SUMMARY
The mitochondrial uniporter (MCU) is an ion channel that mediates Ca2+ uptake into the matrix to regulate metabolism, cell death and cytoplasmic Ca2+ signaling. Matrix Ca2+ concentration is similar to that in cytoplasm, despite an enormous driving force for entry, but the mechanisms that prevent mitochondrial Ca2+ overload are unclear. Here, we show that MCU channel activity is governed by matrix Ca2+ concentration through EMRE. Deletion or charge neutralization of its matrix-localized acidic carboxyl terminus abolishes matrix Ca2+ inhibition of MCU Ca2+ currents, resulting in MCU channel activation, enhanced mitochondrial Ca2+ uptake and constitutively elevated matrix Ca2+ concentration. EMRE-dependent regulation of MCU channel activity requires intermembrane space-localized MICU1, MICU2 and cytoplasmic Ca2+. Thus, mitochondria are protected from Ca2+ depletion and Ca2+ overload by a unique molecular complex that involves Ca2+ sensors on both sides of the inner mitochondrial membrane, coupled through EMRE.
INTRODUCTION
The mitochondrial calcium uniporter is a Ca2+-selective ion channel localized in the inner mitochondrial membrane (IMM) (Gunter et al., 1994; Kirichok et al., 2004) that mediates Ca2+ uptake into the mitochondrial matrix from the cytoplasm to regulate metabolism, cell death and cytoplasmic Ca2+ signaling. Under normal resting conditions the matrix free Ca2+ concentration is similar to that in the cytoplasm (Lukacs and Kapus, 1987; Nicholls, 2009), despite an enormous ~180 mV driving force for Ca2+ entry generated by proton pumping by the respiratory chain, suggesting that the Ca2+ uniporter possesses mechanisms to inactivate it under resting conditions to prevent mitochondrial Ca2+ overload. However, the nature of such mechanisms is unclear. The mitochondrial Ca2+ uniporter is a complex of proteins including the Ca2+ selective pore-forming subunit MCU and accessory proteins including MICU1, MICU2, MCUR1 and EMRE (De Stefani and Rizzuto, 2014; Foskett and Philipson, 2015; Kamer et al., 2014). Previously it was suggested that either MICU1 or MICU2 provided a so-called gatekeeping function that reduces MCU-mediated Ca2+ uptake in situ below a threshold value of 1–2 μM external free Ca2+ (the low cytoplasmic [Ca2+] regime) to prevent mitochondrial Ca2+ loading under basal conditions (Csordas et al., 2013; Mallilankaraman et al., 2012; Patron et al., 2014), most likely by reducing MCU single channel open probability. However, it is unclear if MICU proteins exert their effects from the matrix or inter-membrane space or if Ca2+ binding to their pairs of EF hands is required (Foskett and Madesh, 2014). Furthermore, their regulation of MCU-mediated Ca2+ uptake has not been examined by electrophysiological studies of the uniporter channel directly in its native membrane environment, so the molecular details of channel regulation by MICU1 and MICU2 remain unknown. The importance of understanding this regulatory mechanism is underscored by patients with loss of function mutations in MICU1, who lack inhibition of mitochondrial Ca2+ uptake under basal conditions and exhibit proximal myopathy, learning difficulties and a progressive extrapyramidal movement disorder (Logan et al., 2014).
RESULTS
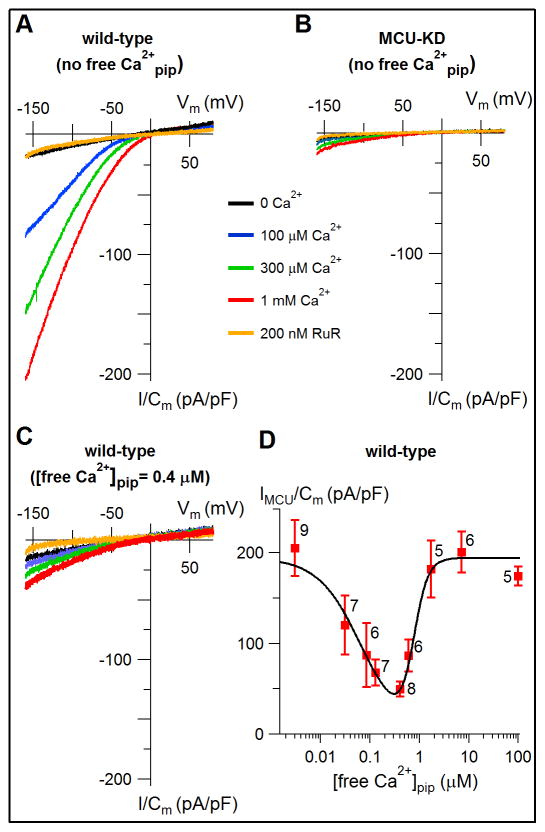
We recorded uniporter Ca2+ currents (IMiCa) using patch clamp electrophysiology of mitoplasts (Fieni et al., 2012; Kirichok et al., 2004; Vais et al., 2015) isolated from human embryonic kidney (HEK) cells. In the whole-mitoplast recording configuration with the pipette solution lacking Ca2+, ruthenium red (RuR, 200 nM)-sensitive Ca2+ currents were observed (Figure 1A) with densities and properties similar to those previously reported for IMiCa with matrix [Ca2+] buffered either at zero or >10 μM (Fieni et al., 2012; Kirichok et al., 2004). Similar currents were nearly abolished in cells with MCU knocked down (Figure 1B), confirming their identity as uniporter currents. Unexpectedly, IMiCa was markedly reduced when matrix [Ca2+] was raised from 0 into a range from 30 nM to ~400 nM (Figure 1C), resulting in a biphasic matrix [Ca2+] dependence with apparent inhibition constant of 60 ± 30 nM and Hill coefficient of 1.0 ± 0.2, and apparent recovery constant of 730 ± 15 nM and Hill coefficient of 3.1 ± 1.6, with peak inhibition of MCU currents by ~75% at ~400 nM (Figure 1D). Of note, resting matrix [Ca2+] is ~100–300 nM (Boyman et al., 2014; Brandenburger et al., 1996; Ivannikov and Macleod, 2013; Lukacs and Kapus, 1987; Nicholls, 2009; Palmer et al., 2006), suggesting that this inhibition of MCU activity may be related to the previously reported inhibition of MCU activity in the low Ca2+ regime attributed to MICU1 and MICU2.
