Abstract
Overweight and obesity are global health concerns. Various effective weight-loss diets have been developed, including the Atkins diet. The Atkins diet is known as an extreme low-carbohydrate diet. This diet reduces body weight and has gained widespread popularity. However, the metabolite profiles of such a diet have been shown to be detrimental to colonic health. Therefore, a concern for the long-term health effects of this diet exists. We encountered a case in which ulcerative colitis developed while the patient was following the Atkins diet.
A man, 172 cm in height and weighing 72 kg, at age 36 years followed a low-carbohydrate weight-loss diet. His weight decreased to 66 kg as desired. Thereafter he noticed bloody stool. Colonoscopy revealed diffuse inflammation limited to the rectum, and he was diagnosed with ulcerative colitis. He underwent an educational hospitalization for ulcerative colitis. A plant-based/semivegetarian diet was provided during hospitalization. Bloody stool disappeared during hospitalization and he achieved remission without medication for inflammatory bowel disease.
This case indicates that an onset of ulcerative colitis can be an adverse event to a low-carbohydrate weight-loss diet.
INTRODUCTION
Overweight and obesity are global health concerns.1 Various effective weight-loss diets have been developed, including the Atkins diet.2–5 The Institute of Medicine’s Acceptable Macro-nutrient Distribution Ranges of carbohydrate, protein, and fat for energy are 45% to 65%, 20% to 35%, and 10% to 35%, respectively.3 The Atkins diet is known as an extreme low-carbohydrate weight-loss diet (LCHWLD) and results in extreme high-fat content: percentages of carbohydrate, protein, and fat are 9%, 29%, and 62%, respectively.3 This diet reduces body weight, blood pressure, and triglyceride levels and has gained widespread popularity.2–5 However, the metabolite profiles of such a diet have been shown to be detrimental to colonic health.6–8 For example, butyrate, a key short-chain fatty acid in colonic homeostasis, significantly decreased in fecal samples.6 Therefore, concerns for the long-term health effects of this diet have been expressed.2,3,6–8 We encountered a case that seemed to substantiate this concern, in which ulcerative colitis developed during adherence to an LCHWLD.
CASE PRESENTATION
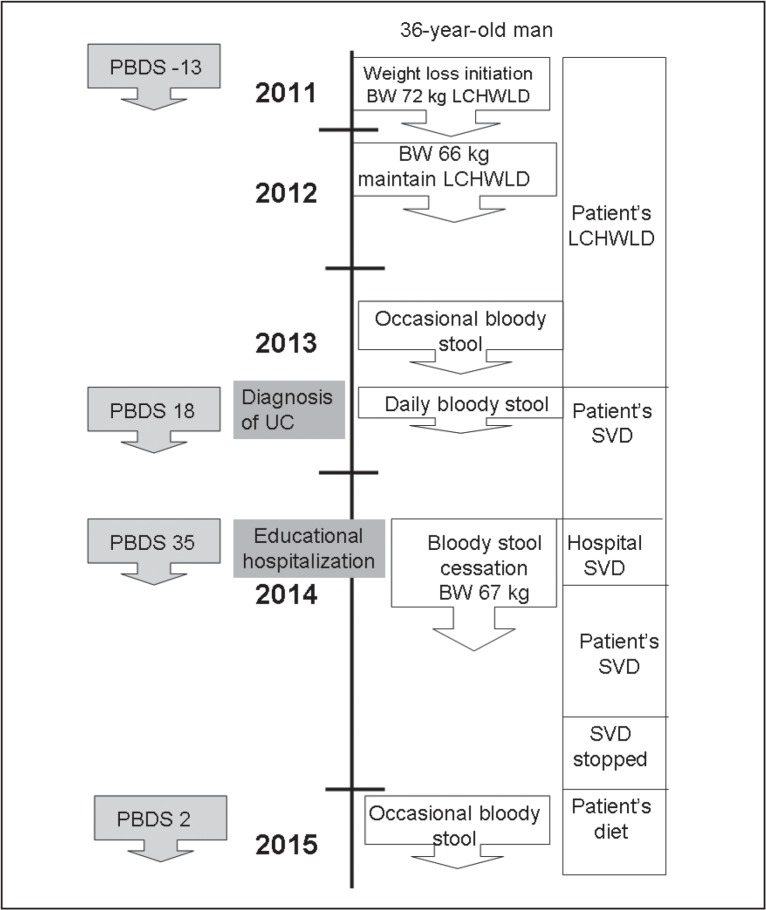
A 38-year-old man, a physician, 172 cm in height and 67 kg in body weight, was referred to the Division of Gastroenterology in late November 2013, with a chief complaint of bloody stool. He had undergone an appendectomy for acute appendicitis at age 27 years. He had no family history of inflammatory bowel disease (IBD). He practiced martial arts in his mid-20s. His maximum body weight was 76 kg. He started to follow an LCHWLD in August 2011 (Figure 1). He decreased his white rice consumption to approximately one-third and consumed meat every day and minced or processed meat 3 to 5 servings per week (Table 1). His body weight decreased from 72 kg to 66 kg as desired, and he continued the diet. He often noticed bloody stool beginning in April 2013.
