Abstract
Less toxic treatment options for patients with myeloperoxidase (MPO)-ANCA–associated GN are needed. Using an established murine model of focal necrotizing GN mediated by autoimmunity to MPO (autoimmune anti–MPO GN), we assessed the capacity for nasal tolerance induced by nasal insufflation of the immunodominant nephritogenic MPO peptide (MPO409–428) to attenuate this disease. Compared with mice that received an irrelevant immunodominant ovalbumin (OVA) peptide, OVA323–339, mice that received MPO409–428 were protected from the development of humoral and cell-mediated autoimmunity to full-length MPO and the development of GN. In mice with established anti–MPO autoimmunity, nasal insufflation of MPO409–428 as a therapeutic attenuated anti–MPO GN. To investigate the nature of this induced tolerance, we isolated CD4+ T cells from the upper airway draining lymph nodes of both OVA323–339- and MPO409–428-tolerized mice. Adoptive transfer of CD4+ T cells from MPO409–428- but not OVA323–339-tolerized mice to animals with established anti–MPO autoimmunity attenuated the subsequent development of GN, confirming that the immunosuppression induced by these T cells is antigen specific. Ex vivo studies showed that nasal tolerance to MPO is mediated by both conventional and induced T regulatory cells. The strong homology between the pathogenic human MPO B cell epitope recognized by ANCA in patients with acute vasculitis and the nephritogenic murine T cell MPO epitope emphasizes the clinical relevance of this study.
Keywords: ANCA, GN, tolerance
ANCA-associated GN is the most common form of crescentic GN. Among patients with ANCA-associated GN, most have ANCA that reacts to myeloperoxidase (MPO). MPO, the major constituent of the azurophilic granules in neutrophils, is a heme-containing enzyme that plays a key role in the generation of injurious oxidant products. These products are potent antimicrobial agents1 but can also mediate tissue injury in GN.2
Cyclophosphamide is still the standard of care for patients with MPO-ANCA–associated GN.3 This drug has adverse side effects, including severe and life-threatening infections, sterility, acute myeloid leukemia, bladder cancer, and hemorrhagic cystitis.4–6 New, more targeted, less toxic therapies are needed. Experimental animal models have provided a depth of understanding of the pathogenesis of MPO-ANCA–associated GN. This disease begins with the loss of tolerance and development of autoimmunity to MPO. In 2012, the dominant disease–inducing murine MPO epitope, MPO409–428, was defined7 and shown to have high homology with the immunodominant human MPO B cell epitope recognized by sera from patients with active MPO-ANCA–associated GN.8
Discovery of an immunodominant nephritogenic MPO peptide offers the opportunity for the development of peptide–based immunomodulatory therapies that may provide antigen-specific immunosuppression capable of restoring immune homeostasis with antigenic specificity.9 Mucosal tolerance is a phenomenon, whereby peripheral tolerance is maintained by administering the antigen of interest through the oral or nasal/respiratory routes.10–12 The induction of tolerance by mucosal administration of autoantigens or immunodominant/immunogenic peptides has been reported in various experimental models of autoimmune diseases, including encephalomyelitis,13,14 myasthenia gravis,15,16 uveitis,16,17 diabetes,18–20 arthritis,21–23 and autoimmune GN.24,25 In several of these studies, nasal administration of the immunodominant peptide was more effective in inducing tolerance than oral peptide administration.22,23,25,26 Despite considerable research in this area, the mechanisms involved in the induction of mucosal tolerance are uncertain.
In these studies, we examined the effects of nasal administration of the mouse immunodominant MPO T cell peptide (mouse sequence numbering MPO409–428; mapped to the heavy-chain region of the MPO molecule) in the prevention and treatment of GN in experimental autoimmune anti–MPO GN.8 Induction of mucosal tolerance is dependent on the dose of antigen administered. In some experimental models of autoimmune diseases, administration of high-dose peptide is more effective to treat established disease by favoring the induction of clonal anergy or deletion. Low-dose therapy prevents the development of autoimmunity by generating a subset of regulatory T cells (Tregs) inducing immunoregulation through their production of IL-10, TGF-β, and IL-4.10,11 To determine the optimal dosage of MPO409–428 in nasal tolerance induction in experimental autoimmune anti–MPO GN, we administered different dosages of MPO409–428 (cumulative dose of 3, 30, or 150 µg divided over three consecutive daily administrations; days −3, −2, and −1) by nasal insufflation before the induction of anti–MPO T cell autoimmunity by subcutaneous MPO immunization (day 0). Nasal insufflation of 150 µg MPO409–428 effectively limited the development of anti–MPO T cell autoimmunity compared with the effect of lower dosages of MPO409–428 (cumulative dose of 3 or 30 µg), which were assessed by the frequency of MPO409–428 or whole-MPO–specific IFN-γ– and IL-17A–producing lymph node (LN) cells draining the immunization site (Supplemental Figure 1, A and B).
