Abstract
Background and Purpose
Early diagnosis of cerebral venous and sinus thrombosis (CVT) is currently a major clinical challenge. We proposed a novel MR black-blood thrombus imaging technique(MRBTI) for detection and quantification of CVT.
Methods
MRBTI was performed on 23 patients with proven CVT and 24 patients with negative CVT confirmed by conventional imaging techniques. Patients were divided into two groups based on the duration of clinical onset: ≤ 7 days (group 1); between 7 and 30 days (group 2). Signal-to-noise ratio (SNR) was calculated for the detected thrombus and contrast-to-noise ratio (CNR) was measured between thrombus and lumen, and also between thrombus and brain tisssue. The feasibility of using MRBTI for thrombus volume measurement was explored and total thrombus volume was calculated for each patient.
Results
In 23 patients with proven CVT, MRBTI correctly identified 113 out of 116 segments with a sensitivity of 97.4%. Thrombus SNR was 153±57 and 261±95 for group 1(n=10) and group 2(n=13), respectively(P<0.01). Thrombus to lumen CNR was 149±57 and 256±94 for group 1 and group 2. Thrombus to brain tissue CNR was 41±36 and 120±63 (P<0.01), respectively. Quantification of thrombus volume was successfully conducted in all patients with CVT, and mean volume of thrombus was 10.5±6.9cc.
Conclusions
The current findings support that with effectively suppressed blood signal, MRBTI allows selective visualization of thrombus as opposed to indirect detection of venous flow perturbation and can be used as a promising first line diagnostic imaging tool.
Keywords: Cerebral Venous Thrombosis, Magnetic Resonance, Thrombus Imaging
Introduction
Cerebral venous thrombosis (CVT), including thrombosis of cerebral veins and major dural sinuses, is a relatively uncommon form of stroke that usually affects young individuals1,2. During the past decades, improved diagnosis and treatment have improved the outcome of CVT3. However, there is often a diagnostic delay in patients with CVT4. This is because the confirmation of the diagnosis always rely on the combination of different imaging modalities, such as computed tomography(CT), magnetic resonance (MR), and MR venography, and conventional X-ray angiography. These methods assess CVT indirectly by imaging venous flow perturbation caused by thrombus. However, given the variation in venous anatomy, it is sometimes difficult to exclude CVT with existing noninvasive imaging studies. The diagnosis dilemma may delay treatment and result in death or permanent disability5,6.
A general solution to these limitations may be the direct visualization of the thrombus itself. Direct thrombus imaging test may be useful to aid in optimizing medical therapy and guiding early invasive management. Magnetic resonance direct thrombus imaging (MRDTI), as a non-contrast-enhanced T1-weighted imaging method, has gained broad interest. Several studies have confirmed a high sensitivity for detecting thrombus in the settings of intracoronary artery7, deep vein thrombosis8, and pulmonary embolus9. By exploiting the short T1 relaxation time of methemoglobin within thrombus, MRDTI depicts subacute thrombus as hyper-intense while maintaining background tissues such as the blood, vessel wall, and surrounding brain tissues as relatively low, iso-intense signal. However, the signal intensity of evolving thrombus may be complicated by coexisting more acute and older thrombus components which may appear iso-intense as well. As a result, part of thrombus could be mistaken as venous blood or surrounding brain tissues10. This would be more challenging when utilizing MRDTI in the cerebral venous system where anatomic variants including sinus atresia/hypoplasia asymmetrical sinus drainage are commonly present.
To address this limitation, we hypothesize that the thrombus can be well isolated from the surrounding tissues including lumen and wall by using so-called black-blood MR techniques. In the current study, we proposed to use a MRBTI technique, namely 3D variable-flip-angle turbo spin echo, to achieve accurate detection of thrombus in the cerebral venous system. The diagnostic performance of MRBTI was assessed in patients with or without CVT.
Subjects and Methods
Patients
Between February 2014 to May 2015, consecutive patients with signs and symptoms suspected of CVT within the past 30 days were prospectively recruited in this study. Exclusion criteria included general contraindications to MR examination, and patients with incomplete conventional imaging examinations (CT, MR, and MRV). Informed consent was obtained from all participants, and all protocols were approved by the Institutional Review Board.
