Abstract
Introduction:
The inadequate reporting of cross-sectional studies, as in the case of the prevalence of metabolic syndrome, could cause problems in the synthesis of new evidence and lead to errors in the formulation of public policies.
Objective:
To evaluate the reporting quality of the articles regarding metabolic syndrome prevalence in Peruvian adults using the STROBE recommendations.
Methods:
We conducted a thorough literature search with the terms "Metabolic Syndrome", "Sindrome Metabolico" and "Peru" in MEDLINE/PubMed, LILACS, SciELO, LIPECS and BVS-Peru until December 2014. We selected those who were population-based observational studies with randomized sampling that reported prevalence of metabolic syndrome in adults aged 18 or more of both sexes. Information was analysed through the STROBE score per item and recommendation.
Results:
Seventeen articles were included in this study. All articles met the recommendations related to the report of the study's rationale, design, and provision of summary measures. The recommendations with the lowest scores were those related to the sensitivity analysis (8%, n= 1/17), participant flowchart (18%, n= 3/17), missing data analysis (24%, n= 4/17), and number of participants in each study phase (24%, n= 4/17).
Conclusion:
Cross-sectional studies regarding the prevalence of metabolic syndrome in peruvian adults have an inadequate reporting on the methods and results sections. We identified a clear need to improve the quality of such studies.
Keywords: Metabolic syndrome, epidemiology, prevalence, adult, Peru
Abstract
Introducción:
El reporte inadecuado de estudios transversales, como en el caso de la prevalencia de síndrome metabólico, podría causar problemas en la síntesis de nueva evidencia y generar errores en la formulación de políticas públicas.
Objetivo:
Evaluar la calidad de reporte de estudios transversales sobre la prevalencia de síndrome metabólico en Perú utilizando las recomendaciones de STROBE.
Métodos:
Se realizó una búsqueda bibliográfica exhaustiva hasta Diciembre 2014 en MEDLINE/PubMed, LILACS, SciELO, LIPECS y BVS-Perú con los términos "Metabolic Syndrome", "Sindrome Metabolico" y "Peru". Se seleccionaron estudios observacionales con base poblacional, muestreo aleatorizado, que reportaran datos de prevalencia en adultos mayores de 18 años de ambos sexos. La información fue analizada a través de STROBE según puntuación por artículo y por recomendación.
Resultados:
Diecisiete artículos fueron incluidos en este estudio. Todos cumplieron con las recomendaciones relacionadas con el reporte de razones y fundamentos de la investigación, reporte del diseño de estudio y la proporción de medidas de resumen. Las recomendaciones con menor puntaje fueron las relacionadas a la descripción del análisis de sensibilidad (8%, n= 1/13), consideración del uso de diagrama de flujo para los participantes (18%, n= 3/17), explicación del análisis de datos ausentes (24%, n= 4/17) y del número de participantes en cada fase (24%, n= 4/17).
Conclusión:
Los estudios transversales sobre prevalencia de síndrome metabólico en adultos del Perú presentan un inadecuado reporte en las secciones de métodos y resultados. Se identifica una clara necesidad de mejorar la calidad de este tipo de estudios.
Introduction
The inadequate reporting of biomedical research is a longstanding and potentially serious global problem that is not entirely evident to many researchers 1. All scientific study must be fully and accurately reported, allowing a proper understanding of their methodology, findings and replication of the same if needed 2,3. However, most of the reports are far from those ideals 2. For this reason, many guidelines that seek to standardize and improve the reporting quality of different types of research were developed in the past few years 4. Strengthening the Reporting of OBservational studies in Epidemiology (STROBE) is a guideline whose recommendations have been established in order to adequately report observational studies (cohort, case-control and cross-sectional studies) 3,5. It should be noted that the STROBE recommendations assess the quality of reporting, but not the research or methodological quality per se 6.
Moreover, an inadequate reporting of cross-sectional studies could lead to problems in the synthesis and adoption of new evidence and generate errors in the justification and formulation of public policies 2, especially in regions with limited resources like Peru or other Latin American countries. For example, the prevalence of Metabolic Syndrome (MS) is relevant to public health issues because it had been associated with an increase of two to three times the risk of presenting a heart attack or stroke 7 and five times higher risk of developing type 2 diabetes mellitus 8,9. Nonetheless, the diversity of criteria for defining MS 10-16 associated with the poor reporting in cross-sectional studies, generate confusion when interpreting the real extent of the problem. For that reason, this study aims to evaluate the reporting quality of cross-sectional studies regarding the prevalence of MS in Peruvian adults, using the STROBE recommendations as an objective tool.