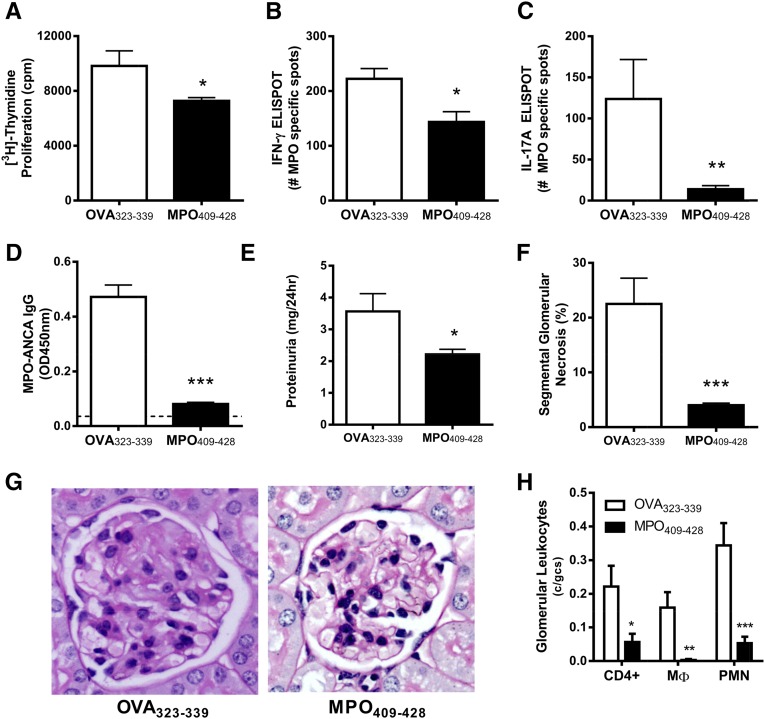
Having determined the optimal dose of MPO409–428 in nasal tolerance, the potential of nasal administration of MPO409–428 to prevent the development of anti-MPO autoimmunity and GN was assessed. Mice were tolerized by daily nasal insufflation of either MPO409–428 or the irrelevant immunodominant ovalbumin (OVA) peptide OVA323–339 (days −3, −2, and −1). Tolerized mice were immunized with MPO409–428 emulsified in Freund’s complete adjuvant, and immune response was boosted with MPO409–428 emulsified in Freund’s incomplete adjuvant (days 0 and 7, respectively). GN was triggered by intravenous injection of a subnephritogenic dose of sheep anti–mouse glomerular basement membrane (GBM) antibody for the purpose of recruiting neutrophils to the glomeruli to deposit MPO (days 14 and 15). Immune responses and renal injury assessed 4 days later before any adaptive immune response were directed against the sheep anti–mouse GBM antibody. Anti-MPO responses were measured by isolating draining LN cells and culturing these cells with whole MPO in vitro. Compared with mice that received OVA323–339, nasal insufflation of mice with MPO409–428 reduced anti–MPO proliferative responses of cells from MPO409–428-immunized mice (Figure 1A) as well as the frequency of IFN-γ– and IL-17A–producing cells (Figure 1, B and C). Furthermore, nasal insufflation of MPO409–428 decreased circulating MPO-ANCA IgG titers (Figure 1D). Collectively, these results indicate that nasal administration of MPO409–428 has the capacity to suppress the development of anti-MPO autoimmunity. The reduced systemic anti–MPO autoimmunity in MPO409–428 nasally insufflated mice limited the development of GN with reduced proteinuria compared with mice receiving OVA323–339 (Figure 1E). This was associated with fewer glomeruli developing segmental glomerular necrosis (Figure 1, F and G) and a similar reduction in the accumulation of effector leukocytes (CD4+ T cells, macrophages, and neutrophils) in glomeruli of mice nasally insufflated with MPO409–428 compared with control OVA323–339–tolerized mice (Figure 1H).
Figure 1.
Nasal insufflation of MPO409–428 prevents the development of anti-MPO GN. LN cells draining MPO immunization sites were restimulated with whole-MPO protein in vitro. (A) Mice that received MPO409–428 nasally (n=8) had reduced MPO–specific T cell proliferation with reduced frequency of (B) IFN-γ– and (C) IL-17A–producing cells compared with OVA323–339 (n=8). (D) Circulating MPO-ANCA titers were diminished in MPO409–428-tolerized mice and similar to naïve C57BL/6 mice (dotted line). Nasal insufflation of MPO409–428 resulted in a marked reduction in (E) albuminuria and (F and G) glomerular segmental necrosis and (H) fewer infiltrating glomerular CD4+ T cells, macrophages, and neutrophils. *P<0.05; **P<0.01; ***P<0.001. gcs, Glomerular cross-section; MΦ, macrophages; OD, optical density; PMN, polymorphonuclear leukocytes.