Figure 1. MCU channel activity is modulated by a mechanism dependent upon matrix [Ca2+].
(A) MCU current density in various bath [Ca2+] (indicated in inset) with 0 Ca2+ (1.5 mM EGTA) in the pipette solution, in response to voltage ramps from −160 to 60 mV. Inhibition by ruthenium red (RuR, 200 nM) in 1 mM bath Ca2+ also shown.
(B) MCU current density measured from MCU-KD cells.
(C) Similar to panel (A), recorded with pipette solution containing 400 nM free Ca2+.
(D) Response of MCU Ca2+current density at Vm = −160 mV, with 1 mM Ca2+ in the bath, as function of free [Ca2+] in pipette (matrix) solution. Data fitted with a biphasic Hill equation (continuous line).
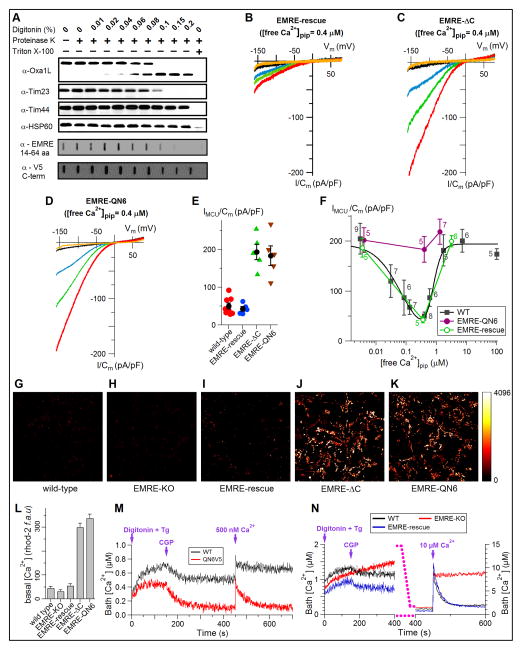
Using a proteinase sensitivity assay (Sato and Mihara, 2010), we determined that MICU1 and MICU2 are localized outside of the matrix, in the intermembrane space (IMS; Figure S1A), in agreement with other reports (Csordas et al., 2013; Kamer and Mootha, 2014; Lam et al., 2015; Petrungaro et al., 2015; Sancak et al., 2013). This suggested that matrix [Ca2+] regulation of IMiCa is mediated by MCU itself or by another matrix-localized Ca2+ binding protein. Plasma membrane Ca2+ channels also have Ca2+ dependent channel inactivation, usually mediated by an associated protein (Christel and Lee, 2012). EMRE is a single pass 10 kD IMM protein that binds to MCU and is required for uniporter activity (Sancak et al., 2013), although its function is unknown. By protease sensitivity biochemistry, we determined that the carboxyl terminus of EMRE faces into the mitochondrial matrix (Figure 2A and Figure S1B). The carboxyl terminus contains a conserved acidic motif that is homologous to the Ca2+ binding “Ca2+ bowl” and “Ca2+ clasp” in Ca2+-activated large conductance (BK) K+ and bestrophin Cl− channels, respectively (Figure S1C). We speculated that it similarly bound Ca2+ in EMRE. Deletion of the EMRE carboxyl terminus or charge neutralization of its seven terminal acidic residues (Figure S1C) was without effect on MICU1 or MICU2 localization (Figure S1D), EMRE topology (Figure S1D and S1E), expression of MCU, MICU1 or MICU2 (Figure S2C) or ability of EMRE to interact with MCU (Figure S3) but it abolished matrix [Ca2+] regulation of uniporter Ca2+ currents (Figure 2B–F). Loss of matrix [Ca2+] inhibition of MCU channel activity in mutant EMRE-expressing cells resulted in constitutively elevated matrix [Ca2+] (Figure 2G–L and Figure S4).
Figure 2. The carboxyl terminus of EMRE confers matrix [Ca2+] sensitivity to MCU activity.
(A) The carboxyl terminus of EMRE resides in the matrix. EMRE-rescue (V5-tagged EMRE over-expressed in EMRE-KO cells) was probed for its amino- (α-EMRE) and carboxyl (α-V5) termini. Carboxyl terminus is protected from protease similarly to matrix-localized proteins.
(B to D) MCU current densities in various bath [Ca2+], recorded from mitoplasts from EMRE KO cells expressing wild-type EMRE (B), EMRE-ΔC (C) or EMRE-QN6 (D), with pipette solution containing 400 nM free Ca2+. Color schemes as in Figure 1.
(E) Summary of experiments shown in B–D. Means and SEMs. Wild-type (Figure 1D) and EMRE-rescue data included for comparison.
