Abstract
Cryptococcus neoformans is a facultative intracellular fungal pathogen that has a polysaccharide capsule and causes life-threatening meningoencephalitis. Its capsule, as well as its ability to survive in the acidic environment of the phagolysosome, contributes to the pathogen's resilience in the host environment. Previously, we reported that downregulation of allergen 1 (ALL1) results in the secretion of a shorter, more viscous exopolysaccharide with less branching and structural complexity, as well as altered iron homeostasis. Now, we report on a homologous coregulated gene, allergen 2 (ALL2). ALL2's function was characterized by generating null mutants in C. neoformans. In contrast to ALL1, loss of ALL2 attenuated virulence in the pulmonary infection model. The all2Δ mutant shed a less viscous exopolysaccharide and exhibited higher sensitivity to hydrogen peroxide than the wild type, and as a result, the all2Δ mutant was more resistant to macrophage-mediated killing. Transcriptome analysis further supported the distinct function of these two genes. Unlike ALL1's involvement in iron homeostasis, we now present data on ALL2's unique function in maintaining intracellular pH in low-pH conditions. Thus, our data highlight that C. neoformans, a human-pathogenic basidiomycete, has evolved a unique set of virulence-associated genes that contributes to its resilience in the human niche.
INTRODUCTION
Cryptococcus neoformans is a major fungal pathogen that causes disease predominantly in patients with AIDS but also in patients with other immune deficiencies. The most common clinical presentation of cryptococcal disease is chronic meningoencephalitis (CME). Worldwide, approximately 1 million new cases of cryptococcal meningitis occur every year, resulting in more than 600,000 deaths (1). Despite advances made in antifungal treatment, intracranial pressure management, and antiretroviral therapy, there is a major challenge to rapidly clear C. neoformans from cerebral spinal fluid (CSF), which is required to achieve a good outcome (2). The pathogen has various highly regulated virulence traits, including capsule, melanin formation, growth at 37°C, and extracellular enzymes (3). Differential gene regulation of these virulence traits can promote microevolution in vivo, which may facilitate evasion of the host immune response. One process by which microevolution can be achieved is phenotypic switching, which generates variants with augmented virulence that also alter host-pathogen interactions (4, 5).
In our previous studies, we documented that RC2, a serotype D C. neoformans clinical strain that is also a standard laboratory strain (6), undergoes phenotypic switching from a smooth to mucoid colony morphology and is associated with downregulation of a defined set of genes, including allergen 1 (ALL1) (7). Loss of ALL1 function resulted in a hypervirulent phenotype in both serotype A- and D-null mutants (all1Δ). Furthermore, it was shown that ALL1 is regulated during capsule induction (8) and by the transcription factor Sp1 and the PKC1 gene under glucose starvation (9), as well as by CIR1 in response to changes in iron concentration (10). Under starvation, VAD1 downregulates ALL1 transcripts via degradation of mRNA (11). Although the precise function of ALL1 is not known, its expression indirectly affects polysaccharide conformation and iron homeostasis (12). Thus, intracisternal infection with the all1Δ mutant results in augmented intracerebral pressure and premature death in infected rats (7). Extensive homology search for ALL1 revealed a highly homologous uncharacterized cryptococcal gene, CNM02200 (JEC21), which was subsequently named ALL2. Similarly to ALL1, ALL2 is also regulated by Sp1 and PKC1 under glucose starvation and during capsule induction (9).
In the present study, we sought to characterize the function of this gene and generated a null mutant of ALL2 (all2Δ) and a double mutant of ALL1 and ALL2 (all1Δ all2Δ). Results obtained imply that these homologous genes differ in their contribution to virulence and associated function. In contrast to the loss of ALL1, the loss of ALL2 attenuates virulence, and the mutants shed more of a less viscous exopolysaccharide (exo-PS). Microarray analysis supports distinct functions for these homologous genes and indicates that, unlike ALL1, the gene ALL2 is involved in maintaining intracellular pH.
MATERIALS AND METHODS
Yeast strains and media.
The C. neoformans strains used in this study are listed in Table S1 in the supplemental material. C. neoformans strains were cultured at 37°C in YNB broth (0.67% yeast nitrogen base without amino acids plus 2% glucose) or grown on YPD broth/agar (20 g/liter glucose, 10 g/liter yeast extract, 20 g/liter peptone [for YPD agar, 20 g/liter agar was added]), unless otherwise stated. For glucose starvation studies, YNB broth supplemented with 0.2% glucose, no glucose, or asparagine salt medium (1 g/liter asparagine, 10 mM sodium phosphate [pH 6.5], and 0.25 g/liter MgSO4) was used. For screening mutants, YPD supplemented with 100 mg/liter nourseothricin (NAT) or 200 mg/liter neomycin G418 (NEO) was used. RNA for microarray analysis was isolated from C. neoformans cells grown in minimal medium (10 mM magnesium sulfate, 29.3 mM potassium phosphate monobasic, 13 mM glycine, and 3 μM thiamine-HCl; adjusted to pH 5.5 and supplemented with 15 mM glucose as the carbon source). Other relevant media are specified below.
Strain construction.
The C. neoformans serotype A and D reference sequences were accessed originally through TIGR (now available at the NCBI) and the Fungal Genome Initiative database at the Broad Institute (http://www.broadinstitute.org/annotation/genome/cryptococcus_neoformans/MultiHome.html).
Generation of all2Δ, all2Δ+PACT1-ALL2, and all1Δ all2Δ mutants.
The ALL2 gene is 1,549 bp long, has four introns, and encodes a putative protein with a molecular mass of 25 kDa. In strain JEC21, ALL2 (CNM02200) is located on chromosome 13. To generate the all2Δ mutant, the coding region of ALL2 (RC2, 926 bp) was replaced with a neomycin resistance marker by homologous recombination as described previously (7). Primers used to generate mutants are listed in Table S2 in the supplemental material. Homologous recombination was confirmed by PCR, Southern blot analysis, and real-time PCR of ALL2 transcripts. For ALL2 complementation, the ACT1 promoter and the ALL2 open reading frame (ORF) were amplified from genomic DNA of RC2 and fused in frame using the NdeI restriction site. ACT1 and ALL2 fragments were cloned in pJAF13 using XbaI and XhoI cloning sites to generate plasmid pJAF13/PACT1-ALL2. The plasmid pJAF13/PACT1-ALL2 was linearized by XhoI and introduced into the all2Δ mutant by biolistic transformation. ALL2-positive clones (all2Δ+PACT1-ALL2) were selected on YPD agar with 100 mg/liter NAT. Gene complementation was confirmed by PCR, and the level of ALL2 expression was determined by reverse transcription-PCR (RT-PCR) of ALL2 transcripts. For generation of the all1Δ all2Δ double mutant, the ALL1 gene was replaced with the NAT resistance marker in RC2-all2Δ by modifying the construct designed previously to disrupt ALL1 (7). Positive clones were selected on YPD agar containing 100 mg/liter NAT and 200 mg/liter NEO. Homologous recombination was confirmed by PCR, Southern blot analysis, and determining the transcription level of ALL1 and ALL2 by RT-PCR.
Phenotypic characterization.
Mutants were phenotypically characterized for (i) baseline and induced capsule size, (ii) growth rate in variable pH and Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 10% NCTC, 1% nonessential amino acids, 1% penicillin-streptomycin, or minimal medium (10 mM magnesium sulfate, 29.3 mM potassium phosphate monobasic, 13 mM glycine, 3 μM thiamine-HCl; adjusted to pH 5.5 and supplemented with 15 mM glucose as the carbon source), (iii) in vitro stress sensitivity (1 M potassium chloride, lithium chloride [100 and 200 mM with glucose and galactose, respectively], 1 M sodium chloride), (iv) hydrogen peroxide sensitivity, (v) macrophage phagocytosis index, and (vi) macrophage-mediated killing assays as described previously (13–16). The intrinsic viscosity of exopolysaccharide and glucuronic acid residue content was determined as previously described (17, 18). All measurements were done in triplicate.
Generation of ALL1::HA-tagged and ALL2:mCherry fusion protein strains.
The ALL1::HA-tagged strain (where HA is hemagglutinin) was generated using the following strategy (19, 20). The ALL1 gene was amplified under its own promoter from RC2 genomic DNA using primers ALL1-prom-F-XbaI and ALL1-HARev (see Table S2 in the supplemental material). The resulting fragment and pJAF1 were digested with XbaI and NcoI and ligated to generate the 5′ALL1-HA/pJAF1 construct. The 3′ untranslated region (3′UTR) of the ALL1 gene was cloned in frame behind ALL1-HA using EcoRV and NcoI to generate 5′ALL1-HA/3′UTR/pJAF1. Next, 1,000 bp downstream of ALL1 was cloned into ALL1-HA/3′UTR/pJAF1 using the restriction sites for BamHI and KpnI to generate 5′ALL1-HA/3′UTR/NEO/ALL1-3′pJAF1. The construct was restricted with XbaI and KpnI, yielding a linearized fragment of 5′ALL1-HA/3′UTR/NEO/ALL1-3′, which was biolistically delivered into RC2 using a standard procedure as described previously (7). Homologous recombination was confirmed by PCR. ALL1::HA was detected by Western blot hybridization using rabbit anti-HA polyclonal antibody and a chemiluminescence kit (Pierce/Life Technologies) according to the manufacturer's instructions.