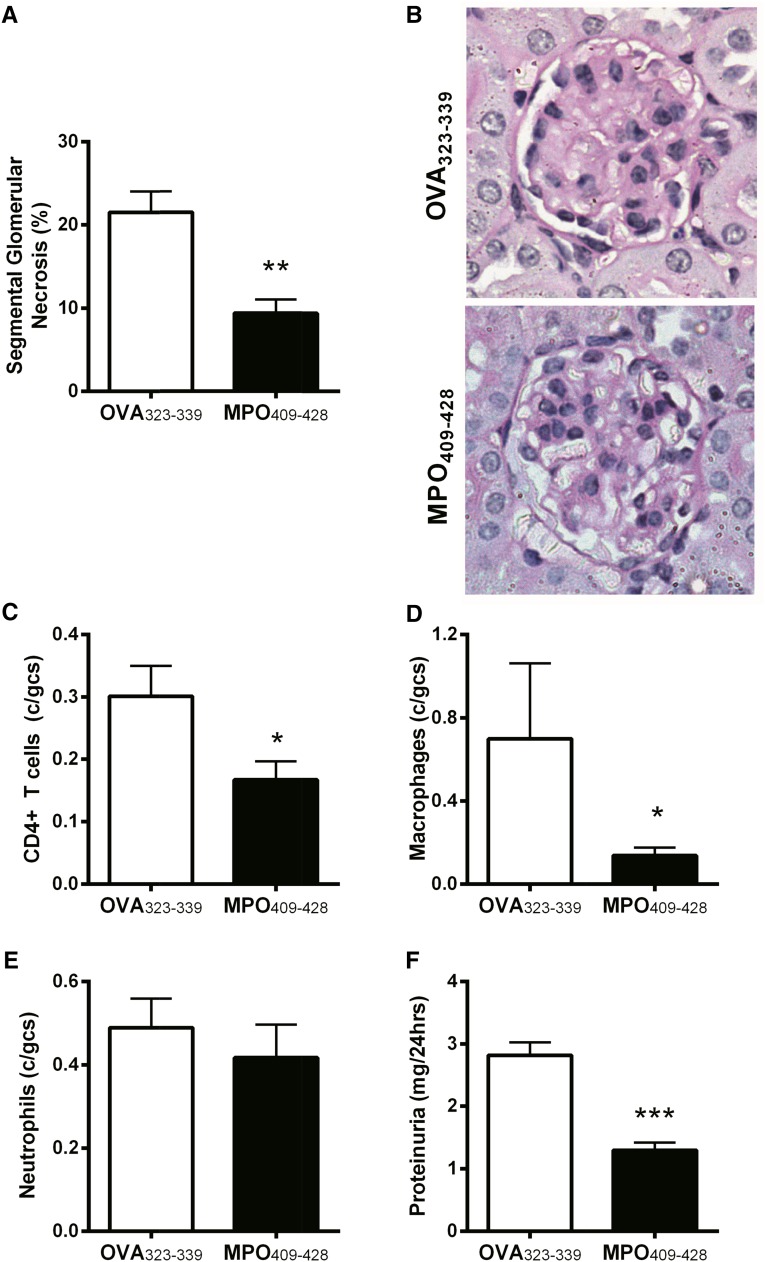
To determine the efficacy of administering MPO409–428 nasally as a potential therapeutic in experimental anti–MPO GN, mice with established MPO autoimmunity were nasally insufflated with OVA323–339 or MPO409–428 (days 10–12), and the severity of GN was assessed on day 20. Compared with nasal insufflation of OVA323–339, MPO409–428 treatment attenuated GN, with fewer glomeruli exhibiting segmental necrosis (Figure 2, A and B) and fewer intraglomerular CD4+ T cells and macrophages (Figure 2, C and D). No difference in glomerular neutrophils was observed between groups (Figure 2E). Functional renal injury as measured by proteinuria paralleled the attenuated histologic injury observed in MPO409–428-treated mice with anti-MPO GN (Figure 2F). Collectively, these results show that nasal insufflation of MPO409–428 can prevent the development of anti-MPO autoimmunity and attenuate anti-MPO GN, even when administered after induction of active anti–MPO autoimmunity.
Figure 2.
Nasal insufflation of MPO409-428 in mice with established MPO autoimmunity (day 14) is therapeutic in the development of anti-MPO GN. (A and B) Nasal insufflation of MPO409–428-attenuated GN (n=8) assessed histologically by the percentage of segmental glomerular necrosis and (C) infiltration of glomerular CD4+ T cells and (D) macrophages compared with OVA323–339-tolerized mice (n=8). (E) No difference in numbers of glomerular neutrophils between groups. (F) Renal function as measured by proteinuria was attenuated in mice nasally tolerized with MPO409–428. gcs, Glomerular cross-section. *P<0.05; **P<0.01; ***P<0.001.
