Abstract
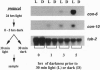
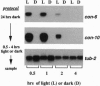
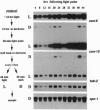
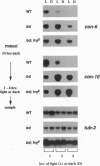
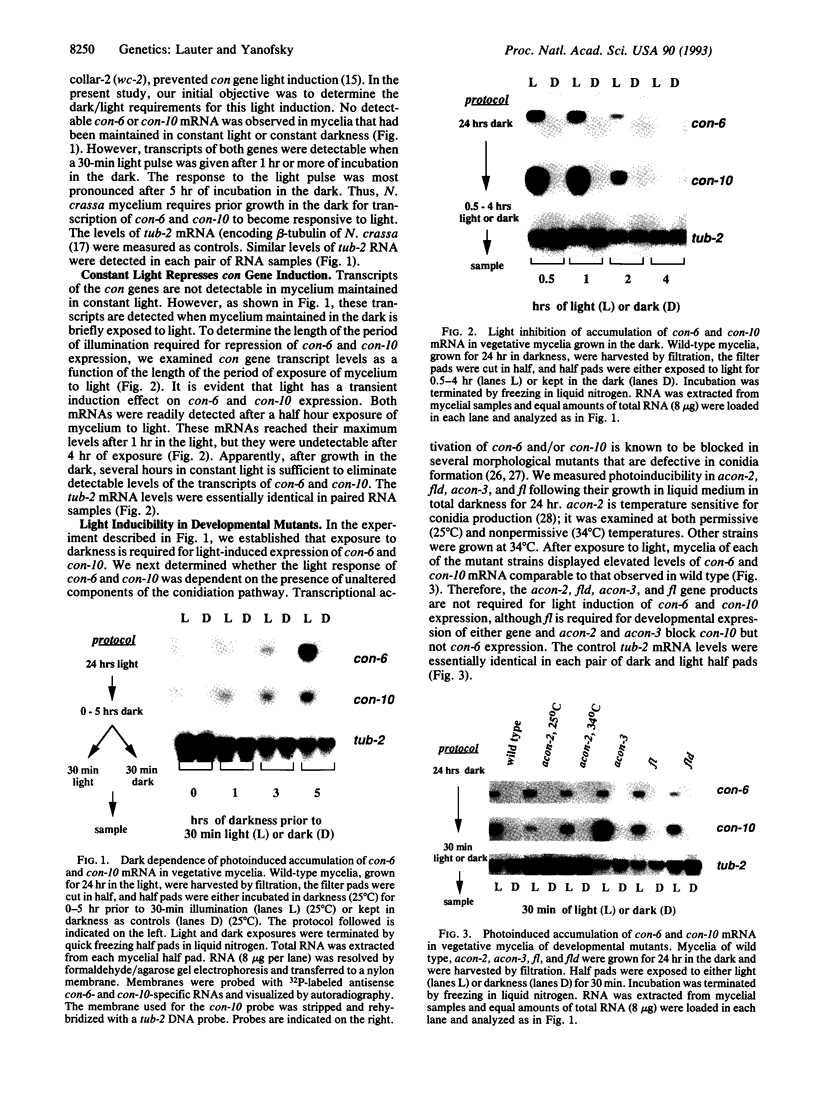
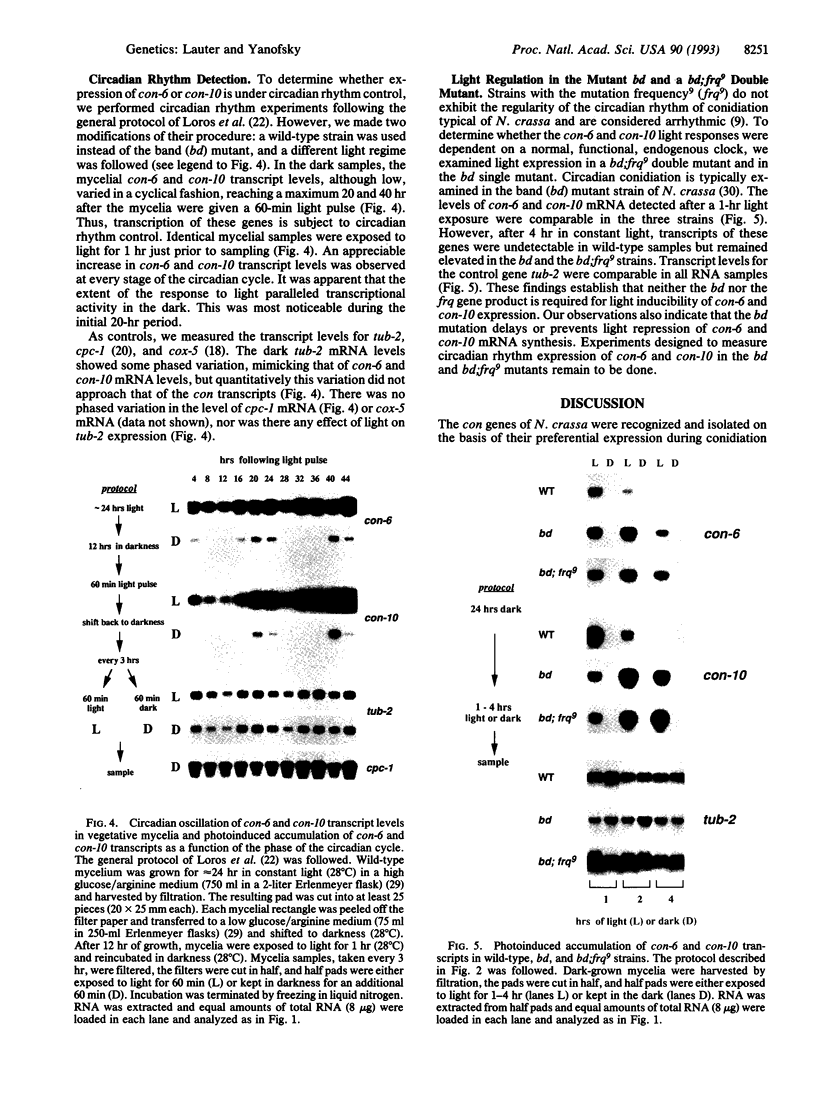
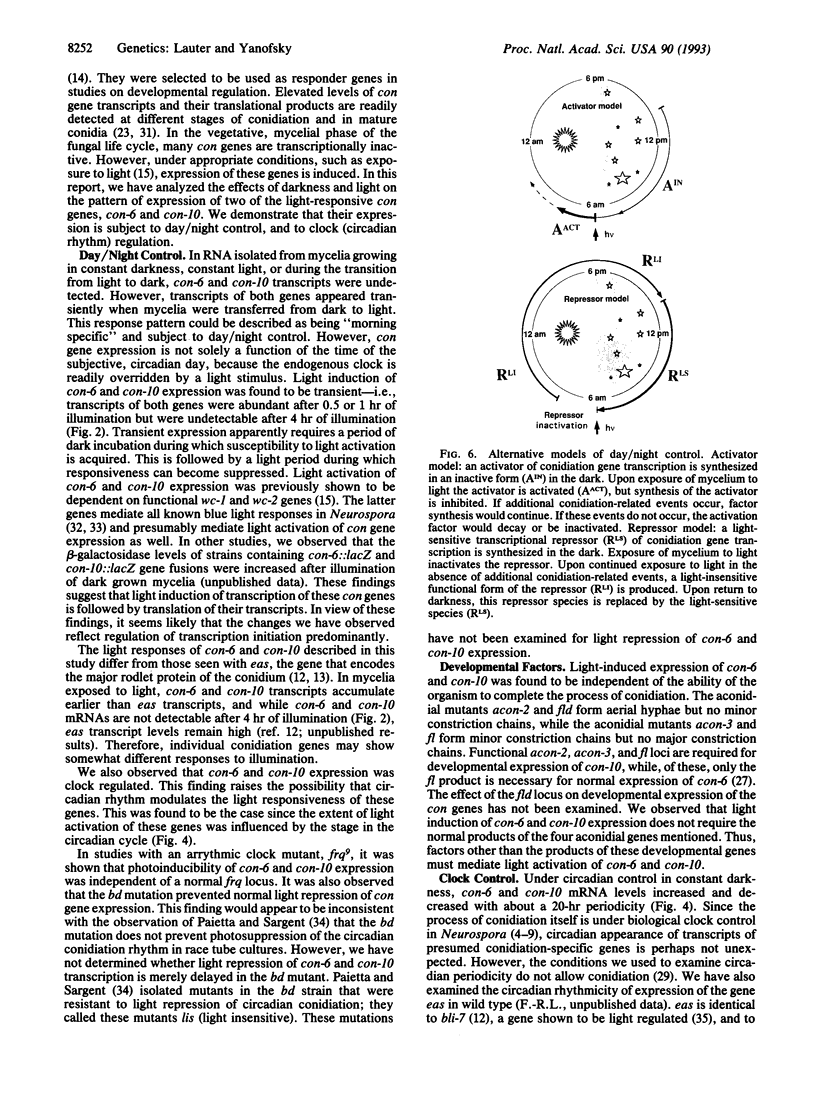
In the filamentous fungus Neurospora crassa, several events in the process of conidiation are influenced by light. Two genes, con-6 and con-10, which were previously shown to be transcriptionally activated during conidiation and by exposure to light, were found to be unexpressed in mycelium maintained in constant darkness or in constant light. However, when mycelium was shifted from darkness to light, transcripts of both genes appeared and were abundant. Upon further illumination both transcripts disappeared--i.e., their continued production was light repressed. When dark-grown mycelium was exposed to a light pulse and reincubated in the dark, expression of con-6 and con-10 exhibited a 20-hr circadian periodicity. Both genes were photoinducible throughout the stages of the circadian cycle. In the mutant strains bd and bd;frq9, con-6 and con-10 were light inducible but were not normally light repressible. Mutant genes such as acon-2, acon-3, and fl that block developmental expression of con-6 and/or con-10 did not prevent their photoinduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beever R. E., Dempsey G. P. Function of rodlets on the surface of fungal spores. Nature. 1978 Apr 13;272(5654):608–610. doi: 10.1038/272608a0. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D., Dunlap J. C., Loros J. J. The Neurospora circadian clock-controlled gene, ccg-2, is allelic to eas and encodes a fungal hydrophobin required for formation of the conidial rodlet layer. Genes Dev. 1992 Dec;6(12A):2382–2394. doi: 10.1101/gad.6.12a.2382. [DOI] [PubMed] [Google Scholar]