To generate ALL2-mCherry fusion protein in ALL1::HA strain for localization studies, plasmid YP164 (a generous gift from the Peter Williamson lab) was used to express a fusion between ALL2 and a synthetic mCherry protein (21). ALL2 under ACT1 promoter was amplified from previously generated plasmid PACT1-ALL2/pJAF13 using primers All2For-NHEI and All2Rev-PST1 and inserted into the plasmid YP164 using standard cloning techniques to generate plasmid ALL2/mCherry/YP142. Further, ALL2-mCherry was amplified from plasmid ALL2/mCherry/YP142 using the primers PAct-XbaFor and mCherry rev-EcoRV, and ALL2-3′UTR was amplified from RC2-SM genomic DNA using the primers All2 3′UTR-For ECORV and All2 3′UTR-Rev XHOI. Both parts were cloned in frame in plasmid pJAF13 using standard cloning techniques. The plasmid ALL2/mCherry/3′UTR/pJAF13 was linearized and transformed in ALL1::HA cells using the standard biolistic transformation technique.
All1p and All2p localization in vivo.
For localization studies, immunofluorescence was performed as described previously (20) with few modifications. Briefly, RC2 wild-type (RC2-wt) cells for All1p::HA and All2p-mCherry were cultured for 16 h in YNB up to an optical density at 600 nm of 0.4 to 0.5. Cells were harvested by centrifugation (5,000 rpm for 1 min), washed once with phosphate-buffered saline (PBS), suspended in 1 ml of 4% paraformaldehyde in PBS, and incubated for 30 min with rotation. The sample was then washed three times with PBS, suspended in 1 ml of lysis buffer (50 mM sodium citrate [pH 6.0], 1 M d-sorbitol, 35 mM β-mercaptoethanol) plus 40 mg/ml lysing enzyme (from Trichoderma harzianum), and incubated for 1 to 2 h at 37°C with occasional inversion. To monitor the protein mobility in variable growth phase, the cells were harvested at different time points during the experiment. After digestion, the cells were washed twice with heparan sulfate (HS) buffer (100 mM HEPES [pH 7.5], 1 M d-sorbitol) and suspended in 200 μl of HS buffer. The washed cell pellet was incubated with HS buffer containing 1% Triton X-100 and incubated for 10 min; it was then washed three times with HS buffer and three times with PBS. Next, the cells were treated with blocking buffer (5% goat serum and 0.02% Triton X-100 in PBS) for 1 h, followed by a high-affinity rabbit anti-HA polyclonal antibody (1:250/1:500 in blocking buffer [BB] obtained from Roche Applied Science) at 4°C in a rotator. Samples were then washed six times with blocking buffer and stained with Alexa Fluor 594-goat anti-rabbit IgG (1 μg/ml in BB) for 1 h in the dark. The cells were washed three times with BB and three times with PBS. Bright-field and fluorescence images were acquired as for the capsule staining assays described above.
Murine virulence studies.
BALB/c mice (female, 6 to 8 weeks old) were obtained from the National Cancer Institute (Bethesda, MD). Mice (n = 10 per group) were infected intratracheally (i.t.) with 5 × 104 or intravenously (i.v.) with 106 of wild type (RC2) or respective mutant cells in PBS. Mouse health was monitored daily, and if moribund, the mice were sacrificed in accordance with Institutional Animal Care and Use Committee (IACUC) regulations at the Albert Einstein College of Medicine (AECOM). To determine CFU studies, infected mice were killed at day 7 or 14, and their lungs were excised. Half of the lung was plated for CFU, and the other half was fixed in 10% formalin for histological analysis. Lung tissues were stained with hematoxylin and eosin, as well as mucicarmine, by the Histopathology Core at AECOM and Stony Brook University. Survival curves were generated and analyzed using GraphPad Prism 6.0.
ALL2 expression in glucose starvation.
RC2 cells were grown overnight in YNB supplemented with 2% glucose, diluted 1:50, and then again grown for 8 h in YNB supplemented with 2% glucose, 0.2% glucose, or no glucose. RNA transcript levels were measured by RT-PCR as previously described (7) in a 10-μl reaction mixture containing SYBR green master mix (Applied Biosystems/Life Technologies) with the primers listed in Table S2 in the supplemental material. An RT-PCR analysis was performed with the appropriate controls, and the relative transcript levels were determined as described earlier (12).
Microarray and GO enrichment analysis.
RC2-wt and mutant cells were grown in minimal medium for 16 h at 37°C with agitation (150 rpm), diluted 1:100, and regrown for 16 h or up to an optical density of 0.6 to 0.7 with agitation (150 rpm) at 37°C. Experiments were performed in duplicates (minimal medium arrays). Total RNA was isolated, and the microarray was performed by the Genome Technology Access Center at Washington University in St. Louis. Microarray analysis and gene ontology (GO) enrichment analysis were performed by the facility as described previously (12). Microarray results were further confirmed by performing RT-PCR on select genes as described above. A functional network was generated using Cryptonet (http://www.inetbio.org/cryptonet/).
Measurement of vacuolar pH.
Intracellular pH (pHi) of cells grown in YNB with variable glucose, as well as total pH, was measured using the pH-sensitive fluorophore BCECF-AM [2′7′-bis-(2-carboxyethyl)-5-(and 6)-carboxyfluorescein]. BCECF accumulates in yeast vacuoles, and excitation spectra are sensitive to pH (22). pHi measurement was performed by collecting the ratio of fluorescence at two excitation wavelengths (450 and 490 nm) and measuring at a single emission wavelength (535 nm) using protocols described elsewhere (23).
ATP measurement.
Cellular ATP levels were measured as previously described (24). Briefly, cells were grown in 50 ml of YNB buffered with 50 mM MES (morpholineethanesulfonic acid) (pH 4 and pH 7) for 16 h with constant agitation of 150 rpm at 37°C. The cultures were centrifuged at 3,000 rpm for 10 min to collect the pellet and washed once with PBS. The cells were counted using a hemocytometer, and a pellet of a similar number of cells was suspended in 1 ml of 50 mM HEPES (pH 7). The cells were further disrupted with a bead-beater (Biospec Products) using prewashed 0.5-mm zirconia beads. Disrupted samples were centrifuged at 8,000 rpm at 4°C for 10 min. The ATP concentrations of supernatants were determined using an ATP bioluminescence assay kit (Sigma-Aldrich, St. Louis, MO). ATP concentrations were normalized with total protein concentrations for each sample. The mean measurements for triplicate samples were reported.
Chronological and replicative life spans.
The chronological life span (CLS) was determined by adaptation of Saccharomyces cerevisiae methods (25) with some modifications. Briefly, equal numbers of C. neoformans cells were grown in YNB medium with or without 2% dextrose for 3 days at 37°C until they reached stationary phase. The cells were then transferred to sterile-distilled H2O, and the number of viable cells was measured by plating appropriate dilutions every 2 to 3 days on Sabouraud dextrose agar (SDA) plates. CFU on the plates were quantitated at 48 h, and the study was terminated when 99% of the cells were dead. All measurements were performed in triplicates.
The replicative life span (RLS) was determined by microdissection using a tetrad dissection Axioscope A1 microscope (Zeiss) as described previously in S. cerevisiae (26) with some modifications for C. neoformans. Briefly, the first buds of C. neoformans cells (n = 20 to 40) were arrayed on a YPD agar plate maintained at 37°C and then evaluated every 1 to 2 h by separating newly arising buds using a 50-μm fiber optic needle (Cora Styles). The RLS of each cell was calculated by determining the sum of the total buds until the cessation of divisions.
Statistical analysis.
Statistical analysis, including Student's t test, Wilcoxon rank sum test, and log-rank test, was performed using Microsoft Excel 2011 and GraphPad Prism 6.0 for Macintosh.
Microarray data accession number.
Microarray data for the mutant and the wt were uploaded to the GEO database and assigned GEO accession no. GSE74219.
RESULTS
Identification of ALL2 and generation of all2Δ and all1Δ all2Δ mutants.
The ALL2 gene is highly conserved among C. neoformans var. neoformans and var. grubii, as well as C. gattii, strains (Fig. 1A). Comparison of All1p and All2p amino acid sequences revealed that they are highly homologous (77% similarity and 61% identity) (Fig. 1B). Homology in the N-terminal part of the protein (176 amino acids [aa]) was 85%, whereas homology in the C-terminal part was only 35% (58 aa). Extensive searches for putative conserved domains or protein motifs using standard motif search programs detected no conserved domains or motifs. Both All1p and All2p exhibit homology (33 to 38%) only with hypothetical proteins in Malassezia sp. and other nonencapsulated plant-pathogenic fungi, such as Sterenum hirsutum and Rhodosporidium toruloides. No homology with proteins of any human-pathogenic fungi was found (see Fig. S1 in the supplemental material). The all2Δ null mutant was generated in the C. neoformans RC2 (serotype D) strain by replacing the coding region of ALL2 (RC2, 926 bp) with a neomycin resistance marker (NEO) of 2,098 bp using biolistic transformation. Successful homologous recombination was confirmed by PCR (see Fig. S2 in the supplemental material) and real-time PCR (see Table S3 in the supplemental material). The double mutant strain (all1Δ all2Δ) was also generated in RC2 using the previously generated all1Δ mutant strain (7) as the background strain.
