Abstract
Pathology laboratories group some tests that they perform on their high throughput biochemistry analysers into profiles of tests that are associated with different organs (e.g. liver function tests—LFT). The components of these profiles are historic and often vary between different laboratories. This can lead to confusion and begs the question of what should be in a particular profile. In community medicine profiles may be used as screening tests but some of the components of the profiles may have low sensitivity and specificity and may produce both false positives and negatives. The LFT may include components which are poor liver function tests but are sensitive to fatty liver and hence elevations may cause unnecessary concern. Harmonisation of clinical chemistry reference intervals and units is occurring now so it is time to consider a similar process for components of a profile. A proposed list of analytes to be performed in the LFT profile is given.
Keywords: Clinical chemistry profiles, Liver function test, Harmonisation
Introduction
Traditionally, pathology laboratories have grouped some tests that they perform on their high throughput biochemistry analysers into profiles of tests that are associated with different organs [e.g. liver function tests (LFT) and renal function tests (RFT)]. Other profiles investigate specific illnesses (e.g. Lipid dysfunction, Glucose dysfunction, cardiac abnormalities and abnormal bone remodelling). Often all these profiles are combined into a panel of about twenty tests called E/LFT. In this review the LFT will be examined but other profiles have similar deficiencies.
The contents of profiles are largely historically dictated. When a new test was developed and was found to be abnormal in a disease associated with a particular organ it was often included in the associated profile without regard to whether it provided any additional diagnostic information.
What constitutes a chemistry profile differs between laboratories in Australasia and it is likely that this variation may lead to confusion with referrers and extra expense to individual laboratories. It is also now more common for a patient to have pathology testing performed at a number of different pathology laboratories over time.
In 2012, the Association of Clinical Biochemists (UK) Clinical Practice Section published a suggested initial standardisation test content of common biochemistry profiles. They proposed in the BMJ publication that it was time to harmonise profiles in the UK [1]. Subsequently, the Harmonisation Committee of the Australasian Association of Clinical Biochemists opted to find out more about Australasian practice. In 2013 this Committee conducted a survey of some of the major laboratory networks to determine the components of their routine LFT profiles.
The sample consisted of ten public hospital and 13 private laboratory networks in Australia and New Zealand. These networks were targeted as they represent the major providers of clinical biochemistry testing in Australasia. It was known that the profile content varied between States and between public and private providers and the sample was chosen to try and capture this diversity.
This document aims to extend the initial work by Smellie and the Association for Clinical Biochemistry in the UK [1], and looks towards a definitive minimum data set which could form the basis for international discussion and consensus.
Screening
There is a difference between the way profiles are used in the hospital setting compared to community (primary care) medicine. In the hospital setting the profiles may be used for rule in/out scenarios of further treatment or discharge, diagnosis of the extent of abnormality and monitoring of the patient’s treatment. In primary practice profiles are used for screening for wellness or otherwise, diagnosis of usually less acute diseases and monitoring of different treatments, for example the use of statins in lipid management. Additionally, patients may also be referred to specialist physicians who may require different or extra tests. These different settings complicate which analytes should be included in a routine profile and how the results should be interpreted. An “ideal” profile should only include analytes that have value in predicting or diagnosing disease and should not have tests that only give the same information as other already selected analytes. The profile is a minimum necessary data set to establish abnormality to which additional tests may be added if and when required.
A major problem with screening test is that they often lack sensitivity and specificity. They produce both false positive and false negative results. When this is combined with a disease that has low prevalence in a community it leads to many false positives which are expensive to investigate and heighten patient anxiety [2]. The potential harms of over-diagnosis have also been well described [3] but often the emphasis has been on the inappropriate use of screening tests using tumour markers such as PSA or newer tests such as eGFR. However more subtle forms of screening have become part of general medical practice. A recent report by Bayram et al. [4] using the Bettering the Evaluation and Care of Health (BEACH) Study investigated the changing patterns of pathology testing by general practitioners (GPs) in Australia between 2000 and 2008. It showed a significant increase in battery tests (with four or five components) compared to single or two component tests. In addition 36.2 % of all test profiles consisted of LFTs and 37.5 % were Multiple Biochemical Analysis (E/LFT) profiles.
The Beach study only included cases where the patient was being investigated in a limited number of morbidities and required that:
-
(i)
The problem was a National Health Priority Area.
-
(ii)
Pathology ordering was common in the management of the morbidity.
-
(iii)
The pathology ordering behaviour of GPs had changed over the time period examined. These accounted for approximately 25 % of laboratory tests performed over the period.
Thus, the six investigations selected were
-
(i)
Type 2 diabetes,
-
(ii)
hypertension
-
(iii)
lipid disorders
-
(iv)
weakness/tiredness
-
(v)
‘health checks’
-
(vi)
overweight/obesity.
