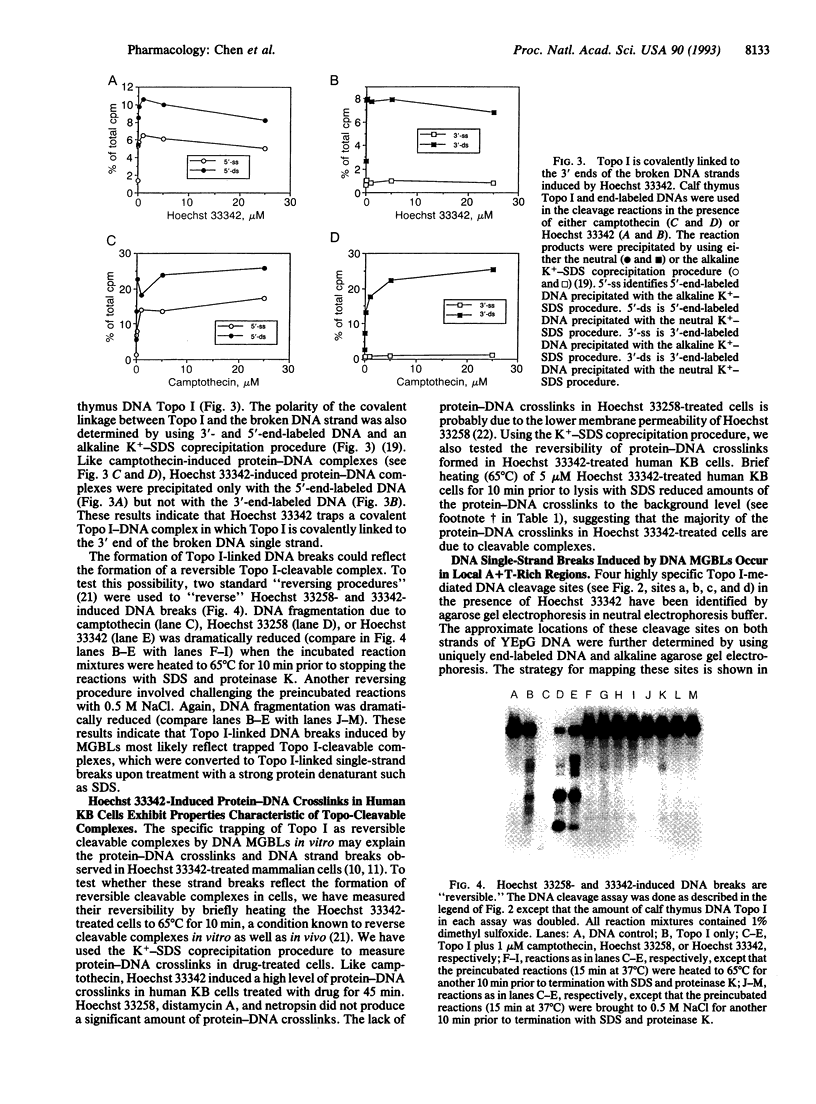
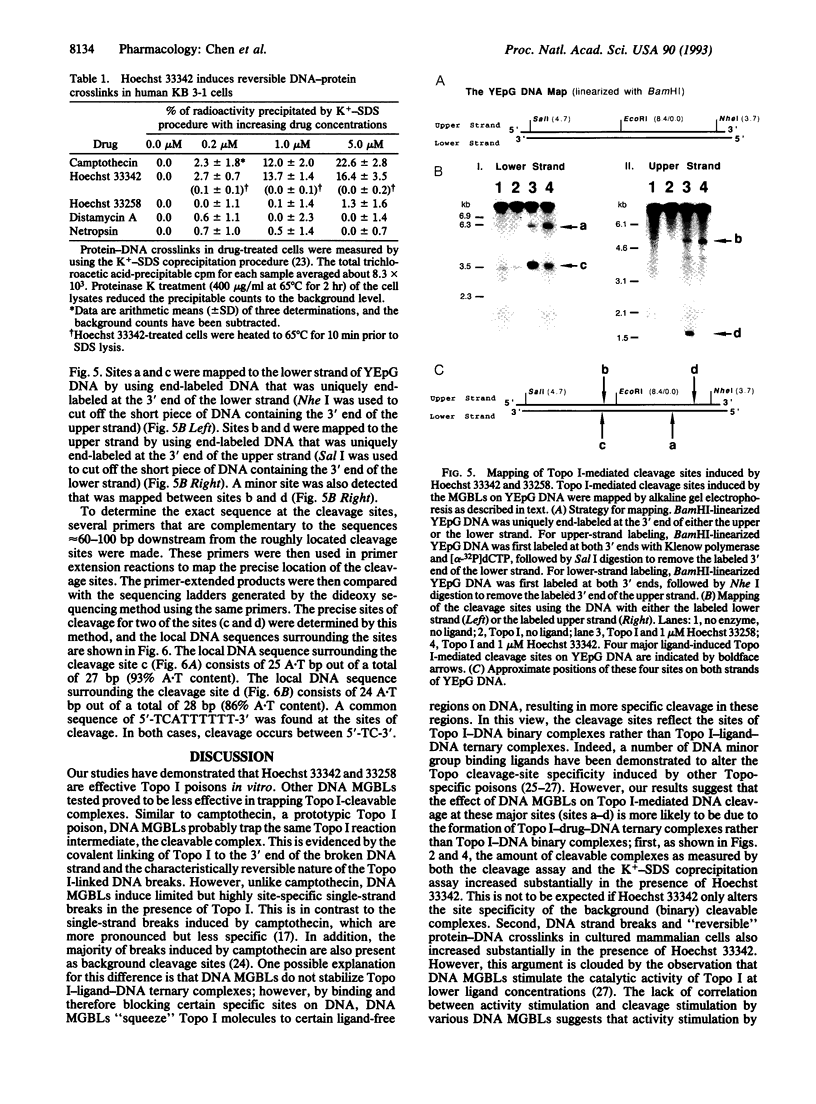
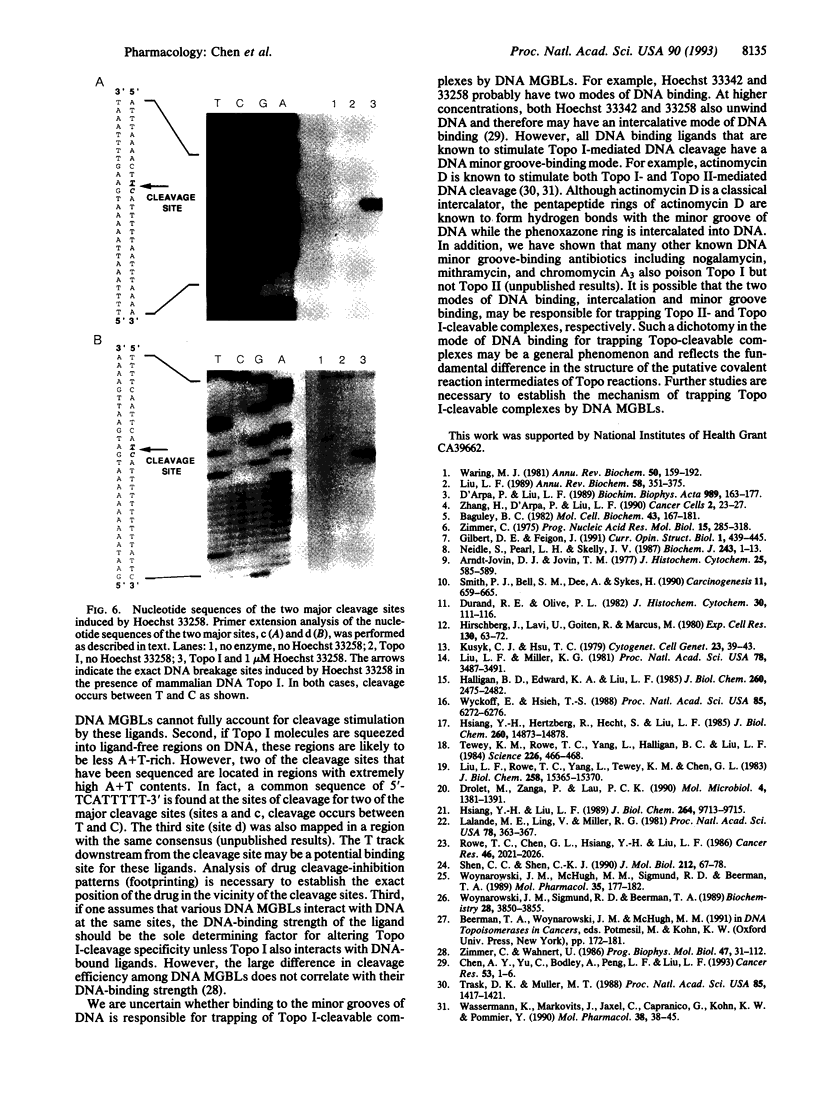
Abstract
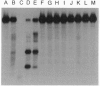
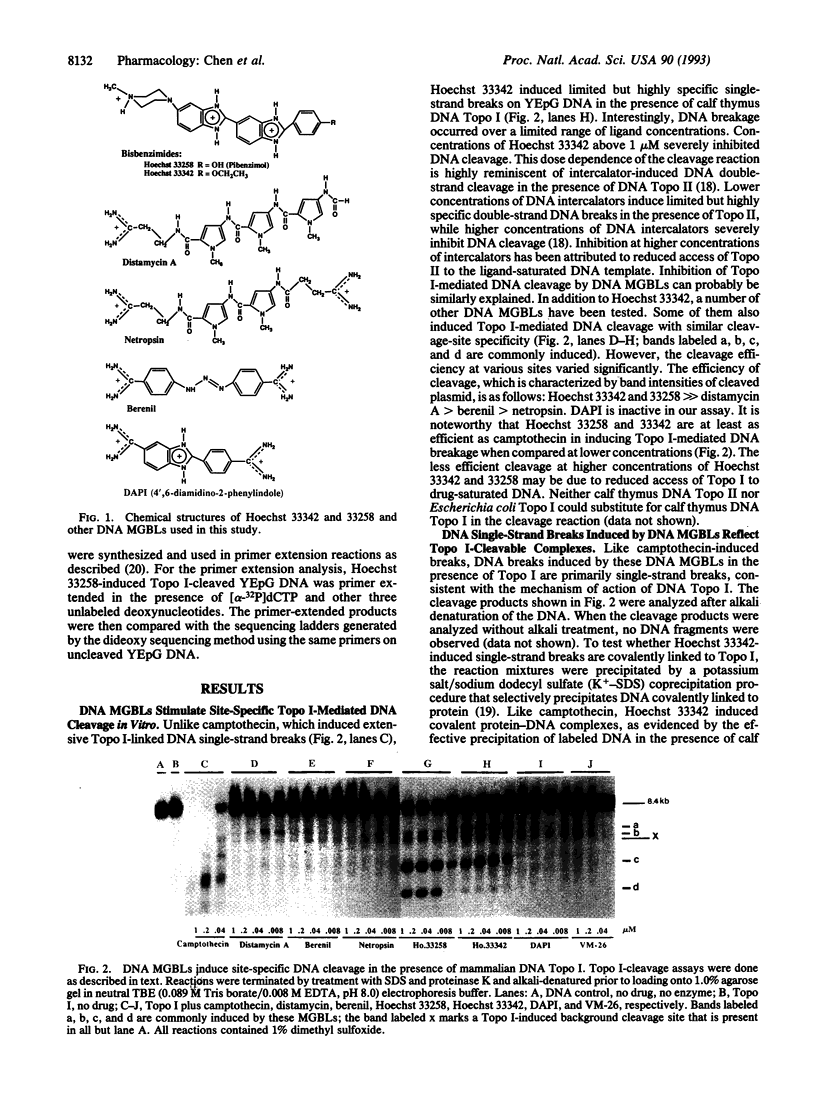
A number of DNA minor groove-binding ligands (MGBLs) are known to exhibit antitumor and antimicrobial activities. We show that DNA topoisomerase (Topo) I may be a pharmacological target of MGBLs. In the presence of calf thymus Topo I, MGBLs induced limited but highly specific single-strand DNA breaks. The 3' ends of the broken DNA strands are covalently linked to Topo I polypeptides. Protein-linked DNA breaks are readily reversed by a brief heating to 65 degrees C or the addition of 0.5 M NaCl. These results suggest that MGBLs, like camptothecin, abort Topo I reactions by trapping reversible cleavable complexes. The sites of cleavage induced by MGBLs are distinctly different from those induced by camptothecin. Two of the major cleavage sites have been sequenced and shown to be highly A + T-rich, suggesting the possible involvement of a Topo I-drug-DNA ternary complex at the sites of cleavage. Different MGBLs also exhibit varying efficiency in inducing Topo I-cleavable complexes, and the order of efficiency is as follows: Hoechst 33342 and 33258 >> distamycin A > berenil > netropsin. The lack of correlation between DNA binding and cleavage efficiency suggest that, in addition to binding to the minor grooves of DNA, MGBLs must also interact with Topo I in trapping Topo I-cleavable complexes.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Jovin T. M. Analysis and sorting of living cells according to deoxyribonucleic acid content. J Histochem Cytochem. 1977 Jul;25(7):585–589. doi: 10.1177/25.7.70450. [DOI] [PubMed] [Google Scholar]