Materials and Methods
This descriptive study was carried out in two stages. First, a systematic literature search was performed to identify the articles to be included in the study. Then, the quality of the reporting was assessed using STROBE. This study followed the recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement for its reporting 17.
Search strategy
We conducted the search in MEDLINE/PubMed (1997 - 4/12/2014), LILACS (1982 - 4/12/2014), SciELO (1999 - 4/12/2014), LIPECS (1987 - 4/12/2014) and BVS-Perú (INS, MINSA and OPS; 1997 - 4/12/2014) after reaching consensus regarding each database search strategy. The terms "Metabolic Syndrome" and "Sindrome Metabolico" were used in combination with the term "Peru" depending upon the database. The terms were used in english for MEDLINE via PubMed; in Spanish for LIPECS, BVS-Perú and OPS; or in both languages for LILACS and SciELO. The last search was completed on December 4, 2014. This was done simultaneously and independently by three researchers (JCT, EFR, OJP) and a list of found items was made. Then, search results were compared and no differences in the outcome were found among the three authors.
Study selection
The selection was made with the purpose of obtaining studies whose external validity allow us to extrapolate their results to different populations of Peru. The following eligibility criteria were defined: 1) population-based observational studies; 2) studies involving random sampling instead of volunteer recruitment; 3) studies that reported prevalence data of MS according to a selected criteria and 4) studies involving adults 18 years or older of both sexes. There were no language restrictions. Full-text articles were evaluated by three researchers (JCT, EFR, OJP) and those who met the inclusion criteria were selected. Additionally, a secondary search through the bibliographical references of the selected articles was made and duplicates were removed. We excluded studies involving pediatric or inpatient population, workers from any institution or patients recruited through health campaigns. Reviews, editorials or short communications were also excluded. A fourth investigator (GM) was consulted in the event of discrepancies and reached a consensus.
Instrument
We used the STROBE recommendations as an objective tool to evaluate the quality of the reports. STROBE presents 32 recommendations for the appropriate reporting of observational studies. These recommendations describe the proper way of reporting the title, abstract, introduction, methods, results, discussion and financing 5. According to the language of each publication, we used the cross-sectional studies suggested-version, available in English 3 or Spanish 18.
For this study, we used 30 of the 32 recommendations from cross-sectional studies. We considered as not-applicable the items 16b (the limits of the ranges for continuous variables of MS -blood pressure, glucose, HDL, triglycerides, or abdominal circumference are already defined for each criterion) and 16c (the objectives of the studies were not to evaluate the report of relative or absolute risk). Additionally, two recommendations were considered as not-applicable for the following cases: 12d if the study had a simple random sampling (single-stage) and 12e if the article fulfilled 12a, 12b and 12c recommendations.
Data extraction
Two formats for data extraction were developed. The first contained information about the general characteristics of each article: first author, publication year, name of the study from which the data came from, publication language, study period, city, type of population, sampling type, age range, sample size and criteria used to define MS. The second format is a list with 30 of the 32 STROBE recommendations.
Three researchers (JCT, EFR, OJP) reviewed the full-text of the articles with their respective protocols, in case the latter were cited, and the data was extracted. Each investigator assessed whether the reports identified met or not the STROBE recommendations. We did not hide the names of the authors nor the title of the articles.
Finally, the corresponding author of each included article was contacted by email. In each email, we presented the purpose of the study and the recommendations fulfilled by the article, based on our analysis according to STROBE. This step was done in order to clarify potential disagrements with our assessment. The inputs of each author were analyzed according to the methodology described above and modifications to our analysis were performed as appropriate. In case of no response, a reminder was sent 7 days after the first email. We waited in total for 14 days for the author to reply, otherwise our analysis was considered as the final result.
Analysis
Two types of scores were reported as follows: score per article and per recommendation. The score per article was defined as the number of the STROBE recommendations adequately reported, divided by the total of recommendations applicable per article and expressed as a percentage. The score per recommendation was defined as the number of articles that met each STROBE recommendation, divided by the total of articles for which the recommendation were applicable and expressed as a percentage.
