Abstract
OBJECTIVE
Progestogen (vaginal progesterone or 17-alpha-hydroxyprogesterone caproate [17OHP-C]) administration to patients at risk for preterm delivery is widely used for the prevention of preterm birth (PTB). The mechanisms by which these agents prevent PTB are poorly understood. Progestogens have immunomodulatory functions; therefore, we investigated the local effects of vaginal progesterone and 17OHP-C on adaptive and innate immune cells implicated in the process of parturition.
STUDY DESIGN
Pregnant C57BL/6J mice received vaginal progesterone (1 mg per 200 μL, n = 10) or Replens (control, 200 μL, n = 10) from 13 to 17 days postcoitum (dpc) or were subcutaneously injected with 17OHP-C (2 mg per 100 μL, n = 10) or castor oil (control, 100 μL, n = 10) on 13, 15, and 17 dpc. Decidual and myometrial leukocytes were isolated prior to term delivery (18.5 dpc) for immunophenotyping by flow cytometry. Cervical tissues were collected to determine matrix metalloproteinase (MMP)-9 activity by in situ zymography and visualization of collagen content by Masson’s trichrome staining. Plasma concentrations of progesterone, estradiol, and cytokines (interferon [IFN]-γ, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, KC/GRO, and tumor necrosis factor-α) were quantified by enzyme-linked immunosorbent assays. Pregnant mice pretreated with vaginal progesterone or Replens were injected with 10 μg of an endotoxin on 16.5 dpc (n = 10 each) and monitored via infrared camera until delivery to determine the effect of vaginal progesterone on the rate of PTB.
RESULTS
The following results were found: (1) vaginal progesterone, but not 17OHP-C, increased the proportion of decidual CD4+ T-regulatory cells; (2) vaginal progesterone, but not 17OHP-C, decreased the proportion of decidual CD8+CD25+Foxp3+ T cells and macrophages; (3) vaginal progesterone did not cause an M1→M2 macrophage polarization but reduced the proportion of myometrial IFNγ+ neutrophils and cervical active MMP-9-positive neutrophils and monocytes; (4) 17OHP-C did not reduce the proportion of myometrial IFNy-positive neutrophils; however, it increased the abundance of cervical active MMP-9-positive neutrophils and monocytes; (5) vaginal progesterone immune effects were associated with reduced systemic concentrations of IL-1β but not with alterations in progesterone or estradiol concentrations; and (6) vaginal progesterone pretreatment protected against endotoxin-induced PTB (effect size 50%, P = .008).
CONCLUSION
Vaginal progesterone, but not 17OHP-C, has local antiinflammatory effects at the maternal-fetal interface and the cervix and protects against endotoxin-induced PTB.
Keywords: decidua, endotoxin, interleukin 1β, macrophages, matrix metalloproteinase-9, myometrium, neutrophils, preterm birth, preterm labor, regulatory T cells
Preterm birth (PTB) is the leading cause of perinatal morbidity and mortality worldwide.1 The rate of PTB in the United States is 11.39%, which is considered high for a developed nation.2 Preterm neonates are at an increased risk for short- and long-term morbidity, and prematurity represents a substantial burden for society and the health care system.3–6 Therefore, the prevention of PTB is a health care priority.
Cervical assessment with ultrasound coupled with the administration of vaginal progesterone represents the main strategy to prevent PTB in nulliparous women and in those without a prior history of prematurity.7–16 17-Alpha-hydroxyprogesterone caproate (17OHP-C) has been recommended to prevent PTB in women with a prior history of prematurity.17–19
Although the term progesterone has been used to refer to natural progesterone and 17OHP-C,20 there is evidence that these compounds have different biological activities in the myometrium,21–24 uterine cervix,25–30 profile of clinical efficacy,7–19,26,31–33 and safety17,34–39; therefore, these terms should not be used interchangeably.
The mechanisms of action whereby progestogens prevent PTB are unknown. There has been considerable interest in the role of progesterone in the maintenance of myometrial quiescence.40–44 However, the realization that a short cervix is a risk factor for preterm delivery45–49 and that a blockage of progesterone action induces cervical ripening in animals and women50–54 has focused investigation on the role of progesterone on this organ.55–61
The current hypothesis is that progesterone acts as an antiinflammatory agent primarily on the uterine cervix.62–66 This hypothesis is largely based on the known antiinflammatory effects of progesterone,67–71 and a microarray study demonstrating the differential expression of inflammatory related messenger ribonucleic acid (mRNA) in the cervix of pregnant mice treated with medroxyprogesterone acetate.55 However, there is no functional evidence that vaginal progesterone has anti-inflammatory effects in vivo.
Inflammation has been implicated in physiological72–99 and pathological parturition.100–129 Pathological inflammation can result from the activation of innate immunity in response to microbial products87,130–138 or activation of the adaptive immune response.139–144 A breakdown of maternal-fetal tolerance is now recognized as a mechanism of disease for spontaneous premature labor/delivery.100,145–151 Therefore, the effects of progesterone in the prevention of preterm delivery may be mediated by the innate and/or adaptive immune system.
Indeed, the administration of RU486 (to block progesterone action152) during late pregnancy in guinea pigs can increase the release of proinflammatory cytokines by the amniochorion, cervix, and decidual-myometrial tissues,153 and this hormone can also increase the proportion of CD4+CD25+ regulatory T cells (Tregs), which are key in the control of the adaptive immune response, in the uterine tissues during mid-pregnancy in mice.154
The objectives of this study were to determine the effects of vaginal progesterone and 17OHP-C on the following: (1) the proportion of CD4+ Tregs and CD8+CD25+Foxp3+ T cells at the maternal-fetal interface (myometrium and decidua); (2) the proportion and phenotype of macrophages (M1-like or M2-like) at the maternal-fetal interface; (3) the proportion of neutrophils and their cytokine production at the maternal-fetal interface; and (4) matrix metalloproteinase (MMP)-9 activity in the cervix.
Finally, we sought to determine whether pretreatment with vaginal progesterone could prevent endotoxininduced PTB.
MATERIALS AND METHODS
Animals
C57BL/6J mice were bred in the animal care facility at the C. S. Mott Center for Human Growth and Development at Wayne State University (Detroit, MI) and housed under a circadian cycle (12 hours of light and 12 hours of dark). Females 8–12 weeks old were mated with male mice of proven fertility. Female mice were examined daily between 8:00 and 9:00 AM for the presence of a vaginal plug, which denoted 0.5 days postcoitum (dpc). Upon observation of vaginal plugs, the female mice were then separated from the males and were housed in different cages. The weight gain of ≤2 g confirmed the pregnancy at 12.5 dpc. Procedures were approved by the Institutional Animal Care and Use Committee at Wayne State University (protocol number A09-08-12).
Progestogen administration
Pregnant females received vaginal progesterone (Crinone 8% vaginal gel; Fleet Laboratories Ltd, Watford, Herts, United Kingdom) at a concentration of 1 mg per 200 μL (n = 10) or 200 μL of Replens (Lil’ Drug Store Products, Inc, Cedar Rapids, IA) as a control (n = 10) from 13 to 17 dpc (Figure 1A).
FIGURE 1. Animal model and identification of decidual CD4+ Tregs and CD8+CD25+Foxp3+ cells.
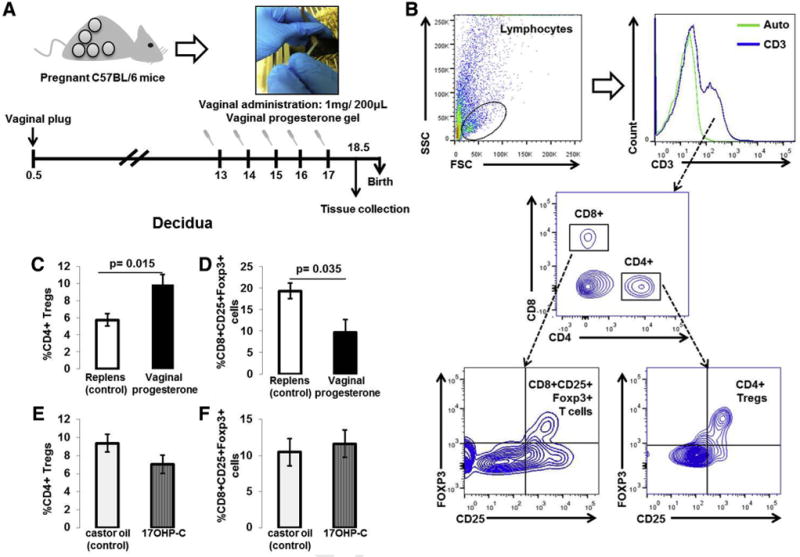
A, Vaginal progesterone administration scheme. B, Gating strategy used to identify CD4+ Tregs (CD4+CD25+Foxp3+ cells) and CD8+CD25+Foxp3+ cells in decidual tissues. CD3+ T cells were gated within the total lymphocyte gate (FSC vs SSC). The green histogram represents the autofluorescence control. CD4+ Tregs and CD8+CD25+Foxp3+ cells were gated within the CD4+ and CD8+ gates, respectively. C, Proportions of decidual CD4+ Tregs in mice treated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc). D, Proportions of decidual CD8+CD25+Foxp3+ cells in mice treated with vaginal progesterone or Replens (control). E, Proportions of decidual CD4+ Tregs in mice injected with 17OHP-C or castor oil (control). F, Proportions of decidual CD8+CD25+Foxp3+ cells in mice injected with 17OHP-C or castor oil (control) (n = 10 each). Data are represented as mean ± SEM.
FSC,; 17OHP-C, 17-alpha-hydroxyprogesterone caproate; SSC, saline sodium citrate; Treg, regulatory T cell.
A second group of mice was injected subcutaneously with 2 mg per 100 μL of 17OHP-C (n = 10; Compounding Solutions, Shelby Township, MI) or 100 μL of castor oil (European Pharmacia Grade; ACROS Organics, Thermo Fisher Scientific, Waltham, MA) as a control (n = 10) on 13, 15, and 17 dpc.
We used this source of the 17OHP-C because it is clinically used at the Detroit Medical Center, and previous studies demonstrated that compounded 17OHP-C had adequate potency compared with the Food and Drug Administration—approved agent.155 The administration of vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc) was performed starting on 13 dpc to mimic the treatment regimen followed by pregnant women with a short cervix.