- Berlin V., Yanofsky C. Isolation and characterization of genes differentially expressed during conidiation of Neurospora crassa. Mol Cell Biol. 1985 Apr;5(4):849–855. doi: 10.1128/mcb.5.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig I. Sex determination: zinc fingers point in the wrong direction. Trends Genet. 1990 May;6(5):135–137. doi: 10.1016/0168-9525(90)90131-o. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Kornhauser J. M., Nelson D. E., Mayo K. E., Takahashi J. S. Regulation of jun-B messenger RNA and AP-1 activity by light and a circadian clock. Science. 1992 Mar 20;255(5051):1581–1584. doi: 10.1126/science.1549784. [DOI] [PubMed] [Google Scholar]
- Lauter F. R., Russo V. E. Blue light induction of conidiation-specific genes in Neurospora crassa. Nucleic Acids Res. 1991 Dec 25;19(24):6883–6886. doi: 10.1093/nar/19.24.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter F. R., Russo V. E. Light-induced dephosphorylation of a 33 kDa protein in the wild-type strain of Neurospora crassa: the regulatory mutants wc-1 and wc-2 are abnormal. J Photochem Photobiol B. 1990 Apr 1;5(1):95–103. doi: 10.1016/1011-1344(90)85008-k. [DOI] [PubMed] [Google Scholar]
- Lauter F. R., Russo V. E., Yanofsky C. Developmental and light regulation of eas, the structural gene for the rodlet protein of Neurospora. Genes Dev. 1992 Dec;6(12A):2373–2381. doi: 10.1101/gad.6.12a.2373. [DOI] [PubMed] [Google Scholar]
- Loros J. J., Denome S. A., Dunlap J. C. Molecular cloning of genes under control of the circadian clock in Neurospora. Science. 1989 Jan 20;243(4889):385–388. doi: 10.1126/science.2563175. [DOI] [PubMed] [Google Scholar]
