Abstract
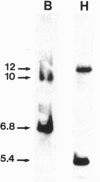
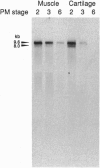
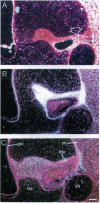
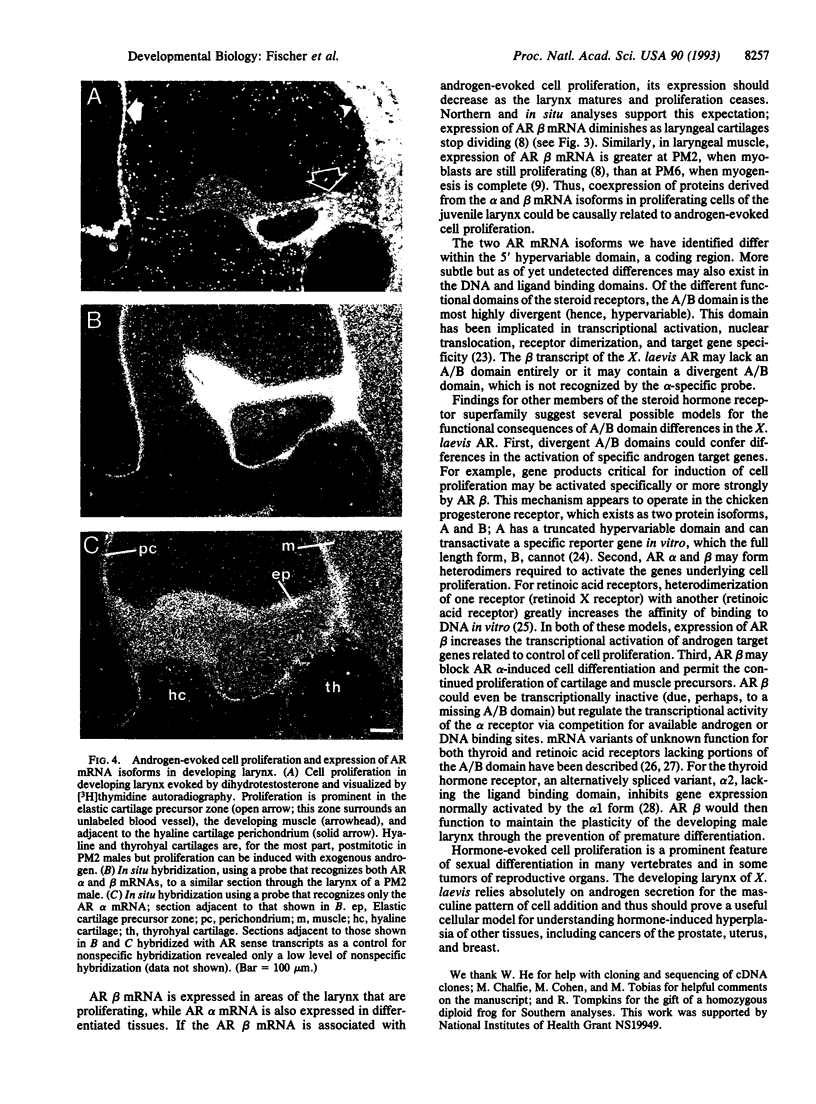
The larynx of male Xenopus laevis undergoes an androgen-driven developmental transformation that enables the adult to produce his complex mate attraction song. During the early postmetamorphic period, androgen directs proliferation and differentiation of laryngeal muscle and cartilage. To explore the cellular and molecular basis of androgen control, we have cloned an androgen receptor cDNA from juvenile larynx. Here we identify two androgen receptor mRNA isoforms, alpha and beta, differing within the A/B, or hypervariable, domain. Northern blot analyses reveal that the beta isoform is transiently expressed during early juvenile stages, whereas the alpha transcript is expressed throughout postmetamorphic life. Using in situ hybridization and [3H]thymidine autoradiography, we examined the expression of androgen receptor mRNA isoforms during androgen-evoked cell proliferation and differentiation. The alpha and beta transcripts are coexpressed in proliferating tissues of the juvenile larynx; in postmitotic differentiated tissues, only the alpha transcript is expressed. Because androgen receptor beta mRNA is specifically expressed during hormone-evoked cell proliferation, we propose that this developmentally regulated mRNA isoform is required for the masculine program of cell addition within the developing vocal organ.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988 Apr 15;240(4850):324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Faber P. W., van Rooij H. C., van der Korput H. A., Baarends W. M., Brinkmann A. O., Grootegoed J. A., Trapman J. Characterization of the human androgen receptor transcription unit. J Biol Chem. 1991 Jun 15;266(17):10743–10749. [PubMed] [Google Scholar]
- Forrest D., Sjöberg M., Vennström B. Contrasting developmental and tissue-specific expression of alpha and beta thyroid hormone receptor genes. EMBO J. 1990 May;9(5):1519–1528. doi: 10.1002/j.1460-2075.1990.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlick D. L., Kelley D. B. The ontogeny of androgen receptors in the CNS of Xenopus laevis frogs. Brain Res. 1986 May;391(2):193–200. doi: 10.1016/0165-3806(86)90283-x. [DOI] [PubMed] [Google Scholar]
- He W. W., Fischer L. M., Sun S., Bilhartz D. L., Zhu X. P., Young C. Y., Kelley D. B., Tindall D. J. Molecular cloning of androgen receptors from divergent species with a polymerase chain reaction technique: complete cDNA sequence of the mouse androgen receptor and isolation of androgen receptor cDNA probes from dog, guinea pig and clawed frog. Biochem Biophys Res Commun. 1990 Sep 14;171(2):697–704. doi: 10.1016/0006-291x(90)91202-4. [DOI] [PubMed] [Google Scholar]
- Hodin R. A., Lazar M. A., Wintman B. I., Darling D. S., Koenig R. J., Larsen P. R., Moore D. D., Chin W. W. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989 Apr 7;244(4900):76–79. doi: 10.1126/science.2539642. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Mendelsohn C., Garnier J. M., Zelent A., Leroy P., Staub A., Chambon P. Murine isoforms of retinoic acid receptor gamma with specific patterns of expression. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2700–2704. doi: 10.1073/pnas.87.