(F) Response of MCU Ca2+current density at Vm = −160 mV, with 1 mM Ca2+ in the bath, as function of free [Ca2+] in pipette (matrix) solution for indicated cells, showing loss of matrix [Ca2+] regulation in mitoplasts expressing EMRE-QN6. Wild-type data fitted with a biphasic Hill equation (continuous line).
(G to K) Basal Rhod-2 fluorescence in cells stably expressing EMRE constructs.
(L) Rhod-2 fluorescence quantification (fluorescence arbitrary units (f.a.u.); Mean ± SEM, n = 3).
(M) Gatekeeping measured by mitochondrial Ca2+ uptake (average traces; n = 3) in permeabilized cells is abolished in cells expressing the EMRE-QN6 mutant, evidenced by MCU-mediated Ca2+ uptake following inhibition of the Na+/Ca2+ exchanger with CGP that reduces baseline bath [Ca2+] to much lower levels than wild-type (WT) cells, and their ability to rapidly take up Ca2+ following addition of 500 nM Ca2+ bolus. Tg: thapsigargin, 2 μM. Digitonin: 40 μg/ml. See text for details.
(N) Similar to (M), demonstrating lack of MCU activity in EMRE KO cells (no Ca2+ uptake following CGP addition or after addition of 10 μM Ca2+ bolus) and normal gatekeeping and Ca2+ uptake in WT and EMRE-rescue cells.
See also Figures S1 to S4.
To determine the relationship between these observations and previously reported regulation of MCU activity by MICU1 and MICU2, we examined MCU activity in intact mitochondria by simultaneous measurements of mitochondrial Ca2+ uptake and mitochondrial membrane potential in permeabilized HEK293 cells as previously described (Mallilankaraman et al., 2012). Addition of digitonin to permeabilize the plasma membrane and thapsigargin to inhibit SERCA activity slowly raised bath [Ca2+] due to leak of Ca2+ from the endoplasmic reticulum (ER). Addition of CGP37157 (CGP) to inhibit the IMM Na+/Ca2+ exchanger and reveal the rate of unidirectional mitochondrial Ca2+ uptake, reversed that trend for all cells with functional MCU (Figure 2M and 2N). In contrast, the slow rise in bath [Ca2+] was unaffected by CGP addition in EMRE-KO cells (Figure 2N), reflecting absence of mitochondrial Ca2+ uptake, as expected since EMRE KO abrogates MCU activity in HEK293 cells (Sancak et al., 2013). In response to addition of a 10 μM Ca2+ bolus, bath [Ca2+] was rapidly reduced by MCU-mediated Ca2+ uptake in WT cells, whereas it remained high with no reduction in EMRE-KO cells (Figure 2N), confirming that EMRE is critical for MCU Ca2+ channel function. Control experiments showed complete abrogation of IMiCa in EMRE-KO cells (n = 6; data not shown). MCU-mediated Ca2+ uptake in response to addition of a 10 μM Ca2+ bolus was restored in EMRE-rescue cells (Figure 2N) and in cells rescued with carboxyl-terminus mutant EMRE (Figure 2M). Unidirectional Ca2+ uptake through MCU observed after CGP addition (Figure 2M and N) reduced bath [Ca2+] for WT cells to a significantly higher level than that for mutant EMRE cells, indicating that inhibition of MCU activity in the low cytoplasmic [Ca2+] regime that terminated Ca2+ uptake in WT cells was lost in cells expressing mutant EMRE. Even in the absence of Na+/Ca2+ exchanger activity in WT cells, no decrease in bath [Ca2+] was detected after the addition of a 0.5 μM Ca2+ bolus, (Figure 2N), whereas bath [Ca2+] was restored to the pre-Ca2+ addition level after the addition of 0.5 μM Ca2+ to carboxyl-terminus mutant EMRE cells (Figure 2N), suggesting also that normal inhibition of MCU activity in the low cytoplasmic [Ca2+] regime was impaired by EMRE carboxyl terminus charge neutralization or deletion. These results indicate that EMRE is a Ca2+ sensor that mediates matrix [Ca2+] regulation of uniporter activity.