Results
Results of the literature search
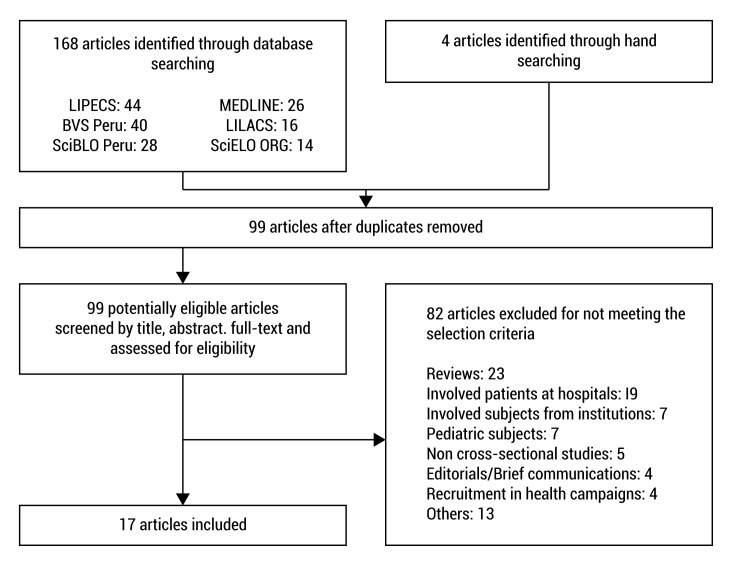
We found 168 articles through the database search and 4 through the secondary search within the references of the included articles. From the 172 articles, 73 were excluded for being duplicates and the remaining 99 were examined in full-text. Of these, 82 were discarded because they did not meet the selection criteria, resulting in a total of 17 articles for data extraction 19-35 (Fig. 1).
Figure 1. Flowchart of the selection of articles .
General features of the reports
Table 1 summarizes the main features of the 17 included articles. Four articles 19,20,23,24 were published in 3 Peruvians journals and 13 21,22,24-33,35 in 11 foreign journals. Of the total, 3 reported belonging to the ENINBSC study 36, 3 to the PERU MIGRANT study 37, 2 to the CARMELA study 33, 2 to the PREVENCION study 38, 2 to the PIRS study and 1 to the FRENT study. These population-based studies were conducted between 1999 and 2008 and involved 12,789 participants from different Peruvian cities, including urban and rural population, and rural-urban migrants. According to the sampling strategy, 4 had simple random sampling (single-stage), while the 13 remaining had multistage randomized sampling. In addition, 12 reports were published in English and 5 in Spanish. Finally, regarding the criteria used to determine prevalence of MS in adults from Peru, 7 articles (41%) used the NCEP-ATP III criteria 12, 2 the JIS criteria 16, 1 the AHA/NHLBI criteria 13, 1 the IDF criteria 14, and the others used more than one criterion.
Table 1. Main features of the articles regarding the prevalence of Metabolic Syndrome of Adults in Peru.
| Reference | Article | Publication Year | Protocol | Publication language | Study Period | City | Population | Sampling Type | Age | Sample Size | MS Criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soto et al 19 | A-1 | 2005 | NS | Spanish | 2004 | Lambayeque | Urban, rural | Randomized, multistage, stratified and by clusters | 30 - 70 | 1,000 | NCEP-ATP III12, ILIBLA |
| Guarnizo et al 20 | A-2 | 2006 | NS | Spanish | 2004 - 2005 | Lambayeque | Urban, rural | Randomized, multistage, stratified and by clusters | 30 - 70 | 621 | NCEP-ATP III12, IDF14, ILIBLA |
| Lorenzo et al 21 | A-3 | 2006 | PIRS | English | 1999 - 2001 | Lima | Urban | Randomized, multistage, stratified and by clusters | 35 - 64 | 346 | rNCEP-ATP III15, IDF14 |
| Seclén et al 22 | A-4 | 2006 | PIRS | English | 1999 - 2001 | Lima | Urban | Randomized, multistage, stratified and by clusters | ≥30 | 612 | NCEP-ATP III12 |
| Pajuelo et al 23 | A-5 | 2007 | ENINBSC | Spanish | 2004 - 2005 | National | Urban, rural | Randomized, multistage, stratified and by clusters | ≥20 | 4,091 | NCEP-ATP III12 |
| Medina-Lezama et al 24 | A-6 | 2007 | PREVENCION | English | 2004 - 2006 | Arequipa | Urban | Randomized, multistage, stratified and by clusters | 20 - 80 | ,1878 | NCEP-ATP III12, AHA/NHLBI13 |
| Baracco et al 25 | A-7 | 2007 | NS | English | 2002 - 2003 | Lima, | Urban, rural | Simple randomization | ≥30 | 271 | NCEP-ATP III12 |
| Schargrodsky et al 26 | A-8 | 2008 | CARMELA | English | 2003 - 2005 | Lima | Urban | Randomized, multistage and stratified | 25 - 64 | 1,652 | NCEP-ATP III12 |
| Cárdenas et al 27 | A-9 | 2009 | ENINBSC | Spanish | 2004 - 2005 | National | Urban, rural | Randomized, multistage, stratified and by clusters | ≥20 | 4,053 | IDF14 |
| Escobedo et al 28 | A-10 | 2009 | CARMELA | English | 2003 - 2005 | Lima | Urban | Randomized, multistage and stratified | 25 - 64 | 1,645 | NCEP-ATP III12 |
