Abstract
BACKGROUND: Patients with non-tuberculous mycobacteria are usually started on conventional antituberculous triple therapy once acid fast bacilli are detected, before the exact type of mycobacteria has been identified. The ability to identify the characteristics of patients with tuberculous and non-tuberculous mycobacteria may be helpful in identifying before treatment those patients more likely to have non-tuberculous infection. METHODS: A retrospective study was conducted of all patients in one unit in whom non-tuberculous mycobacteria were identified in sputum or bronchoalveolar washings in the period 1987-93. The pattern of drug resistance was determined from laboratory records, and all case notes and chest radiographs were reviewed to identify the underlying disease and treatment outcome. All cases were compared with a matched control group of patients with culture positive Mycobacterium tuberculosis diagnosed during the same period. RESULTS: In the period studied there were 70 non-tuberculous and 221 tuberculous isolates. The non-tuberculous bacteria were typed as follows: M xenopi 23 (33%), M kansasii 19 (27%), M fortuitum 14 (20%), others 14 (20%). Of those with non-tuberculous mycobacteria, 83% were white subjects compared with 47% for tuberculosis. Patients with non-tuberculous mycobacteria were older than those with tuberculosis. Pre-existing lung disease or AIDS was present in 81% of patients with non-tuberculous mycobacteria and in 17% of patients with tuberculosis. Sensitivity to rifampicin and ethambutol was seen in 95% of M xenopi and 96% of M kansasii isolates. Relapse occurred in 60% of cases infected with M xenopi, 20% infected with M kansasii, and in 7% of cases with tuberculosis. CONCLUSIONS: In the population studied non-tuberculous mycobacteria occurred most frequently in elderly white subjects with pre-existing lung disease. If mycobacteria are detected in this group, consideration should be given to the possibility of non-tuberculous infection before embarking on treatment. A combination containing rifampicin and ethambutol is effective. The relapse rate for infection with M xenopi is high and prospective studies of the effect of the above combination of antituberculosis drugs are needed.
Full text
PDF
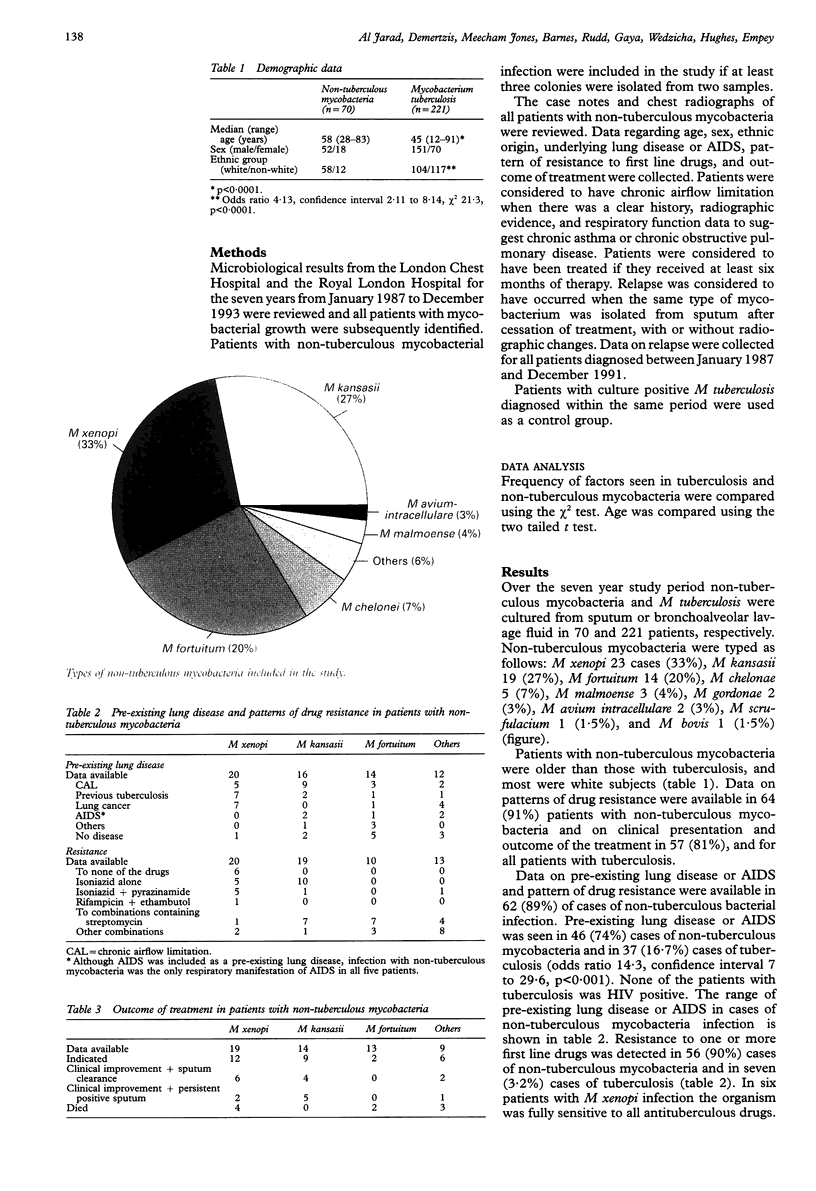

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks J., Hunter A. M., Campbell I. A., Jenkins P. A., Smith A. P. Pulmonary infection with mycobacterium xenopi: review of treatment and response. Thorax. 1984 May;39(5):376–382. doi: 10.1136/thx.39.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. D. Infection with Mycobacterium kansasii. Thorax. 1994 May;49(5):435–436. doi: 10.1136/thx.49.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld R., Bodey G. P., Gröschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976 Jan;136(1):67–70. [PubMed] [Google Scholar]
- Grange J. M., Yates M. D. Infections caused by opportunist mycobacteria: a review. J R Soc Med. 1986 Apr;79(4):226–229. doi: 10.1177/014107688607900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel D. E., Leech J. A., Pollak B. Mycobacterium kansasii: colonization and disease. Br J Dis Chest. 1986 Apr;80(2):131–137. doi: 10.1016/0007-0971(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]