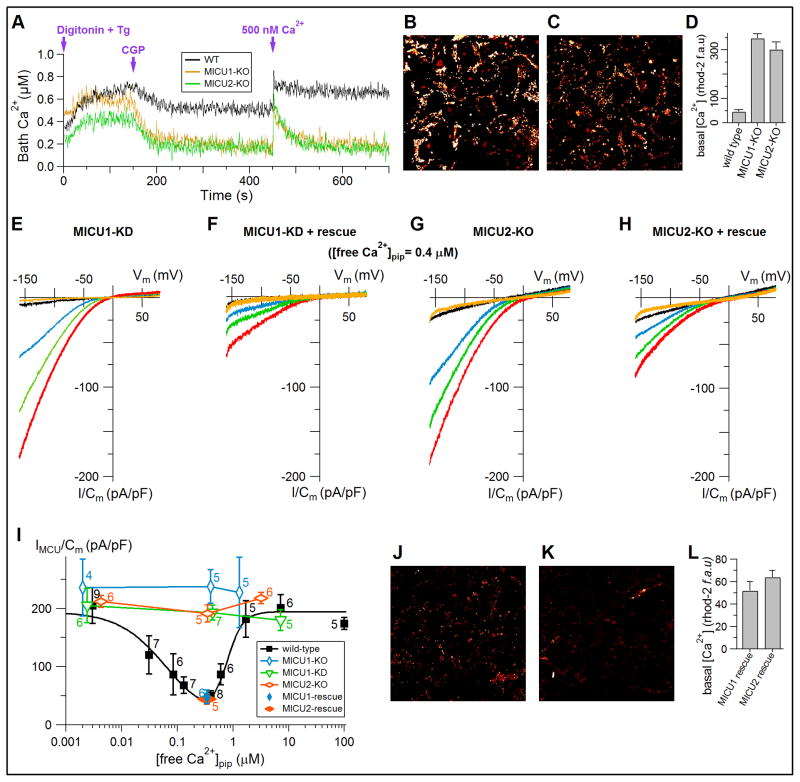
It was originally reported that MICU1 conferred inhibition of MCU-mediated mitochondrial Ca2+ influx in the low cytoplasmic [Ca2+] regime (Mallilankaraman et al., 2012). It was subsequently suggested that MICU2 instead mediates this inhibition of MCU activity (Patron et al., 2014). Measurements of mitochondrial Ca2+ uptake in permeabilized cells confirmed that knockout of either MICU1 or MICU2 abrogated normal inhibition of MCU activity in the low cytoplasmic [Ca2+] regime (Figure 3A), resulting in constitutive matrix Ca2+ loading (Figure 3B–D and Figure S4). To explore the relationship between the EMRE-dependent matrix [Ca2+] regulation of IMiCa and apparent inhibitory functions of MICU proteins, we recorded IMiCa in cells with either MICU protein knocked down. Remarkably, knockdown or knockout of either MICU1 or MICU2 (Figure S2A and 2B) abolished matrix [Ca2+] regulation of IMiCa (Figure 3E and 3F). IMiCa regulation (Figure 3E and 3F) and constitutive mitochondrial Ca2+ loading (Figure 3B and 3C) were rescued by expression of the wild-type proteins (Figure 3F and 3H–L). These results indicate that matrix [Ca2+] regulation of IMiCa is part of the so-called gatekeeping mechanism underlying previously reported inhibition of MCU activity in the low cytoplasmic [Ca2+] regime.
Figure 3. MICU1 and MICU2 are required for matrix [Ca2+] regulation of MCU.
(A) Average traces (n = 3) of bath [Ca2+] showing inhibition of gatekeeping in MICU1-KO and MICU2-KO cells as evidenced by MCU-mediated Ca2+ uptake that reduces baseline bath [Ca2+] to much lower levels than wild-type (WT) cells, and ability of KO cells to rapidly take up Ca2+ following addition of 500 nM Ca2+ bolus.
(B and C) Basal Rhod-2 fluorescence in MICU1-KO (B) and MICU2-KO (C) cells.
(D) Quantification of Rhod-2 fluorescence (mean ± SEM, n = 3).
(E and F) Representative MCU currents in various bath [Ca2+] from mitoplasts from MICU1-KD (E) or MICU1-rescue (F) cells, with pipette solution containing 400 nM free Ca2+. Color scheme as in Fig. 1.
(G and H) Representative MCU currents in various bath [Ca2+] recorded from MICU2-KO (G) or MICU2 rescue (H) mitoplasts, with pipette solution containing 400 nM free Ca2+. Color scheme as in Fig. 1.
(I) Summary of MCU current densities over range of matrix [Ca2+] in mitoplasts from wild-type (WT) and MICU1 and MICU2 KD/KO and rescue cells.
(J and K) Basal Rhod-2 fluorescence in MICU1 (J) and MICU2 (K) rescue cells.
(L) Quantification of Rhod-2 fluorescence (mean ± SEM, n = 3).
See also Figure S2 and S4.
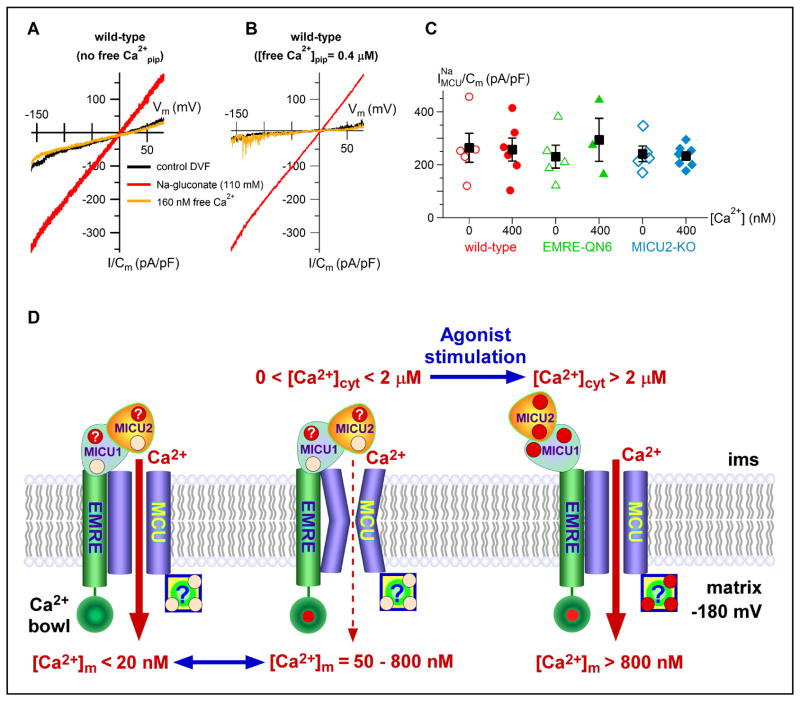
It was suggested that MICU1- (Csordas et al., 2013) or MICU2- (Patron et al., 2014) mediated inhibition of MCU activity does not require Ca2+ binding to their EF hands. To explore the role of cytoplasmic Ca2+ in EMRE regulation of MCU activity, we removed Ca2+ from the bath and recorded MCU activity by measuring Na+ currents (Figure 4A–C) (Fieni et al., 2012; Kirichok et al., 2004). Normal matrix [Ca2+] (400 nM) inhibition of MCU activity was eliminated in the absence of bath Ca2+ (Figure 4A–C), suggesting that Ca2+ binding in the intermembrane space, perhaps to MICU1 and/or MICU2 or MCU itself, is required for EMRE-dependent matrix [Ca2+] regulation of IMiCa. It has been suggested that MICU2 suppresses MCU activity (Patron et al., 2014). However, comparable Na+ currents were recorded in MICU2 KO cells (Figure 4C).