Conventional Imaging Evaluation
Thrombi were defined as intraluminal filling defects detected by conventional imaging techniques. Two readers (JG Duan and XM Ji) performed a consensus reading of all conventional imaging studies for each patient including CT, MR, and MRV with full clinical and outcome information on the patient to obtain a reference standard. The following 14 venous segments were included in evaluations: superior sagittal sinus, inferior sagittal sinus, right transverse sinus, right sigmoid sinus, left transverse sinus, left sigmoid sinus, straight sinus, confluence of sinuses, veins of galen, internal cerebral veins, basal veins of rosenthal, veins of Labbé, right cortical veins, and left cortical veins.
MRBTI
3D variable-flip-angle turbo spin echo11 (SPACE) has an inherent black-blood effect and has recently been proposed for arterial vessel wall imaging12,13. To exploit the short-T1 property of acute thrombus, the sequence was used for MRBTI with a T1-weighted acquisition mode.
All MR studies were conducted on a 3.0T system (MAGNETOM Verio, Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil for signal reception. Typical imaging parameters for included: oblique coronal single-slab coverage, repetition time (TR) = 800 milli-seconds, echo time (TE) = 22 milli-seconds, matrix = 198×192, FOV = 160×200 mm2, slice thickness = 0.6-1.0 mm, slices = 100-200, scan time = 6-8 minutes.
MRBTI Image Evaluation
All MRBTI images were randomized and presented to two independent readers with 10 years (Q.Y.) and 8 years (XF.Q.) of experience in reading. The readers were not involved with the diagnostic and/or therapeutic management of the patients, and were blinded to clinical information and conventional imaging data on which the diagnosis of CVT was based. Source images, free mode multiplanar reformation (MPR), and minimum intensity projection (MinIP) images were used by readers. A third reader with 15 years experience of reading (KC.L.) was involved to resolve any disputes.
Image quality was first rated for each segment using a 4-point scale as follows: 4 = excellent, no relevant artifacts; 3 = good, minimal inhomogeneity, only minor flow artifacts; 2 = adequate, delineated lumen, major flow artifacts; and 1 = non-diagnostic.
Thrombus was visually assessed in each segment based on its characteristic hyper-intense signals relative to the luminal blood and surrounding brain tissues. The presence or absence of thrombus was recorded for each segment. Patients with CVT detected by MRBTI were divided into two groups based on the duration of clinical onset: ≤ 7 days (group 1); between 7 and 30 days (group 2). Signal intensity was measured from thrombus, luminal blood, and gray matter. Signal-to-noise ratio (SNR) was calculated for the detected thrombus and was defined as the ratio of the thrombus signal intensity and standard deviation of the background noise measured in an area outside of the head free of tissue structure and artifact. Contrast-to-noise ratio (CNR) was measured between thrombus and lumen, and also between thrombus and gray matter. CNR was calculated as signal intensity difference between the thrombus and lumen/gray matter divided by the standard deviation of background noise.
In addition, the feasibility of using MRBTI for thrombus volume measurement was explored. Specifically, thrombi in each patient was segmented in a semi-automatic fashion using a commercial software (Object Research System, Montreal, Quebec, Canada) and total thrombus volume was reported for each patient.
Statistical analysis
Differences in SNR and CNRs between group 1 and group 2 were tested with two-tailed independent t-test. A value of P<0.05 was considered to indicate statistical significance. The level of agreement in thrombus detection between the two readers was evaluated by the kappa value on a per-segment basis. The consensus reading of conventional imaging techniques were used as the reference standard for assessing the sensitivity, specificity, negative and positive predictive values of MRBTI. For the patient level analysis, each patient was categorized as correctly diagnosed if at least one venous segment was judged as positive CVT. All statistical analysis was performed using statistical software (SAS version 9.1, SAS Institute Inc., Cary, NC).
Results
Patients characteristics
Sixty-two consecutive patients met the eligibility criteria and fifteen patients were excluded due to incomplete imaging at baseline. Thus, forty-seven patients were enrolled in the MRBTI examination. MRBTI was successfully performed in all 47 patients without complications. The mean age of the patients in the study was 34 years (range, 5 to 84 years), and 28 (60%) were female. Study population characteristics are listed in Table 1.