- Baguley B. C. Nonintercalative DNA-binding antitumour compounds. Mol Cell Biochem. 1982 Apr 2;43(3):167–181. doi: 10.1007/BF00223008. [DOI] [PubMed] [Google Scholar]
- Drolet M., Zanga P., Lau P. C. The mobilization and origin of transfer regions of a Thiobacillus ferrooxidans plasmid: relatedness to plasmids RSF1010 and pSC101. Mol Microbiol. 1990 Aug;4(8):1381–1391. doi: 10.1111/j.1365-2958.1990.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Durand R. E., Olive P. L. Cytotoxicity, Mutagenicity and DNA damage by Hoechst 33342. J Histochem Cytochem. 1982 Feb;30(2):111–116. doi: 10.1177/30.2.7061816. [DOI] [PubMed] [Google Scholar]
- Halligan B. D., Edwards K. A., Liu L. F. Purification and characterization of a type II DNA topoisomerase from bovine calf thymus. J Biol Chem. 1985 Feb 25;260(4):2475–2482. [PubMed] [Google Scholar]
- Hirschberg J., Lavi U., Goitein R., Marcus M. The pleiotropic effects of 33258-Hoechst on the cell cycle in Chinese hamster cells in vitro. Exp Cell Res. 1980 Nov;130(1):63–72. doi: 10.1016/0014-4827(80)90042-7. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Hsiang Y. H., Liu L. F. Evidence for the reversibility of cellular DNA lesion induced by mammalian topoisomerase II poisons. J Biol Chem. 1989 Jun 15;264(17):9713–9715. [PubMed] [Google Scholar]