Figure 4. Matrix [Ca2+] regulation of MCU requires cytoplasmic Ca2+.
(A) Representative MCU-mediated Na+ currents (red trace) recorded in absence of bath Ca2+ with no free Ca2+ in pipette. Addition of 160 nM free Ca2+ to bath completely blocked the Na+ current (orange trace).
(B) Same as in (A), recorded with pipette solution containing 400 nM free Ca2+.
(C) Summary of MCU Na+ current densities in different cell types with pipette solutions with or without 400 nM free Ca2+.
(D) Model for MCU regulation, with Ca2+ sensors on both sides of the IMM: EMRE and an unknown protein (box associated with MCU in the cartoon) sense matrix [Ca2+] ([Ca2+]m); MICU1 and/or MICU2, through their paired EF hands (red dots), represent intermembrane space (ims) cytoplasmic Ca2+ ([Ca2+]cyt) sensor(s). Under normal resting conditions (middle conformation), channel open probability is strongly reduced (gatekeeping) when sensors on both sides of the IMM are liganded with Ca2+ (red dots with question marks signify Ca2+ binding sites possibly liganded). Channel inhibition is effective in the range of [Ca2+]m = 50–800 nM, with maximal inhibition at [Ca2+]m ~ 400 nM (from IMiCa biphasic matrix [Ca2+] dependence with apparent inhibition constant of 60 nM and apparent recovery constant of 730 nM; Figure 1D) with peak inhibition of MCU currents by ~75% at ~400 nM (25% open probability indicated by dashed line in middle conformation)), and [Ca2+]cyt between resting levels and ~ 2 μM (from permeabilized cell measurements here and in (Mallilankaraman et al., 2012). Physiological relief of MCU channel inhibition is achieved by agonist-induced elevated [Ca2+]cyt that drives Ca2+ into the matrix, raising [Ca2+]m to levels (>800 nM) that result in highly cooperative activation (Hill coefficient > 3) of mitochondrial Ca2+ influx by unknown mechanisms (right conformation, with the unknown matrix reactivation mechanism(s) depicted as a Ca2+ binding protein with multiple Ca2+ binding sites, and MICU proteins with EF hands fully liganded). MCU channel inhibition can also be relieved by very low [Ca2+]m (left conformation) as a result of Ca2+ unbinding from EMRE, independent of the Ca2+ binding states of MICU proteins (See also Figure S4B).
DISCUSSION
Our results suggest a novel model employing Ca2+ sensors on both sides of the IMM in which the activity of the mitochondrial uniporter is tuned to provide optimal Ca2+ influx into the mitochondrial matrix under resting conditions. EMRE is a sensor of matrix [Ca2+] that governs the inhibition of uniporter activity under normal conditions, acting to prevent mitochondrial Ca2+ loading under basal conditions. This function requires conserved acidic residues in the matrix-localized carboxyl tail of EMRE as well as MICU1 and MICU2 and Ca2+ on the opposite side of the IMM (Figures 4D and S4B). Of note, EMRE mediates the interaction of MICU proteins with MCU (Sancak et al., 2013), suggesting a possible mechanism for EMRE and MICU proteins to have coupled functions. EMRE-dependent matrix [Ca2+] regulation of IMiCa may also play a role in preventing mitochondrial Ca2+ depletion when cytoplasmic [Ca2+] becomes low or mitochondrial Ca2+ extrusion exceeds influx (Figure 4D and S4B). Under such conditions, Ca2+ may dissociate from EMRE and possibly MICU proteins, relieving inhibition of MCU and enabling uniporter activity to increase to preserve matrix [Ca2+] to support critical mitochondrial functions (Cardenas et al., 2010). Thus, mitochondria are protected both from Ca2+ depletion under low-Ca2+ conditions and from Ca2+ loading under normal resting conditions by a unique molecular complex that involves Ca2+ sensors on both sides of the IMM, governed by EMRE. Our results suggest a model in which agonist-generated cytoplasmic Ca2+ signals raise matrix [Ca2+] due to the non-zero open probability of MCU under basal conditions, to a level (> 1 μM; Figure 1D that relieves EMRE-mediated matrix [Ca2+] inhibition of uniporter activity (Figures 1D, 4D, and S4B), enabling agonist-activated cytoplasmic Ca2+ signals to be conveyed to the matrix to stimulate respiration. The mechanism of matrix [Ca2+]-mediated relief from inhibition is highly cooperative (apparent Hill coefficient ~3, Figure 1D) and may therefore account for observed cooperative Ca2+ activation of the uniporter (Bragadin et al., 1979; Gunter and Pfeiffer, 1990; Zoccarato and Nicholls, 1982) without the need to invoke amplification by Ca2+ binding to MICU1 (Csordas et al., 2013; Perocchi et al., 2010). Importantly, the nature of the mechanism(s) involved in matrix [Ca2+] activation of the uniporter remain to be determined (Figure 4D and S4B).