Figure 1.
Timeline of case.
BW = body weight; LCHWLD = low-carbohydrate weight-loss diet;
PBDS = plant-based diet score; SVD = semivegetarian diet; UC = ulcerative colitis.
Table 1.
Plant-based diet score for Japanese patients with inflammatory bowel disease
| Food group | Comment | Scoring by frequency of consumption | Measured plant-based diet score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Daily | 3–5 servings/week | 1–2 servings/week | Rarely | Patient’s LCHWLD | Patient’s SVD after ulcerative colitis diagnosis | SVD during hospitalization | 10 months after discharge | ||
| Vegetables | PBD, preventive factor for IBD | 5 | 3 | 1 | 0 | 3 | 3 | 5 | 3 |
| Fruits | PBD, preventive factor for IBD | 5 | 3 | 1 | 0 | 1 | 1 | 5 | 1 |
| Pulses (beans, soybeans, peas, etc) | PBD | 5 | 3 | 1 | 0 | 1 | 3 | 5 | 1 |
| Potatoes/starches | PBD | 5 | 3 | 1 | 0 | 0 | 3 | 5 | 0 |
| Rice | Washoku | 5 | 3 | 1 | 0 | 5 | 5 | 5 | 3 |
| Miso soup | Washoku | 5 | 3 | 1 | 0 | 3 | 3 | 5 | 1 |
| Green teaa | Washoku | 5 | 3 | 1 | 0 | 0 | 5 | 0a | 5 |
| Yogurt (plain) | Probiotic | 5 | 3 | 1 | 0 | 0 | 5 | 5 | 3 |
| Beef/pork/chicken | Western diet, risk factor for IBD | −5 | −3 | −1 | 0 | −5 | 0 | 0 | −1 |
| Minced or processed meat | Western diet | −5 | −3 | −1 | 0 | −3 | 0 | 0 | −1 |
| Cheese/butter/margarine | Western diet, risk factor for IBD | −5 | −3 | −1 | 0 | −1 | −1 | 0 | −1 |
| Sweets/ice cream/milk shake | Western diet, risk factor for IBD | −5 | −3 | −1 | 0 | −3 | −3 | 0 | −3 |
| Soft drinks/fruit juice | Western diet, risk factor for IBD | −5 | −3 | −1 | 0 | −3 | −1 | 0 | −3 |
| Alcohol | Risk factor for IBD | −5 | −3 | −1 | 0 | −5 | 0 | 0 | −1 |
| Bread | Risk factor for Japanese IBD | −5 | −3 | −1 | 0 | −5 | −5 | 0 | −5 |
| Fish | Risk factor for Japanese IBD | −2 | −1 | 0 | 0 | −1 | 0 | 0 | 0 |
| Plant-based diet score | −13 | 18 | 35 | 2 | |||||
Green tea is recommended to drink at home but is not provided at the hospital.
IBD = inflammatory bowel disease; LCHWLD = low-carbohydrate weight-loss diet; PBD = component of plant-based diet; SVD = semivegetarian diet; Washoku = component of traditional Japanese diet.