| Gelaye et al29 | A-11 | 2009 | FRENT Study | English | 2006 | Lima, | Urban | Randomized, multistage and stratified | ≥18 | 1,675 | NCEP-ATP III12 |
| Masterson et al 30 | A-12 | 2010 | PERU MIGRANT | English | 2007 - 2008 | Lima, | Rural, urban, Urban, rural, rural-urban migrants | Simple randomization | ≥30 | 985 | AHA/NHLBI13 |
| Medina-Lezama et al 31 | A-13 | 2010 | PREVENCION | English | 2004 - 2006 | Arequipa | Urban | Randomized, multistage, stratified and by clusters | 20 - 80 | 1448 | AHA/NHLBI13, JIS16 |
| Miranda et al 32 | A-14 | 2011 | PERU MIGRANT | English | 2007 - 2008 | Lima, | Rural, urban Urban, rural, rural-urban migrants | Simple randomization | ≥30 | 989 | JIS16 |
| Boissonet et al 33 | A-15 | 2011 | CARMELA | English | 2003 - 2005 | Lima | Urban | Randomized, multistage and stratified | 25 - 64 | 1652 | NCEP-ATP III12 |
| Pajuelo et al 34 | A-16 | 2012 | ENINBSC | Spanish | 2004 - 2005 | <1000, | Urban, rural | Randomized, multistage, stratified and by clusters | ≥20 | 3384 | NCEP-ATP III12 |
| Bernabe-Ortiz et al 35 | A-17 | 2012 | PERU MIGRANT | English | 2007 - 2008 | Lima | Rural-urban Migrants | Simple randomization | ≥30 | 589 | JIS16 |
MS: Metabolic Syndrome NS: Not stated, NCEP-ATP III: National Cholesterol Education Program-Adult Treatment Panel III; ILIBLA: International Lipid Information Bureau-Latin America; IDF: International Diabetes Federation; rNCEP-ATP III: revised NCEP-ATP III; AHA/NHLBI: American Heart Association/National Heart, Lung and Blood Institute; JIS: Joint Interim Statement
Reporting Quality according to the STROBE recommendations
Thirteen (76%) out of the 17 corresponding authors answered the emails, commenting and supporting if they agreed (5/13) or disagreed (8/13) with our analysis. Most of the disagreements were found in the recommendations related to the statistical analysis (sample size modification according to the sampling strategy, and the analysis of sensitivity, subgroups and missing data) and the description of the number of participants for each study phase. Based on these disagreements, each recommendation was reviewed again and answers were sent via email with the respective modifications.
Table 2 shows the number of articles that met each STROBE recommendation. The recommendations that were fully met were those related to the reporting of the reasons and rationale of the investigation (recommendation 2), to the reporting of the study design (recommendation 4) and to provide summary measures (recommendation 15). In addition, 16 out of the 17 articles adequately defined their variables and their respective measurement methods (recommendation 7 and 8). They also reported how they grouped and analyzed the quantitative variables (recommendation 11) and provided confidence intervals for their estimates (recommendation 16a). On the other hand, the recommendations with the lowest scores were those related to the description of the sensitivity analysis (recommendation 12e; 1/13 (8%)), to consider the use of a flowchart for the participants (recommendation 3; 3/17 (18%)), to explain the analysis of the missing data (recommendation 12c; 4/17 (24%)), to specify the number of participants in each study phase (recommendation 13a; 4/17 (24%)), to describe the reasons for the loss of the participants (recommendation 13b; 5/17 (29%)) and to specify the steps taken to identify possible sources of bias (recommendation 9; 7/17 [41%]). In supplementary material is shown the score assigned for each STROBE recommendation applicable per article (supplementary material (603.8KB, pdf) ). Only 3 out of the 17 articles met all the STROBE recommendations.