In the period 2006–2008 the pathology order rate per 100 encounters for any GP visit was 18.7 %. Where the patient was presenting with one of the BEACH study morbidities the pathology ordering rate was 46.4 %.
As these conditions become more prevalent in the community because of the ageing population there will be more testing due to greater doctor encounter rates. However in the BEACH data it is apparent that not only is there an increase in the number of encounters but also in the number of tests being ordered in each encounter.
The study also evaluated the tests ordered against published guidelines. Table 1 below shows the percentages of orders that agreed with the guidelines for the LFT profile. It must be noted that there are numerous guidelines for different investigations and their evaluation may be a consensus or amalgamated document. Additionally, some guidelines may not have been available at the start of the study.
Table 1.
The percentages of orders that agreed with the guidelines for the LFT profile from the Beach study
| Investigation | Recommended in guidelines | How often LFT ordered (%) | Considerations |
|---|---|---|---|
| Type 2 Diabetes | No | 7 | Yes when on glitazones and prior to Metformin |
| Hypertension | Yes | 2.9 | |
| Lipid disorders | Yes | 12.4 | Particularly when on medications |
| Weakness/tiredness | Yes | 20.5 | |
| Health checks | No | 16.2 | |
| Overweight/obesity | Yes | 8.6 |
Many Guidelines for chronic disease treatment include monitoring of patients for the development of complications commonly associated with the disease. This monitoring is effectively a form of case-based screening. Examples are screening for renal disease in diabetes and hypertension, or thyroid disease in diabetic patients. In addition many people are concerned about their health and request a ‘wellness’ screen, when pathology testing is seen to be an inexpensive way of generating a lot of information which will comfort a “worried well patient”. In the BALLETS study [5] described shortly it was shown that LFTs are often carried out in order to reassure the patient or as a “tick-box” response to the patient’s symptoms. This implies that LFTs are routinely carried out for social and psychological, rather than clinical, reasons. In the BEACH data set “health check” accounted for 3.4 % of LFT testing and 2.26 % of E/LFT pathology tests requested [4].
We can see the influence of the nonspecific screening in the data from the BALLETS study [5] Table 2.
Table 2.
Reason for requesting LFT from the BALLETS study
| Documented reason | Percentage (n) |
|---|---|
| Diabetes review | 18.0 (201) |
| Non-specific routine bloods | 15.2 (171) |
| Hypertensive disease review | 11.4 (128) |
| Gastrointestinal symptoms (excluding liver specific) | 10.0 (112) |
| Generalised fatigue or tiredness | 6.2 (69) |
| Cardiovascular disease review | 4.7 (53) |
| Medications review (non-specific) | 4.5 (50) |
| Hyperlipidaemia disease review | 3.8 (42) |
| Neurological symptoms | 2.7 (31) |
| Musculoskeletal symptoms | 2.4 (27) |
If a test is to be used as a screening test then it should be subject to the following questions:
Is the disease prevalent in the community
Is it treatable if detected early
Is the screening test sensitive, non-invasive and inexpensive
Arguably the liver diseases that we are trying to detect in primary care have changed as metabolic syndrome and the possibility of non-alcoholic fatty liver disease (NAFLD) becomes more prevalent in the community. Alcoholic liver disease (ALD) and NAFLD are associated with progression to fibrosis, cirrhosis and hepatoma and with the greater prevalence of obesity in the community these sequelae will become more critical in the future. Thus LFTs should be sensitive enough to detect not only the infectious, genetic and autoimmune diseases but also the insipient fibrosis associated with fatty liver disease.
Biochemical LFTs are certainly inexpensive and relatively non-invasive. The question is their sensitivity. In the case of the autoimmune, metabolic and viral liver diseases there are more specific tests available (serological and specific protein assay) but in the case of NAFLD, apart from a biopsy there are no simple tests.
Is there a clinical intervention available?
When confronted with an abnormal result the clinician has three possible decisions:
-
(i)
The result is a statistical outlier and the patient has no abnormality.
-
(ii)
The result is correct and the patient has a true abnormal result.
-
(iii)
The result is a laboratory error or has been affected by an artefact.
Now we will move to the specific question of the use of LFTs as a screening test and also examine the components of this test profile.
Liver Function Tests
Despite the nearly 6000 papers published on LFTs since 1990 [6] nearly all are based on hospital practice and most are retrospective and concerned with probabilities given a disease state rather than the predictive probability of disease. Until recently there were no prospective studies based in primary care practice where patients were fully investigated following at least one abnormal analyte from a full LFT profile. LFTs result in many false positive results for each true case of disease detected and are therefore not an efficient way to diagnose liver disease.