FIG 1.
(A) ALL2 is conserved among C. neoformans var. neoformans, C. neoformans var. grubii, and C. gattii strains. (B) Homology blast of All1 and All2 protein sequences. All2p has a 77% similarity and a 61% identity to All1p.
Characterization of disruption mutants.
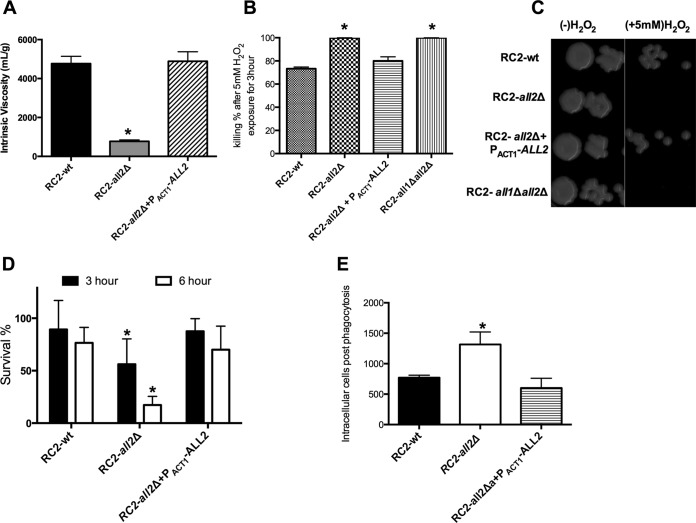
Phenotypic characteristics of the null all2Δ mutant, the all1Δ all2Δ double mutant, and the reconstituted all2Δ+PACT1-ALL2 strain are summarized in Table 1. Baseline capsule size and capsule induction were not affected by loss of ALL2 either in vitro or in vivo. Measurement of the biophysical properties of shed exo-PS demonstrated that exo-PS from the RC2-all2Δ mutant was less viscous than exo-PS from the RC2-wt and the reconstituted strain (Fig. 2A). To quantify shed exo-PS, a phenol-sulfuric colorimetric assay, which quantifies glucuronic acid residues, was used. This experiment showed that RC2-all2Δ cells shed more exo-PS per cell than the wild type (Table 1). Furthermore, RC2-all2Δ cells have no growth defect at neutral pH and display normal melanin production. Mutant cells exhibit comparable sensitivity to lysing enzyme and various high salt concentrations (data not shown). However, all2Δ mutant cells exhibit increased sensitivity to oxidative stress. In the presence of hydrogen peroxide (H2O2), RC2-all2Δ cells demonstrated a larger zone of inhibition in disc diffusion assays than the wild-type cells (65 versus 57 mm). This defect was resolved with gene reconstitution in RC2-all2Δ+PACT1-ALL2 (58 mm) (Table 1). Consistent with this finding, incubation of mutant cells (all2Δ and all1Δ all2Δ) with 5 mM H2O2 for 3 h resulted in significantly enhanced killing (P < 0.001) compared to that seen with the wild type and the reconstituted strain (Fig. 2B and C). Macrophage-mediated killing assays showed significantly enhanced (P < 0.001) killing of RC2-all2Δ cells compared to cells of the wild type and the reconstituted strains (Fig. 2D). Further, antibody-mediated phagocytosis was enhanced (P = 0.042) for the all2Δ mutant (Fig. 2E). Notably, an all2Δ mutation was also generated in the standard laboratory strain H99, a serotype A strain and also a clinical isolate. The phenotype of the mutant in H99 was similar with respect to viscosity and H2O2 sensitivity but not macrophage-mediated phagocytosis (see Table S4 in the supplemental material).
TABLE 1.
Characteristics of the mutantsa
| Characteristic | RC2-SM | RC2-all2Δ | RC2-all2Δ+PACT1-ALL2 | RC2-all1Δall2Δ |
|---|---|---|---|---|
| Mean capsule diam (μm) ± SEM | ||||
| In vitro | 1.2 ± 0.8 | 1.0 ± 0.4 | 1.3 ± 0.65 | 1.5 ± 0.43 |
| In vivo | 10.8 ± 2.6 | 11.7 ± 2.7 | 11.8 ± 1.5 | ND |
| Presence of melanin | Yes | Yes | Yes | Yes |
| Mean growth period (h) ± SEM at: | ||||
| pH 4 | 2.3 ± 0.2 | 2.8 ± 0.1 | 2.8 ± 0.1 | ND |
| pH 7 | 2.9 ± 0.8 | 2.4 ± 0.7 | 2.5 ± 0.6 | ND |
| Mean phagocytosis index ± SEM | 93.2 ± 16.3 | 113.2 ± 20.3 | 72 ± 8.9 | 109.6 ± 32.4 |
| Zone size (mm) on: | ||||
| YNB + disc containing 5 μl of 30% H2O2 | 57 | 65 | 58 | 72 |
| MM + disc containing 5 μl of 30% H2O2 | 54 | 62 | 58 | 64 |
| SAB + disc containing 5 μl of 30% H2O2 | 30 | 40 | 34 | 38 |
| Glucuronic acid content (mg/cell) | 1.40 × 10−13 | 1.57 × 10−12 | 5.32 × 10−14 | ND |
SM, smooth; SEM, standard error of the mean; MM, minimal medium; SAB, Sabouraud broth; ND, not determined.
FIG 2.
(A) Viscosity measurements of the exopolysaccharide shed from wild-type (RC2-wt), mutant (RC2-all2Δ), and reconstituted (RC2-all2Δ+PACT1-ALL2) strains grown for 14 days in minimal medium. Shed capsular polysaccharide of the all2Δ mutant was less viscous. The all2Δ mutant had increased sensitivity to H2O2 in vitro, as documented by incubating 105 or 106 RC2-wt, RC2-all2Δ, and RC2-all2Δ+PACT1-ALL2 cells in DMEM with 5 mM H2O2 for 3 h and quantification of the percent survival (B) and by spotting 5 μl on YPD agar (C). (D) Killing assays were performed by lysing murine macrophages 3 h or 6 h after coincubation and plating the CFU. all2Δ cells were more sensitive to macrophage killing, consistent with their increased sensitivity to H2O2 in vitro. (E) The phagocytosis index determined at 2 h after coincubation was significantly higher in macrophages incubated with RC2-all2Δ cells than in macrophages coincubated with RC2-wt or RC2-all2Δ+PACT1-ALL2 cells. Intracellular phagocytosis index was calculated by visually counting cells on a microscope. All experiments were performed in triplicates. An asterisk denotes a P value of ≤0.05.
Loss of ALL2 results in attenuated virulence in pulmonary cryptococcosis but not in CME.
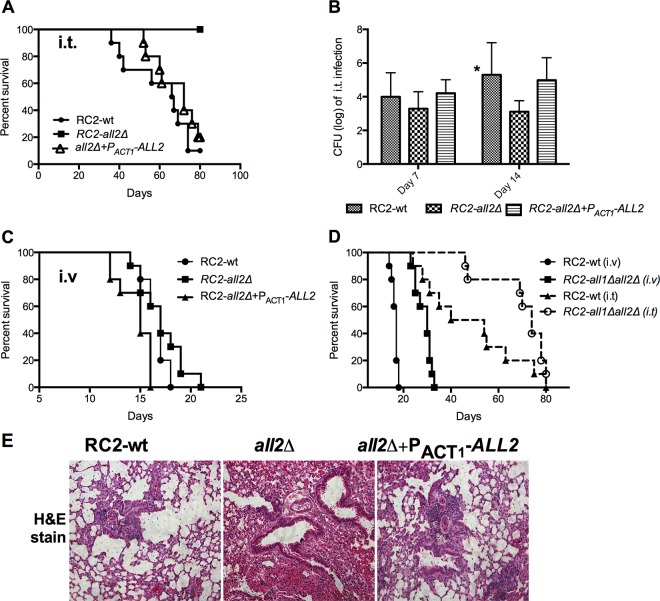
Next, virulence of all2Δ mutants in pulmonary and i.v. murine infection models was compared to that of wild-type and reconstituted strains. Mice infected i.t. with an inoculum of 5 × 104 RC2-all2Δ cells survived significantly longer (P < 0.001) than those infected with the wild-type strain (Fig. 3A), which was consistent with the enhanced macrophage-mediated killing and phagocytosis observed in vitro (Fig. 2D and E). Consistent with the unaltered growth of the mutants, comparable fungal burden was detected in the lung at 7 days postinfection, but then decreased CFU levels were found at 14 days postinfection in the mutant cells (log 5.3 ± 1.9, 3.1 ± 0.6, and 4.9 ± 1.3 for RC2-, RC2-all2Δ-, and RC2-all2Δ+PACT1-ALL2-infected mice, respectively, P < 0.05) (Fig. 3B), which is consistent with their enhanced clearance from the lungs. In contrast, virulence in the i.v. infection model for the all2Δ strain was comparable to that for the wild-type and the reconstituted strains (Fig. 3C). The all1Δ all2Δ double mutant was hypovirulent in both infection models (Fig. 3D).