- Loros J. J., Feldman J. F. Loss of temperature compensation of circadian period length in the frq-9 mutant of Neurospora crassa. J Biol Rhythms. 1986 Fall;1(3):187–198. doi: 10.1177/074873048600100302. [DOI] [PubMed] [Google Scholar]
- Matsuyama S. S., Nelson R. E., Siegel R. W. Mutations specifically blocking differentiation of macroconidia Neurospora crassa. Dev Biol. 1974 Dec;41(2):278–287. doi: 10.1016/0012-1606(74)90306-6. [DOI] [PubMed] [Google Scholar]
- Orbach M. J., Porro E. B., Yanofsky C. Cloning and characterization of the gene for beta-tubulin from a benomyl-resistant mutant of Neurospora crassa and its use as a dominant selectable marker. Mol Cell Biol. 1986 Jul;6(7):2452–2461. doi: 10.1128/mcb.6.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paietta J., Sargent M. L. Isolation and characterization of light-insensitive mutants of Neurospora crassa. Genetics. 1983 May;104(1):11–21. doi: 10.1093/genetics/104.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh J. L., Orbach M. J., Legerton T. L., Yanofsky C. The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-jun-encoded protein. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3728–3732. doi: 10.1073/pnas.85.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D., Radford A., Newmeyer D., Björkman M. Chromosomal loci of Neurospora crassa. Microbiol Rev. 1982 Dec;46(4):426–570. doi: 10.1128/mr.46.4.426-570.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. N., Berlin V., Hager K. M., Yanofsky C. Molecular analysis of a Neurospora crassa gene expressed during conidiation. Mol Cell Biol. 1988 Jun;8(6):2411–2418. doi: 10.1128/mcb.8.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. N., Yanofsky C. Genes expressed during conidiation in Neurospora crassa: characterization of con-8. Nucleic Acids Res. 1989 Jan 11;17(1):197–214. doi: 10.1093/nar/17.