Abstract
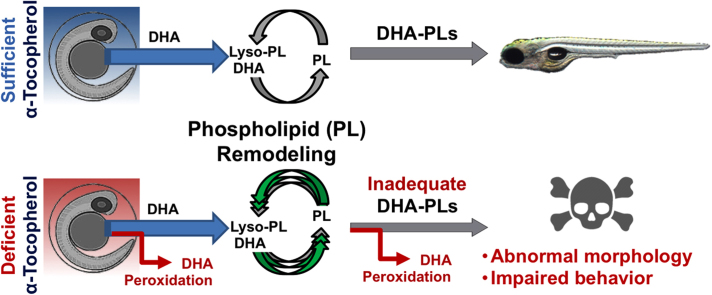
We hypothesized that vitamin E (α-tocopherol) is required by the developing embryonic brain to prevent depletion of highly polyunsaturated fatty acids, especially docosahexaenoic acid (DHA, 22:6), the loss of which we predicted would underlie abnormal morphological and behavioral outcomes. Therefore, we fed adult 5D zebrafish (Danio rerio) defined diets without (E−) or with added α-tocopherol (E+, 500 mg RRR-α-tocopheryl acetate/kg diet) for a minimum of 80 days, and then spawned them to obtain E− and E+ embryos. The E− compared with E+ embryos were 82% less responsive (p<0.01) to a light/dark stimulus at 96 h post-fertilization (hpf), demonstrating impaired locomotor behavior, even in the absence of gross morphological defects. Evaluation of phospholipid (PL) and lysophospholipid (lyso-PL) composition using untargeted lipidomics in E− compared with E+ embryos at 24, 48, 72, and 120 hpf showed that four PLs and three lyso-PLs containing docosahexaenoic acid (DHA), including lysophosphatidylcholine (LPC 22:6, required for transport of DHA into the brain, p<0.001), were at lower concentrations in E− at all time-points. Additionally, H218O labeling experiments revealed enhanced turnover of LPC 22:6 (p<0.001) and three other DHA-containing PLs in the E− compared with the E+ embryos, suggesting that increased membrane remodeling is a result of PL depletion. Together, these data indicate that α-tocopherol deficiency in the zebrafish embryo causes the specific depletion and increased turnover of DHA-containing PL and lyso-PLs, which may compromise DHA delivery to the brain and thereby contribute to the functional impairments observed in E− embryos.
Abbreviations: ARA, arachidonic acid; dpf, days post-fertilization; DHA, docosahexaenoic acid; E−, vitamin E (α-tocopherol)-deficient; E+, vitamin E (α-tocopherol)-sufficient; ECD, electrochemical detection; EM, embryo media; hpf, hours post-fertilization; TOF-MS, hybrid quadrupole time-of-flight mass spectrometry; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; LPS, lysophosphatidylserine; lyso-PL, lysophospholipid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PL, phospholipid; PLA2, phospholipase A2; PS, phosphatidylserine; PUFA, polyunsaturated fatty acid; UPLC, ultra-performance liquid chromatography; ZAAP, zebrafish analysis and acquisition program
Keywords: Brain, Development, Docosahexaenoic acid, H218O, Peroxidation, Vitamin E, Mass spectrometry, Phospholipids
Graphical abstract
Highlights
-
•
α-Tocopherol deficient (E-) embryos are abnormal and have impaired locomotor responses.
-
•
DHA-containing phospholipids and lysophospholipids are depleted in E− embryos.
-
•
E- embryos have increased turnover of DHA-containing phospholipids and lysophospholipids.
-
•
DHA delivery to tissues is compromised, contributing to the functional impairments in E- embryos.
1. Introduction
The zebrafish model presents a highly useful and translational experimental system for investigating the impact of α-tocopherol status on neurodevelopment, as zebrafish and humans have similar dietary antioxidant requirements [1]. α-Tocopherol’s requirement during development may be due primarily to its fundamental role in facilitating healthy brain formation because knockdown of the α-tocopherol transfer protein in zebrafish embryos causes lethal head and/or brain malformations [2]. Further, we have observed that α-tocopherol deficient (E−) zebrafish embryos, when compared to α-tocopherol sufficient (E+) embryos, begin to develop morphologic abnormalities at 48 hours post-fertilization (hpf) [3], the severity of which increase from 48–72 hpf with simultaneous increases in E- embryo mortality.
We propose that that the physiological link between brain health and adequate α-tocopherol relates to the vitamin’s role as the body’s most potent lipophilic antioxidant [4], whereby it protects highly unsaturated membrane lipids that are vulnerable to peroxidation [5]. Foremost among these in terms of susceptibility to peroxidation is docosahexaenoic acid (DHA; 22:6) [6], a long-chain ω-3 poly-unsaturated fatty acid (PUFA) that is highly concentrated in nervous tissue [7] and is critical for neurodevelopment [8], [9], as well as for preservation of lifetime neurological function [10], [11]. Given that the developing brain consumes 60% of fetal oxygen [12], this environment makes adequate antioxidant status especially crucial for protection of vital membrane lipids like DHA.
We recently demonstrated that α-tocopherol deficiency significantly altered the PL composition of the brain in adult E− zebrafish by causing the specific depletion of PL species containing DHA [13]. Intriguingly, we also found that α-tocopherol deficiency disrupts brain lysophospholipid (lyso-PL) status, resulting in an overall depletion of lipid species by approximately 60% [13]. Lyso-PLs are substrates for the synthesis, remodeling, and repair of membrane PLs [14], [15]; thus, the results from our adult studies suggest these processes are compromised in the E− brain. One depleted lyso-PL species in particular, lysophosphatidylcholine (LPC) 22:6, also is essential for normal DHA delivery to the brain [16], [17], where evidence shows that DHA is required for function and maintenance of neural membranes, such as the blood-brain barrier [18], [19]. In addition, inadequate brain DHA has been found to compromise membrane integrity within the developing zebrafish [20], leading to brain deformities and early mortality.
In light of our lipidomics data from adult E− zebrafish brains [13] as well as our past studies showing morphological defects that coincide with the rapid depletion of DHA during development in E− embryos [3], [21], we hypothesized that embryonic α-tocopherol deficiency compromises brain development by decreasing the supply of DHA to the embryonic brain, which perturbs DHA-containing PL and lyso-PL (specifically, LPC 22:6) status and, hence, processes of membrane PL remodeling. Further, given the numerous reports of adverse behavioral outcomes due to embryonic DHA deficiency, we also postulated that the disruption of membrane DHA content may correlate with the onset of behavioral abnormalities in the developing embryonic zebrafish. Herein, we exploited our high-throughput embryonic assessments and lipidomics techniques, as well as developed a method to measure PL turnover in zebrafish embryos using H218O labeling, to gain mechanistic insight on the organism-level effects of developmental α-tocopherol deficiency.
2. Materials and methods
2.1. Materials
1,2-ditridecanoyl-sn-glycero-3-phosphocholine (DT-PC) was obtained from Avanti Polar Lipids Inc. (Alabaster, AL) and used without further purification. H218O (18O, 97%) was obtained from Cambridge Isotope Laboratories (Tewksbury, MA). Butylated hydroxytoluene (BHT) was obtained from TCI America (Portland, OR).