FIG 3.
RC2-all2Δ mutants have attenuated virulence in a murine pulmonary infection model. (A) Mice infected i.t. with the mutant strain survived significantly longer than those infected with the wild-type or reconstituted strain (P = 0.0002). (B) Mice infected with 5 × 104 cells i.t. had comparable lung fungal burdens of log 3.9 ± 1.4, 3.2 ± 1.2, and 4.1 ± 0.8 for RC2-wt, RC2-all2Δ, and RC2-all2Δ+PACT1-ALL2 strain-infected mice, respectively, at 7 days postinfection. However, consistent with the survival difference, RC2-all2Δ strains had a lower lung fungal burden at 14 days postinfection (log 5.3 ± 1.9, 3.1 ± 0.6, and 4.9 ± 1.3 for wild-type, all2Δ, and all2Δ+PACT1-ALL2 strain-infected mice, respectively [P = 0.04]). (C) No difference in survival was noted in an i.v. infection model between the wild-type and RC2-all2Δ mutant strains. (D) The double mutant (RC2-all1Δall2Δ) was hypovirulent in both the i.t. and i.v. models. (E) Histopathology of lungs stained with hematoxylin and eosin (H&E) and infected with 104 cells was examined. On day 14, lung histology revealed that RC2-all2Δ-infected mice had a more pronounced host response, resulting in enhanced clearance of lung fungal burden compared to wild-type- and reconstituted strain-infected mice. Lower numbers of yeast cells and more inflammatory cells were observed in the alveolar spaces of RC2-all2Δ infected mice than for wild-type- and RC2-all2Δ+PACT1-ALL2-infected mice. An asterisk denotes a P value of ≤0.05.
Histopathology analysis of RC2-all2Δ-infected lung tissues at 14 days revealed decreased numbers of cryptococcal cells and pronounced recruitment of inflammatory cells in the lung tissue compared to results for the wild-type-infected lung tissues (Fig. 3E). This is consistent with a more effective host response, which results in enhanced clearance and thus explains the lower fungal burden at day 14. In summary, these findings confirm that ALL2 is a virulence-associated gene and that its loss can alter the pathogenesis of pulmonary cryptococcal infection.
The ALL2-fluorescent fusion protein localizes to the cytoplasm and vacuoles.
Next, we sought to investigate whether All1p and All2p proteins colocalize, since both the ALL1 and the ALL2 transcripts are coregulated (9). For these studies, ALL2 was fused with C. neoformans codon-optimized mCherry under an ACT1 promoter (ALL2-mCherry). The ALL2-mCherry was transformed in cells expressing ALL1 with a hemagglutinin (HA) tag under its native promoter (ALL1::HA). ALL1::HA was detected using antibodies against HA that were conjugated to Alexa Fluor 594 (green). Immunofluorescence staining revealed that All1p and All2p fusion proteins colocalize in the cytoplasm (Fig. 4A), and the protein location was found to be unaffected at multiple time points of cell growth (data not shown). However, protein expression varied widely among yeast cells, and a majority of C. neoformans cells expressed only either All1p or All2p (82%). Phase-contrast microscopy indicated that the All2-mCherry fusion protein also localized to the yeast vacuoles (Fig. 4B). This finding was confirmed by vacuole-specific BCECF-AM staining of yeast cells. Variable pH and glucose did not alter the amount of All2p accumulation in vacuoles. Further, cells recovered from the lungs and the brains of mice 9 days postinfection displayed All2p localization in vacuoles (see Fig. S3 in the supplemental material).
FIG 4.
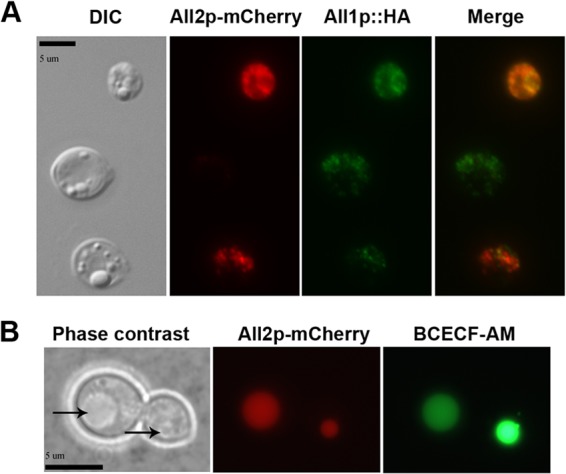
(A) All2p-mCherry fusion proteins localize in the cytoplasm, with accumulation in vacuoles. For coexpression studies, single cells expressing All2p-mCherry (red) and All1p::HA (green) fusion proteins were grown in YNB for 16 h at 37°C and observed by using differential interference contrast microscopy and fluorescence. The imaging revealed the cytoplasmic location and colocalization of both fusion proteins. (B) All1-mCherry fusion proteins were also found to colocalize in yeast vacuoles by phase-contrast microscopy (indicated by an arrow) and BCECF-AM staining (scale bar, 5 μm).
ALL2 is highly expressed under stress conditions, and its loss affects the expression of genes involved in multiple pathways of transport.
Review of published transcriptional profiles of Sp1Δ under glucose starvation documented upregulation of ALL2 in addition to ALL1 (9). Quantification of ALL2 expression by RT-PCR in cells grown for 3 h in asparagine stress media and then transferred to yeast nitrogen base media under glucose (Fig. 5A) or asparagine (Fig. 5B) starvation confirmed elevated expression of ALL2 in both stress conditions. Comparison of transcriptomes from RC2-all2Δ and RC2-wt cells grown in minimal media documented downregulation of 20 genes and upregulation of 263 genes in RC2-all2Δ cells (Table 2; see also Fig. S4 in the supplemental material). Downregulated genes included a hypothetical protein involved in carbohydrate transport and metabolism (CNE05330, 7.6-fold), a d-galactonate transporter (CNE05340, 4.1-fold), and a cluster of proteins that have in common a domain for 5-oxoprolinase (CNE05320, 2.1-fold; CNE05310, 2.5-fold). The 5-oxoprolinase (EC 3.5.2.9) enzyme is ATP dependent and is involved in glutathione metabolism to synthesize l-glutamate from 5-oxoproline.
FIG 5.
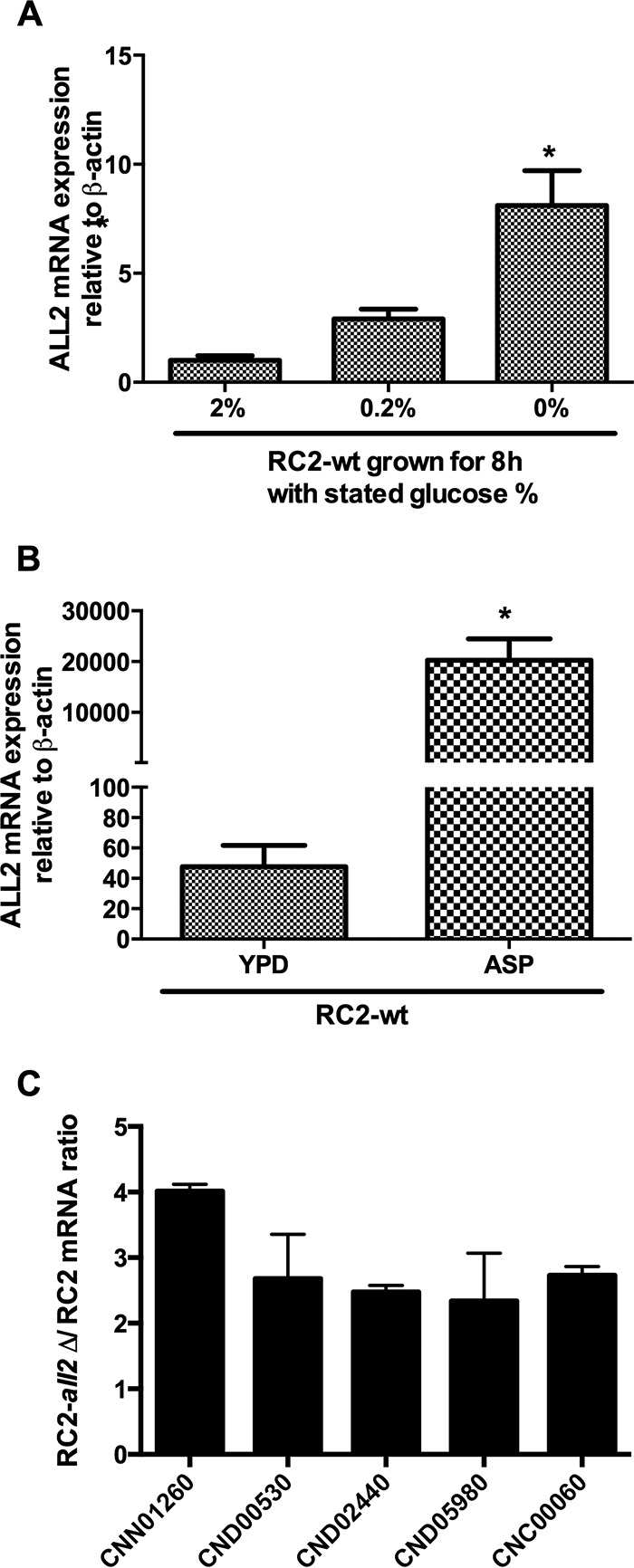
ALL2 transcription was highly induced under glucose starvation (A) and in asparagine stress medium (B). The transcription of ALL2 was measured by RT-PCR in RNA obtained from cells incubated in YNB without glucose and in YNB with 0.2 or 2% glucose for 8 h, as well as from cells incubated in asparagine medium without glucose for 3 h at 37°C. The ACT1 gene was used as a control. (C) RT-PCR analysis confirmed the microarray results showing the upregulation of transport-related genes in RC2-all2Δ cells, including H+-ATPase (CNN01260).