Components of the LFT
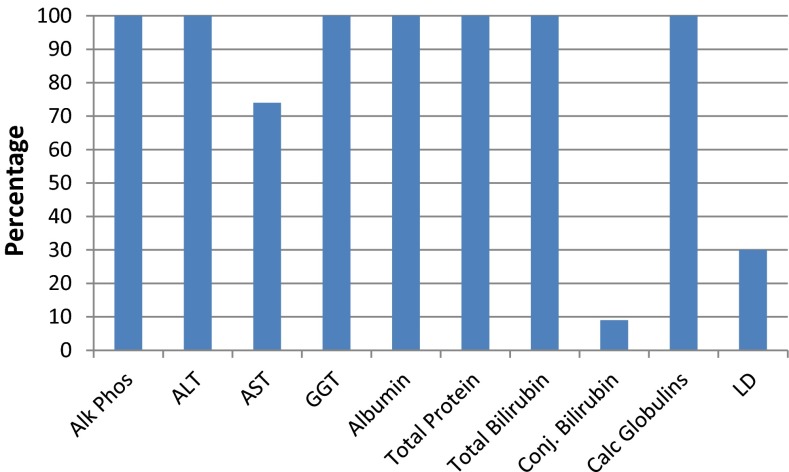
The survey asked the question “which analytes do you routinely include in the LFT?” The results appear in Fig. 1.
Fig. 1.
The components reported in the LFT from the surveyed laboratories
Transaminases
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enter the bloodstream because of hepatocellular damage or death. ALT is found in the hepatocyte cytoplasm whereas AST is found in both the cytoplasm and mitochondria of hepatocytes. AST is also found in other tissues including muscle. Most of the AST activity in serum is the cytosolic isoenzyme [7], however in alcoholism mitochondrial AST (mAST) is preferentially released [8]. AST is also cleared faster from the bloodstream than ALT. This combination of altered clearance and the different tissue sources produces the varying levels seen in different diseases.
ALT elevation is associated with all of the five components that compose the metabolic syndrome which are central (truncal) obesity, hyperglycaemia, and low levels of high-density lipoprotein cholesterol, hypertriglyceridemia, and hypertension. Therefore it is likely that most cases of elevated ALT can be attributed to being overweight (body mass index [BMI] ≥ 25 kg/m2) and obesity (BMI ≥ 30 kg/m2). Whilst both AST and ALT increase with body weight it is more prominent for ALT than AST [9].
Excess alcohol consumption is associated with fatty liver [10, 11]. It has been shown that more than three drinks per day may cause an elevated ALT and AST in both men and women [12]. ALT is not an ideal marker for either the diagnosis of NAFLD or distinguishing simple steatosis from non-alcohol steatohepatitis (NASH) [13]. This was also verified in the NHANES survey where approximately 10 % of subjects have elevations of ALT and AST [14] with fatty liver being one of the most common causes [15].
In fact, obesity is the most likely cause of a raised ALT in men [16] while alcohol was the more likely cause of an elevated ALT in women. Thus ALT is a more sensitive and specific marker of liver disease than AST.
Gamma Glutamyl Transferase (GGT) and Alkaline Phosphatase (ALP)
Hepatobiliary disease is the predominant source of increased serum GGT activity. Increases are associated with all forms of primary and secondary hepatobiliary disorders. Elevated GGT is also associated with toxic alcohol or drug intake by the patient. This elevation with xenobiotics is due to microsomal enzyme induction and is particularly so with anti-epileptic drugs. Elevated GGT activity also occurs in patients with acute and chronic pancreatitis.
ALP has isoenzymes or isoforms from other sites particularly bone, so elevations of ALP are not specific to liver disease. Thus in an adult, an isolated elevated serum ALP suggests bone related disease, but when elevated with GGT it is a marker of possible hepatic pathology.
Elevations of GGT are moderate (2–5 times reference) with diffuse hepatic cell injury due to toxic or infectious hepatitis. Cholestasis due to intrahepatic or extra hepatic biliary obstruction causes higher serum levels (5–30 times reference). Increases occur earlier and persist longer than ALP in cholestatic disorders. The majority of sustained elevated ALP levels are associated with disorders of the liver or bone, or both. Therefore, these organ systems are of prime consideration in the differential diagnosis.
A variety of primary and secondary hepatic conditions may be associated with elevated serum ALP levels. Since production is increased in response to cholestasis, serum ALP activity provides a sensitive indicator of obstructive and space-occupying lesions of the liver. The latter includes neoplastic (primary or metastatic) and infiltrative diseases (granulomatous hepatitis).