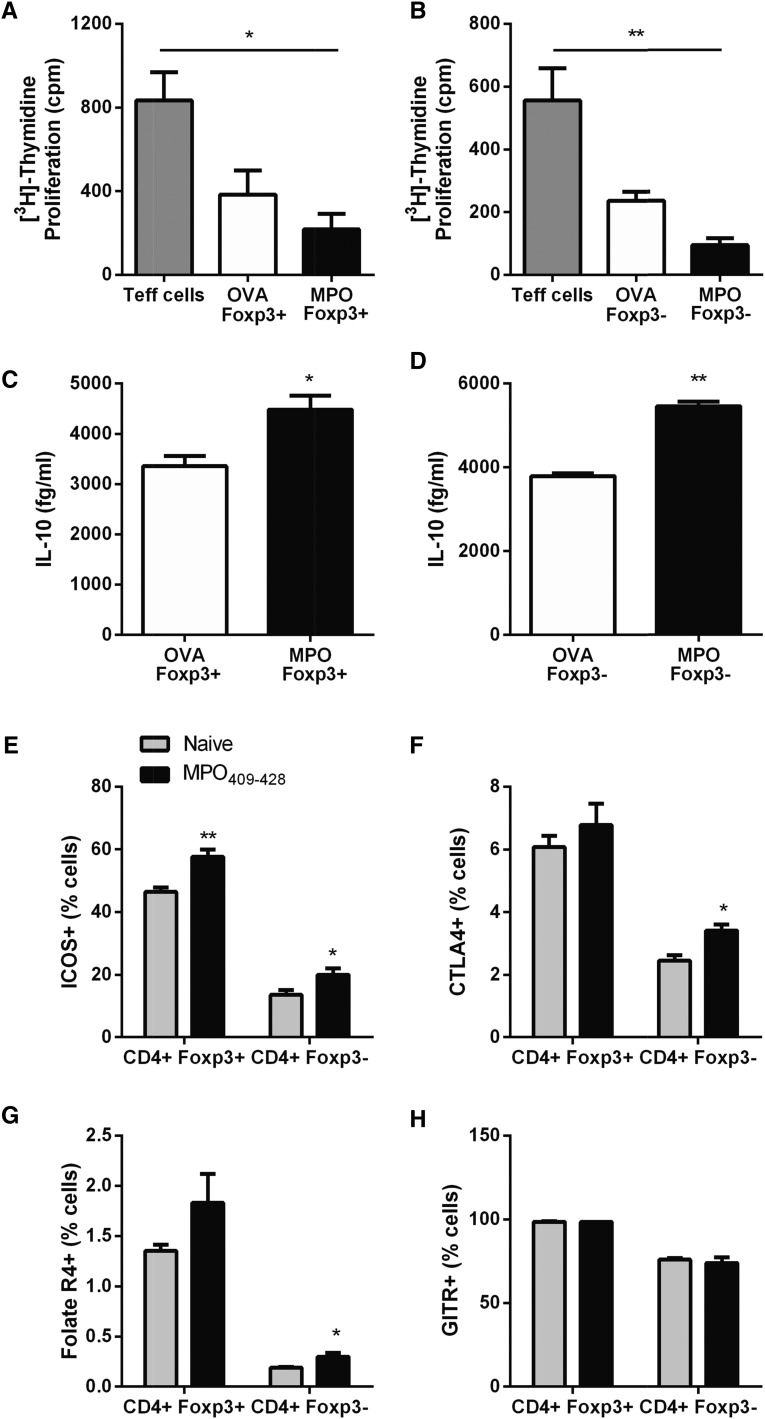
The mechanisms involved in the development of mucosal tolerance to autoantigens are not well understood. It has been previously shown that nasal tolerance induces antigen-specific Tregs in a model of delayed type hypersensitivity responses to OVA as a foreign antigen and that CD4+ T cell transfer from OVA-tolerized mice can prevent the development of delayed type hypersensitivity to OVA in recipient mice.27 To define mechanisms by which nasal administration of MPO409–428 may prevent anti-MPO autoimmunity and attenuate anti-MPO GN, we assessed Treg function in an ex vivo coculture system using Foxp3-GFP mice. Anti–MPO T effectors (Teffs) were generated by immunizing Foxp3-GFP mice subcutaneously with MPO. Draining LNs from MPO immunization sites were harvested 10 days later, and CD4+Foxp3− Teff sorted cells were isolated. These anti–MPO CD4+ Teffs were cocultured with either CD4+Foxp3+ or CD4+Foxp3− cells isolated from upper respiratory tract draining LNs from Foxp3-GFP mice that received either MPO409–428 or OVA323–339 intranasally (150-µg cumulative dose). Cocultured cells were incubated with whole MPO. MPO409–428–tolerized CD4+ T cells, regardless of Foxp3 expression, significantly reduced anti–MPO Teff cell proliferation, whereas OVA323–339–tolerized CD4+Foxp3+ or CD4+Foxp3− cells did not (Figure 3, A and B). Enhanced CD4+ (Foxp3+ and Foxp3−) immunoregulatory cell function observed from mice nasally insufflated with MPO409–428 was also associated with increased production of IL-10 compared with CD4+ cells from OVA323–339-tolerized mice after MPO recall challenge (Figure 3, C and D). Characterization of CD4+Foxp3+ and CD4+Foxp3− cells from upper respiratory draining LNs of MPO409–428-tolerized mice for Treg cell surface markers showed increased expression of inducible T-cell co-stimulator compared with upper respiratory draining LN cells from naïve mice (Figure 3E). Interestingly, among the MPO409–428 nasally tolerized mice, only the CD4+Foxp3− population of upper respiratory draining LN cell expression of cytotoxic T-lymphocyte-associated protein 4 and Folate R4 was significantly increased compared with CD4+ cells from naïve mice, an increase not observed in CD4+Foxp3+ cells (Figure 3, F and G). No difference in glucocorticoid-induced tumour-necrosis-factor-receptor expression was observed between groups (Figure 3H).