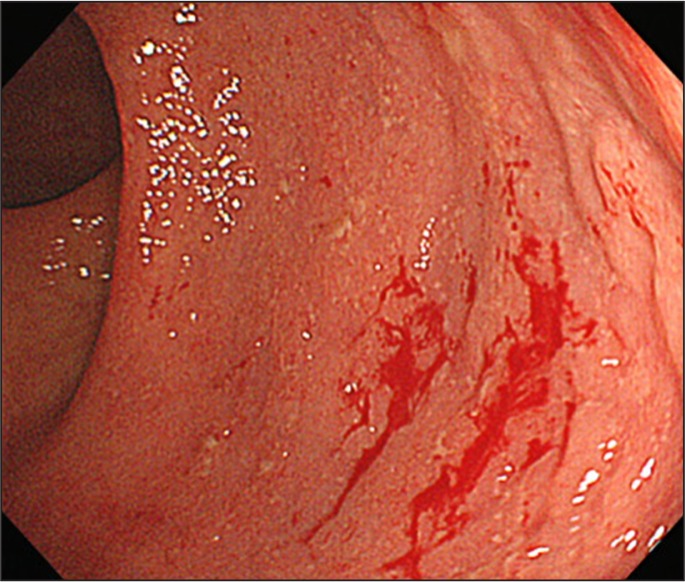
An abnormal fecal occult blood test was identified after a periodic health examination in October 2013. Colonos-copy revealed diffuse inflammation limited to the rectum (Figure 2). The histologic findings were consistent with ulcerative colitis: cryptitis, goblet cell depletion, and inflammatory cell infiltration. Results of routine laboratory studies, including a hematology study, liver and kidney function tests, C-reactive protein, and erythrocyte sedimentation rate, were normal. There was no increase in the number of eosinophils in the peripheral blood. Clostridium difficile antigen and toxin were not detected by a rapid membrane enzyme immunoassay (TECHLAB C diff Quik Chek Complete, TECHLAB Inc, Blacksburg, VA). Stool culture did not reveal any pathogen, including enterohemorrhagic Escherichia coli, Campylobacter jejuni, Salmonella spp, Staphylococcus aureus, and Klebsiella oxytoca. No abnormality was found in a double-contrast barium enema study. Ultimately, a proctitis type of ulcerative colitis was diagnosed.
Figure 2.
Photograph taken during colonoscopy. Diffuse inflammation was observed in the rectum. A small amount of blood was also observed.
The patient knew of a semivegetarian diet to treat IBD,9 and he therefore attempted to replace the LCHWLD with the semivegetarian diet. Although he noticed a decrease in the amount of blood in his feces, bloody stool continued. He did not indicate any stress from his family or his work.
The patient was referred to our Division and underwent an 11-day “educational hospitalization” for ulcerative colitis10 in late March 2014. He had a good appetite and no abdominal pain. Physical examination, including of the abdomen, found no abnormalities. His retrospective food-frequency questionnaire identified plant-based diet score11 was −13 (see Discussion) during an LCHWLD and +18 after the diagnosis of ulcerative colitis (Table 1). A semivegetarian diet (plant-based diet score = 35) was provided during the hospitalization (Table 1). He noticed that the bloody stool disappeared during hospitalization. At the end of the hospitalization, a fecal occult blood test showed a normal result (< 50 ng/mL). No medication was provided during hospitalization. He was advised to continue the semivegetarian diet after discharge.
The patient remained in remission until January 2015, when he again noticed faint blood in his stool every other day. His latest plant-based diet score, 10 months after discharge, was 2 (Table 1, Figure 1). He mentioned that he had been too busy to maintain a plant-based diet but would try to restore his diet to a sound plant-based diet. No medication was prescribed.
DISCUSSION
Progress in medicine has revealed that an imbalance (dysbiosis) or metabolism of gut microflora is a critical factor in various chronic diseases.12–15 It has also been shown that gut microflora is formed by diet.16–19 A recent large-scale prospective cohort study showed that intake of dietary fiber, nondigestible carbohydrates, reduces the risk of overall mortality and death caused by cardiovascular disease, infectious diseases, respiratory diseases, and cancer.20 A low consumption of carbohydrates has been shown to result in a significantly higher risk of all-cause mortality.21–23 These studies imply a critical role of diet and carbohydrates, particularly dietary fiber, in our health. The favorable effects of high amounts of dietary fiber in IBD have recently been stressed.24
An LCHWLD is an extreme low-carbohydrate and extreme high-fat diet that results in decreased consumption of nondigestible carbohydrates.3 LCHWLD can be thought of as an extreme example of dietary westernization. Concerns for the long-term health effects of this diet have been expressed.2,3,6–8 Various minor adverse events associated with LCHWLD have been reported: headache, halitosis, constipation, muscle cramps, general weakness, diarrhea, and rash.2
To our knowledge, there have been no documented cases of newly diagnosed ulcerative colitis during LCHWLD. It is assumed that there are similar cases, but they have not been reported. We expect additional cases will be reported after this initial report. This case report provides additional information regarding the risk of LCHWLD.