GGT is also a sensitive marker of NAFLD, as well as at risk alcohol related liver disease, but GGT elevation due to these diseases often leads to inappropriate follow up.
Total Protein
All laboratories in the survey included total protein in their LFT. This is used to calculate a globulin level to detect opportunistic case finding of paraproteinaemia. The clinical value of this procedure has been both supported [17] and dismissed [18]. In a review on this practice, Beetham et al. [19] concluded that this is a form of screening which does detect treatable B cell malignancies that were not already clinically suspected. However they also found no evidence that this detection improved the quality of life for those patients. There was also considerable variation in the reference interval used to define a raised globulin level and that there needed to be a protocol for further management of patients detected by this screening. The cost of this screening in Australia had been raised as an issue by Watts et al. [17] who concluded that the cost, in 2000, was approximately $1.2 million. They concluded that the screening did not contribute to clinical management as no new patients with myeloma were detected in their series.
Albumin
Albumin is produced only in the liver and therefore it is a marker of parenchymal damage, however levels drop only after more than 50 % of liver function is lost [20]. There is a further problem as low concentrations may in particular reflect impaired production or either renal or gastrointestinal losses.
Total Bilirubin
Bilirubin, the breakdown product of haemoglobin, is a marker of intra and extra-vascular haemolysis and liver disease. Gilbert’s disease, a benign form of hyperbilirubinemia, has a prevalence of approximately 10 % in Caucasians. Bilirubin excretion is compromised only with extensive biliary obstruction or diffuse hepatic cell disruption; therefore, differential elevation of ALP relative to serum bilirubin provides an early indicator for obstructive or space-occupying conditions. Hepatic cell lesions are manifested by hyperbilirubinemia and dominant serum elevation of parenchymal enzymes, such as aminotransferases.
Specificity and Sensitivity of the LFT
Recently the diagnostic value of the traditional battery of chemistry LFTs has been informed by the publication of two landmark studies, BALLETS [5] and ALFIE [21]. The Birmingham and Lambeth Liver Evaluation Testing Strategies [5, 22] was a prospective cohort study of 1290 patients with abnormal LFTs in primary care who were subsequently fully characterised by clinical history, extensive blood testing and ultrasound of their abdomens. They were also followed up 2 years after the initial findings. Statistical tests were used to identify the interactions between clinical features, the initial pattern of abnormal LFTs and the following categories:
Specific viral, genetic and autoimmune diseases of the liver, such as viral hepatitis, haemochromatosis and primary biliary cirrhosis
A range of other serious diseases affecting the liver, such as metastatic cancer and hypothyroidism
‘Fatty liver’ not associated with the above
No disease detected.
The abnormal liver function investigations evaluation (ALFIE) [21, 23] followed up all those who had had an incident batch of LFTs in primary care to subsequent liver disease or mortality over a maximum period of 15 years (approximately 2.3 million tests in 95,000 people). The study was set in primary care in the region of Tayside, Scotland (population approximately 429,000) between 1989 and 2003. The target population consisted of patients with no obvious signs of liver disease and registered with a GP. The health technologies being assessed are primary care LFTs [transaminases, GGT, albumin, ALP, bilirubin below level of jaundice], viral and autoantibody tests, ultrasound and liver biopsy.
The study utilised the epidemiology of liver disease in Tayside (ELDIT) database to determine the outcomes of liver disease. The database links hospital admission data [Scottish Morbidity Record (SMR1)], dispensed medication records, death certificates, biochemistry, virology, immunology and examination of medical records from Tayside hospitals, and diagnosis is obtained by means of diagnostic algorithms.
The data from the ALFIE trials can be used to identify which components of the common LFTs may have value as a screening test Table 3.
Table 3.
Patterns of abnormality in the index LFT
| Analyte | % Abnormal | ALT | AST | Bilirubin | ALP | GGT | Albumin | Globulin | Total Protein |
|---|---|---|---|---|---|---|---|---|---|
| 39.3 | 22.0 | 11.7 | 14.9 | 75.3 | 2.3 | 5.6 | 9.9 | ||
| ALT | 39.3 | 1.00 | 0.88 | 0.22 | 0.30 | 0.37 | 0.23 | 0.18 | 0.31 |
| AST | 22.0 | 0.44 | 1.00 | 0.15 | 0.22 | 0.18 | 0.08 | 0.11 | 0.15 |
| Bilirubin | 11.7 | 0.06 | 0.06 | 1.00 | 0.04 | 0.05 | 0.17 | 0.06 | 0.05 |
| ALP | 14.9 | 0.09 | 0.15 | 0.05 | 1.00 | 0.10 | 0.13 | 0.13 | 0.08 |
| GGT | 75.3 | 0.71 | 0.72 | 0.33 | 0.64 | 1.00 | 0.48 | 0.49 | 0.54 |
| Albumin | 2.3 | 0.01 | 0.01 | 0.03 | 0.02 | 0.01 | 1.00 | 0.07 | 0.08 |
| Globulin | 5.6 | 0.02 | 0.03 | 0.04 | 0.06 | 0.03 | 0.24 | 1.00 | 0.37 |
| Total Protein | 9.9 | 0.08 | 0.08 | 0.06 | 0.07 | 0.07 | 0.47 | 0.65 | 1.00 |
The entries are the proportions of patients with abnormality in the row analyte given that the column analyte is abnormal. For example, the proportion of abnormal ALTs among patients whose AST is abnormal is 0.88; the proportion of abnormal ASTs among those with abnormal ALTs is 0.44 [21]
What is the sensitivity and specificity of this profile in determining liver disease? Table 4
Table 4.