2.2. Zebrafish husbandry and diets
The Institutional Animal Care and Use Committee (IACUC) of Oregon State University approved this protocol (ACUP Number: 4344). Tropical 5D strain zebrafish were housed in the Sinnhuber Aquatic Research Laboratory. Adults were kept at standard laboratory conditions of 28 °C on a 14-h light/10-h dark photoperiod in fish water (FW) consisting of reverse osmosis water supplemented with a commercially available salt (Instant Ocean®) to create a salinity of 600 microsiemens. Sodium bicarbonate was added as needed to adjust the pH to 7.4.
At 55 days post-fertilization (dpf), zebrafish were randomly allocated to one of two diet groups, α-tocopherol deficient (E−) or α-tocopherol sufficient (E+), and fed one of the defined diets for the duration of the study [22]. The defined diets, which contained only fatty acids with 18 or fewer carbons and 2 or 3 double bonds [3], [22], were prepared with the vitamin C source as StayC (500 mg/kg, Argent Chemical Laboratories Inc., Redmond, WA) and without (E−) or with added α-tocopherol (E+, 500 mg RRR-α-tocopheryl acetate/kg diet, ADM, Decatur, IL), as described previously [21], [22]. Diets were stored at −20 °C until fed to the adult zebrafish.
E− and E+ embryos were obtained from adult fish fed either the E− or E+ diet, respectively, for a minimum of 80 days. Embryos were obtained through natural group spawning, collected, and kept in standard embryo media (EM; prepared as described [1] from 0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4 and 4.2 mM NaHCO3; pH 7.2–7.4). Embryos used for biochemical analysis, described below, were euthanized by cold exposure (placed on ice of a minimum of 30 min) prior to sampling. Note that embryos are not fed prior to 120 hours post fertilization (hpf), as each is a complete unit containing only those nutrients present in the yolk when eggs are laid, which the embryo fully utilizes by ~120 hpf. For the experiments described herein, the E+ embryos are considered the control condition.
2.3. Vitamin E analyses
Using high pressure liquid chromatography with electrochemical detection (HPLC-ECD), α-tocopherol was measured both in diet samples and embryos, as described [23]; ascorbic acid content in diet was measured using HPLC-ECD as described [24]. Measured α-tocopherol concentrations in the E− and E+ diets were 0.45±0.01 and 369±2 mg/kg (n=3 replicate samples measured for each diet), respectively; vitamin C was 143±16 mg ascorbic acid/kg. This level of dietary vitamin C has been found to be adequate for the zebrafish [25], [26]. Measured α-tocopherol concentrations in the E− and E+ embryos at 24 hpf were 3.4±0.1 and 105±3 pmol/embryo, respectively, similar to previous reports [3], [21].
2.4. Evaluation of phenotypic and developmental progress
At 24 hpf, embryos were assessed for viability, developmental progression and spontaneous movements (earliest behavior in zebrafish), using the zebrafish acquisition and analysis program (ZAAP). ZAAP is a custom program designed to inventory, acquire, and manage zebrafish data, and was used to collect 18 developmental endpoints, as either present or absent (i.e. binary responses were recorded, described below) [27]. Developmental progression is considered perturbed if zebrafish are more than 12 h delayed compared to control animals. Spontaneous movements are assessed over a 2 min. period and are considered perturbed if there is a lack of embryonic contractions and/or movement. At 96 hpf, larval morphology (body axis, eye, snout, jaw, otic vesicle, notochord, heart, brain, somite, fin, yolk sac, trunk, circulation, pigment, and swim bladder) was evaluated and recorded and behavioral endpoints (motility, tactile response) were thoroughly evaluated in vivo. If the embryo was dead at either 24 or by 96 hpf, the non-mortality endpoints were not included in the evaluations.
2.5. Behavioral assessments
Locomotor activity was measured using Viewpoint Zebrabox [28], [29] in a total of n=128 embryos per diet group. Briefly, at 96 hpf, the plates containing the embryos were placed in a Viewpoint ZebraBox (software version 3.0, Viewpoint Life Sciences, Lyon, France). Embryo locomotor activity was assessed using the “tracking” setting during alternating periods of light and dark, a modification of Ref. [30]. Embryos subjected to this test typically move less during the light periods and more during dark periods, and behavioral differences can be determined by comparing distances moved during the light and/or dark periods. Locomotor activity in response to the light/dark transition was tracked during 3 min. periods of alternating light and dark for a total of 24 min. The integration time was set to 6 s to increase statistical power. A high definition camera (30 frames/s) tracked the total movement (swim distance) in response to the multiple light-dark transitions.
2.6. Zebrafish embryo lipidomics analyses
At 12 hpf, E− and E+ embryos were transferred one embryo per well into 96 well plates containing 100 μL EM per well. Following 24, 48, 72, and 120 hpf, embryos (15 per replicate, n=4 replicates per group) were transferred to 1.5 mL Eppendorf tubes, covered with EM, and kept on ice for 30 min to euthanize the animals. EM was carefully removed to prevent loss of embryos and samples were stored at −80 °C overnight. To extract embryos for lipidomics analyses, solvent (300 μL, 25:10:65 v/v/v methylene chloride: isopropanol: methanol, with 50 μg/mL butylated hydroxytoluene [BHT]) and internal standard (0.5 µg/µL, 1,2-ditridecanoyl-sn-glycero-3-phosphocholine [DT-PC, PC 26:0] in methanol) was added. Next, sample extracts were homogenized with 0.5 mm zirconium oxide beads (Next Advance, Inc., Averill Park, NY, product #ZrOB05) using a counter-top bullet blender for 6 min.; then, following a 15 min. incubation on ice, the extracts were centrifuged at 4 oC at 15,000×g for 13 min. Aliquots (200 μL) of the upper layer were transferred individually to new tubes and stored at −80 °C until analysis.
2.7. H218O incorporation by embryos
To optimize H218O labeling into lipids, a cohort of laboratory embryos (n=180; from adult 5D zebrafish not subjected to dietary manipulation) were incubated with increasing concentrations of H218O (0% to 50% v/v in EM) from 48 to 72 hpf (n=15 embryos per replicate, 2 replicates per concentration) to determine the H218O concentration that yielded the greatest label incorporation. Incubation for 24 h (48–72 hpf) with a 40% v/v concentration of H218O provided optimal labeling of PL species (Supplementary Data). Embryos showed no signs of toxicity when assessed using a phenotypic screen (ZAAP), described above, following exposure to any concentration of the H218O label used in the present experiment. The outcome of this pilot trial informed the H218O labeling study with E− and E+ embryos, in which a cohort of 48 hpf E− and E+ embryos (n=120/group) was incubated with either 40% v/v H218O in EM (n=60/group) or with 40% v/v reverse-osmosis water (H2O) in EM (n=60/group) from 48 to 72 hpf. At 72 hpf, embryos used for the labeling study were extracted as described above for lipidomics sample preparation.