TABLE 2.
ALL2 in acidic growth conditions regulates genes involved in transport and multiple pathways
| GO function and C. neoformans gene | Description | Fold change (mutant/wt) |
|---|---|---|
| Transport | ||
| CND02440 | Peptide transporter | 4.30 |
| CND00530 | Urea transporter | 3.70 |
| CND05980 | Nucleoside transporter | 3.40 |
| CNA07090 | ABC transporter | 2.60 |
| CNE00270 | Amino acid transmembrane transporter | 2.57 |
| CNA05800 | Amino acid transporter | 2.43 |
| CNB02200 | RAN protein binding protein | 2.27 |
| CNC05860 | Metal resistance protein | 2.10 |
| Nucleotide binding | ||
| CNN01260 | Plasma membrane H+-ATPase 1 | 9.50 |
| CNL04860 | ATPase activity | 2.70 |
| CND06300 | Aspartate-tRNA ligase | 2.23 |
| CNG01310 | Arginine-tRNA ligase | 2.23 |
| CNB05640 | Proline-tRNA ligase | 2.21 |
| CNF00070 | Glutamate-tRNA ligase | 2.02 |
| CNK00560 | DNA polymerase | 2.08 |
| CNM01290 | Leucine-tRNA ligase | 2.05 |
| CNN00350 | Glutamate-cysteine ligase | 2.03 |
| Transmembrane transport | ||
| CNC00060 | Sugar transporter | 3.07 |
| CND05980 | Folic acid metabolism | 2.92 |
| CND04690 | Sugar transporter | 2.90 |
| CNI03510 | Allanote transmembrane transporter | 2.88 |
| CNH01210 | Phosphate transmembrane transporter | 2.73 |
| CNC04510 | Siderophore (ion) uptake | 2.71 |
| CNG04630 | Efflux protein EncT | 2.64 |
| CNJ01450 | Multidrug transporter activity | 2.58 |
| CNE01880 | Pantothenate transporter | 2.57 |
| CNF01220 | Allantoate transmembrane transporter | 2.30 |
| CNF00350 | Hypothetical protein | 2.08 |
| CNG01480 | Fructose transmembrane transporter | 2.08 |
| CNC03220 | Receptor glucose binding | 2.07 |
| CNI03490 | Sugar transporter | 2.03 |
| CNC01890 | Na+/H+ exchange | 2.00 |
| CNE05340 | Allantoate membrane transporter | −4.01 |
| Catalytic activity | ||
| CNA02570 | Acetolactate synthase | 3.46 |
| CNG00590 | Folic acid metabolism | 2.92 |
| CNC00360 | Protein phosphatase | 2.83 |
| CNG01150 | Spermidine synthase | 2.78 |
| CNE00720 | Hypothetical protein | 2.46 |
| CNG04420 | α-1,3-Glucan synthase | 2.34 |
| CNN00440 | Purine nucleotide biosynthesis | 2.33 |
| CNA07740 | Acetate coligase | 2.28 |
| CNI03300 | Phosphoribosylformylglycinamidine synthase activity | 2.05 |
| CNB03190 | Hypothetical protein | 2.01 |
| CNE05320 | 5-Oxoprolinase | −2.10 |
| Glucogenesis | ||
| CNI03590 | Phosphoenolpyruvate carboxykinase, putative | 3.02 |
| CNJ00950 | Pyruvate decarboxylase | 2.35 |
| CNB04050 | Glucose 6-phosphate isomerase | 2.20 |
| Protein binding | ||
| CNG00480 | Atomer alpha subunit | 3.64 |
| CNG02370 | tRNA (guanine) methyltransferase | 3.28 |
| CNC02150 | Translation initiation factor | 2.92 |
| CNJ02300 | Structural molecule activity | 2.77 |
| CNH02560 | Hypothetical protein | 2.70 |
| CNG02820 | Protein phosphate regulator | 2.69 |
| CNE01020 | Translation initiation factor | 2.68 |
| CNB00260 | Hypothetical protein | 2.59 |
| CNN01950 | Hypothetical protein | 2.41 |
| CNJ01250 | Hypothetical protein | 2.39 |
| CNA04270 | HMG1 chromatin binding | 2.38 |
| CNA04050 | Elongation factor | 2.35 |
| CNK01990 | Hypothetical protein | 2.25 |
| CNB05570 | ER-to-Golgi body transport | 2.22 |
| CNJ00610 | Argonaute-like protein | 2.13 |
| CNJ00830 | Hypothetical protein | 2.13 |
| CNL05140 | Hypothetical protein | 2.13 |
| CNA04910 | Retrograde transport; endosome to Golgi body | 2.13 |
| CNB04960 | Hypothetical protein | 2.10 |
| CNK02410 | General transcription repressor | 2.10 |
| CNE03290 | Translation initiation factor | 2.10 |
| CNA00630 | Nuclear mRNA splicing | 2.07 |
| CNA06050 | Guanyl-nucleotide exchange factor | 2.01 |
| RNA binding | ||
| CNI01160 | Poly(A) binding | 4.01 |
| CNA01020 | Pre-mRNA splicing factor activity | 2.00 |
| CNE01200 | rRNA processing-related protein | 3.11 |
| CNC04280 | mRNA binding | 2.89 |
| CNE05380 | RNA-directed DNA polymerase activity | 2.10 |
| CNH03600 | Pseudouridine synthase | 2.35 |
| CNJ03440 | RNA-directed DNA polymerase activity | −2.04 |
| CNF04940 | RNA-directed DNA polymerase activity | −2.17 |
Interestingly, the most abundant upregulated gene was a plasma membrane H+-ATPase 1 (CNN01260, 9-fold). Other genes upregulated in the mutant were involved in multiple amino acid biosynthesis pathways: aminoacyl-tRNA biosynthesis (CNF00070, CND06300, CNM01290, CNG01310, and CNB05640), valine, leucine, and isoleucine biosynthesis (CNA02070), and purine (CNK02180, CNN00440), glutamate (CNJ02910), and glutathione (CNN00350 and CNG01150) metabolism (Table 2). Differential expression of the plasma membrane H+-ATPase (CNN01260) and other highly regulated genes was further confirmed by RT-PCR (Fig. 5C). Importantly, the loss of ALL2 or ALL1 did not result in increased expression of the respective homologous genes. Transcriptomes of all1Δ all2Δ and all1Δ mutants grown in minimal media were also compared with that of the all2Δ mutant. An overlap of genes was noted only with all1Δ all2Δ cells but not with all1Δ cells (GEO accession number GSE74219). This further supported our conclusion that ALL1 and ALL2 have distinct functions.
Lastly, gene ontology (GO) analysis of differentially expressed genes in the all2Δ mutant under starvation conditions was consistent with the above findings. This analysis identified specific GO categories, namely, those related to transport, nucleotide binding, transmembrane transport, and catalytic activity among others (Table 3).
TABLE 3.
GO terms identified in the differentially expressed genes
| GO term | Description |
P |
|
|---|---|---|---|
| Hypergeometric test | Bonferroni's test | ||
| GO:0006810 | Transport | 2.2 × 10−5 | 4.2 × 10−3 |
| GO:0000166 | Nucleotide binding | 3.79 × 10−5 | 7.0 × 10−3 |
| GO:0055085 | Transmembrane transport | 4.16 × 10−4 | 7.7 × 10−3 |
| GO:0005215 | Transporter activity | 5.61 × 10−4 | 7.8 × 10−2 |
| GO:0003824 | Catalytic activity | 7.96 × 10−4 | 0.104 |
| GO:0006094 | Glucogenesis | 8.06 × 10−4 | 0.15 |
| GO:0005515 | Protein binding | 1.57 × 10−3 | 0.29 |
| GO:0005524 | ATP binding | 1.69 × 10−3 | 0.31 |
| GO:0003723 | RNA binding | 1.87 × 10−3 | 0.34 |
Intracellular pH, ATP levels, and chronological aging are altered in the all2Δ mutant.