Figure 3.
Nasal insufflation enhances Treg function. Anti–MPO CD4+ Teffs were cocultured with either CD4+Foxp3+ or CD4+Foxp3− Tregs from upper respiratory tract draining LNs of mice nasally administered with MPO409–428 at a ratio of 1 Teff to 4 Tregs. (A and B) Both CD4+Foxp3+ and CD4+Foxp3− T cells suppressed anti–MPO Teff cell proliferation compared with OVA323–339–tolerized CD4+ T cells. (C and D) Enhanced Treg function was associated with enhanced IL-10 production from both MPO409–428–tolerized CD4+Foxp3+ and CD4+Foxp3− T cells. (E–G) Characterization of CD4+ T cells from mice nasally insufflated with MPO409–428 showed a significant increase in ICOS in MPO409–428–tolerized CD4+Foxp3+ cells, whereas ICOS, CTLA4, and Folate R4 expressions were all significantly upregulated in MPO409–428–tolerized CD4+Foxp3− cells compared with CD4+ T cells from naïve C57BL/6 mice. (H) No difference in expression of GITR was observed between groups. *P<0.05; **P<0.01. CTLA4, cytotoxic T-lymphocyte-associated protein 4; GITR, glucocorticoid-induced tumour-necrosis-factor-receptor; ICOS, inducible T-cell co-stimulator.
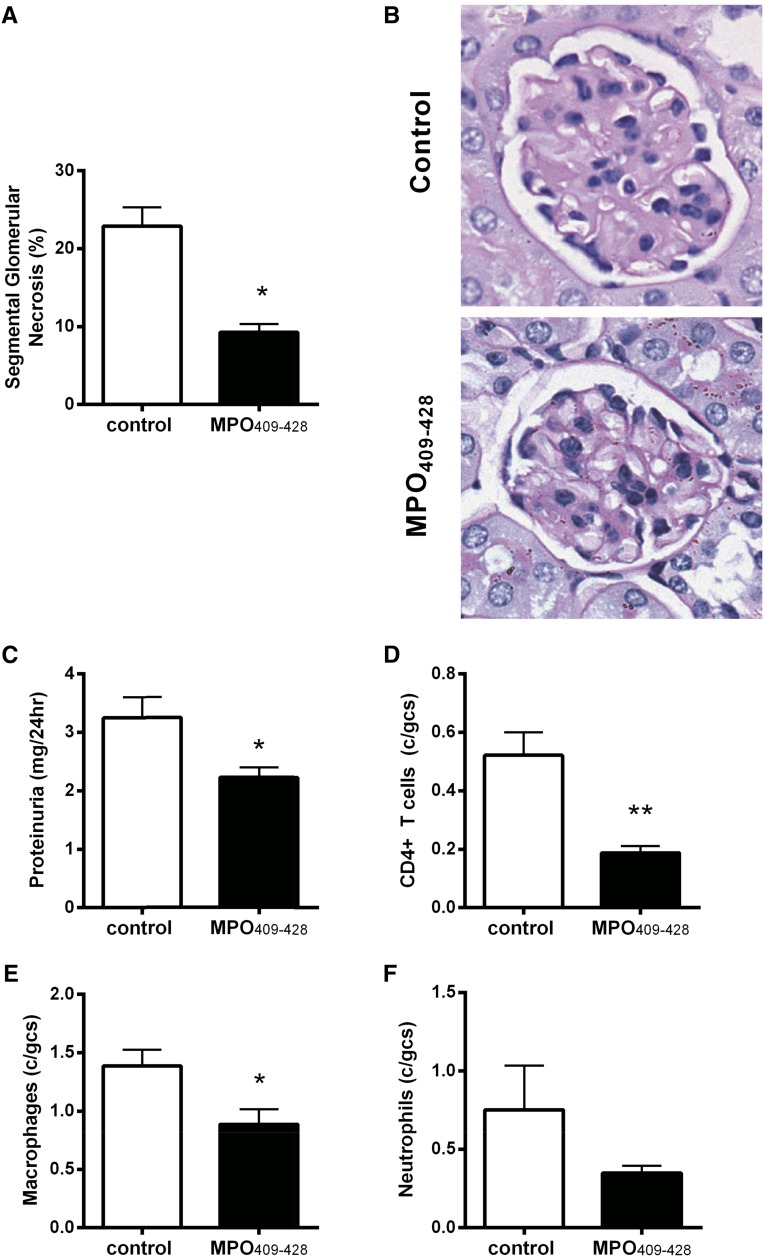
Given that nasal insufflation of MPO409–428 enhances the regulatory function of CD4+ cells and also, generates both conventional Tregs (CD4+Foxp3+) and Type 1 regulatory (CD4+Foxp3−) cells in an antigen-specific manner, these CD4+ cells were adoptively transferred to mice with established anti–MPO autoimmunity (day 10) to determine whether they can functionally suppress the development of anti-MPO GN. MPO-immunized mice that did not receive tolerized MPO409–428 CD4+ T cells were used as controls. Transfer of MPO409–428–tolerized CD4+ T cells reduced the percentage of glomeruli with segmental glomerular necrosis (Figure 4, A and B) and proteinuria (Figure 4C). This was associated with decreased numbers of glomerular CD4+ T cells and macrophages (Figure 4, D and E). No difference in glomerular neutrophils was observed between groups (Figure 4F). To ascertain if the therapeutic nature of adoptively transferring nasally tolerized CD4+ T cells is antigen specific, OVA323–339–tolerized CD4+ T cells were adoptively transferred to mice with established anti–MPO autoimmunity. MPO-immunized mice that did not receive OVA323–339–tolerized CD4+ T cells served as controls. OVA323–339–tolerized CD4+ T cells did not attenuate GN, and disease severity was similar to control mice that did not receive cells (Supplemental Figure 2).
Figure 4.
Adoptive transfer of nasally tolerized MPO409-428 CD4+ T cells to mice with established MPO autoimmunity (day 10) limits the development of anti-MPO GN. (A and B) Adoptive transfer of tolerized MPO409–428 CD4+ T cells to mice with established anti–MPO autoimmunity (n=4) limited the development of GN with reduced segmental glomerular necrosis and (C–E) glomerular leukocyte accumulation (CD4+ T cells, macrophages, and neutrophils) and (F) attenuated functional renal injury as measured by proteinuria compared with MPO-immunized mice that received no cells (n=4). gcs, Glomerular cross-section. *P<0.05; **P<0.01.