Table 1. Baseline study population characteristics.
| n/N (%) | |
|---|---|
| Demographics | |
| Mean age, y(SD) | 34±13 years |
| Sex (% of women) | 28/47(60%) |
| Clinical characteristics | |
| Headache | 27/47 (57%) |
| Papilledema | 12/47 (26%) |
| Focal neurological deficit | 5/47 (11%) |
| Comatose | 1/47 (2%) |
| Duration from onset to BB-MRDTI | |
| 0-7days | 19/47(40%) |
| 7-30days | 28/47(60%) |
| Risk factors | |
| Pregnancy or puerperium (% of women) | 6/47(13%) |
| Oral contraceptives (% of women) | 8/47 (17%) |
| Infection | 7/47(15%) |
Distribution of Venous Thrombosis by Conventional Imaging Techniques
All 47 patients have CT, MR and TOF MRV, five of 47 patients have contrast enhanced CTV. A total of 116 thrombosed venous segments were identified in 23 patients. Thrombosed segments included: superior sagittal sinus (14), right transverse sinus(16), right sigmoid sinus(17), left transverse sinus(11), left sigmoid sinus(9), straight sinus(8), confluence of sinuses(12), veins of galen(4), internal cerebral veins(2), veins of Labbé(1), right cortical veins(11), and left cortical veins(11).
MRBTI Image Quality
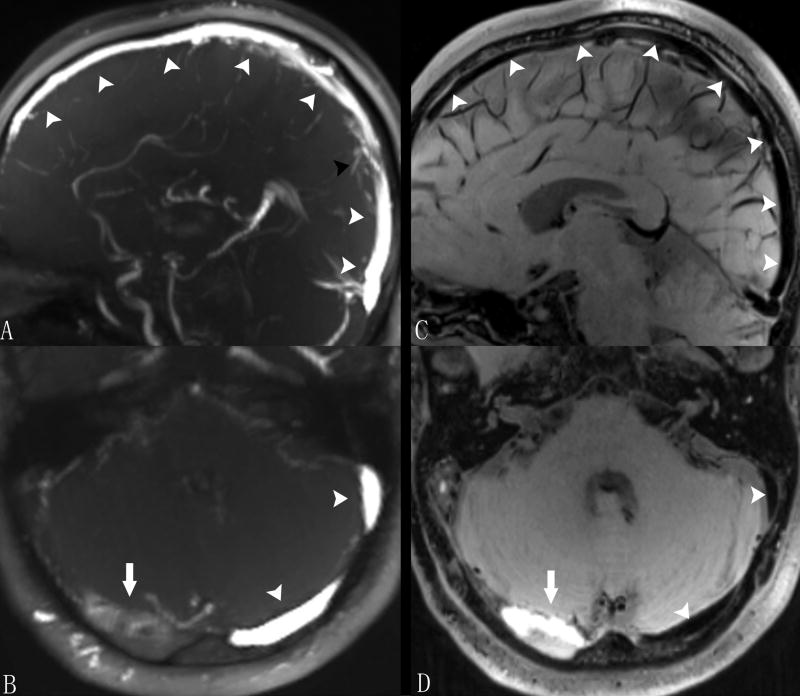
Figure 1 shows a typical example of thrombosis case acquired with conventional TOF technique and the propose MRBTI method. Blood signal was effectively suppressed using MRBTI and thrombi were depicted as hyper-intense with excellent contrast relative to surrounding tissues (Fig. 1B, 1D). In comparison, some flow-dephasing related signal loss was observed in TOF images (Fig 1A, arrowheads). The overall image quality score was 3.5±0.6. Among 658 segments, 647 (98%) were diagnostic (score ≥2). Among the non-diagnostic segments, 7 were due to limited spatial coverage and 4 were due to flow artifacts. These 11 segments were excluded in the following diagnostic performance analyses.
Figure 1.
MRBTI images of a 48-year-old male with sub-acute CVT. On TOF images, normal venous sinuses were depicted with bright venous flow signals (arrowheads in A and B) and minor flow defects (black arrowhead in A) observed in the superior sagittal sinus was not considered as thrombus by radiologists. With blood signal adequately suppressed on MRBTI, normal venous sinus were depicted as black area (arrowheads in C and D) and hyper-intense signal was found in the right transverse sinus suggesting a fresh thrombus (arrow in D). The thrombus was also confirmed on TOF (arrow in B).