Although the precise mechanisms have yet to be determined, epidemiology provides convincing evidence that a plant-based diet is a healthy diet providing therapeutic and/or preventive effects against current major chronic diseases.25–28 We recognize IBD as a lifestyle-related disease mediated mainly by a westernized diet. The greatest environmental factor in IBD is diet-associated gut microflora.29 Diets rich in animal protein and animal fat cause a decrease in beneficial bacteria in the intestine.18,19,30,31 Prebiotics, foods that increase beneficial bacteria in the gut, are mostly extracts of plants.32 Therefore, a semivegetarian diet was designed to combat dietary westernization.9,24,29,33,34 Our semivegetarian diet is a lacto-ovo-vegetarian diet with an additional serving of fish once a week and meat once every 2 weeks.9 This semivegetarian diet is primarily a plant-based diet. Contemporary meals are far from a semivegetarian diet. Dietary intervention to restore dysbiosis in gut microflora is essential in the treatment of IBD. We treat patients with moderate or severe IBD with drugs and a semivegetarian diet. For mild ulcerative colitis, we recommend an educational hospitalization for about 2 weeks to familiarize patients with a semivegetarian diet.10 More than 50% of our patients experience an improvement in symptoms and/or a decrease in amount of fecal occult blood without medication by the end of hospitalization.10
To facilitate an efficient training tool regarding a plant-based diet, we developed a simple scoring method to evaluate the efficacy of a plant-based diet.11 Components of the plant-based diet35 with or without preventive factors for IBD are scored positively: pulses (beans, soybeans, peas), potatoes/starches, vegetables,36 and fruits.36 Components of a westernized diet37 with or without risk factors for IBD are scored negatively: meat,36,38–42 minced or processed meat, cheese/butter/margarine,39,40,42,43 soft drinks,44 and sweets.36,39,40,43,45 Components of the traditional Japanese diet, known as Washoku,46 are scored positively: rice, miso soup, and green tea.39,40 Risk factors for IBD in Japanese individuals are scored negatively: fish41,45 and bread.43 Plain yogurt, a probiotic, is scored positively. Alcohol is scored negatively.47 Scores of 5, 3, and 1 are given for each food group according to the frequency of consumption, as shown in Table 1. A plant-based diet score is developed from the sum of plus and minus scores (Table 1). The maximum positive score is 35 for inpatients and 40 for outpatients because green tea is not provided as a drink at the hospital. The maximum negative score is −37. A higher plant-based diet score indicates a greater adherence to a plant-based diet.
In this case, a plant-based diet score of −13 was obtained during the patient’s LCHWLD; a score of 18, during the patient’s designed semivegetarian diet after the ulcerative colitis diagnosis; and a score of 35, while receiving the hospital’s semivegetarian diet (Table 1). He achieved remission with the hospital’s semivegetarian diet. In this case of mild inflammation, medication was unnecessary to induce remission. Although a plant-based diet was recommended as a dietary pattern as part of his lifestyle, it was not attained in this case. His latest plant-based diet score was only 2, and he had very mild symptoms of ulcerative colitis.
Dietary fiber affects production of short-chain fatty acids, including butyrate, through fermentation of Faecalibacterium prausnitzii and Roseburia spp. Butyrate plays a major role in gut homeostasis owing to a variety of functions, including anti-inflammation, modulation of oxidative stress, energy for colonocytes, maintenance of epithelial permeability, and maintenance in gut barrier integrity.6–8,20,48 Short-chain fatty acid lowers stool pH, resulting in suppression of potentially pathogenic bacteria.49 An LCHWLD is definitely deprived of dietary fiber.3 Short-chain fatty acids including butyrate, stool pH, and gut microflora were not studied in this patient. It is plausible that LCHWLD in a susceptible individual can cause dysbiosis of gut microflora, low production of butyrate, elevation of stool pH, disruption of homeostasis, and onset of ulcerative colitis. The patient’s chronology, onset of ulcerative colitis during LCHWLD, and amelioration associated with shifting of the diet to a semivegetarian diet, indicate that dietary factors played a critical role in this case.
CONCLUSION
Weight loss diets that are low in carbohydrates are popular among obese people despite concern over the long-term adverse health effects. We encountered a case that seemed to substantiate this concern. Namely ulcerative colitis that apparently developed during adherence to an LCHWLD. Although we recognize that ulcerative colitis was not documented before the initiation of an LCHWLD, this case represents the possibility of one adverse reaction and how ulcerative colitis was treated in our patient. This case report provides additional information regarding the risk of an LCHWLD. We recommend a prospective investigation to look further into this possibility.