In conclusion, this study reveals that re-establishing tolerance to MPO with the immunodominant MPO peptide by the nasal route allows for antigen-specific immunomodulation. The successful induction of tolerance to MPO peptide justifies future studies of the mechanisms of how nasal mucosal–associated lymphoid tissue induces tolerance. Relevant to these studies is the high-degree of homology of this nephritogenic peptide with the dominant human MPO peptide found in studies of sera from patients with acute MPO-ANCA–associated vasculitis. This discovery provides proof of concept to underpin preclinical studies to assess whether peptide–induced nasal tolerance may be therapeutic in patients with MPO-ANCA–associated GN.
Concise Methods
Mice and Experimental Design
Eight- to twelve-week-old male C57BL/6 (wild type) and Foxp3-GFP mice (from Alexander Rudensky, University of Washington, Seattle, WA) were bred and housed in specific pathogen–free conditions at Monash Medical Centre Animal Facilities, Monash University. Studies were approved by the Monash University Animal Ethics Committee in accordance with the Australian National Health and Medical Research Council animal experimentation guidelines.
Peptides MPO409–428 (PRWNGEKLYQEARKIVGAMV) and the control irrelevant immunodominant OVA peptide (OVA323–339; ISQAVHAAHAEINEAGR) were synthesized by Mimotopes Australia. To determine the optimal dose of intranasal MPO409–428, three groups of mice (n=4) were given MPO409–428 in 20 μl saline nasally on 3 consecutive days (days −3, −2, and −1) with total cumulative doses of 3, 30, and 150 μg. Control mice received 20 μl saline nasally (n=4). On day 0, mice were immunized with 25 μg MPO409–428 in Freund’s Complete Adjuvant subcutaneously at the base of tail, and they were killed on day 10. Draining LNs from MPO immunization sites were aseptically removed for analysis of systemic immunity.
For prevention of experimental anti–MPO GN, 150 μg MPO409–428 or OVA323–339 in 20 μl saline was administered nasally for 3 consecutive days. Mice were then immunized on day 0 with 100 μg MPO409–428 in Freund’s Complete Adjuvant subcutaneously at the base of tail, and on day 7, they were immunized with 100 μg MPO409–428 in Freund’s Incomplete Adjuvant subcutaneously at the neck. On days 14 and 15, GN was triggered by administration of 1 mg sheep anti–mouse GBM globulin intravenously. Mice were humanely culled on day 18. To treat mice with established anti–MPO autoimmunity, nasal administration of a cumulative dose of 150 μg MPO409–428 or OVA323–339 began 14 days post-MPO409–428 immunization. GN was triggered by sheep anti–mouse GBM globulin on days 17 and 18, and mice were culled on day 22.