Vaginal progesterone administration is generally started around 20–23 weeks of gestation in women with a short cervix,11 which is equivalent to approximately 13 dpc in mice during midgestation. Administration of 17OHP-C or castor oil control started on 13 dpc and continued on alternating days because women receive this synthetic progesterone on a weekly basis.17 The doses of vaginal progesterone and 17OHP-C were similar to those previously reported in studies using the same animal species.29,33,55 All mice were euthanized prior to term delivery (18.5 dpc) and decidual, myometrial, and cervical tissues were harvested.
Leukocyte isolation
Immediately after collection, myometrial and decidual tissues were mechanically disaggregated in a cell dissociating reagent (Accutase; Life Technologies, Grand Island, NY) using scissors for approximately 1–2 minutes, as previously described.156 Samples were then incubated at 37°C for 35 minutes with gentle shaking (MaxQ 4450 benchtop orbital shaker; Thermo Fisher Scientific). The cell suspensions were filtered using a 100 μm cell strainer (Fisher Scientific, Hanover Park, IL) and washed with fatty acyl-CoA synthase (FACS) buffer (bovine-serum albumin 0.1% [Sigma Aldrich, St Louis, MO]), sodium azide 0.05% (Fischer Scientific Bioreagents, Fair Lawn, NJ), and 1× phosphate-buffered saline (PBS; Fischer Scientific Bioreagents)]. The resulting pellet was resuspended in FACS buffer and used for immunophenotyping.
Immunophenotyping
Cell suspensions were incubated with a monoclonal mouse CD16/CD32 antibody (FcγIII/II receptor; BD Biosciences, San Jose, CA) for 10 minutes at 4°C. The cells were then washed with FACS buffer and incubated for 30 minutes at 4°C with the corresponding extracellular and/or intracellular fluorochrome-conjugated antibodies (Supplemental Table). Tregs were determined in decidual and myometrial tissues using the extracellular markers CD3, CD4, CD8, and CD25 and the transcriptional factor Foxp3. Innate leukocyte populations including macrophages, dendritic cells (DCs), natural killer (NK) cells, and neutrophils were also identified in the decidual and myometrial tissues using the extracellular markers CD45, F4/80, CD11c, CD49b, and Ly6G.
Foxp3 staining was performed using the Foxp3/transcription factor staining buffer set (eBioscience, San Diego, CA). For cytokine staining, the Cytofix/Cytoperm fixation/permeabilization solution kit (BD Biosciences) was used, following the manufacturer’s recommendations. Unstained cells were treated with the same protocol and used as autofluorescence controls. Cell suspensions were acquired and analyzed using the LSRFortessa flow cytometer and BD FACSDiva software, version 8.0 (BD Biosciences), respectively. Figures were prepared using FlowJo Software version 10 (FlowJo, LLC, Ashland, OR).
In situ MMP-9 zymography
To determine the MMP-9 activity in cervical tissues, in situ zymography was performed as described by Hadler-Olsen et al.157 Cervical tissue sections were fixed in ethanol and embedded in paraffin; from these blocks, 5 μm thick sections were cut and mounted on FisherBrand Superfrost microscope slides (Fisher Scientific) and heated to 59°C. Slides were further deparaffinized in xylene and rehydrated in graded alcohol baths. The gelatinase reaction was performed using the EnzChek gelatinase/collagenase assay kit (Life Technologies), and to verify the enzyme specificity, tissue sections were preincubated for 1 hour with 200 μL of 10 mM phenanthroline, a metal chelator and general inhibitor of metalloproteinases.
The remaining slides were preincubated with a reaction buffer, and a substrate was prepared by dissolving 1 mg DQ gelatin (Life Technologies) in 1.0 mL of deionized water and diluted 1:50 with reaction buffer. Substrate solution (200 μL) with or without 10 mM phenanthroline was then added to the tissue sections. All slides were incubated in a dark humidity chamber at 37°C for 2 hours, and the negative control slides were incubated at −20°C for 2 hours.
Following incubation, the sections were rinsed twice with deionized water and fixed in 4% neutral buffered formalin for 10 minutes in the dark and then were rinsed with 1× PBS twice prior to mounting with ProLong Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (Life Technologies). The slides were scanned using the Pannoramic MIDI digital slide scanner (PerkinElmer, Inc, Waltham, MA), and annotations were made by laboratory personnel who then utilized 3DHISTECH software (3DHISTECH Kft, Budapest, Hungary) to assess the number of positive cells.
Masson’s trichrome staining
Cervical tissue sections were fixed in 4% paraformaldehyde upon harvesting and stored at 4°C in ethanol before being embedded into paraffin blocks. The embedded tissues were then cut into 5 μm thick sections, placed onto salinized slides, deparaffinized with xylene, and hydrated with ethanol and water. The staining was performed on the Dako AutostainerPlus (Dako, Carpinteria, CA) using Masson’s trichrome stain kit (American MasterTech, Lodi, CA), following the manufacturer’s protocol. Briefly, the sections were mordanted in Bouin solution overnight at room temperature, rinsed in water, stained with Weigert’s hematoxylin for 3 minutes, rinsed again in water, and stained with Biebrich Scarlet-Acid Fuchsin solution for 15 minutes.
After a second rinse, the slides were incubated with phosphomolybdic/phosphotungstic acid for 15 minutes, stained with Aniline Blue stain for 10 minutes, rinsed, and incubated with 1% acetic acid for 5 minutes. The sections were then dehydrated in a series of alcohol baths, and then a coverslip was placed. The images were taken using the Pannoramic MIDI digital slide scanner (PerkinElmer, Inc).
Decidual protein extracts
Decidual tissues were collected from the mice treated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc) at 18.5 dpc and placed in small Petri dishes with sterile 1 × PBS (n = 10 each). Tissues were incubated in a 12-well culture plate (Falcon multiwell plates for cell culture; Becton Dickinson Labware, Franklin Lanes, NJ), using a single well per tissue with 1 mL of Gibco Dulbecco’s modified eagle medium (Life Technologies) supplemented with 1% Gibco antibiotic-antimycotic solution (Life Technologies) for 24 hours at 37 C in 5% CO2. Following incubation, tissues were homogenized using a Tissue Tearor (BioSpec Products, Inc, Bartlesville, OK) and centrifuged at 15,000 × g for 30 minutes at 4°C to obtain a cell-free supernatant that contained the protein extract.
Enzyme-linked immunosorbent assays (ELISAs)
Blood samples, obtained by cardiac puncture from the mice that received vaginal progesterone, Replens (Lil’ Drug Store Products, Inc), 17OHP-C, or castor oil were placed in tubes containing heparin (Sigma-Aldrich). Plasma samples were then obtained by centrifugation. Plasma progesterone and estradiol concentrations were measured using the PROG-EASIA ELISA kit (GenWay Biotech, Inc, San Diego, CA) and the Calbiotech mouse/rat estradiol ELISA kit (Calbiotech Inc, Spring Valley, CA), respectively, according to the manufacturer’s instructions.
The concentrations of interferon (IFN)-γ, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, KC/GRO, and tumor necrosis factor (TNF)-α in plasmawere measuredwith sensitive and specific immunoassays according to the manufacturer’s instructions (Meso Scale Discovery, Gaithersburg, MD). IL-10 was also determined in the decidual protein extracts.
The sensitivities of the assays were as follows: 0.022 pg/mL (IFNγ), 0.104 pg/mL (IL-1β), 0.179 pg/mL (IL-2), 0.098 pg/mL (IL-4), 0.066 pg/mL (IL-5), 0.825 pg/mL (IL-6), 0.425 pg/mL (IL-10), 8.578 pg/mL (IL-12p70), 0.218 pg/mL (KC/GRO), and 0.164 pg/mL (TNFα), respectively. The interassay and intraassay coefficients of variation were below 7% and 15%, respectively.
Endotoxin-induced preterm birth in animals treated with vaginal progesterone or placebo
Pregnant mice were pretreated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc) from 13 to 17 dpc as previously described (n = 10 each). On 16.5 dpc, the mice were challenged with an intraperitoneal injection of 10 μg of an endotoxin (lipopolysaccharides from Escherichia coli, O55:B5; Sigma-Aldrich) in 200 μL of 1 × PBS.
Video recording provided precise measurements of the gestational age, duration of active labor, and rate of stillbirth. Gestational age at birth was calculated from the identification of the vaginal plug (0.5 dpc) through the delivery of the first pup. Active labor was defined as the time elapsed from the delivery of the first pup through the delivery of the last pup. The rate of stillbirth was defined as the number of pups that were born dead of the total number of pups born. PTB was defined as fetal delivery before 18 dpc.
Statistical analysis
Statistical analyses were performed using SPSS, version 21.0 (IBM Corp, Armonk, NY). A Shapiro-Wilk test was performed to determine whether data were normally distributed. Because the data did not have a normal distribution, Mann-Whitney U tests were performed. A χ2 test was used to compare proportions. Graphical data were presented as mean ± SEM. A value of P < .05 was considered statistically significant.
RESULTS
Administration of vaginal progesterone, but not 17OHP-C, increases the proportion of CD4D+ Tregs in decidual tissues
We first determined the proportions of CD4+ Tregs (CD4+CD25+Foxp3+ T cells) and CD8+CD25+Foxp3+ T cells in myometrial and decidual tissues following vaginal progesterone or 17OHP-C administration to pregnant mice. Figure 1B shows the gating strategy used to analyze CD4+ Tregs and CD8+CD25+Foxp3+ T cells in myometrial and decidual tissues.
Vaginal progesterone administration increased the proportion of decidual CD4+ Tregs when compared with the group receiving Replens (control; Lil’ Drug Store Products, Inc) (Figure 1C); however, it decreased the proportion of decidual CD8+CD25+Foxp3+ T cells (Figure 1D)
Administration of 17OHP-C did not have such effects (Figures 1, E and F, P > .05). Moreover, the vaginal progesterone administration did not alter the proportion of myometrial CD4+ Tregs or CD8+CD25+Foxp3+ T cells (Figure 2). Therefore, the administration of vaginal progesterone, but not 17OHP-C, increased the proportion of CD4+ Tregs in the decidual tissues.
FIGURE 2. Proportions of myometrial CD4+ Tregs and CD8+CD25+Foxp3+ cells.