Diet-associated gut dysbiosis is becoming a leading environmental factor in a variety of current chronic diseases. A plant-based diet is advocated to counter these diseases. Ulcerative colitis is not an exception. A novel frontline approach consisting of dietary manipulation (ie, semivegetarian diet) induced remission without medication in this case.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
A Gold Coin
It soon became evident that appendicitis was on its last legs, and that a new complaint had to be discovered to meet the demand. The Faculty was up to the mark, a new disease was dumped on the market, a new word was coined, a gold coin indeed, COLITIS.
— The Story of San Michele, Axel Munthe, 1857–1949, Swedish physician
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Aug 30;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. DOI: http://dx.doi.org/10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004 May 18;140(10):769–77. doi: 10.7326/0003-4819-140-10-200405180-00006. DOI: http://dx.doi.org/10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 3.de Souza RJ, Swain JF, Appel LJ, Sacks FM. Alternatives for macronutrient intake and chronic disease: a comparison of the OmniHeart diets with popular diets and with dietary recommendations. Am J Clin Nutr. 2008 Jul;88(1):1–11. doi: 10.1093/ajcn/88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shai I, Schwarzfuchs D, Henkin Y, et al. Dietary Intervention Randomized Controlled Trial (DIRECT) Group Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008 Jul 17;359(3):229–41. doi: 10.1056/NEJMoa0708681. DOI: http://dx.doi.org/10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 5.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014 Sep 3;312(9):923–33. doi: 10.1001/jama.2014.10397. DOI: http://dx.doi.org/10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 6.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007 Feb;73(4):1073–8. doi: 10.1128/AEM.02340-06. DOI: http://dx.doi.org/10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkworth GD, Noakes M, Clifton PM, Bird AR. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr. 2009 May;101(10):1493–502. doi: 10.1017/S0007114508094658. DOI: http://dx.doi.org/10.1017/S0007114508094658. [DOI] [PubMed] [Google Scholar]
- 8.Russell WR, Gratz SW, Duncan SH, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011 May;93(5):1062–72. doi: 10.3945/ajcn.110.002188. DOI: http://dx.doi.org/10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- 9.Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010 May 28;16(20):2484–95. doi: 10.3748/wjg.v16.i20.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba M, Abe T, Tsuda H, Sugawara T, Tozawa H, Fujiwara K. Educational hospitalization to experience a semi-vegetarian diet in ulcerative colitis [Abstract] J Gastroenterol Hepatol. 2012 Dec;27(Suppl 5):180. [Google Scholar]
- 11.Chiba M, Takayama Y, Sugawara K, et al. Development of a plant-based diet score: its application for Crohn’s disease [Abstract] J Gastroenterol Hepatol. 2014 Nov;29(Suppl 3):126. [Google Scholar]
- 12.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013 May;19(5):576–85. doi: 10.1038/nm.3145. DOI: http://dx.doi.org/10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallus SJ, Brandt LJ. The intestinal microbiota and obesity. J Clin Gastroenterol. 2012 Jan;46(1):16–24. doi: 10.1097/MCG.0b013e31823711fd. DOI: http://dx.doi.org/10.1097/MCG.0b013e31823711fd. [DOI] [PubMed] [Google Scholar]
- 14.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014 Sep;63(9):1513–21. doi: 10.1136/gutjnl-2014-306928. DOI: http://dx.doi.org/10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 15.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011 Aug 23;7(10):569–78. doi: 10.1038/nrrheum.2011.121. DOI: http://dx.doi.org/10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14691–6. doi: 10.1073/pnas.1005963107. DOI: http://dx.doi.org/10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011 Oct 7;334(6052):105–8. doi: 10.1126/science.1208344. DOI: http://dx.doi.org/10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012 Aug;4(8):1095–119. doi: 10.3390/nu4081095. DOI: http://dx.doi.org/10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoetendal EG, de Vos WM. Effect of diet on the intestinal microbiota and its activity. Curr Opin Gastroenterol. 