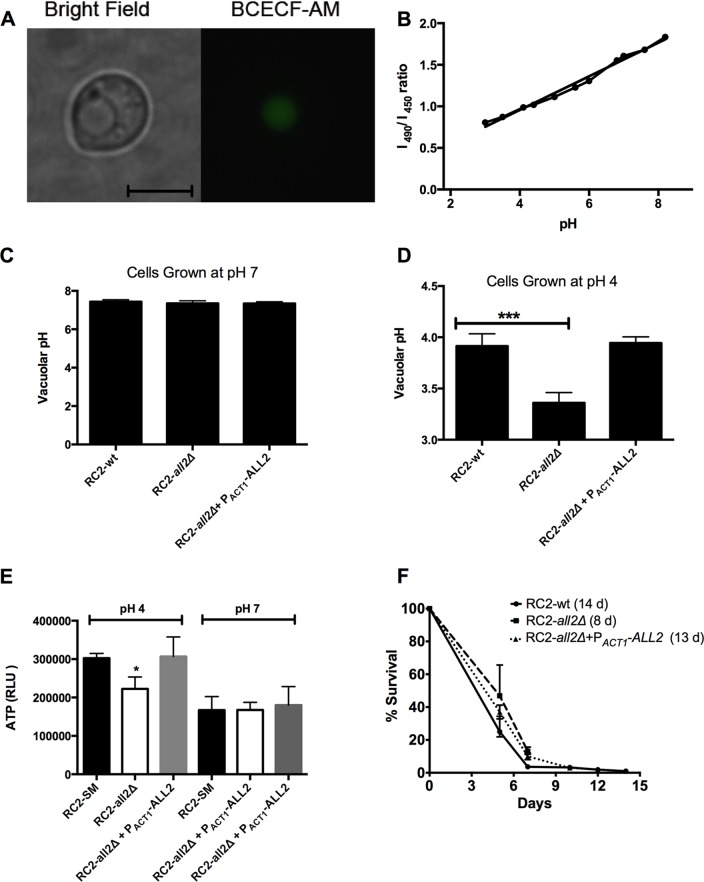
The main intracellular pH (pHi) regulator in all eukaryotic cells is the plasma membrane H+-ATPase pump (27). To measure vacuolar pH, yeast cells were stained with a pH-sensitive fluorophore, BCECF-AM. Vacuole-specific labeling was confirmed by microscopy (Fig. 6A), and calibration curves were generated (Fig. 6B) using the ratio of fluorescence intensity from excitation at 450 to 490 nm (both measured at an emission wavelength of 535 nm) at variable pHs. We measured pHi of yeast cells grown in YNB supplemented with 2 or 0.2% glucose at medium pH 4 or 7. The pHi were comparable in cells grown at pH 7 under both conditions (Fig. 6C). However, in cells grown at an acidic pH, the mutant was not capable of maintaining intracellular pH compared to the wild type and the reconstituted strains. As a result, the pHi of RC2-all2Δ was significantly more acidic than that of the wild type (Fig. 6D). Next, we investigated the actual function of the plasma membrane H+-ATPase 1 (CNN01260) in the all2Δ mutant under different pH conditions. The primary function of ATP synthase in most organisms is ATP synthesis (28). This enzyme is part of complex V ATP synthase in the oxidative phosphorylation pathway in C. neoformans. Accordingly, we quantified ATP cellular levels in RC2-all2Δ cells using an ATP bioluminescence assay kit (Sigma-Aldrich, St. Louis, MO). These experiments demonstrated that RC2-all2Δ cells grown in minimal media or YNB for 16 h produced significantly less cellular ATP per μg protein than the wild-type cells when grown at pH 4 (Fig. 6E) and produced comparable levels of ATP at pH 7 (Fig. 6E). Finally, chronological life span (CLS) was assessed in the mutant and compared to that in the wild type because the CLS, which is a resilience trait of yeast strains and measures their viability under no nutrition, was predicted to shorten in the setting of impaired intracellular pH regulation. Consistent with this hypothesis, we found a significantly shortened CLS in the RC2-all2Δ strain compared to that in the wild type (8 days versus 14 days, P = 0.01, Fig. 6F). It is noteworthy that the replicative life spans of the mutant and the wild type, which measures the reproductive ability as opposed to the viability of the cell, were comparable under 2% glucose and not 0.05% glucose. Under low-glucose conditions, the RC2-all2Δ cells had a significantly shortened RLS compared to that of the wild-type cells (21 versus 28 generations, P = 0.02).
FIG 6.
Intracellular pH measurement of C. neoformans RC2-wt and RC2-all2Δ cells. (A) BCECF-AM successfully stained vacuoles of C. neoformans (scale bar, 5 μm). (B) Calibration curves were generated using ratio of fluorescence intensity from excitation at 450 to 490 nm (both measured at an emission wavelength of 535 nm) at varied pH. The pHi were comparable in cells grown at pH 7 (C) and found to be significantly different between RC2-wt and RC2-all2Δ cells when grown in acidic pH conditions (D). (E) RC2-all2Δ mutant cells exhibited significantly less ATP production per μg of protein than RC2-wt cells at pH 4 but comparable levels at pH 7. (F) The CLS of RC2-wt cells was significantly longer than the CLS of all2Δ cells (14 versus 8 days, P < 0.001), as measured by survival in water. An asterisk denotes a P value of ≤0.05.
DISCUSSION
In this study, we report the role of a novel virulence-associated gene named ALL2, which is coregulated with a highly homologous gene, ALL1, during the process of phenotypic switching of C. neoformans to a hypervirulent mucoid variant.
ALL2p is highly homologous (77% similarity and 61% identity) to the previously described gene ALL1p (7) and is also regulated by the same transcriptional regulator, Sp1 and gene PKC1 (9), both of which are activated under stress. Our data demonstrate, however, that the functions of the two genes are mostly distinct, although both mutants exhibit enhanced exopolysaccharide secretion. The effects of gene loss on virulence and exo-PS viscosity are contrasting. It is conceivable that these two genes may be paralogs, or duplicated genes that over time have come to be different in sequence composition and function.
Loss of ALL1 leads to hypervirulence and early death, whereas the loss of ALL2 leads to hypovirulence and prolonged survival. Interestingly, hypovirulence is observed in the pulmonary infection model only and not in the i.v. infection model. In the latter model, dissemination across the blood-brain barrier occurs immediately, whereas in the i.t. model, fungal infection is initially controlled by alveolar macrophages, and dissemination to the brain occurs secondarily, usually after 1 to 2 weeks. Mice infected with all2Δ cells eventually exhibited lower CFU in lung tissue, consistent with their impaired resilience in the host environment. Similar results were demonstrated in mice infected with all1Δ all2Δ yeast cells, which also lived longer, albeit their mortality was more rapid. This was not expected from partial suppression of all2Δ by all1Δ since their expression levels remain unchanged based on the microarray data.
C. neoformans is a facultative intracellular pathogen (29, 30) that has developed mechanisms to counteract the stress it encounters inside macrophages. Experiments in murine infection models suggest that the outcome of cryptococcal infection correlates with the ability of alveolar macrophages to control intracellular fungal replication (30–32). A higher budding index for intracellularly located than for extracellularly located C. neoformans has been described in murine pulmonary infection (29), supporting the notion that this niche is important in pathogenesis, as it permits fungal growth. Also, murine alveolar macrophages appear to have less killing efficacy than rat macrophages and therefore support enhanced intracellular replication (32). Recent experiments support the concept that human and murine macrophages exhibit comparable efficacies with respect to supporting C. neoformans growth (33). Furthermore, two independent investigations with clinical C. neoformans strains derived from different patient cohorts with CME, although their conclusions differed slightly, support the notion that growth of the yeast in the intracellular niche affects evasion of the immune response and nonsterilization of CSF (33, 34). Our data now indicate that the function of All2p contributes through diverse mechanisms to reduce the efficacy of this key immune cell.
Similarly to the antimicrobial activity against other microorganisms, reactive oxygen species are key mediators of the anticryptococcal activity of macrophages and neutrophils (35–39). Iron catalyzes the generation of hydroxyl radicals from oxidant precursors, such as superoxide anions and hydrogen peroxide (40). Gene ontology analysis of differentially expressed genes has shown that adaptation of C. neoformans to H2O2 treatment is distinct from the response of other fungal organisms to oxidative stress (41). One reason for this is that C. neoformans is encapsulated and both the size and the biophysical properties of the exo-PS of C. neoformans affect its resistance to oxidative stress (13, 17). Our results now demonstrate that the exo-PS of all2Δ cells is augmented and has altered biophysical properties, namely, decreased viscosity compared to the exo-PS of the wild type. In contrast to this, the loss of ALL1 resulted in the secretion of a hyperviscous exo-PS and manifestation of a hypervirulent phenotype in murine models (12). The ALL2 mutant exhibits no significant growth defects in rich or nutrient-limiting media. Hence, we propose that the hypovirulence observed in pulmonary, but not in i.v., infection stems from an altered resistance to host response and from pH rather than nutrient differences in the two infection sites. The inability of all2Δ cells to buffer the low pH inside alveolar macrophages likely contributes to the loss of yeast cell viability in the intracellular niche. A slightly slower growth at lower pH supports this concept.