EXPERIMENTAL PROCEDURES
Cell lines and constructs
Wild-type HEK-293T, MICU1-KO, MICU2-KO and EMRE-KO cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C and 5% CO2. All knockout (KO) cell lines were gifts from Dr. Vamsi Mootha. MICU1 knockdown (KD) cells were generated by lentiviral transduction of shRNAs targeting MICU1; transduced cells were selected and maintained in complete DMEM supplemented with puromycin (2 μg/ml), as described (Mallilankaraman et al., 2012). Full length MICU1, MICU2 and EMRE cDNAs were purchased from Origene Technologies USA. EMRE-ΔC-V5 and EMRE-QN6-V5 constructs were generated by a PCR-based cloning strategy. All constructs were sequence verified and stably transfected into either KD or KO backgrounds.
Uniporter electrophysiology
Mitoplast electrophysiology was performed as described (Fieni et al., 2012; Kirichok et al., 2004; Vais et al., 2015). For IMiCa measurements, patch pipettes with tip diameters significantly less than 1 μm had resistances of 20 – 60 MΩ when filled with (in mM): TMA-OH, 130 mM; Hepes, 100; glutathione, 10; MgCl2, 2; EGTA, 1.5; pH = 7.0 with D-gluconic acid; osmolarity 330–350 mOsm/kg. EGTA was also used to buffer different amounts of CaCl2, for [Ca2+]free ≤ 600 nM. However, EGTA was replaced by 1.5 mM of: dibromo-Bapta (for 600 nM < [Ca2+]free ≤ 4 μM), HEDTA (for 4 μM ≤ [Ca2+]free ≤ 30 μM), or NTA (for [Ca2+]free = 100 μM). In all pipette solutions, [Ca2+]free (< 100 μM) was confirmed by Ca2+-indicator dye fluorimetry. For Na+ current measurements, pipettes were filled with (in mM): Na-gluconate, 110; Hepes, 40; NaCl, 2; EGTA, 1; EDTA, 5; CaCl2, see above; pH = 7.0 with NaOH; osmolarity 330–350 mOsm/kg. Mitoplasts were initially bathed in (in mM): KCl, 150; Hepes, 10; EGTA, 1; pH = 7.2, osmolarity 300 mOsm/Kg (“KCl-DVF” solution). Voltage pulses of 350 – 500 mV amplitude and 15 – 50 ms duration, delivered by the PClamp-10 (Molecular Devices) program, were used to obtain the “whole-mitoplast” configuration. Access resistance (30 – 90 MΩ) and mitoplast capacitance Cm (0.2 – 1 pF) were determined using the membrane test protocol of the PClamp-10 software. Because the volume of a mitoplast is over three orders of magnitude smaller than that of a cell, we assumed that matrix [Ca2+] free rapidly equilibrated with pipette [Ca2+] free. After the whole-mitoplast configuration was obtained, the KCl-DVF bath solution was exchanged with: Hepes-EGTA [(in mM): Hepes, 150; EGTA, 1.5; pH = 7.0 with Tris-base for baseline (control) measurements, followed by Hepes (no EGTA) solutions with 0.1; 0.3; 1 mM CaCl2, successively. Finally, a Hepes-based solution with 1 mM CaCl2 and 200 nM ruthenium red (RuR) was perfused into the bath to record the final baseline (= IRuR) after block of MCU Ca2+ currents. For MCU Na+ current measurements, the Hepes-EGTA control solution was instead exchanged with (in mM): Na-gluconate 110; Hepes, 40; EGTA, 1; EDTA, 5; pH = 7.0, followed by the same solution supplemented with 4 mM CaCl2 ([Ca2+]f ≈ 180 nM, estimated with the MaxChelator software), to block the Na+ currents. Osmolarities of all bath solutions were 297–305 mOsm/Kg, adjusted with sucrose. The voltage protocol, delivered by the PClamp-10 software with a DigiData-1550 interface (Molecular Devices), consisted of stepping from Vm = 0 mV to -160 mV for 20 ms, followed by ramping to 80 mV (rate of 279 mV/s), dwelling at 80 mV for 20 ms and return to 0 mV. Currents were recorded using an Axopatch 200-B amplifier. Data were acquired at room temperature with a sampling rate of 50 kHz and anti-aliasing filtered at 1 kHz. Data analysis was performed with the PClamp-10 software. For quantitative comparisons, current densities defined as:
with ICa and IRuR measured at Vm = −160 mV in the presence of 1 mM Ca2+ in the bath, were used. Wild-type data corresponding to various concentrations of free Ca2+ in the pipette solution (=[Caf]) were fitted with the biphasic Hill equation:
where Hinh and Hrec are the Hill coefficients for inhibition and recovery from inhibition, respectively, while Kinh and Krec are the correspondent mid-point pipette [Caf]. Least-square fitting was done with IGOR Pro software (WaveMetrics).