- Lalande M. E., Ling V., Miller R. G. Hoechst 33342 dye uptake as a probe of membrane permeability changes in mammalian cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):363–367. doi: 10.1073/pnas.78.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Miller K. G. Eukaryotic DNA topoisomerases: two forms of type I DNA topoisomerases from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3487–3491. doi: 10.1073/pnas.78.6.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Rowe T. C., Yang L., Tewey K. M., Chen G. L. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983 Dec 25;258(24):15365–15370. [PubMed] [Google Scholar]
- Neidle S., Pearl L. H., Skelly J. V. DNA structure and perturbation by drug binding. Biochem J. 1987 Apr 1;243(1):1–13. doi: 10.1042/bj2430001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T. C., Chen G. L., Hsiang Y. H., Liu L. F. DNA damage by antitumor acridines mediated by mammalian DNA topoisomerase II. Cancer Res. 1986 Apr;46(4 Pt 2):2021–2026. [PubMed] [Google Scholar]
- Shen C. C., Shen C. K. Specificity and flexibility of the recognition of DNA helical structure by eukaryotic topoisomerase I. J Mol Biol. 1990 Mar 5;212(1):67–78. doi: 10.1016/0022-2836(90)90305-6. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Bell S. M., Dee A., Sykes H. Involvement of DNA topoisomerase II in the selective resistance of a mammalian cell mutant to DNA minor groove ligands: ligand-induced DNA-protein crosslinking and responses to topoisomerase poisons. Carcinogenesis. 1990 Apr;11(4):659–665. doi: 10.1093/carcin/11.4.659. [DOI] [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Trask D. K., Muller M. T. Stabilization of type I topoisomerase-DNA covalent complexes by actinomycin D. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1417–1421. doi: 10.1073/pnas.85.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann K., Markovits J., Jaxel C., Capranico G., Kohn K. W., Pommier Y. Effects of morpholinyl doxorubicins, doxorubicin, and actinomycin D on mammalian DNA topoisomerases I and II. Mol Pharmacol. 1990 Jul;38(1):38–45. [PubMed] [Google Scholar]
- Woynarowski J. M., McHugh M., Sigmund R. D., Beerman T. A. Modulation of topoisomerase II catalytic activity by DNA minor groove binding agents distamycin, Hoechst 33258, and 4',6-diamidine-2-phenylindole. Mol Pharmacol. 1989 Feb;35(2):177–182. [PubMed] [Google Scholar]
- Woynarowski J. M., Sigmund R. D., Beerman T. A. DNA minor groove binding agents interfere with topoisomerase II mediated lesions induced by epipodophyllotoxin derivative VM-26 and acridine derivative m-AMSA in nuclei from L1210 cells. Biochemistry. 1989 May 2;28(9):3850–3855. doi: 10.1021/bi00435a034. [DOI] [PubMed] [Google Scholar]
- Wyckoff E., Hsieh T. S. Functional expression of a Drosophila gene in yeast: genetic complementation of DNA topoisomerase II. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6272–6276. doi: 10.1073/pnas.85.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C. Effects of the antibiotics netropsin and distamycin A on the structure and function of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1975;15(0):285–318. doi: 10.1016/s0079-6603(08)60122-1. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]