Sensitivity and specificity of the components of the LFT in determining liver mortality and morbidity from the ALFIE study
| LFT | Performance measure | All deaths | Liver death | Liver disease | |||
|---|---|---|---|---|---|---|---|
| 1 year | 5 years | 1 year | 5 years | 1 year | 5 years | ||
| ALP | Sensitivity (%) | 17.4 | 11.2 | 48.8 | 37.6 | 42.5 | 32.1 |
| Specificity (%) | 89.8 | 89.7 | 89.6 | 89.6 | 89.7 | 89.8 | |
| PPV (%) | 4.9 | 9.7 | 0.2 | 0.5 | 1.5 | 2.5 | |
| NPV (%) | 97.3 | 91.0 | 99.9 | 99.9 | 99.8 | 99.4 | |
| Albumin | Sensitivity (%) | 20.4 | 11.4 | 26.8 | 19.2 | 15.0 | 6.6 |
| Specificity (%) | 99.8 | 98.9 | 98.0 | 98.0 | 98.0 | 98.0 | |
| PPV (%) | 29.4 | 50.8 | 0.6 | 1.2 | 2.6 | 3.5 | |
| NPV (%) | 98.0 | 91.8 | 99.9 | 99.9 | 99.7 | 99.0 | |
| Transaminase | Sensitivity (%) | 11.5 | 9.5 | 37.5 | 40,2 | 42.1 | 35.8 |
| Specificity (%) | 93.2 | 93.3 | 93.1 | 93.1 | 93.2 | 93.3 | |
| PPV (%) | 4.5 | 11.0 | 0.2 | 0.7 | 2.2 | 3.9 | |
| NPV (%) | 97.3 | 92.0 | 99.9 | 99.9 | 99.8 | 99.5 | |
| GGT | Sensitivity (%) | 37.1 | 27.4 | 76.9 | 71.4 | 72.4 | 61.9 |
| Specificity (%) | 85.3 | 85.7 | 84.6 | 84.7 | 85.1 | 85.4 | |
| PPV (%) | 7.4 | 15.2 | 0.4 | 1.0 | 3.1 | 5.2 | |
| NPV (%) | 97.5 | 91.2 | 99.9 | 99.9 | 99.8 | 99.4 | |
| Bilirubin | Sensitivity (%) | 10.5 | 8.5 | 35.9 | 24.8 | 16.8 | 14.9 |
| Specificity (%) | 93.0 | 93.1 | 92.9 | 92.9 | 92.9 | 93.0 | |
| PPV (%) | 4.3 | 11.0 | 0.2 | 0.5 | 0.9 | 1.7 | |
| NPV (%) | 97.2 | 91.0 | 99.9 | 99.9 | 99.7 | 99.2 | |
In the BALLETS cohort the following causes of abnormal LFTs were determined [5] Table 5.
Table 5.
Prevalence of abnormal LFTs in different conditions identified in the BALLETS study
| Cause | Percentage prevalence (%) |
|---|---|
| NAFLD | 26.4 |
| At-risk alcohol intake | 25.3 |
| Non-fatty liver | 14.0 |
| Fatty liver | 11.3 |
| Primary biliary cirrhosis | 0.81 |
| Hepatitis B | 0.72 |
| Haemochromatosis | 0.90 |
| Other (cancer, drug, abscess) | 0.36 |
| Hepatitis C | 0.17 |
| Primary sclerosing cholangitis | 0.17 |
| Alpha-1-antitrypsin Deficiency | 0.17 |
| Unexplained group | 45.1 |
* ALFIE study not all patients received both an ALT and an AST. It should also be noted that, as the BALLETS study chose patients with an abnormal LFT from the native population, this selection bias will overestimate the sensitivity and underestimate the specificity
Determining the sensitivity of the conventional LFT is more difficult however there have been some recent studies which can inform. We need to be specific about what we are trying to detect in an LFT screening test. It has long been recognised that ALT elevation is poorly correlated with the severity of histologic liver disease in chronic liver disease [24, 25]. Mofrad et al. [26] reported on a cohort of patients with normal ALT levels who were diagnosed as having NAFLD histologically and identified clinically (persistent hepatomegaly) or because the subjects were live liver transplant donors. There was no relation in this group between fibrosis stage (histologic) and ALT level. In this cohort of normal ALTs (15 of 51 had ALTs < 30) three had cirrhosis and 40 % had advanced fibrosis. Sorrentino et al. [27] investigated silent NAFLD in patients with metabolic syndrome and normal LFTs. In this group these authors found histologically that 58/80 patients had varying degrees of NAFLD with 26 having fibrosis and eight with silent cirrhosis. In this study 97.5 % of obese patients with metabolic syndrome had normal LFTs and silent NAFLD. In another study 79 % of patients with steatosis had a normal ALT [28].