Table 2. Number of articles that fulfill each recommendation of the STROBE Statement.
| Section | Subsection | Code | Recommendation | Articles that fulfill each STROBE recommendation |
|---|---|---|---|---|
| n(%) | ||||
| Title and abstract | Title and abstract | 1a | Indicate the study's design with a commonly used term in the title or the abstract | 13 (76) |
| 1b | Provide in the abstract an informative and balanced summary of what was found | 15 (88) | ||
| Introduction | Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported. | 17 (100) |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses. | 13 (76) | |
| Methods | Study design | 4 | Present key elements of study design early in the paper. | 17 (100) |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection. | 10 (59) | |
| Participants | 6 | Cross-sectional study: give the eligibility criteria, and the sources and methods of selection of participants. | 17 (100) | |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable. | 16 (94) | |
| Data sources/measurement | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group. | 16 (94) | |
| Bias | 9 | Describe any efforts to address potential sources of bias. | 7 (41) | |
| Study size | 10 | Explain how the study size was arrived at. | 9 (53) | |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why. | 16 (94) | |
| Statistical methods | 12a | Describe all statistical methods, including those used to control for confounding. | 15 (88) | |
| 12b | Describe any methods used to examine subgroups and interactions. | 15 (88) | ||
| 12c | Explain how missing data was addressed. | 4 (24) | ||
| 12d | Cross-sectional study: If applicable, describe analytical methods taking account of sampling strategy. | 7 (41) | ||
| 12e | Describe any sensitivity analyses. | 1 (6) | ||
| Results | Participants | 13a | Report numbers of individuals at each stage of study-eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 4 (24) |
| 13b | Give reasons for non-participation at each stage. | 5 (299 | ||
| 13c | Consider use of a flow diagram. | 3 (18) | ||
| Descriptive data | 14a | Give characteristics of study participants (eg. Demographic, clinical, social) and information on exposures and potential confounders. | 13 (76) | |
| 14b | Indicate number of participants with missing data for each variable of interest. | 3 (18) | ||
| Outcome data | 15 | Cross-sectional study: report numbers of outcome events or summary measures. | 17 (100) | |
| Main results | 16a | Give unadjusted estimates and , if applicable, confounder-adjusted estimates and their precision (eg. 95% confidence interval). Make clear which confounders were adjusted for and why they were included. | 16 (94) | |
| 16b | Report category boundaries when continuous variables were categorized. | NA | ||
| 16c | If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period. | NA | ||
| Other analyses | 17 | Report other analyses done - eg. analyses of subgroups and interactions, and sensitivity analyses. | 15 (85) | |
| Discussion | Key results | 18 | Summarize key results with reference to study objectives. | 16 (94) |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias. | 9 (53) | |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence. | 14 (82) | |
| Generalisability | 21 | Discuss the generalizability (external validity) of the study results. | 13 (76) | |
| Other information | Funding | 22 | Give the sources of funding and the role of the funders for the present study and, if applicable, for the original study on which present article is based. | 13 (76) |
Discussion
The results of the analysis show that the fulfillment of the STROBE recommendations for the reporting of cross-sectional studies on the prevalence of MS was mixed, being the ones related to the methodology and results the lowest. These deficiencies are particularly critical for methodologically well-conducted studies and properly analyzed. For that reason, every scientific report, to be reliable, must provide a clear, complete and transparent presentation of what was planned, made and found, in order to facilitate the adequate interpretation and diffusion of their findings 3.
Of the 17 articles involving 12,789 people, 14 (11,804 people) showed limitations when reporting the methodology related to the statistical analysis, including sensitivity analysis, missing data and sources of bias. Also, the report of the results was not clear regarding the description of the participants flow and the reasons of loss in each study phase. These weaknesses are not unique to this scenario as further investigations, in fields other than MS, have used the recommendations of STROBE to analyze the report of other observational studies and had also described limitations in the same areas 39-45. On the other hand, 7 (41%) out of the 17 articles were published before the creation of STROBE and this could explain their weaknesses in its reporting in contrast to those published after (average score pre-STROBE 60%; average score post-STROBE: 77%). However, it has been shown that the quality of reporting of observational studies still remains suboptimal several years after the creation of STROBE 46 and that, despite some authors refer to follow the recommendations of STROBE, more than half do it inappropriately 6. Still, 3 articles (985 people) 30,32,35 mentioned using STROBE to write its report and when analyzed, it was certified that they met all recommendations.