Although both ALL1 and ALL2 transcripts are expressed during logarithmic growth in vitro (9), they achieve maximal expression under starvation in the stationary growth phase. Localization studies with labeled protein constructs revealed some colocalization of All2p with All1p in the cytoplasm, although they did not accumulate in vacuoles as described for the membrane transporter CTR1 (42) and the copper transporter CTR4 (21). It appears that mCherry has a tendency to accumulate in vacuoles after degradation (21), and our interpretation is that vacuolar localization of All2p is not specific. This could indicate that All2p undergoes a significant level of degradation as previously reported for H+-ATPase in S. cerevisiae (43) and ctr4-ctr5 in Schizosaccharomyces pombe (44). Despite coexpression and, most importantly, coregulation by the same transcription factors, our data suggest distinct functions of ALL2 and ALL1 except for the fact that both mutants exhibit augmented exo-PS shedding. Also, respective GO analysis of the mutants identified regulated genes in the transmembrane transport category. In support of these conclusions, no change in the expression levels in either mutant to compensate for the loss of the other gene was observed. Also, efforts to coprecipitate the two proteins were not successful (data not shown).
Transcriptome comparison of all2Δ and wild-type cells revealed differential expression of multiple transporters and nucleotide binding proteins, with significant downregulation of 5-oxoprolinase and the highest upregulation of a plasma membrane, H+-ATPase. 5-Oxoprolinase is an enzyme involved in the gamma-glutamyl cycle for glutathione metabolism and catalyzes the cleavage of 5-oxoproline to form l-glutamate in a reaction coupled to the hydrolysis of ATP to ADP and inorganic phosphate (28). Glutathione metabolism is crucial for amino acid transport in vitro, as well as in vivo (45); however, in S. cerevisiae, deletion of 5-oxoprolinase results in the accumulation of 5-oxoproline (46). The protein localizes to the cytoplasm and an abundant increase in response to stress is observed.
Plasma membrane H+-ATPase, CNM01260, encodes a protein that has a high homology to an isoform of Pma1p, a plasma membrane H+-ATPase that is involved in pumping protons out of the cell. This enzyme is a regulator of intracellular pH and maintains the electrochemical proton gradient necessary for nutrient uptake. GO analysis indicates that this gene is associated with several processes, including ATP catabolic and biosynthesis process, cation transport, nucleotide binding, ATP binding, hydrolase activity, and ATPase activity. The gene is an integral component of the plasma membrane (http://www.ebi.ac.uk/QuickGO/GProtein?ac=F5HCX1).
Mutations in PMA1 that encode H+-ATPase of S. cerevisiae lead to decreased levels of ATPase, which has been correlated with decreased proton efflux and decreased uptake of amino acids (47). How exactly All2p and Pma1 interact remains to be determined. Our data indicate that upregulation of H+-ATPase gene expression in the all2Δ mutant does not compensate for the loss of PMA1 because the mutant still exhibited low ATP production and was inefficient in maintaining intracellular pH in acidic conditions. Maintaining intracellular pH is crucial under starvation conditions in the phagosome because, in contrast to Candida albicans and Candida glabrata (48, 49), C. neoformans does not interfere with phagosome maturation. Thus, the C. neoformans containing phagosome undergoes acidification through fusion with lysosomes, and C. neoformans needs to buffer pH to grow inside the mature acidic vacuole (50, 51). Of note is that although PMA1 is abundant among fungi, All2p has homologues only in basidiomycetes that prefer plants as their host.
Interestingly, no significant regulation of ALL2 was observed in C. neoformans currently implicated in pH homeostasis. For instance, Rim101 is a conserved pH-responsive transcription factor that allows the microorganism to respond to pH changes (16). However, neither All2 nor Rim101 appears to be significantly regulated by the other in respective transcriptome studies (16). In a more recent study, Rim101 was shown to share downstream targets with Pka1, and not surprisingly, ALL2 was found to be upregulated in the pka1Δ mutant (52). Impaired ability to maintain proper intracellular pH also explains the significantly shortened chronological life span of the mutants compared to that of the wild type. In S. cerevisiae, chronological aging has been shown to result in an acidic intracellular environment (25), and the inability of the mutants to neutralize pH results in a shortened chronological life span but has no effect on the replicative life span. C. neoformans is acquired by inhaling spores from the environment. Most clinical manifestations of infections represent reactivation of latent disease (53). The intracellular environment of macrophages in small granulomas is likely the site where the fungus survives nonreplicating for decades, similar to the pathogenesis of latent tuberculosis. Chronological aging is a distinct trait of resilience as it measures the ability of a fungus to survive in a nonreplicating state. Although this trait has not been studied with respect to pathogenesis, we propose that this form of resilience is critical for the establishment of latency of cryptococcal infection.
It has been reported previously that the overexpression of H+-ATPase is compensated by the downregulation of H+-ATPase activity in vivo (54) and does not always enhance growth (55). In S. cerevisiae, PTK2 and HRK1 genes encode protein kinases that have been identified to activate yeast plasma membrane production in response to glucose activation (56). Of note, ALL1 and ALL2 are regulated by the protein kinase Pkc1 signaling pathway (9). In contrast to the plasma membrane H+-ATPase of S. cerevisiae, which is upregulated by glucose (57), ALL1 and ALL2 are upregulated in the setting of low glucose levels.
In summary, we conclude that the attenuated virulence of the all2Δ mutant in the pulmonary infection model may result from the mutant's inefficiency to maintain intracellular pH in an acidic phagosome environment. The all2Δ mutant has no dramatic growth defect but produces significantly lower levels of ATP than the wild type, which affect the energetic state of the all2Δ mutant in vivo. We reason that this changes its resilience and the outcome of infection, similarly to Leishmania donovani, where intracellular ATP levels correlate with parasite proliferation (58).
We propose that ALL2 should be considered a target for antifungal drug discovery and further studied. Our studies highlight the uniqueness of C. neoformans, one of the few basidiomycetes that have evolved to be a successful human fungal pathogen. Specifically, these data show that its unique ability to escape host response and survive in the intracellular niche relies on genes, such as ALL2, that are unique to this fungus and contribute to its exceptional resilience.
Supplementary Material
Funding Statement
This work was supported by NIH awards R01 AI059681 and R21 A1087564 to B.C.F. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01046-15.
REFERENCES
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, Harrison T. 2007. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton AJ, Goodley J. 1996. Virulence factors of Cryptococcus neoformans. Curr Top Med Mycol 7:19–42. [PubMed] [Google Scholar]
- 4.Fries BC, Goldman DL, Cherniak R, Ju R, Casadevall A. 1999. Phenotypic switching in Cryptococcus neoformans results in changes in cellular morphology and glucuronoxylomannan structure. Infect Immun 67:6076–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fries BC, Taborda CP, Serfass E, Casadevall A. 2001. Phenotypic switching of Cryptococcus neoformans occurs in vivo and influences the outcome of infection. J Clin Invest 108:1639–1648. doi: 10.1172/JCI13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franzot SP, Mukherjee J, Cherniak R, Chen LC, Hamdan JS, Casadevall A. 1998. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun 66:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain N, Li L, Hsueh YP, Guerrero A, Heitman J, Goldman DL, Fries BC. 2009. Loss of allergen 1 confers a hypervirulent phenotype that resembles mucoid switch variants of Cryptococcus neoformans. Infect Immun 77:128–140. doi: 10.1128/IAI.01079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes BC, Skowyra ML, Spencer SJ, Gish SR, Williams M, Held EP, Brent MR, Doering TL. 2011. Toward an integrated model of capsule regulation in Cryptococcus neoformans. PLoS Pathog 7:e1002411. doi: 10.1371/journal.ppat.1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adler A, Park YD, Larsen P, Nagarajan V, Wollenberg K, Qiu J, Myers TG, Williamson PR. 2011. A novel specificity protein 1 (SP1)-like gene regulating protein kinase C-1 (Pkc1)-dependent cell wall integrity and virulence factors in Cryptococcus neoformans. J Biol Chem 286:20977–20990. doi: 10.1074/jbc.M111.230268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung WH, Sham A, White R, Kronstad JW. 2006. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans. PLoS Biol 4:e410. doi: 10.1371/journal.pbio.0040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panepinto J, Liu L, Ramos J, Zhu X, Valyi-Nagy T, Eksi S, Fu J, Jaffe HA, Wickes B, Williamson PR. 2005. The DEAD-box RNA helicase Vad1 regulates multiple virulence-associated genes in Cryptococcus neoformans. J Clin Invest 115:632–641. doi: 10.1172/JCI200523048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain N, Cordero RJ, Casadevall A, Fries BC. 2013. Allergen 1 regulates polysaccharide structure in Cryptococcus neoformans. Mol Microbiol 88:713–727. doi: 10.1111/mmi.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. 2008. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol 10:2043–2057. doi: 10.1111/j.1462-5822.2008.