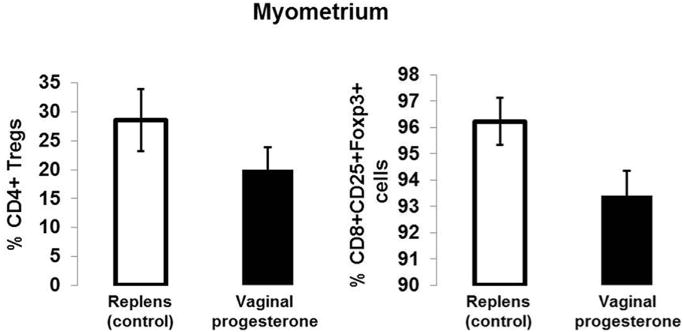
Proportions of myometrial CD4+ Tregs (CD4+CD25+Foxp3+ cells) and CD8+CD25+Foxp3+ cells in mice treated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc) (n = 10 each). Data are represented as mean ± SEM.
Treg, regulatory T cell.
To explore whether IL-10 (an antiinflammatory cytokine and a differentiation factor of Tregs158) could mediate an increase in CD4+ Tregs, we determined the concentration of this cytokine in decidual tissues. No differences were observed in the concentration of IL-10 between the decidual protein extracts upon vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc) administration (Supplemental Figure). These results do not support a role for IL-10 in the increase of decidual CD4+ Tregs upon administration of vaginal progesterone.
Administration of vaginal progesterone, but not 17OHP-C, decreases the proportion of macrophages in decidual tissues
To further characterize the decidual microenvironment following vaginal progesterone or 17OHP-C administration, the proportion of innate immune cells was determined. The gating strategy used to analyze NK cells (CD45+CD49b+ cells), DCs (CD45+CD11c+ cells), neutrophils (CD45+Ly6G+ cells), and macrophages (CD45+F4/80+ cells) in decidual tissues is shown in Figure 3A.
FIGURE 3. Immunophenotyping of innate immune cells in decidual tissues.
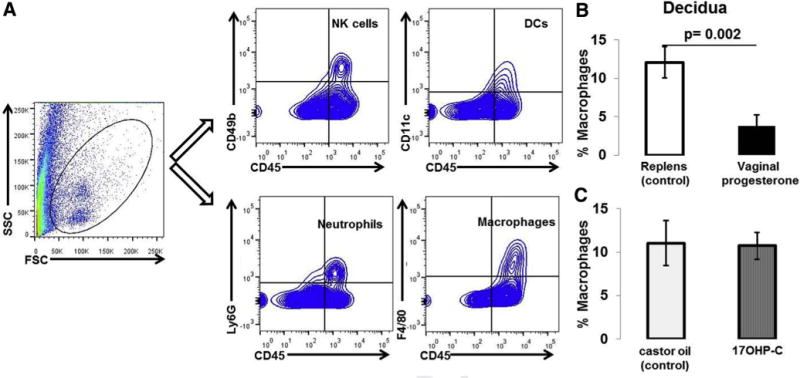
A, Gating strategy used to identify NK cells (CD45+CD49b+ cells), DCs (CD45+CD11c+ cells), neutrophils (CD45+Ly6G+ cells), and macrophages (CD45+F4/80 cells) in decidual tissues. B, Proportions of decidual macrophages in mice treated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc). C, Proportions of decidual macrophages in mice injected with 17OHP-C or castor oil (control) (n = 10 each). Data are represented as mean ± SEM.
DC, dendritic cell; FSC,; NK, natural killer; 17OHP-C, 17-alpha-hydroxyprogesterone caproate; SSC, saline sodium citrate.
Vaginal progesterone administration reduced the proportion of macrophages in decidual tissues when compared with Replens (control; Lil’ Drug Store Products, Inc) (Figure 3B). In contrast, 17OHP-C administration did not alter the proportion of decidual macrophages (Figure 3C). No differences were found in the proportions of decidual neutrophils, NK cells, or DCs between these 2 groups of mice (data not shown).
To characterize the phenotype of macrophages that were reduced in decidual tissues upon vaginal progesterone administration, we determined the expression of M1-like and M2-like markers including inducible NO synthase (iNOS), IFNγ, Arg1 cells, and IL-4.159 The gating strategy used to determine M1-like (CD11b+Ly6G-F4/80+iNOS+ or IFNγ cells) and M2-like (CD11b+Ly6G-F4/80+Arg1+ or IL4+ cells) macrophages in decidual tissues is shown in Figure 4A.
FIGURE 4. M1- and M2-like macrophages in decidual tissues.
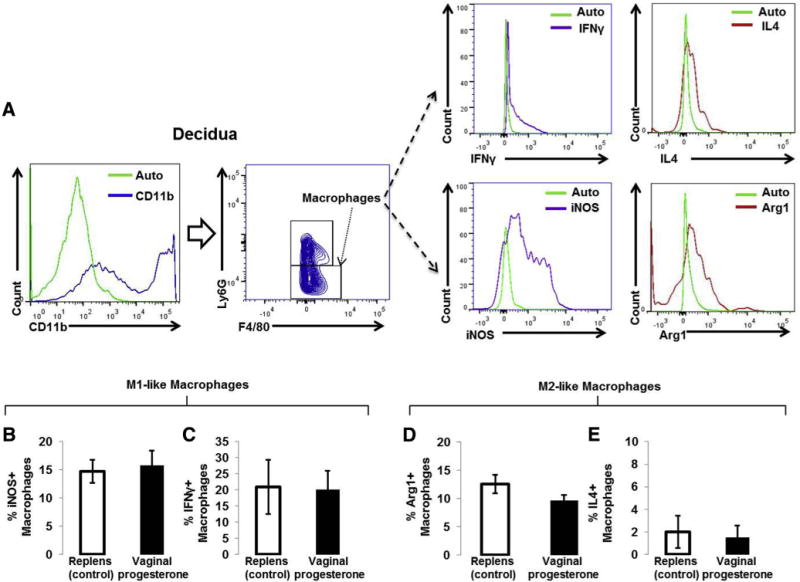
A, Gating strategy used to identify M1-like (CD11b+ Ly6G-F4/80+ IFNγ -positive or iNOS-positive cells) and M2-like (CD11b Ly6G-F4/80+ IL4-positive or Arg1-positive cells) macrophages. The green histogram represents the autofluorescence control. B and C, Proportions of M1-like (CD11b+Ly6G-F4/80+ IFNγ-positive or iNOS-positive cells) macrophages in decidual tissues from mice treated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc). D and E, Proportions of M2-like (CD11b+Ly6G-F4/80+ IL4-positive or Arg1-positive cells) macrophages in decidual tissues from mice treated with vaginal progesterone or Replens (control) (n = 10 each). Data are represented as mean ±SEM.
IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase.
We hypothesized that vaginal progesterone administration would reduce the proportion of M1-like macrophages and/or would cause an M1→M2 macrophage polarization. Administration of vaginal progesterone did not change the proportion of M1-like (Figure 4, B and C) or M2-like (Figure 4, D and E) macrophages. Vaginal progesterone administration reduced the proportion of decidual macrophages, yet these results do not support the hypothesis that vaginal progesterone reduces M1-like macrophages or causes an M1→M2 macrophage polarization.
Administration of vaginal progesterone, but not 17OHP-C, reduces the proportion of IFNγ+ neutrophils in myometrium
Uterine/myometrial macrophages and neutrophils have been implicated in the onset of term and preterm labor.84,87 We therefore sought to determine whether vaginal progesterone or 17OHP-C administration alters the proportion of these innate immune cells in myometrial tissues.
The gating strategy used to determine macrophages, neutrophils, and their expression of IFNγ or IL-4 was similar to the strategy used in Figure 4A. Administration of vaginal progesterone tended to reduce the proportion of myometrial macrophages; however, this reduction did not reach statistical significance (Figure 5A). Although the administration of vaginal progesterone did not reduce the proportion of total neutrophils (Figure 5B), it decreased the proportion of IFNγ -positive neutrophils (CD11b+Ly6G+F4/80– cells) in myometrium (Figure 5C). 17OHP-C administration did not reduce the proportion of IFNγ -positive neutrophils in myometrium (data not shown). These results demonstrate that vaginal progesterone administration reduced the proportion of proinflammatory neutrophils in myometrium.
FIGURE 5. Macrophages and neutrophils in myometrium.
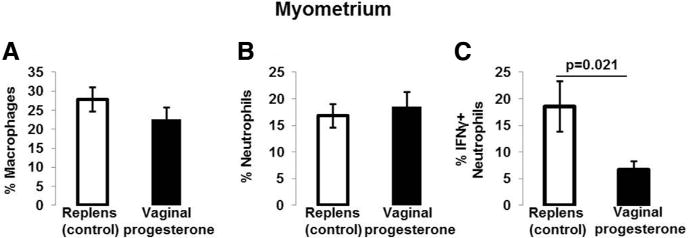
A, Proportions of myometrial macrophages in mice treated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc). B, Proportions of myometrial neutrophils in mice treated with vaginal progesterone or Replens (control). C, Proportions of myometrial IFNγ -positive neutrophils (CD11b+Ly6G+F4/80− cells) in mice treated with vaginal progesterone or Replens (control) (n = 10 each). Data are represented as mean ±SEM.
IFN, interferon.
Administration of vaginal progesterone, but not 17OHP-C, reduces the abundance of active MMP-9-positive cells in the cervix
We further investigated whether vaginal progesterone and 17OHP-C had effects on MMP-9 activity and collagen content in the cervical tissues. Administration of vaginal progesterone or 17OHP-C increased MMP-9 activity (green staining) (Figure 6, A and B) and reduced collagen content (blue staining; Figure 6, C and D) in the cervical tissues.
FIGURE 6. MMP-9 activity and collagen content in cervical tissues.