The elaboration of an optimal research report is responsibility of the main authors, but several mechanisms (editorial policies editorial board, external reviewers) play a role in the process of publishing and also aim to an appropriate report 47. In order to achieve this objective, biomedical journals should adhere to reporting guidelines such as STROBE; also, editors and reviewers should be trained to demand its proper use 2. In the case of biomedical journals in Latin America and the Caribbean, only 28% recommends on its website any specific guideline to improve the quality of reporting 47. In this instance, although we have no data regarding the editorial policies of the journals at the time of publication of the selected articles, currently 43% of the 14 journals (2 of 3 peruvian journals and 4 of 11 foreign journals) suggest the authors to follow the STROBE recommendations as an editorial policy. The quality of reporting of observational and cross-sectional studies could be improved if journals introduce an active policy of adherence to reporting guidelines such as STROBE 46,48. Furthermore, an open access initiative that seeks to spread this kind of reporting guidelines is the EQUATOR Network 49 (http://www.equator-network.org/).
Even though there are barriers to provide more details of what has been done and found, such as the article's length allowed in biomedical journals or the cost of its publication, the prior publication of the study protocol or the publishing of supplementary data online (eg. https://figshare.com/) are some alternatives to overcome these limitations.
Considering the above, it should be noted that the scientific reports play a greater purpose in addition to the generation of new knowledge. In particular, epidemiological studies have different audiences, uses and implications. For a more technical audience, studies should report detailed estimates of the burden of the diseases that allow prioritization of public policies. On the other hand, in case of a more general audience, they should provide a consistent message about a particular situation. On both platforms, technical and general audiences, we found that the articles analyzed regarding MS in Peru have significant restrictions in its report that limit the appropriate use of their findings.
The limitations of our study are that some of our results might have been different if they were assesed by other researchers, however, to avoid subjective decisions, each corresponding author was contacted to verify our analysis, obtaining a high rate response (76%). Furthermore, we can not assume that every STROBE recommendation has the same impact on the quality of the report, thus assigning an equitable score to each recommendation could be considered as arbitrary. However, we decided to use this strategy to help readers have a global view of the quality of the reports.
Conclusion
Cross-sectional studies on the prevalence of MS in peruvian adults show an inadequate reporting of important areas such as methods and results. This finding identifies a clear need to improve the reporting quality of such studies in order to fulfill its role to adequately inform relevant subjects for the implementation of public health policies.
Electronic supplementary material
Table s1. Recommendations from the STROBE Statement fulfilled by each article.
Acknowledgements:
We want to thank Carlos Boissonnet, Haydeé Cardenas, Julio A. Chirinos, Jorge Escobedo, Bizu Gelaye, Mirella Guarnizo, Carlos Lorenzo and Jaime Pajuelo for their willingness to discuss and verify the information submitted in their original reports.
Footnotes
Funding: GM and JJM are affiliated to CRONICAS Centre of Excellence in Chronic Diseases at Universidad Peruana Cayetano Heredia, which is funded by the National Heart, Lung And Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract No. HHSN268200900033C
References
- 1.Lang T, Secic M. How to report statistics in medicine. Philadelphia: American College of Physicians; 2006. [Google Scholar]
- 2.Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014;383(9913):267–276. doi: 10.1016/S0140-6736(13)62228-X. [DOI] [PubMed] [Google Scholar]
- 3.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannocci A, Saulle R, Colamesta V, D'Aguanno S, Giraldi G, Maffongelli E, et al. What is the impact of reporting guidelines on Public Health journals in Europe? The case of STROBE, CONSORT and PRISMA. J Public Health (Oxf) 2015;37(4):737–740. doi: 10.1093/pubmed/fdu108. [DOI] [PubMed] [Google Scholar]