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain N, Li L, McFadden DC, Banarjee U, Wang X, Cook E, Fries BC. 2006. Phenotypic switching in a Cryptococcus neoformans variety gattii strain is associated with changes in virulence and promotes dissemination to the central nervous system. Infect Immun 74:896–903. doi: 10.1128/IAI.74.2.896-903.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandal P, Banerjee U, Casadevall A, Nosanchuk JD. 2005. Dual infections with pigmented and albino strains of Cryptococcus neoformans in patients with or without human immunodeficiency virus infection in India. J Clin Microbiol 43:4766–4772. doi: 10.1128/JCM.43.9.4766-4772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Meara TR, Norton D, Price MS, Hay C, Clements MF, Nichols CB, Alspaugh JA. 2010. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog 6:e1000776. doi: 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordero RJ, Frases S, Guimaraes AJ, Rivera J, Casadevall A. 2011. Evidence for branching in cryptococcal capsular polysaccharides and consequences on its biological activity. Mol Microbiol 79:1101–1117. doi: 10.1111/j.1365-2958.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 19.Reilly MC, Aoki K, Wang ZA, Skowyra ML, Williams M, Tiemeyer M, Doering TL. 2011. A xylosylphosphotransferase of Cryptococcus neoformans acts in protein O-glycan synthesis. J Biol Chem 286:26888–26899. doi: 10.1074/jbc.M111.262162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly MC, Levery SB, Castle SA, Klutts JS, Doering TL. 2009. A novel xylosylphosphotransferase activity discovered in Cryptococcus neoformans. J Biol Chem 284:36118–36127. doi: 10.1074/jbc.M109.056226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterman SR, Park YD, Raja M, Qiu J, Hammoud DA, O'Halloran TV, Williamson PR. 2012. Role of CTR4 in the virulence of Cryptococcus neoformans. mBio 3:e00285-12. doi: 10.1128/mBio.00285-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plant PJ, Manolson MF, Grinstein S, Demaurex N. 1999. Alternative mechanisms of vacuolar acidification in H+-ATPase-deficient yeast. J Biol Chem 274:37270–37279. doi: 10.1074/jbc.274.52.37270. [DOI] [PubMed] [Google Scholar]
- 23.Diakov TT, Tarsio M, Kane PM. 2013. Measurement of vacuolar and cytosolic pH in vivo in yeast cell suspensions. J Vis Exp doi: 10.3791/50261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia J, Shea J, Alvarez-Vasquez F, Qureshi A, Luberto C, Voit EO, Del Poeta M. 2008. Mathematical modeling of pathogenicity of Cryptococcus neoformans. Mol Syst Biol 4:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. 2009. A molecular mechanism of chronological aging in yeast. Cell Cycle 8:1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffen KK, Kennedy BK, Kaeberlein M. 2009. Measuring replicative life span in the budding yeast. J Vis Exp doi: 10.3791/1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serrano R. 1988. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim Biophys Acta 947:1–28. doi: 10.1016/0304-4157(88)90017-2. [DOI] [PubMed] [Google Scholar]
- 28.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J. 1994. Molecular biology of the cell, 3rd ed Taylor and Francis, New York, NY. [Google Scholar]
- 29.Feldmesser M, Kress Y, Novikoff P, Casadevall A. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun 68:4225–4237. doi: 10.1128/IAI.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldmesser M, Tucker S, Casadevall A. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol 9:273–278. doi: 10.1016/S0966-842X(01)02035-2. [DOI] [PubMed] [Google Scholar]
- 31.Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. 2007. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect Immun 75:2729–2739. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao X, Mednick A, Alvarez M, van Rooijen N, Casadevall A, Goldman DL. 2005. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J Immunol 175:3244–3251. doi: 10.4049/jimmunol.175.5.3244. [DOI] [PubMed] [Google Scholar]
- 33.Sabiiti W, Robertson E, Beale MA, Johnston SA, Brouwer AE, Loyse A, Jarvis JN, Gilbert AS, Fisher MC, Harrison TS, May RC, Bicanic T. 2014. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J Clin Invest 124:2000–2008. doi: 10.1172/JCI72950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alanio A, Desnos-Ollivier M, Dromer F. 2011. Dynamics of Cryptococcus neoformans-macrophage interactions reveal that fungal background influences outcome during cryptococcal meningoencephalitis in humans. mBio 2:e00158-11. doi: 10.1128/mBio.00158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alspaugh JA, Granger DL. 1991. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun 59:2291–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Granger DL, Hibbs JB Jr, Perfect JR, Durack DT. 1988. Specific amino acid (l-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest 81:1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray HW, Cartelli DM. 1983. Killing of intracellular Leishmania donovani by human mononuclear phagocytes: evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest 72:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. 1996. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med 184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker L, Lowrie DB. 1981. Killing of Mycobacterium microti by immunologically activated macrophages. Nature 293:69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- 40.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. 1995. Free radicals and phagocytic cells. FASEB J 9:200–209. [DOI] [PubMed] [Google Scholar]
- 41.Upadhya R, Campbell LT, Donlin MJ, Aurora R, Lodge JK. 2013. Global transcriptome profile of Cryptococcus neoformans during exposure to hydrogen peroxide induced oxidative stress. PLoS One 8:e55110. doi: 10.1371/journal.pone.0055110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding C, Yin J, Tovar EM, Fitzpatrick DA, Higgins DG, Thiele DJ. 2011. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol Microbiol 81:1560–1576. doi: 10.1111/j.1365-2958.2011.07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira T, Mason AB, Slayman CW. 2001. The yeast Pma1 proton pump: a model for understanding the biogenesis of plasma membrane proteins. J Biol Chem 276:29613–29616. doi: 10.1074/jbc.R100022200. [DOI] [PubMed] [Google Scholar]
- 44.Ioannoni R, Beaudoin J, Mercier A, Labbe S. 2010. Copper-dependent trafficking of the Ctr4-Ctr5 copper transporting complex. PLoS One 5:e11964. doi: 10.1371/journal.pone.0011964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffith OW, Bridges RJ, Meister A. 1978. Evidence that the gamma-glutamyl cycle functions in vivo using intracellular glutathione: effects of amino acids and selective inhibition of enzymes. Proc Natl Acad Sci U S A 75:5405–5408. doi: 10.1073/pnas.75.11.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar A, Bachhawat AK. 2010. OXP1/YKL215c encodes an ATP-dependent 5-oxoprolinase in Saccharomyces cerevisiae: functional characterization, domain structure and identification of actin-like ATP-binding motifs in eukaryotic 5-oxoprolinases. FEMS Yeast Res 10:394–401. doi: 10.1111/j.1567-1364.2010.00619.x. [DOI] [PubMed] [Google Scholar]
- 47.Vallejo CG, Serrano R. 1989. Physiology of mutants with reduced expression of plasma membrane H+-ATPase. Yeast 5:307–319. doi: 10.1002/yea.320050411. [DOI] [PubMed] [Google Scholar]
- 48.Lee SC, Kress Y, Zhao ML, Dickson DW, Casadevall A. 1995. Cryptococcus neoformans survive and replicate in human microglia. Lab Invest 73:871–879. [PubMed] [Google Scholar]
- 49.Seider K, Brunke S, Schild L, Jablonowski N, Wilson D, Majer O, Barz D, Haas A, Kuchler K, Schaller M, Hube B. 2011. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol 187:3072–3086. doi: 10.4049/jimmunol.1003730. [DOI] [PubMed] [Google Scholar]
- 50.Levitz SM, Nong SH, Seetoo KF, Harrison TS, Speizer RA, Simons ER. 1999. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect Immun 67:885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC. 2011. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio 2:e00055-11. doi: 10.1128/mBio.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Meara TR, Xu W, Selvig KM, O'Meara MJ, Mitchell AP, Alspaugh JA. 2014. The Cryptococcus neoformans Rim101 transcription factor directly regulates genes required for adaptation to the host. Mol Cell Biol 34:673–684. doi: 10.1128/MCB.01359-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Hermoso D, Janbon G, Dromer F. 1999. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol 37:3204–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gevaudant F, Duby G, von Stedingk E, Zhao R, Morsomme P, Boutry M. 2007. Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. Plant Physiol 144:1763–1776. doi: 10.1104/pp.107.103762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haruta M, Burch HL, Nelson RB, Barrett-Wilt G, Kline KG, Mohsin SB, Young JC, Otegui MS, Sussman MR. 2010. Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J Biol Chem 285:17918–17929. doi: 10.1074/jbc.M110.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goossens A, de La Fuente N, Forment J, Serrano R, Portillo F. 2000. Regulation of yeast H+-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol Cell Biol 20:7654–7661. doi: 10.1128/MCB.20.20.7654-7661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campetelli AN, Monesterolo NE, Previtali G, Santander VS, Amaiden MR, Arce CA, Valdez-Taubas J, Casale CH. 2013. Activation of H+-ATPase by glucose in Saccharomyces cerevisiae involves a membrane serine protease. Biochim Biophys Acta 1830:3593–3603. doi: 10.1016/j.bbagen.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Luque-Ortega JR, Rivero-Lezcano OM, Croft SL, Rivas L. 2001. In vivo monitoring of intracellular ATP levels in Leishmania donovani promastigotes as a rapid method to screen drugs targeting bioenergetic metabolism. Antimicrob Agents Chemother 45:1121–1125. doi: 10.1128/AAC.45.4.1121-1125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perfect JR, Lang SD, Durack DT. 1980. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol 101:177–194. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.