2.8. UPLC-TOF-MS/MS analyses
Lipidomics analyses of the embryo extracts were carried out to identify lipids, which changed as a result of the dietary manipulation. Liquid chromatography/mass spectrometry (LC/MS) was performed using a UPLC with a 1.8 µm particle 100×2.1 mm2 id HSS T3 column (Waters, Milford, MA) coupled to a hybrid quadrupole time-of-flight mass spectrometer (UPLC-TOF-MS/MS; SCIEX 5600) operated in information dependent MS/MS acquisition mode, as described [13]. Data were generated from the UPLC separation of each lipid extract using TOF accurate mass detection and MS/MS fragment characterization. Data was imported into PeakView software (Version 1.2, SCIEX) for relative quantification and lipid identification. Lipid species were confirmed by validated UPLC retention times, high resolution MS, MS/MS fragmentation, and isotopic distribution using the PeakView database. Peak intensities were normalized using DT-PC (internal standard) intensities and then used for relative quantification between E+ and E− embryo samples and expressed per embryo based on the numbers of embryos extracted.
For assessment of H218O labeling, extracts were analyzed using the UPLC-TOF-MS/MS by following the lipidomics protocol. The incorporation of the H218O label was determined by comparing [M+H]+ and [M+2+H]+ mass spectral peak intensities from isotopic distributions of individual lipid species. Mass spectral peak identities were confirmed as described above, using validated UPLC retention times, high resolution MS, and MS/MS fragmentation. The ratio of [M+2+H]+/[M+H]+ was used as a metric to measure the extent of H218O label incorporation (minus the ratio found for unlabeled-H2O incubated embryos).
2.9. Statistical analyses
Heatmaps of lipidomics data were generated using Metaboanalyst software [31]. Sample intensities were normalized against the intensity of internal standard (DT-PC) and then scaled with Pareto scaling. All lipidomics statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad, La Jolla, CA). Multiple, unpaired student's t-tests with Sidak-Bonferroni post-test for multiple comparisons (p<0.05) were performed for datasets from each time-point (24, 48, 72, and 120 hpf), for each individual lipid species to identify differences between specific lipids in E− vs. E+ embryos. Lipid species that were significantly different for at least three of the four time-points between the E− and E+ diet conditions were then analyzed using two-way ANOVA comparisons (diet vs. time) with either a Bonferroni’s or Tukey's post-test for multiple comparisons (as recommended by the software) to determine significant (p<0.05) differences during the developmental time-course.
For analysis of significance of the H218O incorporation, data from embryos incubated with the H218O label were analyzed using repeated measures two-way ANOVA with a Tukey’s or Sidak’s post-test (p<0.05; as recommended by the software) for multiple comparisons.
Statistical analyses for morphological and behavioral endpoints were performed using code developed in R (R Developmental Core Team 2014 http://www.R-project.org). For morphological assessments at 24 and 96 hpf, binary responses were recorded as either absent (0) or present (1) for each of the 18 endpoints.
For statistical analyses of behavior, raw data files were processed using custom R scripts (R Developmental Core Team 2014, http://www.R-project.org) with methodologies based on those previously described [32], to average the total distance traveled for each integration time-point, then the area under the curve was computed. The overall area under the cover was compared to the control (E+ defined diet) using a combination of percent change (30% difference from the control) and a Kolmogorov-Smirnov test (p<0.05).
3. Results
3.1. Embryonic α-tocopherol deficiency leads to behavioral impairments in E− embryos
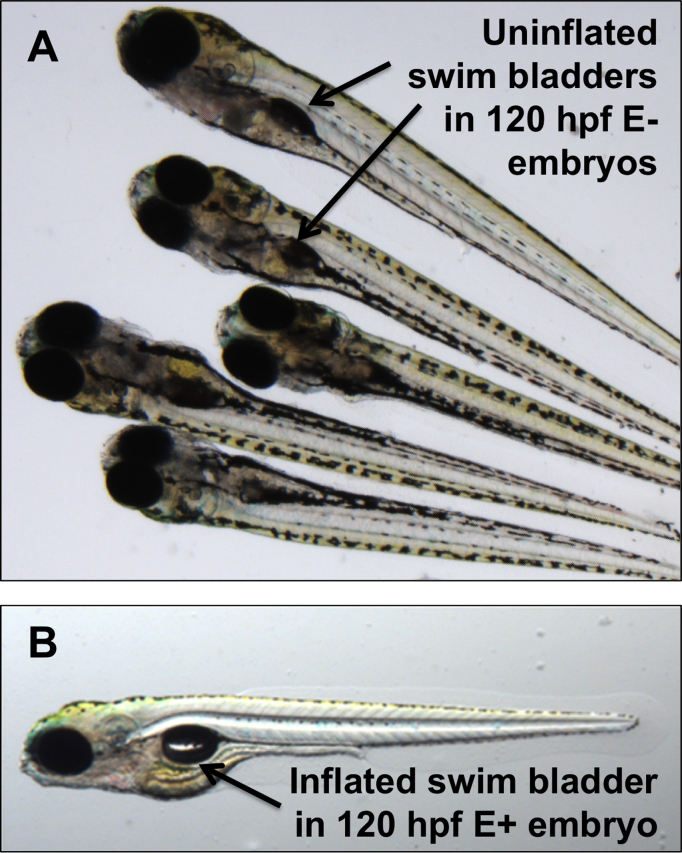
Morphological assessments in the present study were in accord with past observations [3]. Approximately 75–88% of the E− embryo cohorts evaluated either died or developed morphological abnormalities after hatching from the chorion, between 48 and 72 hpf. The most prevalent abnormalities noted at 72 hpf were pericardial and yolk sac edema, as found previously [3]. By 120 hpf, the majority of E− embryos also demonstrated failure to inflate the swim bladder (Fig. 1), even when other abnormalities were absent. Despite this high level of morbidity and mortality in the E− condition, a sub-set of each spawn (12–25% on average) remained morphologically normal when compared to E+ embryos at 96 hpf, and appeared to sustain no developmental abnormalities. Therefore, we performed behavioral assessments to determine if these “normal” E− embryos suffered from behavioral impairments that were not evident in the phenotypic screen.
Fig. 1.
E− embryos have morphological defects and increased mortality compared to E+ embryos. Representative pictures from the two diet groups at 120 hours post-fertilization (hpf). A. Uninflated swim bladders in elsewise morphologically normal E− embryos that survived to this stage. B. An E+ embryo provided for comparison. Morphological evaluations were made using the zebrafish acquisition and analysis program (ZAAP). Phenotypic differences in E− embryos became evident between 48 and 72 hpf, with most mortalities occurring by 72 hpf. By 120 hpf, 75–88% of E− embryos from each spawn were malformed or dead.
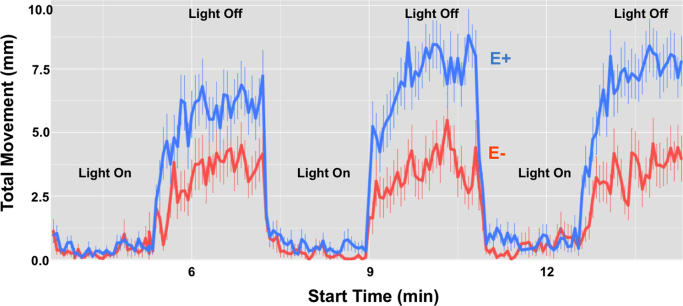
We evaluated the behavioral impact of developmental α-tocopherol deficiency with a high-throughput locomotor response assay and a modification of the protocol previously described [30]. Any dead or malformed embryos at 96 hpf were excluded from the data analysis. E− embryos were 82% less active in the dark phases than were E+ embryos (Fig. 2). Of note, the included E− embryos displayed normal motility and tactile responses during the phenotypic screen that were comparable to evaluations of the E+ condition, indicating E− embryos have a functional motor system.
