolotin, DA, Chen, X et al. (2013). High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med 19: 1534–1541. [DOI] [PubMed] [Google Scholar]
- Kobayashi, E, Mizukoshi, E, Kishi, H, Ozawa, T, Hamana, H, Nagai, T et al. (2013). A new cloning and expression system yields and validates TCRs from blood lymphocytes of patients with cancer within 10 days. Nat Med 19: 1542–1546. [DOI] [PubMed] [Google Scholar]
- Howie, B, Sherwood, AM, Berkebile, AD, Berka, J, Emerson, RO, Williamson, DW et al. (2015). High-throughput pairing of T cell receptor α and β sequences. Sci Transl Med 7: 301ra131. [DOI] [PubMed] [Google Scholar]
- Perko, R, Kang, G, Sunkara, A, Leung, W, Thomas, PG and Dallas, MH (2015). Gamma delta T cell reconstitution is associated with fewer infections and improved event-free survival after hematopoietic stem cell transplantation for pediatric leukemia. Biol Blood Marrow Transplant 21: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Santos, B, Serre, K and Norell, H (2015). γδ T cells in cancer. Nat Rev Immunol 15: 683–691. [DOI] [PubMed] [Google Scholar]
- Dash, P, McClaren, JL, Oguin, TH 3rd, Rothwell, W, Todd, B, Morris, MY et al. (2011). Paired analysis of TCRα and TCRβ chains at the single-cell level in mice. J Clin Invest 121: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, GC, Dash, P, McCullers, JA, Doherty, PC and Thomas, PG (2012). T cell receptor αβ diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci Transl Med 4: 128ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryksin, AV and Matsumura, I (2010). Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. Biotechniques 48: 463–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldrich, AP, Le Nours, J, Pellicci, DG, Gherardin, NA, McPherson, KG, Lim, RT et al. (2013). CD1d-lipid antigen recognition by the γδ TCR. Nat Immunol 14: 1137–1145. [DOI] [PubMed] [Google Scholar]
- Yoshio Ogawa, MN (2013). Gamma-delta T cells may function as carrier vehicles in adenovirus vector-based gene therapy. J Cancer Sci Ther 5: 384–390. [Google Scholar]
- Holst, J, Szymczak-Workman, AL, Vignali, KM, Burton, AR, Workman, CJ and Vignali, DA (2006). Generation of T-cell receptor retrogenic mice. Nat Protoc 1: 406–417. [DOI] [PubMed] [Google Scholar]
- Bettini, ML, Bettini, M, Nakayama, M, Guy, CS and Vignali, DA (2013). Generation of T cell receptor-retrogenic mice: improved retroviral-mediated stem cell gene transfer. Nat Protoc 8: 1837–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P, Yan, F, Doronina, VA, Escuin-Ordinas, H, Ryan, MD and Brown, JD (2012). 2A peptides provide distinct solutions to driving stop-carry on translational recoding. Nucleic Acids Res 40: 3143–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak, AL, Workman, CJ, Wang, Y, Vignali, KM, Dilioglou, S, Vanin, EF et al. (2004). Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol 22: 589–594. [DOI] [PubMed] [Google Scholar]
- Bettini, ML, Bettini, M and Vignali, DA (2012). T-cell receptor retrogenic mice: a rapid, flexible alternative to T-cell receptor transgenic mice. Immunology 136: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, EB, Guillonneau, C, Gras, S, La Gruta, NL, Vignali, DA, Doherty, PC et al. (2011). Structural basis for enabling T-cell receptor diversity within biased virus-specific CD8+ T-cell responses. Proc Natl Acad Sci USA 108: 9536–9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartok, I, Holland, SJ, Kessels, HW, Silk, JD, Alkhinji, M and Dyson, J (2010). T cell receptor CDR3 loops influence alphabeta pairing. Mol Immunol 47: 1613–1618. [DOI] [PubMed] [Google Scholar]
- Siegers, GM, Swamy, M, Fernández-Malavé, E, Minguet, S, Rathmann, S, Guardo, AC et al. (2007). Different composition of the human and the mouse gammadelta T cell receptor explains different phenotypes of CD3gamma and CD3delta immunodeficiencies. J Exp Med 204: 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, AE, Holzapfel, KL, Xing, Y, Cunningham, NR, Maltzman, JS, Punt, J et al. (2011). T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 208: 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, P, Han, X, Zhang, Q, Yang, Z, Fuss, IJ, Myers, TG et al. (2014). Dynamic changes in E-protein activity regulate T reg cell development. J Exp Med 211: 2651–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au-Yeung, BB, Zikherman, J, Mueller, JL, Ashouri, JF, Matloubian, M, Cheng, DA et al. (2014). A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proc Natl Acad Sci USA 111: E3679–E3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, H, Sohara, Y, Moats, RA, Nelson, MD Jr, Groshen, SG, Ye, W et al. (2007). The activity of zoledronic Acid on neuroblastoma bone metastasis involves inhibition of osteoclasts and tumor cell survival and proliferation. Cancer Res 67: 9346–9355. [DOI] [PubMed] [Google Scholar]