- 5.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Costa BR, Cevallos M, Altman DG, Rutjes AW, Egger M. Uses and misuses of the STROBE statement: bibliographic study. BMJ Open. 2011;1(1):e000048. doi: 10.1136/bmjopen-2010-000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 8.Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease. Diabetes Care. 2004;27(11):2676–2681. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 9.Reaven GM. The metabolic syndrome: time to get off the merry-go-round? J Intern Med. 2011;269(2):127–136. doi: 10.1111/j.1365-2796.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 10.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 11.Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–131. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- 12.Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C, American Heart Association et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 16.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Loannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Declaración de la Iniciativa STROBE (Strengthening the Reporting of Observational studies in Epidemiology): directrices para la comunicación de estudios observacionales. Gac Sanit. 2008;22(2):144–150. doi: 10.1157/13119325. [DOI] [PubMed] [Google Scholar]
- 19.Soto V, Vergara E, Neciosup E. Prevalencia y factores de riesgo de síndrome metabólico en población adulta del Departamento de Lambayeque, Perú - 2004. Rev Peru Med Exp Salud Publica. 2005;22(4):254–261. [Google Scholar]
- 20.Guarnizo PM, Loayza RG, Calvay SM, Ynami-Vizcaya M, Lázaro AH. Síndrome metabólico en una población pesquera y otra agropecuaria de la costa del Perú. Rev Soc Per Med Inter. 2006;29(3):685–691. [Google Scholar]
- 21.Lorenzo C, Serrano-Ríos M, Martínez-Larrad MT, González-Sánchez JL, Seclén S, Villena A, et al. Geographic variations of the International Diabetes Federation and the National Cholesterol Education Program-Adult Treatment Panel III definitions of the metabolic syndrome in nondiabetic subjects. Diabetes Care. 2006;29(3):685–691. doi: 10.2337/diacare.29.03.06.dc05-1796. [DOI] [PubMed] [Google Scholar]
- 22.Seclén S, Villena A, Larrad MT, Gamarra D, Herrera B, Pérez CF, et al. Prevalence of the metabolic syndrome in the mestizo population of Peru. Metab Syndr Relat Disord. 2006;4(1):1–6. doi: 10.1089/met.2006.4.1. [DOI] [PubMed] [Google Scholar]
- 23.Pajuelo J, Sánchez J. El síndrome metabólico en adultos, en el Perú. An Fac Med. 2007;68(1):38–46. [Google Scholar]
- 24.Medina-Lezama J, Zea-Diaz H, Morey-Vargas OL, Bolaños-Salazar JF, Muñoz-Atahualpa E, Postigo-MacDowall M, et al. Prevalence of the metabolic syndrome in Peruvian Andean hispanics: the PREVENCION study. Diabetes Res Clin Pract. 2007;78(2):270–281. doi: 10.1016/j.diabres.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Baracco R, Mohanna S, Seclén S. A comparison of the prevalence of metabolic syndrome and its components in high and low altitude populations in peru. Metab Syndr Relat Disord. 2007;5(1):55–62. doi: 10.1089/met.2006.0019. [DOI] [PubMed] [Google Scholar]
- 26.Schargrodsky H, Hernández-Hernández R, Champagne BM, Silva H, Vinueza R, Silva Ayçaguer LC, et al. CARMELA: assessment of cardiovascular risk in seven Latin American cities. Am J Med. 2008;121(1):58–65. doi: 10.1016/j.amjmed.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 27.Cárdenas QH, Sánchez AJ, Roldán AL, Mendoza TF. Prevalencia del síndrome metabólico en personas a partir de 20 años de edad. Perú, 2005. Rev Esp Salud Publica. 2009;83(2):257–265. doi: 10.1590/s1135-57272009000200009. [DOI] [PubMed] [Google Scholar]
- 28.Escobedo J, Schargrodsky H, Champagne B, Silva H, Boissonnet CP, Vinueza R, et al. Prevalence of the metabolic syndrome in Latin America and its association with sub-clinical carotid atherosclerosis: the CARMELA cross sectional study. Cardiovasc Diabetol. 2009;8:52. doi: 10.1186/1475-2840-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelaye B, Revilla L, Lopez T, Sanchez S, Williams MA. Prevalence of metabolic syndrome and its relationship with leisure time physical activity among Peruvian adults. Eur J Clin Invest. 2009;39(10):891–898. doi: 10.1111/j.1365-2362.2009.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masterson CRM, Smeeth L, Gilman RH, Miranda JJ. Physical activity and cardiovascular risk factors among rural and urban groups and rural-to-urban migrants in Peru: a cross-sectional study. Rev Panam Salud Publica. 2010;28(1):1–8. doi: 10.1590/s1020-49892010000700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina-Lezama J, Pastorius CA, Zea-Diaz H, Bernabe-Ortiz A, Corrales-Medina F, Morey-Vargas OL, et al. Optimal definitions for abdominal obesity and the metabolic syndrome in Andean Hispanics: the PREVENCION study. Diabetes Care. 2010;33(6):1385–1388. doi: 10.2337/dc09-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda JJ, Gilman RH, Smeeth L. Differences in cardiovascular risk factors in rural, urban and rural-to-urban migrants in Peru. Heart. 2011;97(10):787–796. doi: 10.1136/hrt.2010.218537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boissonnet C, Schargrodsky H, Pellegrini F, Macchia A, Marcet CB, Wilson E, et al. Educational inequalities in obesity, abdominal obesity, and metabolic syndrome in seven Latin American cities: the CARMELA Study. Eur J Cardiovasc Prev Rehabil. 2011;18(4):550–556. doi: 10.1177/1741826710389418. [DOI] [PubMed] [Google Scholar]