Fig. 2.
E− embryos have impaired behavior compared to E+ embryos when assessed using a locomotor response assay. Embryos were analyzed in 96-well plates, one embryo per well with 128 embryos per diet condition. Locomotor activity following a series of light/dark stimuli (one stimulus every 3 min for 24 min total) was measured as movement (mm) over time (seconds); criteria for statistical significance between conditions was an area-under-curve (AUC) difference of >30% and p<0.05 (Kolmogorov-Smirnov test). At 96 hpf, E- embryos (red) were 82% less responsive to the light than were E+ embryos (blue) (E- AUC:459; E+ AUC:2468; p<0.01). Dead or malformed embryos were removed prior to data analysis (apparently normal embryos: n=54 E−; n=94 E+). The abnormal behavioral response in the E− embryos is indicative of underlying perturbations in neurological processes, as there were no observed differences in swimming behavior between E− and E+ conditions to suggest motor impairments in the former. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Untargeted lipidomics analysis shows significant changes in lipid profiles between E− and E+ embryos
Based on our recent finding that α-tocopherol deficiency results in significant changes in the phospholipid (PL) and lysophospholipid (lyso-PL) composition and status of brains from E− adult zebrafish [13], we hypothesized that embryonic α-tocopherol deficiency also would alter PL and lyso-PL levels in E− embryos. To examine alterations in lipid distribution and relative abundances between E− and E+ embryos, we used an UPLC-TOF-MS/MS lipidomics technique. Four PL classes were identified in embryo extracts, including 115 individually validated PLs and lyso-PLs. PL classes were detected in either positive (phosphatidylcholine [PC], phosphatidylethanolamine [PE], lysophosphatidylcholine [LPC], lysophosphatidylethanolamine [LPE]) or in negative ion modes (phosphatidylserine [PS] and lysophosphatidylserine [LPS], phosphatidylinositol [PI], and lysophosphatidylinositol [LPI]); PC and PE were detected in both ion modes.
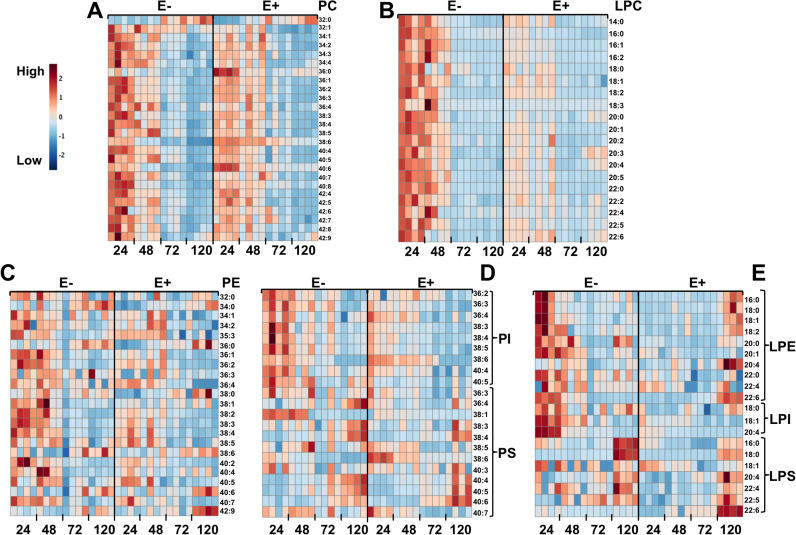
Identities of all PL and lyso-PLs were confirmed using exact mass matching and MS/MS fragmentation patterns; for example, PE species generated the characteristic losses of 43 and 140 Da (positive ion mode) known to be associated with the PE head group, while PS species showed −87 and −184 Da losses (negative ion mode). Significant changes in the PL and lyso-PL profiles of E− compared to E+ embryos were evident at each developmental time-point (Fig. 3). A global evaluation of these differences revealed that, while E− embryos tended to have relatively higher levels of many PLs and lyso-PLs during early development (24–48 hpf), levels of these same lipids were markedly lower in the E− than in the E+ condition by 120 hpf.
Fig. 3.
E− and E+ embryos display significantly different PL and lyso-PL composition profiles during development. Heatmaps of identified PL or lyso-PL are shown. Sample intensities were normalized against an internal standard (DT-PC 13:0/13:0) and then scaled with Pareto scaling. Figures were generated using Metabolanalyst software. Heatmap trends (high [red], low [blue]) indicate that the E− embryos contain, overall, greater quantities of lyso-PL species early in development (24 hpf) when compared to E+ embryos, but many of these species, notably those containing DHA (22:6), are markedly depleted by 120 hpf. PL trends are highly varied; however, E− embryos contain lesser quantities of PL species with DHA (22:6). A. PC, phosphatidylcholine; B. LPC, lysophosphatidylcholine; C. PE, phosphatidylethanolamine; D. PI, phosphatidylinositol and PS, phosphatidylserine E. LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol and LPS, lysophosphatidylserine.
3.3. Embryonic α-tocopherol deficiency causes the specific depletion of phospholipid and lysophospholipid species containing DHA moieties
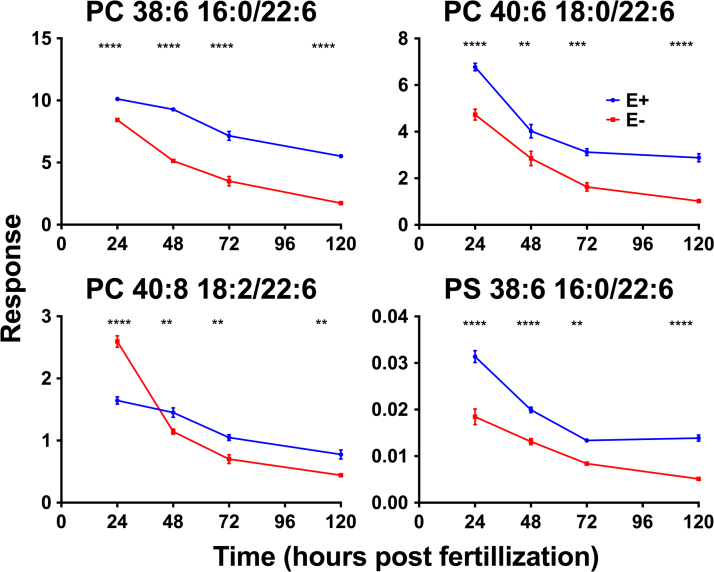
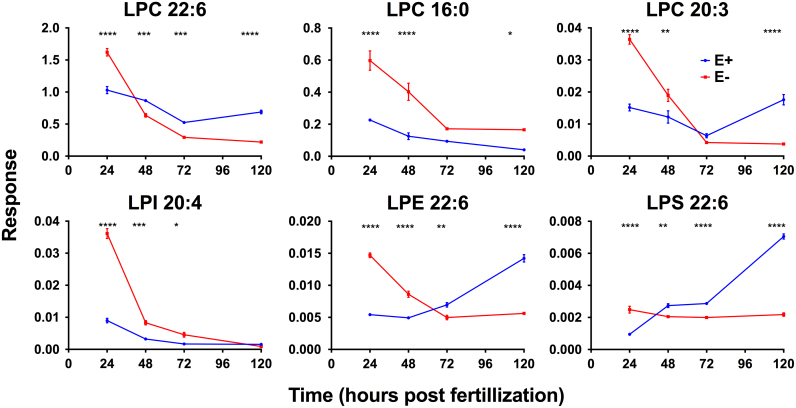
We hypothesized that E− embryos would contain lower levels of DHA-containing PLs and lyso-PLs (e.g. PC 38:6), as past investigations found the overall depletion of DHA in developing E− embryos [21]. Comprehensive analyses of the time-course lipidomics dataset showed that, while levels of only four out of the 75 identified PLs were significantly different between the E− and E+ embryos at all four developmental time-points, each contained DHA (22:6) and each was lower in the E− condition by 48 hpf (Fig. 4). Further, in-depth evaluation of differences in the lyso-PL composition between E− and E+ embryos demonstrated similar findings. Of the 40 identified lyso-PLs, levels of only six were significantly different during multiple (three out of four) developmental time-points, and the three lyso-PLs showing the most significant differences between E− and E+ conditions contained DHA (22:6) (Fig. 5).