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D., Sassoon D., Segil N., Scudder M. Development and hormone regulation of androgen receptor levels in the sexually dimorphic larynx of Xenopus laevis. Dev Biol. 1989 Jan;131(1):111–118. doi: 10.1016/s0012-1606(89)80042-9. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Melton D. A. Expression of Xenopus N-CAM RNA in ectoderm is an early response to neural induction. Development. 1987 Mar;99(3):311–325. doi: 10.1242/dev.99.3.311. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig R. J., Lazar M. A., Hodin R. A., Brent G. A., Larsen P. R., Chin W. W., Moore D. D. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989 Feb 16;337(6208):659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- Krotoski D. M., Reinschmidt D. C., Tompkins R. Developmental mutants isolated from wild-caught Xenopus laevis by gynogenesis and inbreeding. J Exp Zool. 1985 Mar;233(3):443–449. doi: 10.1002/jez.1402330313. [DOI] [PubMed] [Google Scholar]
- Leroy P., Krust A., Zelent A., Mendelsohn C., Garnier J. M., Kastner P., Dierich A., Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991 Jan;10(1):59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sullivan P. M., Willard H. F., French F. S., Wilson E. M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988 Apr 15;240(4850):327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Griffin J. E., Wilson J. D., McPhaul M. J. Definition of the human androgen receptor gene structure permits the identification of mutations that cause androgen resistance: premature termination of the receptor protein at amino acid residue 588 causes complete androgen resistance. Mol Endocrinol. 1990 Aug;4(8):1105–1116. doi: 10.1210/mend-4-8-1105. [DOI] [PubMed] [Google Scholar]
- Marin M. L., Tobias M. L., Kelley D. B. Hormone-sensitive stages in the sexual differentiation of laryngeal muscle fiber number in Xenopus laevis. Development. 1990 Nov;110(3):703–711. doi: 10.1242/dev.110.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissant C., Houdebine L. M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990 Feb;8(2):148–149. [PubMed] [Google Scholar]
- Sassoon D., Kelley D. B. The sexually dimorphic larynx of Xenopus laevis: development and androgen regulation. Am J Anat. 1986 Dec;177(4):457–472. doi: 10.1002/aja.1001770404. [DOI] [PubMed] [Google Scholar]
- Sassoon D., Segil N., Kelley D. Androgen-induced myogenesis and chondrogenesis in the larynx of Xenopus laevis. Dev Biol. 1986 Jan;113(1):135–140. doi: 10.1016/0012-1606(86)90115-6. [DOI] [PubMed] [Google Scholar]
- Tobias M. L., Kelley D. B. Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. J Neurosci. 1987 Oct;7(10):3191–3197. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M. L., Marin M. L., Kelley D. B. Development of functional sex differences in the larynx of Xenopus laevis. Dev Biol. 1991 Sep;147(1):251–259. doi: 10.1016/s0012-1606(05)80022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., Gronemeyer H., Turcotte B., Gaub M. P., Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988 May 12;333(6169):185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., George F. W., Griffin J. E. The hormonal control of sexual development. Science. 1981 Mar 20;211(4488):1278–1284. doi: 10.1126/science.7010602. [DOI] [PubMed] [Google Scholar]