Closely linked to the issue of the value of LFTs is the question of appropriate reference intervals (RI) for the components such as ALT, AST and GGT. Reference intervals for LFTs, like most analytes, are often historical and have been derived from populations that include a high prevalence of obesity which now occurs in many western populations. Thus the true RI may be lower than those derived from populations that include obese people. Excluding alcoholism or chronic liver disease does not exclude covert fatty liver. Elevating the RI for the transaminases to reduce the likelihood of false positives due to non-alcoholic steatohepatitis (NASH) reduces the sensitivity of the LFT to detect these organic liver diseases.
Primary Care LFT Profile
Thus given the high false-positive rate of LFTs and the fact that an abnormal result does not signal any particular disease, we recommend a more selective approach to this particular ‘screening test’. We need to consider a strategy where a screening LFT is performed followed by a more elaborate set of diagnostic tests when the LFT is detected as abnormal. This additional testing could be reflex testing which is automatically performed on the original sample before a third level of more specific testing is undertaken.
Summarising the major implications from the BALLETS study we find that:
Liver function tests should be used sparingly in primary care.
When a chronic disease affecting the liver is suspected, a panel of two analytes (ALT and ALP) should be used, supplemented by bilirubin if an acute disease or poisoning is suspected.
When the clinician wishes to exclude a non-liver disease or simply reassure the patient, a selection should be made from a ‘dropdown’ menu of tests, and tests that provide a clear pointer to the next appropriate step should be favoured.
However the BALLETS study largely considered ALD and NAFLD as unimportant, and optimised their LFT strategy to detect other infectious, genetic and autoimmune liver disorders. Recently reporting on work commissioned by the Association for Clinical Biochemistry, Smellie [1] has suggested a four component LFT (total bilirubin, albumin, ALT and ALP). This profile supports the BALLETS ‘’definition’’ of liver disease and would ignore the potentially significant consequences of ALD and NAFLD.
GGT was the most sensitive test to detect NAFLD in the BALLETS study [9] where 26 % of all abnormal LFT’s were attributed to NAFLD. The ALFIE study also demonstrated that GGT was the most sensitive component of the LFT profile. GGT is also the most sensitive test in ALD but it also has a high false-positive rate.
LFTs are often undertaken to meet perceived patient need for a blood test, but as they are neither specific nor indicative of any particular disease they are among the least suitable tests for this purpose. Obesity and raised ALT provide strong evidence for a presumptive diagnosis of ‘fatty’ liver. Abnormal LFTs and ‘fatty’ liver provoke only short-term anxiety in a patient and neither is associated with sustained weight loss. Even a small amount of weight loss reduces liver fat. As stated earlier liver function tests are often carried out for social and psychological, rather than clinical, reasons. There is no good evidence that single abnormal LFTs or ultrasound findings promote healthy behaviour [7]. McLernon et al. [23] found that LFTs did not improve the Negative Predictive Factor (NPV) for short term mortality and only modestly improved a very low Positive Predictive Factor (PPV) for estimating the all-cause mortality in patients with no apparent liver disease but with an abnormal LFT.
What Should be the Components of a Routine LFT in Community Practice?
There is much debate about the value of pathology tests generally and inappropriate testing specifically [29]. Many profiles in primary care in Australian laboratories are used for screening purposes not necessarily targeted only at the organ for which the profile exists. Biochemical LFTs have poor sensitivity and specificity for true liver disease. They are therefore difficult to interpret by a non-specialist who may ignore a result which is lost in the noise or over investigate a non-significant result. Adding more non-specific and non-sensitive components to the profile does not help and further degrades the value of the test. The AST level no doubt can be of use both to detect non liver disease such as muscle disease and also to sub-classify liver disease by the calculation of the Di Ritis factor [30], but these applications are understood only by a small group of experts. No laboratories routinely report the Di Ritis factor so it is left to the astute clinician to calculate and interpret this parameter.