Fig. 4.
Four specific PLs containing DHA (22:6) are significantly lower in E− embryos. Lipidomic analysis of lipid extracts from E− and E+ embryo samples (n=15/sample; 4 samples/group) taken at 24, 48, 72, and 120 hpf. Lipid species were confirmed by high-resolution MS, MS/MS fragmentation, and isotopic distribution, and then compared using the PeakView database. Peak intensities were used for relative quantification between E− and E+ conditions. The data shown are means±SEM. Two-way ANOVA, Bonferroni's post-test for multiple comparisons (**p<0.01, ***p<0.001, ****p<0.0001).
Fig. 5.
E− embryos show enhanced depletion of many lyso-PL species during development when compared with E+ embryos. Data were generated and analyzed as described in Fig. 4. Notably, the lyso-PL species showing the greatest difference between the E− and E+ conditions all contained DHA (means±SEM). Two-way ANOVA, Bonferroni's post-test for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). LPC 16:0 and LPC 20:3 levels at 72 hpf were significantly altered in E− vs. E+ embryos when analyzed using unpaired t-tests and Sidak-Bonferroni post-test (p<0.001, higher in E−; and p=0.0176, lower in E−, respectively), as were LPI 20:4 levels at 120 hpf (p<0.0001, lower in E−).
3.4. Increased H218O label incorporation demonstrates enhanced membrane phospholipid remodeling in E− embryos
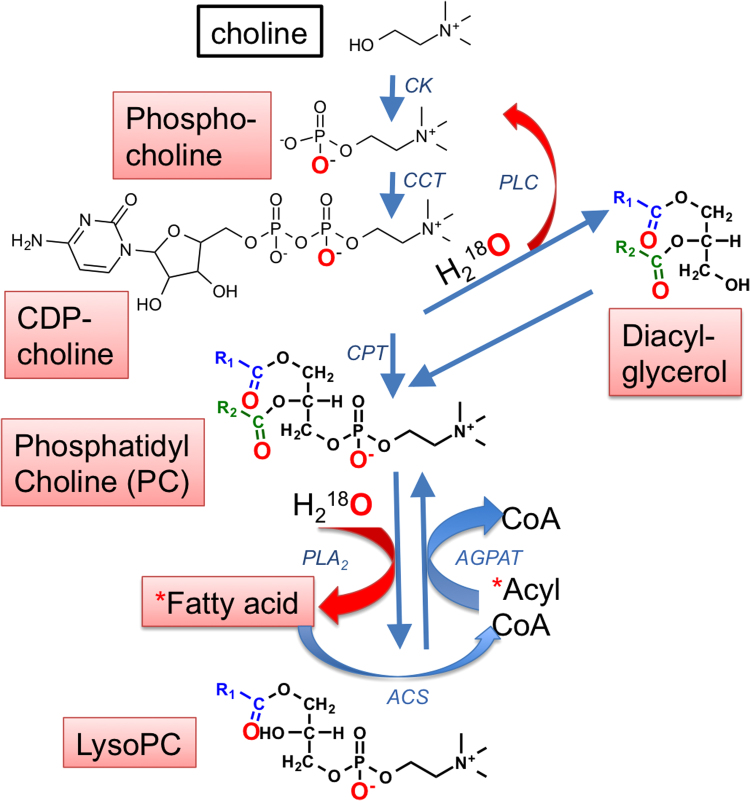
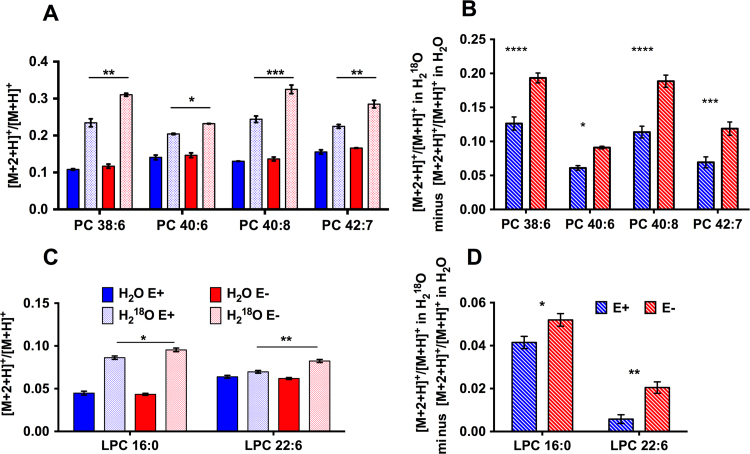
The 18O-labeling technique measures ester hydrolysis of parent PLs in the presence of H218O, which results in the incorporation of 18O into the carboxyl group of the resulting lyso-PL fatty acid; upon reacylation, the 18O is incorporated into the newly formed ester bond of the new PL [33], [34], as outlined for PC (Fig. 6). The time-dependent increase in the incorporation of the 18O label into PL species can, therefore, be taken as a metric of acyl turnover, and, subsequently, processes of PL remodeling and repair [35]. Thus, we divided a cohort of 48 hpf E− and E+ embryos (n=120/group), then incubated half with either 40% v/v H218O (n=60/group) or with 40% v/v H2O (n=60/group) for 24 hours from 48 to 72 hpf. This time-frame was chosen because it coincided with the onset of morbidity and mortality outcomes in E− embryos, which we hypothesized may be due to underlying perturbations in PL remodeling and/or repair mechanisms. Label incorporation was significantly elevated in E− embryos in four PC lipids specifically containing long-chain polyunsaturated fatty-acids, particularly DHA (22:6) (Fig. 7A and B). The concomitant increases in H218O labeling of both LPC 16:0 and LPC 22:6 in E− embryos (Fig. 7C and D), show these lyso-PCs are involved during the remodeling of PC 38:6.
Fig. 6.