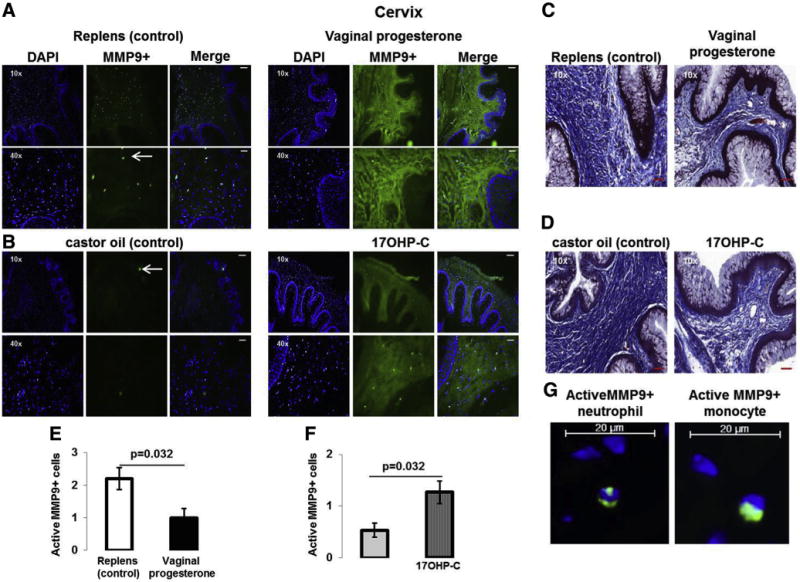
A, MMP-9 activity (green staining) in mice treated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc). B, MMP-9 activity (green staining) in mice injected with 17OHP-C or castor oil (control). Nuclei were stained with DAPI. White arrows represent active MMP-9-positive cells. Scale bars in ×10 and ×40: 200 μm and 50 μm, respectively. C, Masson’s trichrome staining of the cervical tissues from mice treated with vaginal progesterone or Replens (control). Scale bars in ×10 and ×40: 200 μm and 50 μm, respectively. D, Masson’s trichrome staining of the cervical tissues from mice injected with 17OHP-C or castor oil (control). Collagen fibers are stained in blue. Scale bars in ×10 and ×40: 200 μm and 50 μm, respectively. E, Semiquantification of active MMP-9-positive cells in the cervices from mice treated with vaginal progesterone or Replens (control). F, Semiquantification of active MMP-9-positive cells in cervices from mice injected with 17OHP-C or castor oil (control) (n = 5 each). Data are represented as mean ±SEM. G, Magnified image of active MMP-9-positive neutrophils and monocytes in cervical tissues from control mice. Scale bars: 20 μm.
DAPI, 4′,6-diamidino-2-phenylindole; MMP, matrix metalloproteinase; 17OHP-C, 17-alpha-hydroxyprogesterone caproate.
While analyzing the images, we observed that the cervices in the Replens group (control; Lil’ Drug Store Products, Inc) had an abundant number of active MMP-9-positive cells (white arrows). Semiquantification of these cells revealed that vaginal progesterone administration reduced the abundance of active MMP-9-positive cells when compared with Replens (control) (Figure 6E).
In contrast, 17OHP-C administration increased the abundance of active MMP-9-positive cells when compared with castor oil (control) (Figure 6F). Magnification of active MMP-9-positive cells in Replens (control) revealed these cells to be neutrophils and monocytes (Figure 6G). Therefore, vaginal progesterone and 17OHP-C increased MMP-9 activity and reduced collagen content in the cervix. However, only vaginal progesterone reduced the infiltration of active MMP-9-positive neutrophils and monocytes.
Administration of vaginal progesterone or 17OHP-C is not associated with changes in the systemic concentrations of progesterone or estradiol
To investigate whether the immune effects of vaginal progesterone or 17OHP-C were associated with a change in the systemic levels of sex steroids, we quantified the concentrations of progesterone and estradiol in the plasma. Administration of vaginal progesterone or 17OHP-C did not change the systemic concentrations of progesterone or estradiol (Figure 7, A and B). These results demonstrate that the local immunomodulatory effects of vaginal progesterone in decidual, myometrial, and cervical tissues were not associated with systemic changes in sex steroids.
FIGURE 7. Plasma concentrations of progesterone and estradiol.
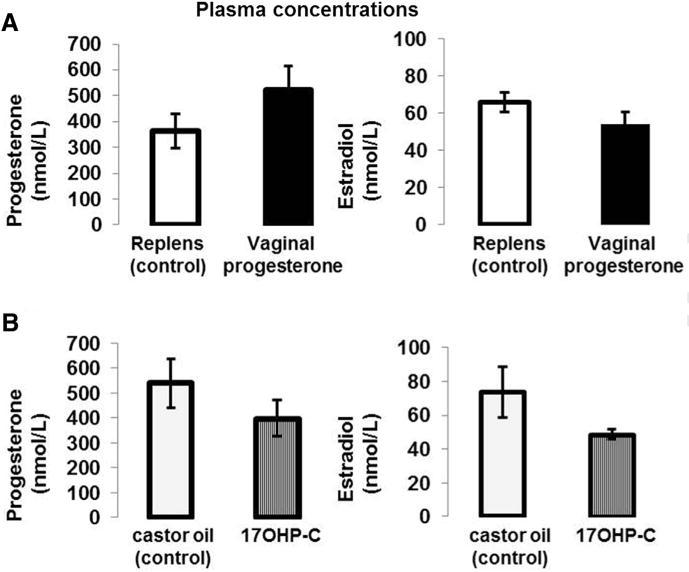
A, Progesterone and estradiol concentrations in mice treated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc). B, Progesterone and estradiol concentrations in mice injected with 17OHP-C or castor oil (control). Plasma samples were collected at 18.5 dpc (n = 10 each). Data are represented as mean ± SEM.
dpc, days postcoitum; 17OHP-C, 17-alpha-hydroxyprogesterone caproate.
Administration of vaginal progesterone, but not 17OHP-C, reduces the systemic concentration of IL-1β
Preterm labor is associated with a systemic inflammatory response125,126 and the systemic or intraamniotic administration of IL-1β leads to PTB in mice.160,161 Therefore, we evaluated whether the administration of vaginal progesterone or 17OHP-C had an effect on the systemic concentration of IL-1β. Vaginal progesterone reduced by 20% the plasma concentrations of IL-1β (Figure 8A); however, the administration of 17OHP-C did not alter the concentration of this cytokine (Figure 8B).
FIGURE 8. Plasma concentration of IL-1β.
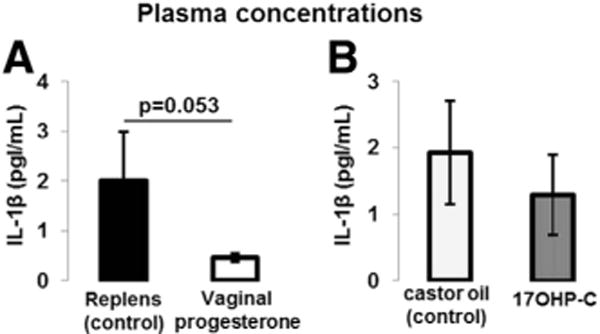
A, IL-1β concentrations in mice treated with vaginal progesterone or Replens (control; Lil’ Drug Store Products, Inc). B, IL-1β concentrations in mice injected with 17OHP-C or castor oil (control). Plasma samples were collected at 18.5 dpc (n = 10 each). Data are represented as mean ± SEM.
dpc, days postcoitum; IL, interleukin; 17OHP-C, 17-alpha-hydroxyprogesterone caproate.
Pretreatment with vaginal progesterone conferred partial protection (50%) against endotoxin-induced preterm birth
Finally, we evaluated the efficacy of vaginal progesterone in preventing endotoxin-induced preterm birth. Mice pretreated with vaginal progesterone had lower rates of endotoxin-induced preterm birth than mice pretreated with Replens (control; Lil’ Drug Store Products, Inc) (40% vs 90%, P = .008; Table). These results demonstrate that vaginal progesterone administration may be an effective treatment for reducing inflammation-associated preterm labor.
TABLE.
Vaginal progesterone administration decreases the rate of endotoxin-induced preterm birth
| Replens (control) plus endotoxin | Vaginal progesterone plus endotoxin | P value | |
|---|---|---|---|
| Number of mice | 10 | 10 | — |
| Preterm birth, %a,b | 90 | 40 | .008 |
| Gestational length, dc,d | 17.7 ± 0.6 | 18.4 ± 0.8 | .038 |
| Duration of labor, mine,d | 37 ± 20.7 | 40.5 ± 30.1 | NS |
Replens is manufactured by Lil’ Drug Store Products, Inc. dpc, days postcoitum; NS, not significant. PTB, preterm birth.
The rate of PTB was defined as the percentage of dams delivering at <18.0 dpc among all births;
χ2 test;
Days elapsed from the detection of a vaginal plug (0.5 dpc) to the delivery of the first pup. Data are shown as mean ± SD;
Mann-Whitney U test;
Time elapsed from the delivery of the first pup to the last pup. Data are shown as mean ± SD.
COMMENT
Principal findings of the study
The principal findings of the study included the following: (1) the administration of vaginal progesterone, but not 17OHP-C, increased the proportion of decidual CD4+ Tregs and decreased the proportions of CD8+CD25+Foxp3+ T cells and macrophages in decidual tissues; (2) administration of vaginal progesterone did not cause an M1→M2 macrophage polarization; however, it reduced the proportion of IFNγ -positive neutrophils in the myometrium and active MMP-9-positive neutrophils and monocytes in the cervix; (3) in contrast, the administration of 17OHP-C increased the abundance of active MMP-9-positive neutrophils and monocytes in the cervix; (4) the immune effects of vaginal progesterone were associated with reduced systemic concentrations of IL-1β but not with alterations in progesterone or estradiol concentrations; and (5) pretreatment with vaginal progesterone was associated with a 50% reduction in endotoxin-induced PTB.
Vaginal progesterone increases the proportion of decidual CD4D+ Tregs
Lymphocytes with immunoregulatory properties were described more than 4 decades ago162–166; however, the lack of specific markers for these cells precluded their characterization using immunophenotypic techniques. CD4+ Tregs are an important subset of T cells, which express CD25 and Foxp3.167–170
The 2 main Treg subsets are thymic Tregs and peripheral Tregs.168,171 These cells play a central role in immune responses through their suppressive activity of both self- and noneself-antigens,172–174 and this suppressive function largely is due to their expression of the transcription factor Foxp3.168,171
During pregnancy, there is an expansion of antigen-specific CD4+ Tregs that exhibit suppressive functions. This is thought to promote maternal-fetal tolerance and pregnancy maintenance.175–178 A breakdown of this tolerance during late pregnancy is considered a mechanism of disease for spontaneous preterm labor,100,151 which might be due to the diminished suppressive function of CD4+ Tregs in preterm labor.179–182 Indeed, we recently presented evidence that the administration of endotoxin, which causes PTB in mice, leads to a reduction of CD4+ Tregs at the maternal-fetal interface.144
Because progesterone plays a central role in pregnancy maintenance40–44 and increases CD4+ Tregs with a suppressive function during midgestation,154 we hypothesized that administration of vaginal progesterone and 17OHP-C from midgestation to late gestation would lead to an expansion of CD4+ Tregsat the maternalfetal interface. In the study herein, administration of vaginal progesterone, but not 17OHP-C, increased the proportion of decidual CD4+ Tregs.