For adoptive transfer of tolerized CD4+ T cells to mice with established anti–MPO autoimmunity, donor Foxp3-GFP mice were nasally insufflated with MPO409–428 or OVA323–339 as above. Four days after initial administration of MPO409–428, upper airway draining LNs were collected, single-cell suspensions were obtained, and CD4+ T cells were isolated by magnetic cell sorting (CD4+ T Cell Isolation Kit; Miltenyi Biotec, North Ryde, Australia); 5×106 MPO409–428–tolerized CD4+ T cells or OVA323–339–tolerized CD4+ T cells were adoptively transferred to MPO-immunized mice on day 10, and control MPO–immunized mice received no cells. GN was triggered and culled as described above.
Assessment of Systemic Autoimmune Responses to MPO
For in vitro measurement of MPO–specific cell proliferation, draining LNs from experimental mice were harvested, and single-cell suspensions were prepared. Cells were seeded at 5×105 cells per well. IFN-γ and IL-17A production was assessed by ELISPOT (Mouse IFN-γ ELISPOT Kit and Mouse IL-17A ELISPOT Kit; BD Biosciences), with draining LN cells restimulated with 5 μg/ml either MPO409–428 or recombinant MPO for 18 hours. IFN-γ– and IL-17A–producing cells were enumerated with an automated ELISPOT reader system.28 For the T cell proliferation assay, cells were restimulated with 10 μg/ml MPO409–428 or recombinant MPO and incubated for 72 hours. During the last 18 hours, 0.5 μCi [3H]-thymidine (PerkinElmer) was added. Cells were harvested, and [3H]-thymidine incorporation was measured as previously described.29 Measurement of circulating MPO-ANCA titers in sera was measured by ELISA as previously described.30
Assessment of Renal Injury
Mice were housed individually in cages for urine collection over the final 24 hours of the experiment. Proteinuria was measured by Bradford’s method using Bradford’s reagent and calculated from the 24-hour urine volume and the urinary protein concentration (expressed as milligrams per 24 hours). The proportions of glomerular segmental necrosis were determined by evaluating, using coded slides, a minimum of 30 glomerular cross-sections per mouse on periodic acid–Schiff reagent–stained kidneys. For assessment of glomerular macrophages, neutrophils, and CD4+ T cell infiltrations, frozen periodate lysine paraformaldehyde–fixed kidneys were cut at 6 μm and stained using a three–layer immunoperoxidase technique. The primary mAbs used were FA/11 (anti-CD68) for macrophages, RB6–8C5 (anti–Gr-1) for neutrophils, and GK1.5 (anti–CD4+ T cells) for CD4+ T cells. A minimum of 30 glomeruli per mouse was assessed, and results are expressed as cells per glomerular cross-section.
Statistical Analyses
Data are given as means±SEMs. An unpaired t test was used for statistical analysis, and one-way ANOVA and Tukey’s post hoc test were used for multiple group comparisons (GraphPad Prism; GraphPad Software Inc., San Diego, CA). Differences were considered to be statistically significant at P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
These studies were supported by grants from the National Health and Medical Research Council of Australia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015010089/-/DCSupplemental.