H218O incorporation into PC lipids. The CDP-choline pathway [33], [34] is a representative metabolic pathway outlining reactions that allow for H218O incorporation into PCs and/or lyso-PCs. From the top, choline kinase (CK) phosphorylates choline to phosphocholine, which is converted to cytidine-diphosphocholine (CDP-choline) by CTP: phosphocholine cytidylyltransferase (CCT). Choline phosphotransferase (CPT) catalyzes PC synthesis. Fatty acyl chains are cleaved from the PC sn-2 position by phospholipase A2 (PLA2) to generate lyso-PC and free fatty acids. Free fatty acids are labeled (*), rapidly converted to acyl-CoA by acyl-CoA synthetase (ACS) and used for acylation of lyso-PL in a reaction catalyzed by acylglycerolphosphate acyltransferases (AGPAT). PC may be cleaved by PLC (phospholipase C) to yield diacylglycerides (DAG) and phosphocholine. Hydrolysis reactions in which incorporation of the 18O label may occur are shown in red, as are the indicated oxygens that may be labeled following PL remodeling.
Fig. 7.
H218O incorporation into PCs and lyso-PCs is greater in E− than in E+ embryos. A and C. Ratios of [M+2+H]+/[M+H]+ from H2O-incubated E− vs. E+ embryos were similar (solid bars; no significant differences) in PCs (A) and lyso-PCs (C); ratios comparing label incorporation in H218O-incubated E− vs. E+ embryo (patterned bars) revealed enhanced labeling in E− embryos of DHA-containing PCs, as well as in LPC 16:0 and 22:6, suggesting increased PL remodeling. PC species (means±SEM) were identified and confirmed as described in Fig. 4; PC 38:6 (16:0/22:6); PC 40:6 (18:0/22:6); PC 40:8 (18:2/22:6); PC 42:7 (20:3/22:4). All PLs and lyso-PLs showed significant (p<0.001) label incorporation in H2O- vs. H218O-incubated embryos within the same diet condition, with the exception of E+ embryos LPC 22:6 in H2O- vs. H218O-incubated (no significant difference, indicating no significant remodeling in E+ embryos). Asterisks indicate statistical differences between H218O E− and E+ groups (red and blue patterned bars, respectively). B and D. [M+2+H]+/[M+H]+ in H218O minus [M+2+H]+/[M+H]+ in H2O (mean±SEM) in E− vs. E+ embryos for PCs (B) and lyso-PCs (D). A–D: Two-way ANOVA with Tukey's (A,C) or Sidak's (B,D) post-test for multiple comparisons (*p<0.05, ** p<0.01, ***p<0.001, ****p<0.0001).
4. Discussion
Our discoveries regarding the effects of α-tocopherol deficiency during zebrafish embryonic development show that E- embryos have such severe morphologic defects that the survivors display altered behaviors. Moreover, increasing defect severity is accompanied by depletion of a relatively limited set of PLs and lyso-PLs containing DHA, which causes increased PL remodeling.
In agreement with our past reports [3], E− embryos begin to accumulate morphological abnormalities between 48 and 72 hpf and the majority of embryos are severely malformed or dead by 120 hpf. Most E− embryos surviving to 120 hpf fail to inflate the swim bladder, even when other developmental deformities are absent (Fig. 1A). We do not at this time know if such an observation represents a defective swim bladder or merely a delay in its inflation; however, both are portents of poor survival outcomes during later stages of development.
Developmental α-tocopherol deficiency may compromise brain function, as apparently “normal” E− embryos are 82% less active during dark phases than are E+ embryos (Fig. 2), suggesting a significantly impaired neurobehavioral response [30], [36]. The E− embryos demonstrated a normal, robust tactile (touch) response comparable to that of E+ embryos when subjected to a phenotypic screen, indicating that the reason underlying the impaired behavioral response is associated with compromised brain, rather than muscle, function. It also is possible that the E− embryos suffer compromised visual acuity; however, eye morphology and movements in the E− group were apparently normal during the phenotypic screen. Given that the E− embryos did demonstrate greater locomotor activity during the dark when compared to the light phases, we believe that compromised visual acuity was not the main cause of the impaired behavior observed in the E− condition. Instead, we argue that the perturbed behavior in E− embryos is primarily due to consequences of inadequate brain α-tocopherol and the subsequent changes in the brain PL and lyso-PL composition.
Our lipidomics data supports the hypothesis that the behavioral abnormalities in the E− group are, at least in part, a consequence of disrupted PL and lyso-PL status during development. While significant differences between E− and E+ embryos are noticeable in the global PL and lyso-PL pool at each developmental time-point (Fig. 3), we found that only four PLs, all containing DHA, are significantly decreased throughout development from 24 to 120 hpf in E− when compared to E+ embryos (Fig. 4). Two of these, PC 38:6 (also lower by 30% in adult E− vs. E+ zebrafish brains [13]) and PC 40:6, are plasma biomarkers that are depleted in humans who progress to dementia [37], raising the provocative possibility that lower levels of these PLs cause compromised neurodevelopment.
Relatedly, we found that six lyso-PLs are significantly altered in E− embryos during the developmental time-course (Fig. 5). Of these, three contained DHA (LPC 22:6, LPE 22:6, and LPS 22:6) and were lower in the E− condition by 120 hpf, despite being higher in E− embryos at 24 hpf. Moreover, while levels of the DHA-containing lyso-PLs increased during development in E+ embryos, all decreased (rather substantially in the cases of LPC 22:6 and LPE 22:6) in E− embryos, suggesting developmental α-tocopherol deficiency leads to the rapid depletion of available DHA-containing lyso-PLs, in agreement with observed depletion of total DHA in developing E- embryos [21], for maintenance of membrane DHA-containing PLs. This may be particularly distressing to the brain, which sequesters substantial amounts of DHA during neurodevelopment [38], especially as LPC 22:6 is the preferred transport and uptake form of DHA across the blood-brain barrier [19], [39]. It is possible that the decrease in LPC 22:6 during development in the E− embryos represents an impaired ability to provide adequate DHA to the brain, and that by 96 hpf (the time at which behavior was assessed) this may adversely affect neurological function. Similarly, as the major storage form of DHA in nervous tissue is within membrane PE lipids [40], [41], lower LPE 22:6 levels in the E− embryos suggests a simultaneous decrease in the brain DHA reservoir that also could underlie abnormal behavior.
Other changes in the lyso-PL composition of the E− embryos are worthy of comment: for example, LPI 20:4, the only inositol-containing PL to be significantly different between E− and E+ embryos, contains arachidonic acid (ARA, 20:4 ω-6). An important function of ARA is phospholipid-mediated signal transduction. In the brain, phospholipase A2 (PLA2), which removes ARA from the sn-2 position of membrane PLs, is activated by glutamatergic, serotonergic, cholinergic, and dopaminergic signaling, releasing ARA as a secondary messenger [42], [43]. ARA also is the major substrate for synthesis of bioactive eicosanoids, endogenous stimulators of myriad cellular responses [44], such as inflammation, that act through signal transduction pathways mediated by inositol PLs [45], [46].The relevance of altered levels of PI lipids and the perturbed brain function as well as morphological deformities observed in E− embryos represents, therefore, an area that warrants additional research.