Altogether these findings suggest that vaginal progesterone administration during late gestation fosters local maternal-fetal tolerance by increasing the proportion of decidual CD4+ Tregs.
Vaginal progesterone reduces the proportion of decidual CD8+CD25+Foxp3+ T cells
In addition to increasing the proportion of CD4+ Tregs, vaginal progesterone administration to pregnant mice reduced the proportion of CD8+CD25+Foxp3+ T cells in decidual tissues. This finding is consistent with previous reports demonstrating that progesterone regulates CD8+ T cell cytokine release and cytotoxicity during pregnancy.183,184 CD8+CD25+ T cells expressing Foxp3 seem to share phenotypic, functional, and mechanistic actions with the classical CD4+ Tregs; therefore, they were named CD8+ Tregs.185 CD8+Foxp3+ T cells increased in vivo in response to inflammation induced by IL-6.186 These cells inhibit T-cell responses in vitro and Th17 cell-mediated immune arthritis in vivo.186
During midgestation, CD8+Foxp3+ T cells expressing CD103 are found in the spleen in which they suppress immune responses via ICOS-B7h.187 Recently we reported that splenic CD8+CD25+Foxp3+ T cells, which produce IL-10, increased in endotoxin-induced PTB.144 In addition, we found that CD8+CD25+Foxp3+ T cells are present in both decidual tissues and maternal circulation during term pregnancy and that their proportions are increased by exogenous administration of IL-6, which restores parturition on time in Il6−/− mice.188 This supports a role for these cells in the proinflammatory milieu that is associated with the process of labor.
As a whole, these data suggest that CD8+CD25+Foxp3+ T cells have a proinflammatory phenotype rather than a suppressive phenotype and that vaginal progesterone administration reduces the proportion of these cells in the decidua, thereby having an antiinflammatory role.
Vaginal progesterone decreases the proportion of decidual macrophages
Macrophages/monocytes play central roles in the maintenance of pregnancy and term and preterm parturition including uterine contractility, cervical ripening, and the rupture of membranes as well as in uterine involution during the postpartum period.78,84,189–199 Macrophage/monocyte neutralization using an anti-F4/80 antibody prevents endotoxin-induced PTB,195 which demonstrates that macrophages/monocytes participate in the process of microbial-induced preterm labor. Macrophages/monocytes express progesterone receptors200,201; therefore, it is possible that the infiltration and/or function of these cells are regulated by progesterone.
In the study herein, we found that administration of vaginal progesterone decreased the proportion of macrophages in the decidual tissues. These data are consistent with previous reports demonstrating that the administration of progesterone reduces the infiltration and migration of macrophages/mono-cytes into the reproductive tissues.56,64 Altogether these data suggest that vaginal progesterone regulates the infiltration of macrophages/monocytes into the decidual tissues, which fosters an antiinflammatory microenvironment at the maternal-fetal interface.
Vaginal progesterone reduces the proportion of IFNγ -positive neutrophils in myometrium
Neutrophils play an important role during term and preterm parturition because they release proinflammatory mediators that are associated with the onset of labor.72,78,84,202–204 In the myometrium, the mRNA expression of CXCL8, a neutrophil chemokine, is higher in women who underwent labor than in those who did not undergo labor at term, suggesting a role for neutrophils in myometrial contractions.78,82
Recently we were able to support this hypothesis by demonstrating that the percentage and total number of myometrial neutrophils increase in endotoxin-induced PTB.144 Indeed, myometrial neutrophils express inflammatory cytokines such as IL-6, IL-8, TNFα, IFNγ, and IL-4,77,144,205 which is a characteristic phenotype of activated neutrophils.206,207 Therefore, we hypothesize that vaginal progesterone administration to pregnant mice would reduce the infiltration of activated neutrophils into the maternal-fetal interface.
In accordance with our hypothesis, we found that vaginal progesterone administration reduces the proportion of IFNγ -positive neutrophils in myometrium. Previous in vitro studies demonstrated that incubation with progesterone reduces the release of chemokine ligand-8, which attracts neutrophils, in human myometrial biopsies or rabbit uterine cervical fibroblasts.62,208 Collectively these data suggest that vaginal progesterone administration to pregnant mice reduces the infiltration of activated neutrophils into the myometrial tissues, which may be mediated by chemokine ligand-8.
Vaginal progesterone, but not 17OHP-C, reduces active MMP-9-positive neutrophils and monocytes in the cervix
We next evaluated whether vaginal progesterone or 17OHP-C administration had effects on MMP-9 activity and collagen content. MMPs are a super-family of zinc enzymes that participate in the degradation of the extracellular matrix.209–211 MMP-9 (also known as gelatinase B) was discovered in polymorphonuclear leukocytes and monocytes.212 During pregnancy, MMP-9 is expressed by resident cells and infiltrating leukocytes at the maternal-fetal interface and has been associated with the process of labor.74,95,97,213–217
The expression of active MMP-9 is increased at term pregnancy in humans and rabbits,63,218 and it was localized in human infiltrating leukocytes and murine columnar epithelial cells and fibroblasts.196,218 In vitro experimentation has demonstrated that the incubation of columnar epithelial cells or fibroblasts with progesterone inhibits MMP-9 activity.63,196 However, in vitro incubation of human myometrial muscle cells with progesterone is not able to reduce IL-1β or TNFα-induced MMP-9 activity.219
Alternatively, in vitro incubation with progesterone reduces collagen synthesis in a 3-dimensional culture system with human cervical fibroblasts.220 Herein the in vivo administration of vaginal progesterone increased MMP-9 activity and reduced collagen content in the cervical stroma. Vaginal progesterone also reduced the infiltration of active MMP-9-positive neutrophils and monocytes. In contrast, 17OHP-C administration increased MMP-9 activity in the cervical stroma, reduced collagen content, and increased infiltration of active MMP-9-positive neutrophils and monocytes.
Collectively these data demonstrate that administration of vaginal progesterone or 17OHP-C increases MMP-9 activity in the cervical stroma and decreases collagen content, yet administration of natural progesterone reduces the infiltration of neutrophils and monocytes expressing active MMP-9. Infiltration may be the key element in determining changes in the biomechanical properties of the cervix, which favor parturition.
Pretreatment with vaginal progesterone reduces the rate of endotoxin-induced preterm birth
Vaginal progesterone administration to women with a sonographic short cervix reduces the rate of PTB.7,9,11,221 In addition, pretreatment by injection of natural or medroxyprogesterone acetate prevents endotoxin-induced PTB in mice, which is associated with the down-regulation of the mRNA expression of the inflammatory cytokines Il1β and Tnf.23,33
It is interesting that the systemic administration of IL-1b induces PTB in mice, and pretreatment with the IL-1 receptor antagonist abrogrates this effect.160 In the current study, pretreatment with vaginal progesterone reduced the frequency of endotoxin-induced PTB by 50% and reduced the systemic concentrations of IL-1β. Altogether these data suggest that pretreatment with vaginal progesterone fosters a local and systemic antiinflammatory response, preventing endotoxin-induced preterm birth.
Although previous studies had reported that systemic administration of progesterone reduces the rate of endotoxin-induced preterm birth by 28%,23 the current study is the first to demonstrate that vaginal progesterone has this effect. The fact that vaginal progesterone does not prevent endotoxin-induced preterm delivery in all cases is not unexpected, given that even in women with a short cervix, the administration of vaginal progesterone reduced the rate of preterm delivery by only 45%.11 The mechanisms responsible for the protection against preterm birth in some animals and women, and not in others, remain to be determined.
Administration of 17OHP-C to women with multiple gestations has been reported to increase the rates of midtrimester fetal loss38 and PTB before 32 weeks.39 Similarly, pretreatment with 17OHP-C before endotoxin exposure has adverse effects on pregnant mice, including behavioral changes (lethargy or piloerection) and maternal death.33 For these reasons, we did not study the effect of 17OHP-C on endotoxin-induced preterm birth. However, in contrast to previous reports with 17OHP-C, vaginal progesterone followed by endotoxin did not result in demonstrable maternal morbidity or death.
A previous study demonstrated that progesterone binds with more avidity to progesterone receptors than 17OHP-C; however, both progestogens are comparable in eliciting the transactivation of reporter genes as assessed by luciferase activity in the T47D-2963.1 and T47Dco carcinoma cell lines.222 Progesterone and 17OHP-C also induced similar stimulation of endogenous alkaline phosphatase activity.222 The equivalent biological effect per unit mass of 17OHP-C and progesterone in preventing preterm delivery induced by an inhibitor of nitric oxide synthase has also been shown in CD-1 mice.223
These findings suggest that the progestational activity of 17OHP-C and progesterone as measured by these assays are similar. However, this does not seem to translate into changes in the immune cell composition at the maternal-fetal interface. Specifically, the total exposure to 17OHP-C was greater than the total exposure to vaginal progesterone. Yet we observed antiinflammatory effects only with vaginal progesterone.
Conclusion
Our results demonstrate that the administration of vaginal progesterone fosters an antiinflammatory microenvironment at the maternal-fetal interface by increasing CD4+ Tregs and reducing CD8+CD25+Foxp3+ T cells, macrophages, and IFNγ-positive neutrophils. In addition, the administration of vaginal progesterone decreases the infiltration of active MMP-9-positive neutrophils and monocytes in the cervix, marginally reduces the plasma concentration of IL-1β, and reduces the frequency of endotoxin-induced PTB. Administration of 17OHP-C did not have the same effects as vaginal progesterone. These results provide insight into the mechanisms whereby vaginal progesterone prevents preterm birth.
Supplementary Material
Acknowledgments
We gratefully acknowledge Akshata Naik, Elly Sanchez-Rodriguez, Dr Eleazar Soto, Marcia Arenas-Hernandez, Nakisha Rutledge, Tamara Roumayah, Yang Jiang, Amapola Balancio, Stella Dewar, Dr Zhong Dong, Lorri McLuckie, Rona Wang, and Sunjay Modi for their contributions to the execution of this study and to Maureen McGerty for her critical readings of the manuscript.
This study was supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal, and Child Health and by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services.
Footnotes
The authors report no conflict of interest.
Procedures were approved by the Institutional Animal Care and Use Committee at Wayne State University (protocol A09-08-12).