We argue therefore that harmonisation of profiles is an initial stage that may be combined with a move to diagnosis or condition-based testing, although the need for some organ profiles is likely to remain. It has also been shown that much information as is useful can be obtained from a smaller number of components in these common profiles [5]. The additional tests which are included do not necessarily add any clinically useful information and may in fact lead to over investigation.
In cases where there is a high degree of suspicion of a particular disease the option to include tests not contained in a profile should always be available to the referrer and this is also most likely when specialists are ordering pathology tests.
If the primary purpose of ordering an LFT is to identify covert underlying liver disease then a profile with a high sensitivity is required. Both the BALLETS and ALFIE studies found that GGT was the test most often elevated in all forms of liver disease. The BALLETS study advocated [31] the use of just ALP and ALT as these were the most specific for the categories they were seeking, the specific viral, genetic and autoimmune liver diseases. But this combination would not detect many of the ALD and NAFLD cases which themselves can lead to fibrosis and cirrhosis.
Why Change from the Current LFTs?
The argument is often made that the marginal cost of doing these tests is low and an additional test such as an AST may only cost a few cents. But this ignores two issues, the true cost of testing and the impact of using screening tests with poor sensitivity and specificity. The true cost of screening large numbers of the population requires building a significant capacity in terms of analysers, space, and staff to perform these assays. It is difficult to estimate just how many LFTs are performed each day in Australasia but it is perhaps in hundreds of thousands. The number of these that are misleading may be half as we have seen so the cost of the additional investigations, follow-up and consequent patient anxiety needs to be taken into consideration and not just the marginal cost. An investigation into pathology usage [4] recommended that the clinical indications for ordering Full Blood Counts, thyroid function tests (TFTs), E/LFT and LFTs in the long-term monitoring of chronic conditions needs clarification. Further research or review of literature to determine the pre-test probability of underlying disease may be useful in developing guidance on the use of these tests.
We advocate a review of current profile components and a change to the way these are used in primary care. This will lead to a reduced cost to the community in terms of the actual cost of the tests and the impact of unnecessary follow on testing. How this is achieved needs dialogue with users of the tests as a single radical move to the minimum data set as suggested in this document could prove culturally difficult for physicians who have become reliant on a particular set or sets of results in a profile, although conversely a single radical change would avoid several incremental ones. This is perhaps a topic for debate if agreement can be reached on the principle of harmonising profiles, and on the definition of what the ultimate profiles should contain. Whilst the drivers and restricting factors may differ between different international health economies the physiological and pathological factors remain the same and improving the cost-effectiveness of laboratory testing is an ambition shared by all of these health economies.
It is likely that chronic liver disease will become a significant burden on the health system particularly NAFLD and ALD as these lifestyle diseases contribute to the number of people with underlying fatty liver disease. The current LFT profiles are not sensitive to these diseases and in fact clinical examination for hepatomegaly and obesity and a measurement of triglycerides may be just as effective in detecting liver disease. There needs to be a more sensitive cost effective marker of liver fibrosis used which is applied instead of the current LFTs. There are some promising markers [13] but their current cost is prohibitive and without an effective screening test to identify possible candidates to use these tests on there is a big gap in our current armoury.
Recommendation
Summarising the above we recommend that until better markers are available the current LFT profile should consist of those analytes proposed by Smellie1 but with addition of GGT. Thus the recommended profile is:
Total Bilirubin
ALT
ALP
Albumin
GGT
If the GGT alone is abnormal the physician should perform a physical examination and request a clinical history including all drugs and complementary therapeutics taken and the patient’s true alcohol intake.