Thrombus Signal Intensity
All thrombi were depicted as hyper-intense relative to surrounding tissues, as demonstrated in Fig. 1D (arrow). Thrombus SNR was 153±57 and 261±95 for group 1(n=10) and group 2(n=13), respectively. Thrombus to lumen CNR was 149±57 and 256±94 for group 1 and group 2. Thrombus to brain tissue CNR was 41±36 and 120±63 (P<0.01), respectively. The difference between the two groups were significant in all above signal measurements (Fig. 2).
Figure 2.
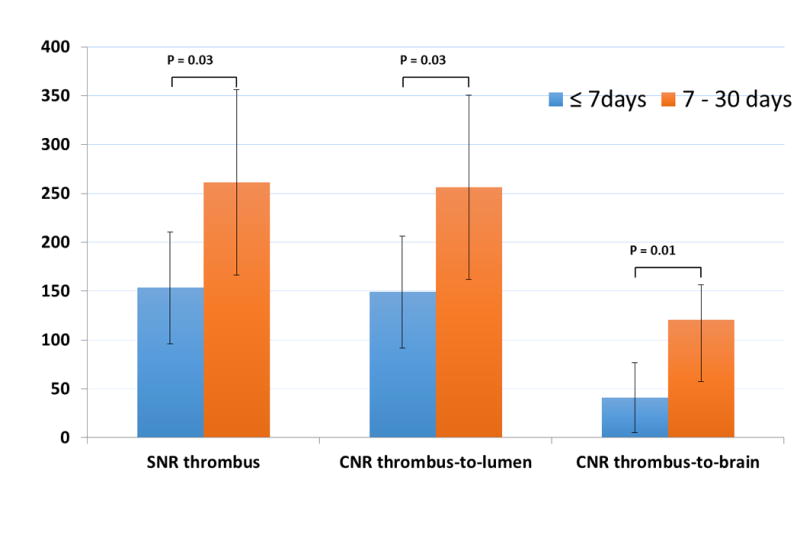
High thrombus SNR, thrombus-to-lumen CNR, and thrombus-to-gray matter CNR were obtained with MRBTI in both thrombus groups. They were all significantly different between the two groups.
Diagnostic Performance of MRBTI
MRBTI correctly identified 113 out of 116 segments with CVT with a sensitivity of 97.4%. In 527 out 531 segments, CVT was ruled out correctly with a specificity of 99.3%. A detailed overview of the diagnostic performance of MRDTI compared with standard of reference is summarized in Table 2.
Table 2. Diagnostic Performance of MRDTI for Detection of CVT.
| Patient based N=47 |
Segment based N=647 |
|
|---|---|---|
| CVT by consensus reading, No. | 23 | 116 |
| CVT by MRDTI, No. | 23 | 113 |
| False-positive, No. | 1 | 4 |
| False-negative, No. | 0 | 3 |
| Sensitivity, % (95% CI) | 100(85.2-100) | 97.4(92.6-99.5) |
| Specificity, % (95% CI) | 95.8(78.9-99.9) | 99.25(98.1- 99.8) |
| Positive predictive value, % (95% CI) | 95.8 (78.9-99.9) | 96.6(91.5- 99.1) |
| Negative predictive value, % (95% CI) | 100(85.2-100) | 99.4(98.4-99.9) |
Note.—Data are percentages, with raw data in parentheses and 95% confidence Abbreviations: CI, confidence interval.