Presented in part at the 62nd Annual Scientific Meeting of the Society for Reproductive Investigation, San Francisco, CA, March 25–28, 2015; the 34th annual meeting of the American Society for Reproductive Immunology, Long Beach, NY, June 2–5, 2014; and the 61st Annual Scientific Meeting of the Society for Gynecological Investigation, Florence, Italy, March 26–29, 2014.
References
- 1.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–65. [PubMed] [Google Scholar]
- 3.Lubow JM, How HY, Habli M, Maxwell R, Sibai BM. Indications for delivery and short-term neonatal outcomes in late preterm as compared with term births. Am J Obstet Gynecol. 2009;200:e30–3. doi: 10.1016/j.ajog.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–52. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrman RE, Butler AS, editors. Preterm Birth: causes, consequences, and prevention. Washington, (DC): 2007. Societal costs of preterm birth. [PubMed] [Google Scholar]
- 6.Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol. 2014;210:426.e1–9. doi: 10.1016/j.ajog.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH, Fetal Medicine Foundation Second Trimester Screening Group Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 8.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–86. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 9.DeFranco EA, O’Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien JM, Defranco EA, Adair CD, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2009;34:653–9. doi: 10.1002/uog.7338. [DOI] [PubMed] [Google Scholar]
- 11.Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero R. Vaginal progesterone to reduce the rate of preterm birth and neonatal morbidity: a solution at last. Womens Health (Lond Engl) 2011;7:501–4. doi: 10.2217/whe.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124.e1–19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conde-Agudelo A, Romero R, Nicolaides K, et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208:42.e1–18. doi: 10.1016/j.ajog.2012.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R, Yeo L, Chaemsaithong P, Chaiworapongsa T, Hassan SS. Progesterone to prevent spontaneous preterm birth. Semin Fetal Neonatal Med. 2014;19:15–26. doi: 10.1016/j.siny.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 18.Meis PJ. Society for Maternal-Fetal Medicine. 17-Hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol. 2005;105:1128–35. doi: 10.1097/01.AOG.0000160432.95395.8f. [DOI] [PubMed] [Google Scholar]
- 19.Society for Maternal-Fetal Medicine Publications Committee, with assistance of Vincenzo Berghella. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206:376–86. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Romero R, Stanczyk FZ. Progesterone is not the same as 17alpha-hydroxyprogesterone caproate: implications for obstetrical practice. Am J Obstet Gynecol. 2013;208:421–6. doi: 10.1016/j.ajog.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sexton DJ, O’Reilly MW, Friel AM, Morrison JJ. Functional effects of 17alpha-hydroxyprogesterone caproate (17P) on human myometrial contractility in vitro. Reprod Biol Endocrinol. 2004;2:80. doi: 10.1186/1477-7827-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruddock NK, Shi SQ, Jain S, et al. Progesterone, but not 17-alpha-hydroxyprogesterone caproate, inhibits human myometrial contractions. Am J Obstet Gynecol. 2008;199:391.e1–7. doi: 10.1016/j.ajog.2008.06.085. [DOI] [PubMed] [Google Scholar]
- 23.Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190:693–701. doi: 10.1016/j.ajog.2003.10.693. [DOI] [PubMed] [Google Scholar]
- 24.Anderson L, Martin W, Higgins C, Nelson SM, Norman JE. The effect of progesterone on myometrial contractility, potassium channels, and tocolytic efficacy. Reprod Sci. 2009;16:1052–61. doi: 10.1177/1933719109340926. [DOI] [PubMed] [Google Scholar]
- 25.Facchinetti F, Paganelli S, Comitini G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196:453.e1–4. doi: 10.1016/j.ajog.2006.09.009. Discussion 21. [DOI] [PubMed] [Google Scholar]
- 26.Durnwald CP, Lynch CD, Walker H, Iams JD. The effect of treatment with 17 alpha-hydroxyprogesterone caproate on changes in cervical length over time. Am J Obstet Gynecol. 2009;201:410.e1–5. doi: 10.1016/j.ajog.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien JM, Defranco EA, Adair CD, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2009;34:653–9. doi: 10.1002/uog.7338. [DOI] [PubMed] [Google Scholar]
- 28.Pessel C, Moni S, Zork N, et al. The effect of intramuscular progesterone on the rate of cervical shortening. Am J Obstet Gynecol. 2013;209:269.e1–7. doi: 10.1016/j.ajog.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 29.Nold C, Maubert M, Anton L, Yellon S, Elovitz MA. Prevention of preterm birth by progestational agents: what are the molecular mechanisms? Am J Obstet Gynecol. 2013;208:223.e1–7. doi: 10.1016/j.ajog.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nold C, Maubert M, Anton L, Elovitz MA. Prevention of preterm birth with progestational agents: reveling molecular mechanisms. Am J Obstet Gynecol. 2013;208:S9. doi: 10.1016/j.ajog.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maher MA, Abdelaziz A, Ellaithy M, Bazeed MF. Prevention of preterm birth: a randomized trial of vaginal compared with intramuscular progesterone. Acta Obstet Gynecol Scand. 2013;92:215–22. doi: 10.1111/aogs.12017. [DOI] [PubMed] [Google Scholar]
- 32.Grobman WA, Thom EA, Spong CY, et al. 17 Alpha-ydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207:390.e1–8. doi: 10.1016/j.ajog.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elovitz MA, Mrinalini C. The use of progestational agents for preterm birth: lessons from a mouse model. Am J Obstet Gynecol. 2006;195:1004–10. doi: 10.1016/j.ajog.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien JM, Steichen JJ, Phillips JA, Creasy GW. Two year infant outcomes for children exposed to supplemental intravaginal progesterone gel in utero: secondary analysis of a multicenter, randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2012;206:S223. [Google Scholar]
- 35.Rebarber A, Istwan NB, Russo-Stieglitz K, et al. Increased incidence of gestational diabetes in women receiving prophylactic 17alpha-hydroxyprogesterone caproate for prevention of recurrent preterm delivery. Diabetes Care. 2007;30:2277–80. doi: 10.2337/dc07-0564. [DOI] [PubMed] [Google Scholar]
- 36.Waters TP, Schultz BA, Mercer BM, Catalano PM. Effect of 17alpha-hydroxyprogesterone caproate on glucose intolerance in pregnancy. Obstet Gynecol. 2009;114:45–9. doi: 10.1097/AOG.0b013e3181a9454b. [DOI] [PubMed] [Google Scholar]
- 37.Gyamfi C, Horton AL, Momirova V, et al. The effect of 17-alpha hydroxyprogesterone caproate on the risk of gestational diabetes in singleton or twin pregnancies. Am J Obstet Gynecol. 2009;201:392.e1–5. doi: 10.1016/j.ajog.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Combs CA, Garite T, Maurel K, Das A, Porto M. Obstetrix Collaborative Research N. Failure of 17-hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2010;203:248.e1–9. doi: 10.1016/j.ajog.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Senat MV, Porcher R, Winer N, et al. Prevention of preterm delivery by 17 alpha-hydroxyprogesterone caproate in asymptomatic twin pregnancies with a short cervix: a randomized controlled trial. Am J Obstet Gynecol. 2013;208:194.e1–8. doi: 10.1016/j.ajog.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 40.Csapo AI, Pinto-Dantas CA. The effect of progesterone on the human uterus. Proc Natl Acad Sci USA. 1965;54:1069–76. doi: 10.1073/pnas.54.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito Y, Sakamoto H, MacLusky NJ, Naftolin F. Gap junctions and myometrial steroid hormone receptors in pregnant and postpartum rats: a possible cellular basis for the progesterone withdrawal hypothesis. Am J Obstet Gynecol. 1985;151:805–12. doi: 10.1016/0002-9378(85)90525-3. [DOI] [PubMed] [Google Scholar]
- 42.Mesiano S. Myometrial progesterone responsiveness and the control of human parturition. J Soc Gynecol Investig. 2004;11:193–202. doi: 10.1016/j.jsgi.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Zakar T, Mesiano S. How does progesterone relax the uterus in pregnancy? N Engl J Med. 2011;364:972–3. doi: 10.1056/NEJMcibr1100071. [DOI] [PubMed] [Google Scholar]
- 44.Garfield RE, Shi L, Shi SQ. Use of progesterone and progestin analogs for inhibition of preterm birth and other uterine contractility disorders. Facts Views Vis Obgyn. 2012;4:237–44. [PMC free article] [PubMed] [Google Scholar]
- 45.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 46.Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length < or = 15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–67. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 47.Owen J, Yost N, Berghella V, et al. Midtrimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–8. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 48.de Carvalho MH, Bittar RE, Brizot Mde L, Bicudo C, Zugaib M. Prediction of preterm delivery in the second trimester. Obstet Gynecol. 2005;105:532–6. doi: 10.1097/01.AOG.0000154157.22500.1d. [DOI] [PubMed] [Google Scholar]
- 49.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362–7. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 50.Chwalisz K, Shi SO, Neff G, Elger J. The effect of antigestagen ZK 98, 199 on the uterine cervix. Acta Endocrinol. 1987;283:113. [Google Scholar]
- 51.Antiprogesterones Norman J. Br J Hosp Med. 1991;45:372–5. [PubMed] [Google Scholar]
- 52.Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstet Gynecol. 1998;92:804–9. doi: 10.1016/s0029-7844(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 53.Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone—a randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand. 1999;78:793–8. [PubMed] [Google Scholar]
- 54.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 55.Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? Am J Obstet Gynecol. 2008;198:314.e1–8. doi: 10.1016/j.ajog.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 56.Yellon SM, Burns AE, See JL, Lechuga TJ, Kirby MA. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol Reprod. 2009;81:1–6. doi: 10.1095/biolreprod.108.074997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersson S, Minjarez D, Yost NP, Word RA. Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J Clin Endocrinol Metab. 2008;93:2366–74. doi: 10.1210/jc.2007-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuon RJ, Shi SQ, Maul H, et al. Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle. Am J Obstet Gynecol. 2010;202:455.e1–9. doi: 10.1016/j.ajog.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holt R, Timmons BC, Akgul Y, Akins ML, Mahendroo M. The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology. 2011;152:1036–46. doi: 10.1210/en.2010-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yellon SM, Dobyns AE, Beck HL, Kurtzman JT, Garfield RE, Kirby MA. Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth. PLoS One. 2013;8:e81340. doi: 10.1371/journal.pone.0081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.House M, Tadesse S, Norwitz ER, Kaplan D. Progesterone inhibits cervical tissue formation in a 3D culture system in a dose-dependent fashion. Am J Obstet Gynecol. 2013;208:S103. [Google Scholar]
- 62.Ito A, Imada K, Sato T, Kubo T, Matsushima K, Mori Y. Suppression of interleukin 8 production by progesterone in rabbit uterine cervix. Biochem J. 1994;301(Pt 1):183–6. doi: 10.1042/bj3010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imada K, Ito A, Sato T, Namiki M, Nagase H, Mori Y. Hormonal regulation of matrix metalloproteinase 9/gelatinase B gene expression in rabbit uterine cervical fibroblasts. Biol Reprod. 1997;56:575–80. doi: 10.1095/biolreprod56.3.575. [DOI] [PubMed] [Google Scholar]
- 64.Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci. 2009;16:257–64. doi: 10.1177/1933719108325757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukuyama A, Tanaka K, Kakizaki I, et al. Anti-inflammatory effect of proteoglycan and progesterone on human uterine cervical fibroblasts. Life Sci. 2012;90:484–8. doi: 10.1016/j.lfs.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 66.Kim MG, Shim JY, Pak JH, et al. Progesterone modulates the expression of interleukin-6 in cultured term human uterine cervical fibroblasts. Am J Reprod Immunol. 2012;67:369–75. doi: 10.1111/j.1600-0897.2011.01094.x. [DOI] [PubMed] [Google Scholar]