Analysis of uniporter gatekeeping by simultaneous measurement of mitochondrial Ca2+ uptake and mitochondrial membrane potential in permeabilized cells
Simultaneous measurement of mitochondrial Ca2+ uptake and mitochondrial membrane potential (ΔΨm) in permeabilized HEK293 cells was performed as described (Mallilankaraman et al., 2012), with experimental data converted to and represented as bath [Ca2+]. In brief, cells were trypsinized, counted and washed in a Ca2+-free buffer, centrifuged and transferred to an intracellular-like medium (in mM: 120 KCl, 10 NaCl, 1 KH2PO4, 20 HEPES-Tris, 2 succinate, pH 7.2, protease inhibitors, 2 μM thapsigargin, 40 μg/ml digitonin) in a spectrofluorometer. FuraFF (0.5 μM) was added at 0 s and JC-1 (800 nM) at 20 s to measure extra-[Ca2+]m and ΔΨm at 37°C. Fluorescence was monitored in a temperature-controlled (37°C) multi-wavelength-excitation dual wavelength-emission spectrofluorometer (Delta RAM, Photon Technology International) using 490-nm excitation/535-nm emission for the monomer, 570-nm excitation/595-nm emission for the J-aggregate of JC1and 340-nm/380-nm for FuraFF. In this study, the size of the Ca2+ boluses added to stimulate subsequent mitochondrial Ca2+ uptake did not change ΔΨm.
Qualitative measurement of basal [Ca2+]m
Qualitative measurement of basal [Ca2+]m were determined as we previously described (Mallilankaraman et al., 2012). Briefly, cells (5×105/well) grown on 0.2% gelatin-coated coverslips in six-well dishes were loaded with 2 μM rhod-2 AM for 50 min in extracellular medium containing 2 mM Ca2+. Coverslips were mounted in an open perfusion microincubator (PDMI-2; Harvard Apparatus) and imaged at 37 °C. All confocal images, obtained at 561 nm excitation using a 63× oil objective, were rapid 100 msec “snapshots” to avoid phototoxicity. All imaging used identical gain and exposure settings that enabled threshold detection of basal Rhod-2 fluorescence in control WT cells. Accordingly, the contribution of low-level diffuse background cytoplasmic Rhod-2 was essentially eliminated. Addition of the mitochondrial uncoupler CCCP (2–10 μM) was added to ensure that punctate fluorescence emanated from mitochondria (Figure S4). Images were analyzed using ImageJ software (NIH) as described (Mallilankaraman et al., 2012).
Topology analysis
Topology analyses of MICU1, MICU2 and EMRE were performed by proteinase K protection assay as described (Sato et al., 1998). Mitochondria were isolated by differential centrifugation as described (Sato et al., 1998). Immediately after isolation, the mitochondrial pellet was resuspended in MIM buffer (280 mM sucrose with 10 mM Hepes; pH 7.2). Mitochondria (40 μg per condition) were placed in MIM buffer containing varying concentrations of digitonin (0–2%) and constant concentration of Proteinase-K (100 μg/ml) for 15 min at room temperature. Samples without proteinase-K or with 1% Triton X-100 served as controls. Proteinase-K was inactivated by addition of 10 μM PMSF, and samples were subjected to Western or dot blotting.
Western blotting
Cells kept on ice were lysed with 1x RIPA lysis buffer (Millipore) supplemented with protease inhibitors (Complete, Roche). Membranes were blocked in 5% fat-free milk 1 hr at RT, incubated O/N at 4 °C with primary antibody, and then for 2 hr at RT with a secondary antibody conjugated to horseradish peroxidase (HRP). Chemiluminescence detection used ECL-plus reagent (Pierce). To ensure equal protein loading across gels, membranes were stripped (Restore western blot stripping buffer; Pierce) and re-probed with a loading control antibody. For dot blots, protein samples were spotted onto nitrocellulose membranes using a slot blot apparatus and immunoblotting was performed as described above. Antibodies for: MICU1 (ab102830, abcam; 1:200) and (HPA037480, Sigma; 1:300); MICU2 (ab101465, abcam 1:500) and (HPA045511; Sigma); MCU (HPA016480, Sigma; 1:500); EMRE (sc-86337; Santacruz) and (HPA032117, Sigma; 1:200); Tom20 (ab56783; abcam); Tim23 (611223; BD Biosciences); Tim44 (ab168649; abcam); Oxa1L (66128-1-Ig; proteintech); HSP60 (ab13532; abcam); anti-V5 (R960-25, Life technologies; 1:1000); anti-actin (A2228, Sigma 1:2000); anti-flag (TA 50011, Origene 1:500); anti-mouse IgG-HRP (7076S, Cell Signaling; 1:1000) and anti-rabbit IgG-HRP (7074S, Cell Signaling; 1:1000) were used in the study. All experiments were done in triplicate.
Co-immunoprecipitation
HEK293 cells (EMRE KO, EMRE KO + QN6 EMRE-V5 and EMRE KO + wild-type EMRE-V5) were grown to confluency in 150 mm plates. Cells were washed twice with PBS and lysed with 1 ml cold lysis buffer (50 mM HEPES-KOH pH 7.4, 150 mM NaCl, 0.2% n-dodecyl-β-D-maltoside, 0.5 mM EGTA, 0.3 mM CaCl2, one Complete protease inhibitor tablet (Roche) per 30 ml lysis buffer). Cells were lysed at 4°C for 30 min and centrifuged at 16,000 × g. Cleared lysates were diluted to normalize for protein concentration. Appropriate volumes of lysates were added, corrected for expression levels based on western blotting and densitometric analysis. Anti-V5 Agarose (30 μl; Sigma #A 7345) was added to diluted lysates and rocked 16 hr at 4°C. Beads were washed with 1 ml PBS 3x and boiled in 90 μl 2x SDS sample buffer. 20 μl of each immunoprecipitate and 30 μg of total cell lysates were loaded on Tris-Glycine gels for Western Blotting. To maintain a constant range [Ca2+] in lysis and wash buffers, CaCl2 and EGTA were added to buffers according to http://maxchelatorStanford.edu/CaEGTA-TS.htm. In addition, [Ca2+] in each preparation was measured with Fura-2. Solutions contained 150–450 nM free Ca2+.