References
- 1.Klebanoff SJ: Myeloperoxidase: Friend and foe. J Leukoc Biol 77: 598–625, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Odobasic D, Kitching AR, Semple TJ, Holdsworth SR: Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol 18: 760–770, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group : KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012 [Google Scholar]
- 4.Radis CD, Kahl LE, Baker GL, Wasko MC, Cash JM, Gallatin A, Stolzer BL, Agarwal AK, Medsger TA, Jr., Kwoh CK: Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study. Arthritis Rheum 38: 1120–1127, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Huong DL, Amoura Z, Duhaut P, Sbai A, Costedoat N, Wechsler B, Piette JC: Risk of ovarian failure and fertility after intravenous cyclophosphamide. A study in 84 patients. J Rheumatol 29: 2571–2576, 2002 [PubMed] [Google Scholar]
- 6.Pryor BD, Bologna SG, Kahl LE: Risk factors for serious infection during treatment with cyclophosphamide and high-dose corticosteroids for systemic lupus erythematosus. Arthritis Rheum 39: 1475–1482, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Ooi JD, Chang J, Hickey MJ, Borza DB, Fugger L, Holdsworth SR, Kitching AR: The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc Natl Acad Sci U S A 109: E2615–E2624, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, McGregor J, Burkart M, Hogan SL, Hu Y, Winnik W, Nachman PH, Stegeman CA, Niles J, Heeringa P, Kitching AR, Holdsworth S, Jennette JC, Preston GA, Falk RJ: Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 123: 1773–1783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller SD, Turley DM, Podojil JR: Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol 7: 665–677, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Xiao BG, Link H: Mucosal tolerance: A two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol 85: 119–128, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Staines NA, Derry CJ, Marinova-Mutafchieva L, Ali N, Davies DH, Murphy JJ: Constraints on the efficacy of mucosal tolerance in treatment of human and animal arthritic diseases. Ann N Y Acad Sci 1029: 250–259, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Weiner HL: Current issues in the treatment of human diseases by mucosal tolerance. Ann N Y Acad Sci 1029: 211–224, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Li HL, Liu JQ, Bai XF, vn der Meide PH, Link H: Dose-dependent mechanisms relate to nasal tolerance induction and protection against experimental autoimmune encephalomyelitis in Lewis rats. Immunology 94: 431–437, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JQ, Bai XF, Shi FD, Xiao BG, Li HL, Levi M, Mustafa M, Wahren B, Link H: Inhibition of experimental autoimmune encephalomyelitis in Lewis rats by nasal administration of encephalitogenic MBP peptides: Synergistic effects of MBP 68-86 and 87-99. Int Immunol 10: 1139–1148, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Im SH, Barchan D, Fuchs S, Souroujon MC: Mechanism of nasal tolerance induced by a recombinant fragment of acetylcholine receptor for treatment of experimental myasthenia gravis. J Neuroimmunol 111: 161–168, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Phipps PA, Stanford MR, Sun JB, Xiao BG, Holmgren J, Shinnick T, Hasan A, Mizushima Y, Lehner T: Prevention of mucosally induced uveitis with a HSP60-derived peptide linked to cholera toxin B subunit. Eur J Immunol 33: 224–232, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Jiang HR, Taylor N, Duncan L, Dick AD, Forrester JV: Total dose and frequency of administration critically affect success of nasal mucosal tolerance induction. Br J Ophthalmol 85: 739–744, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aspord C, Thivolet C: Nasal administration of CTB-insulin induces active tolerance against autoimmune diabetes in non-obese diabetic (NOD) mice. Clin Exp Immunol 130: 204–211, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, Kaufman DL: Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med 183: 1561–1567, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, Thivolet C: A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci U S A 94: 4610–4614, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers LK, Seyer JM, Stuart JM, Kang AH: Suppression of murine collagen-induced arthritis by nasal administration of collagen. Immunology 90: 161–164, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia G, Komagata Y, Slavin AJ, Maron R, Weiner HL: Suppression of collagen-induced arthritis by oral or nasal administration of type II collagen. J Autoimmun 13: 315–324, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Higuchi K, Kweon MN, Fujihashi K, McGhee JR, Kiyono H: Comparison of nasal and oral tolerance for the prevention of collagen induced murine arthritis. J Rheumatol 27: 1038–1044, 2000 [PubMed] [Google Scholar]
- 24.Reynolds J, Abbott DS, Karegli J, Evans DJ, Pusey CD: Mucosal tolerance induced by an immunodominant peptide from rat alpha3(IV)NC1 in established experimental autoimmune glomerulonephritis. Am J Pathol 174: 2202–2210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds J, Prodromidi EI, Juggapah JK, Abbott DS, Holthaus KA, Kalluri R, Pusey CD: Nasal administration of recombinant rat alpha3(IV)NC1 prevents the development of experimental autoimmune glomerulonephritis in the WKY rat. J Am Soc Nephrol 16: 1350–1359, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Li HL, Shi FD, Bai XF, Huang YM, van der Meide PH, Xiao BG, Link H: Nasal tolerance to experimental autoimmune myasthenia gravis: Tolerance reversal by nasal administration of minute amounts of interferon-gamma. Clin Immunol Immunopathol 87: 15–22, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Unger WW, Jansen W, Wolvers DA, van Halteren AG, Kraal G, Samsom JN: Nasal tolerance induces antigen-specific CD4+CD25- regulatory T cells that can transfer their regulatory capacity to naive CD4+ T cells. Int Immunol 15: 731–739, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Tan DS, Gan PY, O’Sullivan KM, Hammett MV, Summers SA, Ooi JD, Lundgren BA, Boyd RL, Scott HS, Kitching AR, Chidgey AP, Holdsworth SR: Thymic deletion and regulatory T cells prevent antimyeloperoxidase GN. J Am Soc Nephrol 24: 573–585, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gan PY, Summers SA, Ooi JD, O’Sullivan KM, Tan DS, Muljadi RC, Odobasic D, Kitching AR, Holdsworth SR: Mast cells contribute to peripheral tolerance and attenuate autoimmune vasculitis. J Am Soc Nephrol 23: 1955–1966, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gan PY, Steinmetz OM, Tan DS, O’Sullivan KM, Ooi JD, Iwakura Y, Kitching AR, Holdsworth SR: Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol 21: 925–931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.