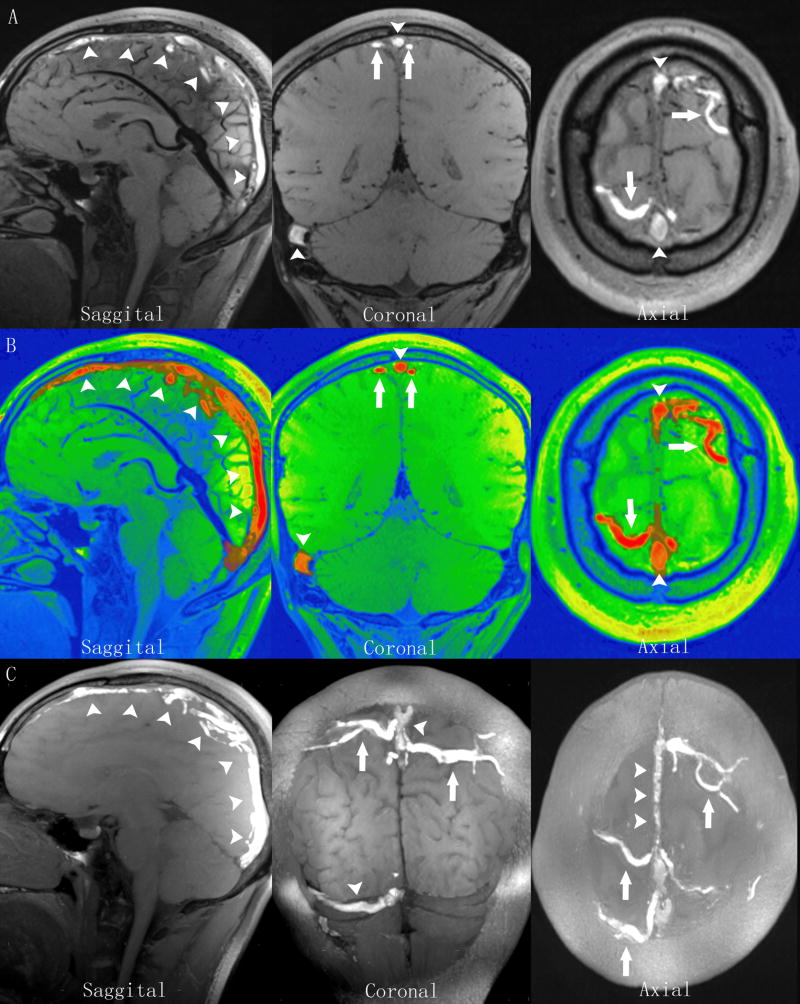
High isotropic-resolution MRBTI was able to detect hyper-intense thrombi in different segments. Fig. 3 demonstrates detection of thrombi in the superior sagittal sinus, the right transverse and sigmoid sinuses, and the cortical veins.
Figure 3.
MRBTI of a 27-year-old male patient with sub-acute CVT. A: MRBTI demonstrated hyper-intense signal intensity in the superior sagittal sinus (arrowheads), the right transverse and sigmoid sinuses (arrowheads), and the cortical veins (arrows) suggesting intraluminal thrombus formation. B: All thrombi semi-automatically outlined by software based on their high signal contrast were rendered with red color and volume was 21.5 cc. C: sagittal, coronal and axial sections of maximum intensity projection (MIP) reformations of MRBTI better depicted the thrombosed segments with hyper-intense signals.
Quantification of Thrombus Volume
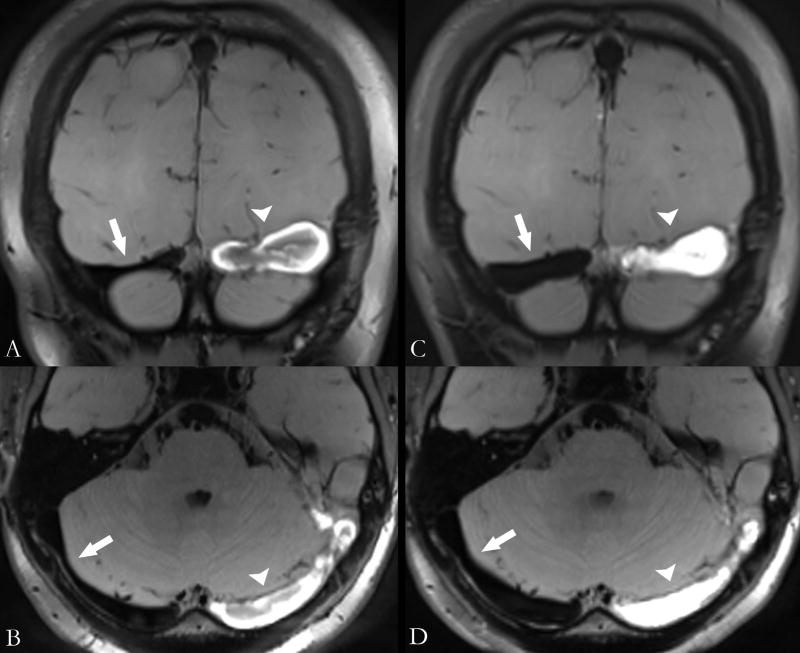
Quantification of thrombus volume was successfully conducted in all patients with CVT. Mean volume of thrombus was 10.5±6.9cc. There was no significant difference between the two groups (8.6±7.2cc vs 11.9±6.5cc, P=0.28). Figure 4 demonstrated thrombus volume quantification in one patient who underwent series scans on day 7 and day 14 (22.4cc vs 12.5cc). The complicated signal intensity pattern of evolving thrombus was also revealed.