Supplementary Material
Acknowledgments
This work was supported by NIH GM56328 to J.K.F. We thank Dr. V. Mootha for knockout cell lines, and Dr. T. Hoshi for use of a spectrofluorometer. We also thank Drs. F. Fieni and Y. Kirichok for advice and training in mitoplast electrophysiology.
Footnotes
AUTHOR CONTRIBUTIONS
H.V. performed the electrophysiology. K.M. performed permeabilized cell experiments, made cell lines and created cDNA constructs. D-O.D.M helped analyze data. R.P. performed Rhod-2 imaging and some permeabilized cell experiments. J.T. and H.H. performed biochemistry. J.K.F, H.V., K.M. J.T. and D-O.D.M wrote the manuscript. The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boyman L, Chikando AC, Williams GS, Khairallah RJ, Kettlewell S, Ward CW, Smith GL, Kao JP, Lederer WJ. Calcium movement in cardiac mitochondria. Biophys J. 2014;107:1289–1301. doi: 10.1016/j.bpj.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragadin M, Pozzan T, Azzone GF. Kinetics of Ca2+ carrier in rat liver mitochondria. Biochemistry. 1979;18:5972–5978. doi: 10.1021/bi00593a033. [DOI] [PubMed] [Google Scholar]
- Brandenburger Y, Kennedy ED, Python CP, Rossier MF, Vallotton MB, Wollheim CB, Capponi AM. Possible role for mitochondrial calcium in angiotensin II- and potassium-stimulated steroidogenesis in bovine adrenal glomerulosa cells. Endocrinology. 1996;137:5544–5551. doi: 10.1210/endo.137.12.8940382. [DOI] [PubMed] [Google Scholar]
- Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christel C, Lee A. Ca2+-dependent modulation of voltage-gated Ca2+ channels. Biochim Biophys Acta. 2012;1820:1243–1252. doi: 10.1016/j.bbagen.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell metabolism. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Rizzuto R. Molecular control of mitochondrial calcium uptake. Biochem Biophys Res Commun. 2014;449:373–376. doi: 10.1016/j.bbrc.2014.04.142. [DOI] [PubMed] [Google Scholar]
- Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nature communications. 2012;3:1317. doi: 10.1038/ncomms2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, Madesh M. Regulation of mitochondrial Ca2+ uniporter by MICU1 and MICU2. Biochemical and Biophysical Research Communications. 2014;449:377–383. doi: 10.1016/j.bbrc.2014.04.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, Philipson B. The mitochondrial Ca2+ uniporter complex. J Mol Cell Cardiol. 2015;78:3–8. doi: 10.1016/j.yjmcc.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Ivannikov MV, Macleod GT. Mitochondrial free Ca2+ levels and their effects on energy metabolism in Drosophila motor nerve terminals. Biophys J. 2013;104:2353–2361. doi: 10.1016/j.bpj.2013.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer KJ, Mootha VK. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 2014;15:299–307. doi: 10.1002/embr.201337946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer KJ, Sancak Y, Mootha VK. The uniporter: from newly identified parts to function. Biochem Biophys Res Commun. 2014;449:370–372. doi: 10.1016/j.bbrc.2014.04.143. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Lam SS, Martell JD, Kamer KJ, Deerinck TJ, Ellisman MH, Mootha VK, Ting AY. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat Methods. 2015;12:51–54. doi: 10.1038/nmeth.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM, Kriek M, Phadke R, Johnson CA, Roberts NY, et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet. 2014;46:188–193. doi: 10.1038/ng.2851. [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Kapus A. Measurement of the matrix free Ca2+ concentration in heart mitochondria by entrapped fura-2 and quin2. Biochem J. 1987;248:609–613. doi: 10.1042/bj2480609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim Biophys Acta. 2009;1787:1416–1424. doi: 10.1016/j.bbabio.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D, Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrungaro C, Zimmermann KM, Kuttner V, Fischer M, Dengjel J, Bogeski I, Riemer J. The Ca2+-dependent release of the Mia40-induced MICU1-MICU2 dimer from MCU regulates mitochondrial Ca2+ uptake. Cell metabolism. 2015;22:721–733. doi: 10.1016/j.cmet.2015.08.019. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Mihara K. Mammalian Oxa1 protein is useful for assessment of submitochondrial protein localization and mitochondrial membrane integrity. Anal Biochem. 2010;397:250–252. doi: 10.1016/j.ab.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Sato Y, Miyazaki S, Shikano T, Mitsuhashi N, Takeuchi H, Mikoshiba K, Kuwabara Y. Adenophostin, a potent agonist of the inositol 1,4,5-trisphosphate receptor, is useful for fertilization of mouse oocytes injected with round spermatids leading to normal offspring. Biol Reprod. 1998;58:867–873. doi: 10.1095/biolreprod58.3.867. [DOI] [PubMed] [Google Scholar]
- Vais H, Tanis JE, Muller M, Payne R, Mallilankaraman K, Foskett JK. MCUR1, CCDC90A, Is a Regulator of the Mitochondrial Calcium Uniporter. Cell metabolism. 2015;22:533–535. doi: 10.1016/j.cmet.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccarato F, Nicholls D. The role of phosphate in the regulation of the independent calcium-efflux pathway of liver mitochondria. European journal of biochemistry/FEBS. 1982;127:333–338. doi: 10.1111/j.1432-1033.1982.tb06875.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.