- 67.Miller L, Alley EW, Murphy WJ, Russell SW, Hunt JS. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukoc Biol. 1996;59:442–50. doi: 10.1002/jlb.59.3.442. [DOI] [PubMed] [Google Scholar]
- 68.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–59. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 69.Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087–94. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- 70.Butts CL, Shukair SA, Duncan KM, et al. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol. 2007;19:287–96. doi: 10.1093/intimm/dxl145. [DOI] [PubMed] [Google Scholar]
- 71.Tait AS, Butts CL, Sternberg EM. The role of glucocorticoids and progestins in inflammatory, autoimmune, and infectious disease. J Leukoc Biol. 2008;84:924–31. doi: 10.1189/jlb.0208104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–81. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- 73.Liggins G. Cervical ripening as an inflammatory reaction. In: Ellwood E, Anderson A, editors. The cervix in pregnancy and labor: clinical and biochemical investigations. Edinburgh (United Kingdom): Churchill Livingstone; 1981. [Google Scholar]
- 74.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–9. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 75.Sennstrom MK, Brauner A, Lu Y, Granstrom LM, Malmstrom AL, Ekman GE. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur J Obstet Gynecol Reprod Biol. 1997;74:89–92. doi: 10.1016/s0301-2115(97)02757-7. [DOI] [PubMed] [Google Scholar]
- 76.Sennstrom MB, Ekman G, Westergren-Thorsson G, et al. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6:375–81. doi: 10.1093/molehr/6.4.375. [DOI] [PubMed] [Google Scholar]
- 77.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66:445–9. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- 78.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–5. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 79.Stjernholm-Vladic Y, Stygar D, Mansson C, et al. Factors involved in the inflammatory events of cervical ripening in humans. Reprod Biol Endocrinol. 2004;2:74. doi: 10.1186/1477-7827-2-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hassan SS, Romero R, Haddad R, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–86. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 81.Hassan SS, Romero R, Tarca AL, et al. Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2007;197:250.e1–7. doi: 10.1016/j.ajog.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200:104.e1–11. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 83.Hertelendy F, Romero R, Molnar M, Todd H, Baldassare JJ. Cytokine-initiated signal transduction in human myometrial cells. Am J Reprod Immunol. 1993;30:49–57. doi: 10.1111/j.1600-0897.1993.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 84.Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–36. [PubMed] [Google Scholar]
- 85.Belt AR, Baldassare JJ, Molnar M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999;181:359–66. doi: 10.1016/s0002-9378(99)70562-4. [DOI] [PubMed] [Google Scholar]
- 86.Mittal P, Romero R, Tarca AL, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38:617–43. doi: 10.1515/JPM.2010.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Lye SJ. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. J Cell Mol Med. 2013;17:90–102. doi: 10.1111/j.1582-4934.2012.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elliott CL, Loudon JA, Brown N, Slater DM, Bennett PR, Sullivan MH. IL-1beta and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am J Reprod Immunol. 2001;46:260–7. doi: 10.1034/j.1600-0897.2001.d01-11.x. [DOI] [PubMed] [Google Scholar]
- 89.Sindram-Trujillo A, Scherjon S, Kanhai H, Roelen D, Claas F. Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am J Reprod Immunol. 2003;49:261–8. doi: 10.1034/j.1600-0897.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 90.Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394.e1–24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig. 2006;13:97–103. doi: 10.1016/j.jsgi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 92.Esplin MS, Peltier MR, Hamblin S, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26:661–71. doi: 10.1016/j.placenta.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 93.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009;80:122–31. doi: 10.1016/j.jri.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Nhan-Chang CL, Romero R, Tarca AL, et al. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol. 2010;202:462.e1–41. doi: 10.1016/j.ajog.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205:235.e15–24. doi: 10.1016/j.ajog.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 96.Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204:364.e9–16. doi: 10.1016/j.ajog.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 97.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69:212–30. doi: 10.1111/aji.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Unal ER, Cierny JT, Roedner C, Newman R, Goetzl L. Maternal inflammation in spontaneous term labor. Am J Obstet Gynecol. 2011;204:223.e1–5. doi: 10.1016/j.ajog.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 99.Cierny JT, Unal ER, Flood P, et al. Maternal inflammatory markers and term labor performance. Am J Obstet Gynecol. 2014;210:447.e1–6. doi: 10.1016/j.ajog.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 100.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 102.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–30. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 104.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 105.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 106.Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol. 1996;88:1034–40. doi: 10.1016/s0029-7844(96)00339-0. [DOI] [PubMed] [Google Scholar]
- 107.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92:77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 108.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 109.Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–7. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 110.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 111.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 112.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120–8. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 113.Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 114.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294.e1–6. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 116.Kallapur SG, Kramer BW, Knox CL, et al. Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep. J Immunol. 2011;187:2688–95. doi: 10.4049/jimmunol.1100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cobo T, Palacio M, Martinez-Terron M, et al. Clinical and inflammatory markers in amniotic fluid as predictors of adverse outcomes in preterm premature rupture of membranes. Am J Obstet Gynecol. 2011;205:126.e1–8. doi: 10.1016/j.ajog.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 118.Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med. 2012;17:12–9. doi: 10.1016/j.siny.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Horvath B, Lakatos F, Toth C, Bodecs T, Bodis J. Silent chorioamnionitis and associated pregnancy outcomes: a review of clinical data gathered over a 16-year period. J Perinat Med. 2014;42:441–7. doi: 10.1515/jpm-2013-0186. [DOI] [PubMed] [Google Scholar]
- 120.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17. doi: 10.3109/14767058.2014.954243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–72. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–58. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125.e1–15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 124.Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. 2014;210:325.e1–10. doi: 10.1016/j.ajog.2013.10.882. [DOI] [PubMed] [Google Scholar]
- 125.Gervasi MT, Chaiworapongsa T, Naccasha N, et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1124–9. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- 126.Gervasi MT, Chaiworapongsa T, Naccasha N, et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:171–5. doi: 10.1080/jmf.11.3.171.175. [DOI] [PubMed] [Google Scholar]
- 127.Mittal P, Romero R, Tarca AL, et al. A molecular signature of an arrest of descent in human parturition. Am J Obstet Gynecol. 2011;204:177.e15–33. doi: 10.1016/j.ajog.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brennan DJ, McGee SF, Rexhepaj E, O’Connor DP, Robson M, O’Herlihy C. Identification of a myometrial molecular profile for dystocic labor. BMC Pregnancy Childbirth. 2011;11:74. doi: 10.1186/1471-2393-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chaemsaithong P, Madan I, Romero R, et al. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med. 2013;41:665–81. doi: 10.1515/jpm-2013-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abrahams VM, Bole-Aldo P, Kim YM, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–96. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 131.Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–55. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 132.Koga K, Cardenas I, Aldo P, et al. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cardenas I, Means RE, Aldo P, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–57. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cardenas I, Mulla MJ, Myrtolli K, et al. Nod1 activation by bacterial iE-DAP induces maternal-fetal inflammation and preterm labor. J Immunol. 2011;187:980–6. doi: 10.4049/jimmunol.1100578. [DOI] [PubMed] [Google Scholar]
- 135.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–55. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krikun G, Trezza J, Shaw J, et al. Lipopolysaccharide appears to activate human endometrial endothelial cells through TLR-4-dependent and TLR-4-independent mechanisms. Am J Reprod Immunol. 2012;68:233–7. doi: 10.1111/j.1600-0897.2012.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, Beaman KD. Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol. 2013;191:5702–13. doi: 10.4049/jimmunol.1301604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoang M, Potter JA, Gysler SM, et al. Human fetal membranes generate distinct cytokine profiles in response to bacterial Toll-like receptor and nod-like receptor agonists. Biol Reprod. 2014;90:39. doi: 10.1095/biolreprod.113.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim JS, Romero R, Kim MR, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52:457–64. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ito M, Nakashima A, Hidaka T, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84:75–85. doi: 10.1016/j.jri.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 141.Xu Y, Tarquini F, Romero R, et al. Peripheral CD300a+CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am J Reprod Immunol. 2012;67:184–97. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wegorzewska M, Nijagal A, Wong CM, et al. Fetal intervention increases maternal T cell awareness of the foreign conceptus and can lead to immune-mediated fetal demise. J Immunol. 2014;192:1938–45. doi: 10.4049/jimmunol.1302403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wegorzewska M, Le T, Tang Q, MacKenzie TC. Increased maternal T cell microchimerism in the allogeneic fetus during LPS-induced preterm labor in mice. Chimerism. 2015:1–6. doi: 10.1080/19381956.2014.1002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol. 2015 doi: 10.1038/cmi.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim MJ, Romero R, Kim CJ, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the fetomaternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–27. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–11. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee J, Romero R, Xu Y, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510–26. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee J, Romero R, Xu Y, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee J, Romero R, Chaiworapongsa T, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70:265–84. doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee J, Romero R, Xu Y, et al. Detection of anti-HLA antibodies in maternal blood in the second trimester to identify patients at risk of antibody-mediated maternal anti-fetal rejection and spontaneous preterm delivery. Am J Reprod Immunol. 2013;70:162–75. doi: 10.1111/aji.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gomez-Lopez N, St Louis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014;11:571–81. doi: 10.1038/cmi.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frydman R, Lelaidier C, Baton-Saint-Mleux C, Fernandez H, Vial M, Bourget P. Labor induction in women at term with mifepristone (RU 486): a double-blind, randomized, placebo-controlled study. Obstet Gynecol. 1992;80:972–5. [PubMed] [Google Scholar]