Figure 4.
MRBTI of a 23-year-old female patient who was imaged on Day 7 (A and B) and Day 14 (C and D) after symptomatic onset. MRBTI demonstrated hyper-intense signal intensity in left transverse sinus suggesting intraluminal thrombus formation. The thrombus on Day 7 (arrowheads) and Day 14 (arrows) exhibited different hyper-intense signal patterns. CVT volume was 22.4cc and 12.5cc on day 7 and day 14, respectively. Notice the right transverse sinus with suppressed lumen signals became larger in size in response to the gradual occlusion of the left transverse sinus.
Discussion
The presented results demonstrated MRBTI can early detect CVT with a high diagnostic accuracy. This study was, to the best of our knowledge, the first evaluation of MRBTI for direct visualization of cerebral venous thrombosis.
Neuroimaging plays a key role in the diagnosis of CVT2,14. CTV and MRV have been widely used for detecting cerebral venous changes that may be related to thrombosis15-18. Instead of directly imaging thrombus, most of these techniques rely on visualization of altered blood flow in the veins resulting from thrombotic vessel lumen. Anatomic variants of normal venous anatomy, including sinus atresia/hypoplasia, asymmetrical sinus drainage, and normal sinus filling defects may mimic sinus thrombosis19,20 and compromise the diagnostic confidence using these methods. For exmaple, arachnoid granulations protruding into the sinus lumen may produce a focal filling defect on MRV that can simulate focal thrombosis. Contrast-enhanced MR venography with elliptic centric ordering has been widely used as venographic method which may assists in distinguishing anatomic variants from cerebral venous thrombosis21. However, it has limited utility in patients with renal impairment because of the requirement of gadolinium.
Unlike conventional imaging techniques, MRDTI directly targets the thrombus itself and depicts thrombus as hyper-intense and other tissues as iso-intense based on strong T1 contrast weighting. Despite the sufficient contrast for thrombus detection with conventional MRDTI, the volume of thrombus could be underestimated due to sometimes heterogeneous appearance in acute and/or subacute thrombus. To overcome the limitation, we proposed to apply T1-weighted SPACE to CVT detection by utilizing its intrinsic blood nulling capability. Our results demonstrated that CVT was well isolated from the surrounding tissues including lumen and wall with this MRBTI method, and the entire thrombus volume is readily appreciated. In addition, sinus anatomy structures, such as sinus wall, arachnoid granulations, and surrounding tissues, can be well visualized. On the other hand, the black-blood contrast helps reduce the false positive diagnosis due to flow artifacts commonly observed on TOF, as shown in Fig. 1. This suggests that the black-blood feature was a major contributor to the high detection accuracy of CVT in this study.
The feasibility of quantifying thrombus volume was demonstrated in this work. Due to the high signal contrast of thrombus and clear spatial depiction of venous structure as mentioned above, the volume of CVT can be quantified with the aid of software in a semiautomatic fashion. Such a quantitative method may make MRBTI as a robust technique for monitoring thrombus progression22.
The non-contrast nature of MRBTI is highly relevant to clinics. The technique is free of the risk for allergic reactions and can be used for repeated follow-up exams if necessary. A substantial patient population including pregnant and post-partum women and the elderly with severe kidney insufficiency will greatly benefit from such a radiation free, non-contrast imaging technique23. The MRBTI technique can potentially serve as a first-line diagnostic imaging test.