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. S., David M., Werner S., RajBhandary U. L. Nuclear genes for cytochrome c oxidase subunits of Neurospora crassa. Isolation and characterization of cDNA clones for subunits IV, V, VI, and possibly VII. J Biol Chem. 1986 Jan 15;261(2):869–873. [PubMed] [Google Scholar]
- Sachs M. S., Yanofsky C. Developmental expression of genes involved in conidiation and amino acid biosynthesis in Neurospora crassa. Dev Biol. 1991 Nov;148(1):117–128. doi: 10.1016/0012-1606(91)90322-t. [DOI] [PubMed] [Google Scholar]
- Sargent M. L., Briggs W. R., Woodward D. O. Circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiol. 1966 Oct;41(8):1343–1349. doi: 10.1104/pp.41.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Kaltenborn S. H. Effects of medium composition and carbon dioxide on circadian conidiation in neurospora. Plant Physiol. 1972 Jul;50(1):171–175. doi: 10.1104/pp.50.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. W., Matsuyama S. S., Urey J. C. Induced macroconidia formation in Neurospora crassa. Experientia. 1968 Nov 15;24(11):1179–1181. doi: 10.1007/BF02147840. [DOI] [PubMed] [Google Scholar]
- Sommer T., Chambers J. A., Eberle J., Lauter F. R., Russo V. E. Fast light-regulated genes of Neurospora crassa. Nucleic Acids Res. 1989 Jul 25;17(14):5713–5723. doi: 10.1093/nar/17.14.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. L., Hager K. M., Garrett-Engele C., Yanofsky C. Timing of synthesis and cellular localization of two conidiation-specific proteins of Neurospora crassa. Dev Biol. 1992 Aug;152(2):255–262. doi: 10.1016/0012-1606(92)90133-2. [DOI] [PubMed] [Google Scholar]
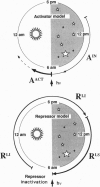
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]