It should be noted that not all lyso-PLs were depleted in E− embryos. Levels of LPC 16:0 were higher in the E− than in the E+ condition throughout development. The increase in LPC 16:0 during development in the E− group, along with the reported concomitant decrease in LPC 22:6 (Fig. 5), supports our hypothesis that membrane turnover of DHA-containing PLs (especially DHA 38:6, which contains both 16:0 and 22:6 fatty acyl chains) is enhanced in E− embryos. Hypothetically, if DHA is depleted by lipid peroxidation, then the saturated lyso-PLs (e.g. LPC 16:0) would be expected to increase following PLA2 action [47], [48].
This evidence agrees with the outcomes of our H218O labeling experiment, which revealed that E− embryos had greater incorporation of the H218O label into several DHA-containing PLs (Fig. 7), as well as LPC 22:6, a required substrate for membrane PC remodeling. In the E− group, H218O incorporation also was elevated significantly in LPC 16:0 (Fig. 7C and D), further supporting our hypothesis that PC 38:6 (and by extension other DHA-containing PLs) is depleted from the membrane PL pool due to increased turnover. In addition, LPC 22:6 label incorporation was not increased significantly in the E+ embryos (Fig. 7C), indicating that in the presence of adequate α-tocopherol, the DHA-containing lyso-PL reserve (with DHA presumably at the sn-2 position for incorporation into remodeled membrane PLs) remains stable. While our 24 h incubation time and high H218O label concentration protocol may reveal only the most gross changes in PL remodeling processes due to α-tocopherol deficiency, the fact that we found such selective labeling of DHA-containing PLs, species that also were decreased in the E− condition, makes the outcomes of this experiment all the more significant.
The alterations in both PL and lyso-PL levels and label incorporation we observed in the E− relative to the E+ embryos are, potentially, the result of a depletion of DHA-containing lipids and an increased requirement for DHA to remodel and repair affected PLs. Hypothetically, with respect to our recent finding of increased DHA peroxidation in adult E− zebrafish brains, [13] insufficient α-tocopherol concentrations during development may allow lipid peroxidation to deplete not only brain DHA-PLs, but DHA throughout the body, thereby limiting DHA delivery (e.g. via LPC 22:6) to the brain. We have evidence of increased DHA peroxidation in E− embryos [21], suggesting that this is the cause of the selective depletion and enhanced remodeling of DHA-containing lipids reported in the present study. While we did search our lipidomics data specifically for oxidized DHA (and other PUFA) derivatives, the amount of sample material proved to be too small to allow for accurate identification and quantification of these oxidized lipids. Larger sample sizes and more targeted analyses will be necessary to confirm that greater lipid peroxidation in the E− condition leads to the ensuing depletion and enhanced remodeling of DHA-containing PLs that we observed.
In summary, the results of this study show that embryonic α-tocopherol deficiency causes: 1) morphological abnormalities, 2) an impaired locomotor response suggestive of compromised neurological function, 3) significant changes to the PL and lyso-PL profile of developing zebrafish embryos, and 4) a selective decrease in DHA-containing PL and lyso-PL species during the developmental time-course that matches evidence of their enhanced turnover in E− embryos. Our data demonstrate that critical lipids are protected by α-tocopherol and suggest that their depletion may be involved in the mechanism(s) leading to observed behavioral defects and increased mortality.
Acknowledgments
The authors thank Carrie L. Barton, Greg D. Gonnerman and Scott W. Leonard for providing excellent technical assistance. This work was supported by National Institutes of Health Grants S10RR027878, NICHD HD062109 (M.G.T. and R.L.T.), and NIEHS ES000210. M.Q.M is supported in part by National Science Foundation Grant DGE 0965820.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.01.004.
Appendix A. Supplementary material
Supplementary material
References
- 1.Westerfield M. University of Oregon Press; Eugene: 2007. The Zebrafish Book; A Guide for the Laboratory use of Zebrafish (Danio Rerio) [Google Scholar]
- 2.Miller G.W., Ulatowski L., Labut E.M., Lebold K.M., Manor D., Atkinson J., Barton C.L., Tanguay R.L., Traber M.G. The alpha-tocopherol transfer protein is essential for vertebrate embryogenesis. PLoS One. 2012;7:e47402. doi: 10.1371/journal.pone.0047402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller G.W., Labut E.M., Lebold K.M., Floeter A., Tanguay R.L., Traber M.G. Zebrafish (Danio rerio) fed vitamin E-deficient diets produce embryos with increased morphologic abnormalities and mortality. J. Nutr. Biochem. 2012;23:478–486. doi: 10.1016/j.jnutbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton G.W., Joyce A., Ingold K.U. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet. 1982;2:327. doi: 10.1016/s0140-6736(82)90293-8. [DOI] [PubMed] [Google Scholar]
- 5.Traber M.G., Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L., Davis T.A., Porter N.A. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 2009;131:13037–13044. doi: 10.1021/ja9029076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green P., Glozman S., Kamensky B., Yavin E. Developmental changes in rat brain membrane lipids and fatty acids. The preferential prenatal accumulation of docosahexaenoic acid. J. Lipid Res. 1999;40:960–966. [PubMed] [Google Scholar]
- 8.Harris W.S., Baack M.L. Beyond building better brains: bridging the docosahexaenoic acid (DHA) gap of prematurity. J. Perinatol. 2015;35:1–7. doi: 10.1038/jp.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H.F., Su H.M. Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic-pituitary-adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. J. Nutr. Biochem. 2013;24:70–80. doi: 10.1016/j.jnutbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Cederholm T., Salem N., Jr, Palmblad J. Omega-3 fatty acids in the prevention of cognitive decline in humans. Adv. Nutr. 2013;4:672–676. doi: 10.3945/an.113.004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia H.S., Agrawal R., Sharma S., Huo Y.X., Ying Z., Gomez-Pinilla F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS One. 2011;6:e28451. doi: 10.1371/journal.pone.0028451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuzawa C.W., Chugani H.T., Grossman L.I., Lipovich L., Muzik O., Hof P.R., Wildman D.E., Sherwood C.C., Leonard W.R., Lange N. Metabolic costs and evolutionary implications of human brain development. Proc. Natl. Acad. Sci. USA. 2014;111:13010–13015. doi: 10.1073/pnas.1323099111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J., Leonard S.W., Kasper K., McDougall M., Stevens J.F., Tanguay R.L., Traber M.G. Novel function of vitamin E in regulation of zebrafish (Danio rerio) brain lysophospholipids discovered using lipidomics. J. Lipid Res. 2015;56:1182–1190. doi: 10.1194/jlr.M058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hishikawa D., Hashidate T., Shimizu T., Shindou H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014;55:799–807. doi: 10.1194/jlr.R046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shindou H., Hishikawa D., Harayama T., Eto M., Shimizu T. Generation of membrane diversity by lysophospholipid acyltransferases. J. Biochem. 2013;154:21–28. doi: 10.1093/jb/mvt048. [DOI] [PubMed] [Google Scholar]