There were several limitations in the present study. First, because of the relatively small numbers of patients include in this single center study, the sensitivity and specificity of the MRBTI technique need to be fully investigated before it is accepted as standard imaging technique for CVT detection. However, CVT is a rare disease, thus rendering the recruitment of large patient cohort difficult. Thus, a multi-center evaluation of the performance of this MRBTI technique is desirable. Second, hyper acute or chronic thrombosis do not have a short T1 relaxation time and exhibits iso-intense signal on T1-weighted images. However, with the blood signal adequately suppressed on MRBTI, normal venous anatomy was depicted as hypo-intense black area. Therefore, thrombus with iso-intense signal can still be readily detected. In addition, the presence of high signal intensity in chronic CVT patient could potentially be used as a conclusive sign of a recurrent CVT. Third, despite using highly efficient SPACE technique for data acquisition, the imaging time of the current protocol is still long (6-8 minutes) and further acceleration of data acquisition is highly desirable. Finally, the reference gold standard used in this study was a combination of conventional imaging techniques (CT, MR, and MRV) with clinical information. The combined use of these imaging techniques gave a more comprehensive reference that mitigates known deficiencies found in any single technique. Despite this comprehensive gold standard, it is difficult to generalize our sensitivity and specificity findings since we relied on the interpretation of these images by two readers at a single center. Follow up studies designed as larger clinical trials can aid in determining a more general sensitivity and specificity. Furthermore, the ease of directly imaging the thrombus with the proposed MRBTI technique may simplify the evaluation and diagnosis of CVT.
In conclusion, the current findings support that MRBTI allows selective visualization of thrombus with high accuracy and holds promise to provide a valuable alternative to current techniques, opening a new place for MRBTI in CVT diagnostics.
Acknowledgments
Sources of Funding: The study was partially support by National Institutes of Health Grant number (2R01HL096119), AHA(15SDG25710441), National Science Foundation of China (No. 81322022, 81325007, 81120108012), Program for New Century Excellent Talents in University (No.13-0918), Cheung Kong Scholars Programme(T2014251).
Debiao Li reports receiving research support from Siemens Medical Solutions. Xiaoming Bi is employee of Siemens AG Healthcare.
Footnotes
Conflict(s) of Interest/Disclosure(s): The remaining authors report no conflicts.
References
- 1.Stam J. Thrombosis of the Cerebral Veins and Sinuses. N Engl J Med. 2005;352:1791–1798. doi: 10.1056/NEJMra042354. [DOI] [PubMed] [Google Scholar]
- 2.Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. The Lancet Neurology. 2007;6:162–170. doi: 10.1016/S1474-4422(07)70029-7. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho JM, Zuurbier SM, Stam J. Declining Mortality in Cerebral Venous Thrombosis A Systematic Review. Stroke. 2014;45:1338–1341. doi: 10.1161/STROKEAHA.113.004666. [DOI] [PubMed] [Google Scholar]
- 4.Ferro JM. Prognosis of Cerebral Vein and Dural Sinus Thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 5.Saposnik G, Barinagarrementeria F, Brown RD, Bushnell CD, Cucchiara B, Cushman M, et al. American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 6.Chiewvit P, Piyapittayanan S, Poungvarin N. Cerebral venous thrombosis: diagnosis dilemma. Neurol Int. 2011;3:e13. doi: 10.4081/ni.2011.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen CHP, Perera D, Makowski MR, Wiethoff AJ, Phinikaridou A, Razavi RM, et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 2011;124:416–424. doi: 10.1161/CIRCULATIONAHA.110.965442. [DOI] [PubMed] [Google Scholar]