- Bäckman, U, Svensson, A, Christofferson, RH and Azarbayjani, F (2008). The bisphosphonate, zoledronic acid reduces experimental neuroblastoma growth by interfering with tumor angiogenesis. Anticancer Res 28(3A): 1551–1557. [PubMed] [Google Scholar]
- Gnant, M and Clézardin, P (2012). Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev 38: 407–415. [DOI] [PubMed] [Google Scholar]
- Di Carlo, E, Bocca, P, Emionite, L, Cilli, M, Cipollone, G, Morandi, F et al. (2013). Mechanisms of the antitumor activity of human Vγ9Vδ2 T cells in combination with zoledronic acid in a preclinical model of neuroblastoma. Mol Ther 21: 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, H, Matsuda, K, Srikoon, P, Kariya, R, Hattori, S, Taura, M et al. (2013). Potent antitumor activity of zoledronic acid-induced Vγ9Vδ2 T cells against primary effusion lymphoma. Cancer Lett 331: 174–182. [DOI] [PubMed] [Google Scholar]
- Sprini, D, Di Stefano, L, Rini, GB, Cianferotti, L and Napoli, N (2014). Vγ9Vδ2 T lymphocytes activation in osteoporotic patients treated with bisphosphonates. Clin Cases Miner Bone Metab 11: 126–128. [PMC free article] [PubMed] [Google Scholar]
- Wada, I, Matsushita, H, Noji, S, Mori, K, Yamashita, H, Nomura, S et al. (2014). Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med 3: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T, Terao, S, Acharya, B, Naoe, M, Yamamoto, S, Okamura, H et al. (2010). The antitumour effect of {gamma}{delta} T-cells is enhanced by valproic acid-induced up-regulation of NKG2D ligands. Anticancer Res 30: 4509–4513. [PubMed] [Google Scholar]
- Pardoll, DM (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, ME, Wunderlich, JR, Shelton, TE, Even, J and Rosenberg, SA (2003). Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 26: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien, YH, Meyer, C and Bonneville, M (2014). γδ T cells: first line of defense and beyond. Annu Rev Immunol 32: 121–155. [DOI] [PubMed] [Google Scholar]
- Gros, A, Robbins, PF, Yao, X, Li, YF, Turcotte, S, Tran, E et al. (2014). PD-1 identifies the patient-specific CD8⁺ tumor-reactive repertoire infiltrating human tumors. J Clin Invest 124: 2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing, JR, Wilson, RK, Zhang, J, Mardis, ER, Pui, CH, Ding, L et al. (2012). The Pediatric Cancer Genome Project. Nat Genet 44: 619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, G, Parker, M, Kranenburg, TA, Lu, C, Chen, X, Ding, L et al. (2012). Novel mutations target distinct subgroups of medulloblastoma. Nature 488: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J, Benavente, CA, McEvoy, J, Flores-Otero, J, Ding, L, Chen, X et al. (2012). A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 481: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J, Ding, L, Holmfeldt, L, Wu, G, Heatley, SL, Payne-Turner, D et al. (2012). The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, AJ and Bollard, CM (2015). The coming of age of adoptive T-cell therapy for viral infection after stem cell transplantation. Ann Transl Med 3: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio, F, Hanley, PJ and Bollard, CM (2014). The time is now: moving toward virus-specific T cells after allogeneic hematopoietic stem cell transplantation as the standard of care. Cytotherapy 16: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantourout, P and Hayday, A (2013). Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 13: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc, MP, Giudicelli, V, Ginestoux, C, Jabado-Michaloud, J, Folch, G, Bellahcene, F et al. (2009). IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res 37(Database issue): D1006–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, J (2008). A simple way to treat PCR products prior to sequencing using ExoSAP-IT. Biotechniques 44: 834. [DOI] [PubMed] [Google Scholar]
- Workman, CJ, Dugger, KJ and Vignali, DA (2002). Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol 169: 5392–5395. [DOI] [PubMed] [Google Scholar]
- Persons, DA, Allay, JA, Allay, ER, Smeyne, RJ, Ashmun, RA, Sorrentino, BP et al. (1997). Retroviral-mediated transfer of the green fluorescent protein gene into murine hematopoietic cells facilitates scoring and selection of transduced progenitors in vitro and identification of genetically modified cells in vivo. Blood 90: 1777–1786. [PubMed] [Google Scholar]
- Heemskerk, MH, Hoogeboom, M, de Paus, RA, Kester, MG, van der Hoorn, MA, Goulmy, E et al. (2003). Redirection of antileukemic reactivity of peripheral T lymphocytes using gene transfer of minor histocompatibility antigen HA-2-specific T-cell receptor complexes expressing a conserved alpha joining region. Blood 102: 3530–3540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.