- 34.Pajuelo J, Sánchez-Abanto J, Torres H, Miranda M. Prevalencia del síndrome metabólico en pobladores peruanos por debajo de 1000 y por encima de los 3000 msnm. An Fac Med. 2012;73(2):101–106. [Google Scholar]
- 35.Bernabe-Ortiz A, Benziger CP, Gilman RH, Smeeth L, Miranda JJ. Sex differences in risk factors for cardiovascular disease: the PERU MIGRANT study. PLoS One. 2012;7(4):e35127. doi: 10.1371/journal.pone.0035127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Instituto Nacional de Salud del Perú . Encuesta Nacional de Indicadores Nutricionales, Bioquímicos, Socioeconómicos y Culturales relacionados con las enfermedades crónicas degenerativas. Ministerio de Salud del Peru; 2006. [31 12 2014]. Available from: http://www.minsa.gob.pe/portada/Especiales/2007/nutricion/publicaciones/INFORME_FINAL_ENIN.pdf. [Google Scholar]
- 37.Miranda JJ, Gilman RH, García HH, Smeeth L. The effect on cardiovascular risk factors of migration from rural to urban areas in Peru: PERU MIGRANT Study. BMC Cardiovasc Disord. 2009;9:23. doi: 10.1186/1471-2261-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medina-Lezama J, Chirinos JA, Zea Díaz H, Morey O, Bolanos JF, Munoz-Atahualpa E, et al. Design of PREVENCION: a population-based study of cardiovascular disease in Peru. Int J Cardiol. 2005;105(2):198–202. doi: 10.1016/j.ijcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 39.Egger M, Altman DG, Vandenbroucke JP, of the STROBE group Commentary: strengthening the reporting of observational epidemiology the STROBE statement. Int J Epidemiol. 2007;36(5):948–950. doi: 10.1093/ije/dym199. [DOI] [PubMed] [Google Scholar]
- 40.Fung AE, Palanki R, Bakri SJ, Depperschmidt E, Gibson A. Applying the CONSORT and STROBE statements to evaluate the reporting quality of neovascular age-related macular degeneration studies. Ophthalmology. 2009;116(2):286–296. doi: 10.1016/j.ophtha.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Müller M, Egger M. Strengthening the reporting of observational epidemiology (STROBE) in sexual health. Sex Transm Infect. 2009;85(3):162–164. doi: 10.1136/sti.2007.028985. [DOI] [PubMed] [Google Scholar]
- 42.Papathanasiou AA, Zintzaras E. Assessing the quality of reporting of observational studies in cancer. Ann Epidemiol. 2010;20(1):67–73. doi: 10.1016/j.annepidem.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Galera LJ, Lahoz GR, Roig LF. Comunicación de los resultados de la investigación observacional: análisis mediante la guía Strobe. Rev Esp Salud Publica. 2011;85(6):583–591. doi: 10.1590/S1135-57272011120000007. [DOI] [PubMed] [Google Scholar]
- 44.Bastuji-Garin S, Sbidian E, Gaudy-Marqueste C, Ferrat E, Roujeau J-C, Richard M-A, et al. Impact of STROBE statement publication on quality of observational study reporting: interrupted time series versus before-after analysis. PLoS One. 2013;8(8):e64733. doi: 10.1371/journal.pone.0064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeelani A, Malik W, Haq I, Aleem S, Mujtaba M, Syed N. Cross-sectional studies published in Indian journal of community medicine: evaluation of adherence to strengthening the reporting of observational studies in epidemiology statement. Ann Med Health Sci Res. 2014;4(6):875–878. doi: 10.4103/2141-9248.144889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pouwels KB, Widyakusuma NN, Groenwold RH, Hak E. Quality of reporting of confounding remained suboptimal after the STROBE guideline. J Clin Epidemiol. 2016;69:217–224. doi: 10.1016/j.jclinepi.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Glujovsky D, Villanueva E, Reveiz L, Murasaki R. Adherencia a las guías de informe sobre investigaciones en revistas biomédicas en América Latina y el Caribe. Rev Panam Salud Publica. 2014;36:214–237. [PubMed] [Google Scholar]
- 48.Hopewell S, Ravaud P, Baron G, Boutron I. Effect of editors' implementation of CONSORT guidelines on the reporting of abstracts in high impact medical journals: interrupted time series analysis. BMJ. 2012;344:e4178. doi: 10.1136/bmj.e4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popham K, Calo WA, Carpentier MY, Chen NE, Kamrudin SA, Le YC, et al. Reporting guidelines: optimal use in preventive medicine and public health. Am J Prev Med. 2012;43(4):e31–42. doi: 10.1016/j.amepre.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table s1. Recommendations from the STROBE Statement fulfilled by each article.