- 8.Tan M, Mol GC, van Rooden CJ, Klok FA, Westerbeek RE, Iglesias del Sol A, et al. Magnetic resonance direct thrombus imaging differentiates acute recurrent ipsilateral deep vein thrombosis from residual thrombosis. Blood. 2014;124:623–627. doi: 10.1182/blood-2014-04-566380. [DOI] [PubMed] [Google Scholar]
- 9.van Langevelde K, Srámek A, Vincken PWJ, van Rooden JK, Rosendaal FR, Cannegieter SC. Finding the origin of pulmonary emboli with a total-body magnetic resonance direct thrombus imaging technique. Haematologica. 2013;98:309–315. doi: 10.3324/haematol.2012.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moody AR. Magnetic resonance direct thrombus imaging. J Thromb Haemost. 2003;1:1403–1409. doi: 10.1046/j.1538-7836.2003.00333.x. [DOI] [PubMed] [Google Scholar]
- 11.Mugler JP. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging. 2014;39:745–767. doi: 10.1002/jmri.24542. [DOI] [PubMed] [Google Scholar]
- 12.Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging. 2011;34:22–30. doi: 10.1002/jmri.22592. [DOI] [PubMed] [Google Scholar]
- 13.Fan Z, Zhang Z, Chung YC, Weale P, Zuehlsdorff S, Carr J, et al. Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD) J Magn Reson Imaging. 2010;31:645–654. doi: 10.1002/jmri.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khandelwal N, Agarwal A, Kochhar R, Bapuraj JR, Singh P, Prabhakar S, et al. Comparison of CT Venography with MR Venography in Cerebral Sinovenous Thrombosis. American Journal of Roentgenology. 2006;187:1637–1643. doi: 10.2214/AJR.05.1249. [DOI] [PubMed] [Google Scholar]
- 15.Meckel S, Reisinger C, Bremerich J, Damm D, Wolbers M, Engelter S, et al. Cerebral Venous Thrombosis: Diagnostic Accuracy of Combined, Dynamic and Static, Contrast-Enhanced 4D MR Venography. American Journal of Neuroradiology. 2010;31:527–535. doi: 10.3174/ajnr.A1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linn J, Ertl-Wagner B, Seelos KC, Strupp M, Reiser M, Bruckmann H, et al. Diagnostic value of multidetector-row CT angiography in the evaluation of thrombosis of the cerebral venous sinuses. American Journal of Neuroradiology. 2007;28:946–952. [PMC free article] [PubMed] [Google Scholar]
- 17.Hu HH, Campeau NG, Huston J, III, Kruger DG, Haider CR, Riederer SJ. High-Spatial-Resolution Contrast-enhanced MR Angiography of the Intracranial Venous System with Fourfold Accelerated Two-dimensional Sensitivity Encoding 1. Radiology. 2007;243:853–861. doi: 10.1148/radiol.2433060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idbaih A, Boukobza M, Crassard I, Porcher R, Bousser MG, Chabriat H. MRI of clot in cerebral venous thrombosis: high diagnostic value of susceptibility-weighted images. Stroke. 2006;37:991–995. doi: 10.1161/01.STR.0000206282.85610.ae. [DOI] [PubMed] [Google Scholar]
- 19.Boukobza M, Crassard I, Bousser MG, Chabriat H. MR Imaging Features of Isolated Cortical Vein Thrombosis: Diagnosis and Follow-Up. American Journal of Neuroradiology. 2008;30:344–348. doi: 10.3174/ajnr.A1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang L, Korogi Y, Sugahara T, Ikushima I, Shigematsu Y, Takahashi M, et al. Normal structures in the intracranial dural sinuses: delineation with 3D contrast-enhanced magnetization prepared rapid acquisition gradient-echo imaging sequence. American Journal of Neuroradiology. 2002;23:1739–1746. [PMC free article] [PubMed] [Google Scholar]
- 21.Farb RI, Scott JN, Willinsky RA, Montanera WJ, Wright GA, terBrugge KG. Intracranial Venous System: Gadolinium-enhanced Three-dimensional MR Venography with Auto-triggered Elliptic Centric-ordered Sequence—Initial Experience1. Radiology. 2003;226:203–209. doi: 10.1148/radiol.2261020670. [DOI] [PubMed] [Google Scholar]
- 22.Coutinho JM, Stam J. How to treat cerebral venous and sinus thrombosis. J Thromb Haemost. 2010;8:877–883. doi: 10.1111/j.1538-7836.2010.03799.x. [DOI] [PubMed] [Google Scholar]
- 23.Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Cantú C, Bousser MG, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40:2356–2361. doi: 10.1161/STROKEAHA.108.543884. [DOI] [PubMed] [Google Scholar]