- 16.Hachem M., Geloen A., Van A.L., Foumaux B., Fenart L., Gosselet F., Da Silva P., Breton G., Lagarde M., Picq M., Bernoud-Hubac N. Efficient Docosahexaenoic Acid Uptake by the Brain from a Structured Phospholipid. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9228-9. [DOI] [PubMed] [Google Scholar]
- 17.Lagarde M., Hachem M., Bernoud-Hubac N., Picq M., Vericel E., Guichardant M. Biological properties of a DHA-containing structured phospholipid (AceDoPC) to target the brain. Prostaglandins Leukot. Essent. Fat. Acids. 2015;92:63–65. doi: 10.1016/j.plefa.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Zvi A., Lacoste B., Kur E., Andreone B.J., Mayshar Y., Yan H., Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen L.N., Ma D., Shui G., Wong P., Cazenave-Gassiot A., Zhang X., Wenk M.R., Goh E.L., Silver D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 20.Guemez-Gamboa A., Nguyen L.N., Yang H., Zaki M.S., Kara M., Ben-Omran T., Akizu N., Rosti R.O., Rosti B., Scott E., Schroth J., Copeland B., Vaux K.K., Cazenave-Gassiot A., Quek D.Q., Wong B.H., Tan B.C., Wenk M.R., Gunel M., Gabriel S., Chi N.C., Silver D.L., Gleeson J.G. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat. Genet. 2015;47:809–813. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebold K.M., Kirkwood J.S., Taylor A.W., Choi J., Barton C.L., Miller G.W., La Du J., Jump D.B., Stevens J.F., Tanguay R.L., Traber M.G. Novel liquid chromatography-mass spectrometry method shows that vitamin E deficiency depletes arachidonic and docosahexaenoic acids in zebrafish (Danio rerio) embryos. Redox Biol. 2013;2:105–113. doi: 10.1016/j.redox.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebold K.M., Jump D.B., Miller G.W., Wright C.L., Labut E.M., Barton C.L., Tanguay R.L., Traber M.G. Vitamin E deficiency decreases long-chain PUFA in zebrafish (Danio rerio) J. Nutr. 2011;141:2113–2118. doi: 10.3945/jn.111.144279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podda M., Weber C., Traber M.G., Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J. Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 24.Frei B., England L., Ames B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebold K.M., Lohr C.V., Barton C.L., Miller G.W., Labut E.M., Tanguay R.L., Traber M.G. Chronic vitamin E deficiency promotes vitamin C deficiency in zebrafish leading to degenerative myopathy and impaired swimming behavior. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2013;157:382–389. doi: 10.1016/j.cbpc.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkwood J.S., Lebold K.M., Miranda C.L., Wright C.L., Miller G.W., Tanguay R.L., Barton C.L., Traber M.G., Stevens J.F. Vitamin C deficiency activates the purine nucleotide cycle in zebrafish. J. Biol. Chem. 2012;287:3833–3841. doi: 10.1074/jbc.M111.316018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truong L., Harper S.L., Tanguay R.L. Evaluation of embryotoxicity using the zebrafish model. Methods Mol. Biol. 2011;691:271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saili K.S., Corvi M.M., Weber D.N., Patel A.U., Das S.R., Przybyla J., Anderson K.A., Tanguay R.L. Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology. 2012;291:83–92. doi: 10.1016/j.tox.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Truong L., Saili K.S., Miller J.M., Hutchison J.E., Tanguay R.L. Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2012;155:269–274. doi: 10.1016/j.cbpc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noyes P.D., Haggard D.E., Gonnerman G.D., Tanguay R.L. Advanced morphological-behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 2015;145:177–195. doi: 10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia J., Sinelnikov I.V., Han B., Wishart D.S. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–W257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truong L., Reif D.M., St Mary L., Geier M.C., Truong H.D., Tanguay R.L. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 2014;137:212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fagone P., Jackowski S. Phosphatidylcholine and the CDP-choline cycle. Biochim. Biophys. Acta. 2013;1831:523–532. doi: 10.1016/j.bbalip.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid P.C., Johnson S.B., Schmid H.H. Remodeling of rat hepatocyte phospholipids by selective acyl turnover. J. Biol. Chem. 1991;266:13690–13697. [PubMed] [Google Scholar]
- 35.Kuwae T., Schmid P.C., Schmid H.H. Alterations of fatty acyl turnover in macrophage glycerolipids induced by stimulation. Evidence for enhanced recycling of arachidonic acid. Biochim. Biophys. Acta. 1997;1344:74–86. doi: 10.1016/s0005-2760(96)00135-x. [DOI] [PubMed] [Google Scholar]
- 36.Macaulay L.J., Bailey J.M., Levin E.D., Stapleton H.M. Persisting effects of a PBDE metabolite, 6-OH-BDE-47, on larval and juvenile zebrafish swimming behavior. Neurotoxicol. Teratol. 2015;52:119–126. doi: 10.1016/j.ntt.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mapstone M., Cheema A.K., Fiandaca M.S., Zhong X., Mhyre T.R., MacArthur L.H., Hall W.J., Fisher S.G., Peterson D.R., Haley J.M., Nazar M.D., Rich S.A., Berlau D.J., Peltz C.B., Tan M.T., Kawas C.H., Federoff H.J. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Innis S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007;137:855–859. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 39.Betsholtz C. Lipid transport and human brain development. Nat. Genet. 2015;47:699–701. doi: 10.1038/ng.3348. [DOI] [PubMed] [Google Scholar]
- 40.Favreliere S., Barrier L., Durand G., Chalon S., Tallineau C. Chronic dietary n-3 polyunsaturated fatty acids deficiency affects the fatty acid composition of plasmenylethanolamine and phosphatidylethanolamine differently in rat frontal cortex, striatum, and cerebellum. Lipids. 1998;33:401–407. doi: 10.1007/s11745-998-0221-y. [DOI] [PubMed] [Google Scholar]
- 41.Kitson A.P., Stark K.D., Duncan R.E. Enzymes in brain phospholipid docosahexaenoic acid accretion: a PL-ethora of potential PL-ayers. Prostaglandins Leukot. Essent. Fat. Acids. 2012;87:1–10. doi: 10.1016/j.plefa.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Bazinet R.P., Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 43.Duncan R.E., Bazinet R.P. Brain arachidonic acid uptake and turnover: implications for signaling and bipolar disorder. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:130–138. doi: 10.1097/MCO.0b013e328336b615. [DOI] [PubMed] [Google Scholar]
- 44.Gabbs M., Leng S., Devassy J.G., Monirujjaman M., Aukema H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015;6:513–540. doi: 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viaud J., Mansour R., Antkowiak A., Mujalli A., Valet C., Chicanne G., Xuereb J.M., Terrisse A.D., Séverin S., Gratacap M.P., Gaits-Iacovoni F., Payrastre B. Phosphoinositides: important lipids in the coordination of cell dynamics. Biochimie. 2015 doi: 10.1016/j.biochi.2015.09.005. pii: S0300-9084(15)00280-1, http://dx.doi.org/10.1016/j.biochi.2015.09.005. [Epub ahead of print] Review. PubMed PMID: 26391221. [DOI] [PubMed] [Google Scholar]
- 46.Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 47.Ren R., Hashimoto T., Mizuno M., Takigawa H., Yoshida M., Azuma T., Kanazawa K. A lipid peroxidation product 9-oxononanoic acid induces phospholipase A2 activity and thromboxane A2 production in human blood. J. Clin. Biochem. Nutr. 2013;52:228–233. doi: 10.3164/jcbn.12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nigam S., Schewe T. Phospholipase A(2)s and lipid peroxidation. Biochim. Biophys. Acta. 2000;1488:167–181. doi: 10.1016/s1388-1981(00)00119-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material