- 153.Gomez-Lopez N, Tong WC, Arenas-Hernandez M, et al. Chemotactic activity of gestational tissues through late pregnancy, term labor, and RU486-induced preterm labor in guinea pigs. Am J Reprod Immunol. 2014;20:12333. doi: 10.1111/aji.12333. [DOI] [PubMed] [Google Scholar]
- 154.Mao G, Wang J, Kang Y, et al. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology. 2010;151:5477–88. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- 155.Chang J, Zhao Y, Zhao W, Venkataramanan R, Caritis SN, Obstetrical-Fetal Pharmacology Research Units Network Quality assessment of compounded 17-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2014;210:47.e1–7. doi: 10.1016/j.ajog.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Arenas-Hernandez M, Sanchez-Rodriguez EN, Mial NT, Robertson SA, Gomez-Lopez N. Isolation of leukocytes from the murine tissues at the maternal-fetal interface. J Vis Exp. 2015;99:e52866. doi: 10.3791/52866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hadler-Olsen E, Kanapathippillai P, Berg E, Svineng G, Winberg JO, Uhlin-Hansen L. Gelatin in situ zymography on fixed, paraffin-embedded tissue: zinc and ethanol fixation preserve enzyme activity. J Histochem Cytochem. 2010;58:29–39. doi: 10.1369/jhc.2009.954354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Murai M, Turovskaya O, Kim G, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–84. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 160.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–5. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 161.Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3:121–6. doi: 10.1177/107155769600300304. [DOI] [PubMed] [Google Scholar]
- 162.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–5. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 163.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- 164.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–14. [PMC free article] [PubMed] [Google Scholar]
- 165.Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. J Immunol. 1972;108:586–90. [PubMed] [Google Scholar]
- 166.Gershon RK, Lance EM, Kondo K. Immuno-regulatory role of spleen localizing thymocytes. J Immunol. 1974;112:546–54. [PubMed] [Google Scholar]
- 167.Itoh M, Takahashi T, Sakaguchi N, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 168.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 169.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 170.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 171.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest. 2003;112:1437–43. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fowell D, Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993;177:627–36. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–91. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 174.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 175.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 176.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA. 2010;107:9299–304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–6. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kisielewicz A, Schaier M, Schmitt E, et al. A distinct subset of HLA-DR+-regulatory T cells is involved in the induction of preterm labor during pregnancy and in the induction of organ rejection after transplantation. Clin Immunol. 2010;137:209–20. doi: 10.1016/j.clim.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 180.Steinborn A, Schmitt E, Kisielewicz A, et al. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin Exp Immunol. 2012;167:84–98. doi: 10.1111/j.1365-2249.2011.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol. 2012;90:935–44. doi: 10.1038/icb.2012.33. [DOI] [PubMed] [Google Scholar]
- 182.Gomez-Lopez N, Laresgoiti-Servitje E. T regulatory cells: regulating both term and preterm labor? Immunol Cell Biol. 2012;90:919–20. doi: 10.1038/icb.2012.48. [DOI] [PubMed] [Google Scholar]
- 183.Blois SM, Joachim R, Kandil J, et al. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J Immunol. 2004;172:5893–9. doi: 10.4049/jimmunol.172.10.5893. [DOI] [PubMed] [Google Scholar]
- 184.Solano ME, Kowal MK, O’Rourke GE, et al. Progesterone and HMOX-1 promote fetal growth by CD8+ T cell modulation. J Clin Invest. 2015;125:1726–38. doi: 10.1172/JCI68140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cosmi L, Liotta F, Lazzeri E, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–14. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 186.Nakagawa T, Tsuruoka M, Ogura H, et al. IL-6 positively regulates Foxp3+CD8+ T cells in vivo. Int Immunol. 2010;22:129–39. doi: 10.1093/intimm/dxp119. [DOI] [PubMed] [Google Scholar]
- 187.Riella LV, Dada S, Chabtini L, et al. B7h (ICOS-L) maintains tolerance at the fetomaternal interface. Am J Pathol. 2013;182:2204–13. doi: 10.1016/j.ajpath.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gomez-Lopez N, Olson DM, Robertson SA. Interleukin-6 controls uterine Th9 cells and CD8+ T regulatory cells to accelerate parturition in mice. Immunol Cell Biol. 2015 doi: 10.1038/icb.2015.63. in press. [DOI] [PubMed] [Google Scholar]
- 189.Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999;61:879–83. doi: 10.1095/biolreprod61.4.879. [DOI] [PubMed] [Google Scholar]
- 190.Mackler AM, Green LM, McMillan PJ, Yellon SM. Distribution and activation of uterine mononuclear phagocytes in peripartum endometrium and myometrium of the mouse. Biol Reprod. 2000;62:1193–200. doi: 10.1095/biolreprod62.5.1193. [DOI] [PubMed] [Google Scholar]
- 191.Mackler AM, Ducsay TC, Ducsay CA, Yellon SM. Effects of endotoxin and macrophage-related cytokines on the contractile activity of the gravid murine uterus. Biol Reprod. 2003;69:1165–9. doi: 10.1095/biolreprod.103.015586. [DOI] [PubMed] [Google Scholar]
- 192.Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol. 2005;66:161–73. doi: 10.1016/j.jri.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 193.Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod. 2006;74:236–45. doi: 10.1095/biolreprod.105.044891. [DOI] [PubMed] [Google Scholar]
- 194.Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181:1470–9. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- 195.Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011;179:838–49. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gonzalez JM, Dong Z, Romero R, Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One. 2011;6:e26877. doi: 10.1371/journal.pone.0026877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Payne KJ, Clyde LA, Weldon AJ, Milford TA, Yellon SM. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012;87:106. doi: 10.1095/biolreprod.112.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hamilton S, Oomomian Y, Stephen G, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86:39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- 199.Gomez-Lopez N, Bijland MT, David MO, Robertson SA. Maternal monocyte-derived cell depletion promotes preterm delivery in mice. Reprod Sci. 2013;20:321A. [Google Scholar]
- 200.Werb Z, Foley R, Munck A. Interaction of glucocorticoids with macrophages. Identification of glucocorticoid receptors in monocytes and macrophages. J Exp Med. 1978;147:1684–94. doi: 10.1084/jem.147.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lu J, Reese J, Zhou Y, Hirsch E. Progesterone-induced activation of membrane-bound progesterone receptors in murine macrophage cells. J Endocrinol. 2015;224:183–94. doi: 10.1530/JOE-14-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–20. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 203.Winkler M, Fischer DC, Ruck P, et al. Parturition at term: parallel increases in interleukin-8 and proteinase concentrations and neutrophil count in the lower uterine segment. Hum Reprod. 1999;14:1096–100. doi: 10.1093/humrep/14.4.1096. [DOI] [PubMed] [Google Scholar]
- 204.Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–46. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 205.Yeaman GR, Collins JE, Currie JK, Guyre PM, Wira CR, Fanger MW. IFN-gamma is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145–53. [PubMed] [Google Scholar]
- 206.Ellis TN, Beaman BL. Murine polymorphonuclear neutrophils produce interferon-gamma in response to pulmonary infection with Nocardia asteroides. J Leukoc Biol. 2002;72:373–81. [PubMed] [Google Scholar]
- 207.Ethuin F, Gerard B, Benna JE, et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–71. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 208.Kelly RW, Illingworth P, Baldie G, Leask R, Brouwer S, Calder AA. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum Reprod. 1994;9:253–8. doi: 10.1093/oxfordjournals.humrep.a138491. [DOI] [PubMed] [Google Scholar]
- 209.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA. 1962;48:1014–22. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54. [PubMed] [Google Scholar]
- 211.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–33. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 212.Sopata I, Wize J. A latent gelatin specific proteinase of human leucocytes and its activation. Biochim Biophys Acta. 1979;571:305–12. doi: 10.1016/0005-2744(79)90100-1. [DOI] [PubMed] [Google Scholar]
- 213.Vadillo-Ortega F, Gonzalez-Avila G, Furth EE, et al. 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol. 1995;146:148–56. [PMC free article] [PubMed] [Google Scholar]
- 214.Vadillo-Ortega F, Hernandez A, Gonzalez-Avila G, Bermejo L, Iwata K, Strauss JF., 3rd Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinases-1 levels in amniotic fluids from pregnancies complicated by premature rupture of membranes. Am J Obstet Gynecol. 1996;174:1371–6. doi: 10.1016/s0002-9378(96)70687-7. [DOI] [PubMed] [Google Scholar]
- 215.Athayde N, Edwin SS, Romero R, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol. 1998;179:1248–53. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 216.Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab. 2002;87:1353–61. doi: 10.1210/jcem.87.3.8320. [DOI] [PubMed] [Google Scholar]
- 217.Castillo-Castrejon M, Meraz-Cruz N, Gomez-Lopez N, et al. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor. Am J Reprod Immunol. 2014;71:86–93. doi: 10.1111/aji.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002;67:889–94. doi: 10.1095/biolreprod.102.005116. [DOI] [PubMed] [Google Scholar]
- 219.Roh CR, Oh WJ, Yoon BK, Lee JH. Up-regulation of matrix metalloproteinase-9 in human myometrium during labour: a cytokine-mediated process in uterine smooth muscle cells. Mol Hum Reprod. 2000;6:96–102. doi: 10.1093/molehr/6.1.96. [DOI] [PubMed] [Google Scholar]
- 220.House M, Tadesse-Telila S, Norwitz ER, Socrate S, Kaplan DL. Inhibitory effect of progesterone on cervical tissue formation in a three-dimensional culture system with human cervical fibroblasts. Biol Reprod. 2014;90:18. doi: 10.1095/biolreprod.113.112540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–24. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 222.Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN. Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins. Am J Obstet Gynecol. 2007;197:599.e1–7. doi: 10.1016/j.ajog.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tiboni GM, Del Corso A, Marotta F. Progestational agents prevent preterm birth induced by a nitric oxide synthesis inhibitor in the mouse. Vivo. 2008;22:447–50. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