References
- 1.Smellie WSA. Time to harmonise common laboratory test profiles. BMJ. 2012;344:e1169. doi: 10.1136/bmj.e1169. [DOI] [PubMed] [Google Scholar]
- 2.Woolf SH, Harris R. The harms of screening: new attention to an old concern. JAMA. 2012;307(6):565–566. doi: 10.1001/jama.2012.100. [DOI] [PubMed] [Google Scholar]
- 3.Moynihan R, Doust J, Henry D. Preventing over diagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi: 10.1136/bmj.e3502. [DOI] [PubMed] [Google Scholar]
- 4.Bayram C, Britt H, Miller G, Valenti L. Evidence-practice gap in GP pathology test ordering: a comparison of BEACH pathology data and recommended testing. Sydney: The University of Sydney; 2009. pp. 1–188. [Google Scholar]
- 5.Lilford RJ, Bentham L, Girling A, Litchfield I, Lancashire R, Armstrong D, et al. Birmingham and Lambeth liver evaluation testing strategies (BALLETS): a prospective cohort study. Health Technol Assess. 2013;17:1–307. doi: 10.3310/hta17280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367–1384. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- 7.Rej R. Aspartate aminotransferase activity and isoenzyme proportion in human liver tissues. Clin Chem. 1978;24:1971–1979. [PubMed] [Google Scholar]
- 8.Ishii H, Okuno F, Shigeta Y, Tsuchiya M. Enhanced serum glutamic oxaloacetic transaminase activity of mitochondrial origin in chronic alcoholics. Curr Alcohol. 1979;5:101–108. [PubMed] [Google Scholar]
- 9.Siest G, Soicsheiele F, Galteau MM, Panek E, Steinmetz J, Fagnani F, et al. Aspartate aminotransferase and alanine aminotransferase activities in plasma: statistical distributions, individual variations, and reference values. Clin Chem. 1975;21:1077–1087. [PubMed] [Google Scholar]
- 10.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 11.Jackson R, Beaglehole R. Alcohol consumption guidelines: relative safety vs absolute risks and benefits. Lancet. 1995;346:716. doi: 10.1016/S0140-6736(95)91496-X. [DOI] [PubMed] [Google Scholar]
- 12.Loo R, Bettencourt R, Barrett-Connor E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment Pharmacol Ther. 2009;30:1137–1149. doi: 10.1111/j.1365-2036.2009.04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce SG, Thosani NC, Pan J. Noninvasive biomarkers for the diagnosis of steatohepatitis and advanced fibrosis in NAFLD. Biomark Res. 2013;1:1–7. doi: 10.1186/2050-7771-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 15.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH, Ha MH, Christiani DC. Body weight, alcohol consumption and liver enzyme activity—a 4 year follow-up study. Int J Epidemiol. 2001;30:766–770. doi: 10.1093/ije/30.4.766. [DOI] [PubMed] [Google Scholar]
- 17.Watts B, Burnett L, Chester D. Measurement of total protein is not a useful inclusion in liver function test profiles. Clin Chem. 2001;46:1022–1023. [PubMed] [Google Scholar]
- 18.Hayden K, van Heyningen C. Measurement of total protein is a useful inclusion in liver function test profiles. Clin Chem. 2001;47:793–794. [PubMed] [Google Scholar]
- 19.Beetham R, Howie N, Soutar R. Can opportunistic case-finding of paraproteins be clinically justified? Ann Clin Biochem. 2005;42:245–253. doi: 10.1258/0004563054255515. [DOI] [PubMed] [Google Scholar]
- 20.Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry and molecular diagnostics. 5. St Louis: Elsevier; 2012. [Google Scholar]
- 21.Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al. Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE) Health Technol Assess. 2009;13:1–156. doi: 10.3310/hta13250. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56:234–240. doi: 10.1016/j.jhep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 23.McLernon DJ, Dillon JF, Sullivan FM, Roderick P, Rosenberg WM, Ryder SD, et al. The utility of liver function tests for mortality prediction within one year in primary care using the algorithm for liver function investigations (ALFI) PLoS One. 2012;7(12):e50965. doi: 10.1371/journal.pone.0050965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mardini H, Record C. Detection assessment and monitoring of hepatic fibrosis: biochemistry or biopsy? Ann Clin Biochem. 2005;42:441–447. doi: 10.1258/000456305774538210. [DOI] [PubMed] [Google Scholar]
- 25.Kallai L, Hahn A, Roder V, Zupanic V. Correlation between histological findings and serum transaminase values in chronic diseases of the liver. Acta Med Scand. 1964;175:49–56. doi: 10.1111/j.0954-6820.1964.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 26.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of non-alcoholic liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 27.Sorrentino P, Tarantino G, Conca P, Perrella A, Terracciano ML, Vecchione R, et al. Silent nonalcoholic fatty liver disease—a clinical-histological study. J Hepatology. 2004;41:751–757. doi: 10.1016/j.jhep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the united states: impact of ethnicity. Hepathology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 29.Isouard G. Quality of pathology service: new strategic directions required. IJHCQA. 2013;26(6):510–521. doi: 10.1108/IJHCQA-10-2011-0058. [DOI] [PubMed] [Google Scholar]
- 30.Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117–130. [PMC free article] [PubMed] [Google Scholar]
- 31.Lilford RJ, Bentham LM, Armstrong ML, Neuberger J, Girling AJ. What is the best strategy for investigating abnormal liver function tests in primary care? Implications from a prospective study. BMJ Open. 2013;3:300–309. doi: 10.1136/bmjopen-2013-003099. [DOI] [PMC free article] [PubMed] [Google Scholar]