2014 Mar;30(2):189–95. doi: 10.1097/MOG.0000000000000048. DOI: http://dx.doi.org/10.1097/MOG.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 20.Park Y, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch Intern Med. 2011 Jun 27;171(12):1061–8. doi: 10.1001/archinternmed.2011.18. DOI: http://dx.doi.org/10.1001/archinternmed.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010 Sep 7;153(5):289–98. doi: 10.1059/0003-4819-153-5-201009070-00003. DOI: http://dx.doi.org/10.7326/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012 Jun 26;344:e4026. doi: 10.1136/bmj.e4026. DOI: http://dx.doi.org/10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noto H, Goto A, Tsujimoto T, Noda M. Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PLoS One. 2013;8(1):e55030. doi: 10.1371/journal.pone.0055030. DOI: http://dx.doi.org/10.1371/journal.pone.0055030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiba M, Tsuji T, Nakane K, Komatsu M. High amount of dietary fiber not harmful but favorable for Crohn disease. Perm J. 2015 Winter;19(1):58–61. doi: 10.7812/TPP/14-124. DOI: http://dx.doi.org/10.7812/TPP/14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McEvoy CT, Temple N, Woodside JV. Vegetarian diets, low-meat diets and health: a review. Public Health Nutr. 2012 Dec;15(12):2287–94. doi: 10.1017/S1368980012000936. DOI: http://dx.doi.org/10.1017/S1368980012000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Cancer Research Fund. American Institute for Cancer Research . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 27.Tuso PJ, Ismail MH, Ha BP, Bartolotto C. Nutritional update for physicians: plant-based diets. Perm J. 2013 Spring;17(2):61–6. doi: 10.7812/TPP/12-085. DOI: http://dx.doi.org/10.7812/TPP/12-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlich MJ, Singh PM, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013 Jul 8;173(13):1230–8. doi: 10.1001/jamainternmed.2013.6473. DOI: http://dx.doi.org/10.1001/jamainternmed.2013.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiba M, Tsuda H, Abe T, Sugawara T, Morikawa Y. Missing environmental factor in inflammatory bowel disease: diet-associated gut microflora. Inflamm Bowel Dis. 2011 Aug;17(8):E82–3. doi: 10.1002/ibd.21745. DOI: http://dx.doi.org/10.1002/ibd.21745. [DOI] [PubMed] [Google Scholar]
- 30.Hentges DJ, Maier BR, Burton GC, Flynn MA, Tsutakawa RK. Effect of a high-beef diet on the fecal bacterial flora of humans. Cancer Res. 1977 Feb;37(2):568–71. [PubMed] [Google Scholar]
- 31.Benno Y, Suzuki K, Suzuki K, Narisawa K, Bruce WR, Mitsuoka T. Comparison of the fecal microflora in rural Japanese and urban Canadians. Microbiol Immunol. 1986;30(6):521–32. doi: 10.1111/j.1348-0421.1986.tb02978.x. DOI: http://dx.doi.org/10.1111/j.1348-0421.1986.tb02978.x. [DOI] [PubMed] [Google Scholar]
- 32.Van Loo J, Cummings J, Delzenne N, et al. Functional food properties of non-digestible oligosaccharides: a consensus report from the ENDO project (DGXII AIRII-CT94-1095) Br J Nutr. 1999 Feb;81(2):121–32. doi: 10.1017/s0007114599000252. [DOI] [PubMed] [Google Scholar]
- 33.Chiba M, Ohno H, Ishii H, Komatsu M. Plant-based diets in Crohn’s disease [Letter] Perm J. 2014 Fall;18(4):94. doi: 10.7812/TPP/14-117. DOI: http://dx.doi.org/10.7812/TPP/14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiba M, Yoshida T, Komatsu M. From low-residue diets to plant-based diets in inflammatory bowel disease. Dig Dis Sci. 2014 Dec;59(12):3129–30. doi: 10.1007/s10620-014-3399-5. DOI: http://dx.doi.org/10.1007/s10620-014-3399-5. [DOI] [PubMed] [Google Scholar]
- 35.Haddad EH, Sabaté J, Whitten CG. Vegetarian food guide pyramid: a conceptual framework. Am J Clin Nutr. 1999 Sep;70(3 Suppl):615S–619S. doi: 10.1093/ajcn/70.3.615s. [DOI] [PubMed] [Google Scholar]
- 36.D’Souza S, Levy E, Mack D, et al. Dietary patterns and risk for Crohn’s disease in children. Inflamm Bowel Dis. 2008 Mar;14(3):367–73. doi: 10.1002/ibd.20333. DOI: http://dx.doi.org/10.1002/ibd.20333. [DOI] [PubMed] [Google Scholar]
- 37.Report of the working group on arteriosclerosis—volume II. Bethesda, MD: National Heart, Lung, and Blood Institute; 1981. Sep 8, [Google Scholar]
- 38.Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr. 1996 May;63(5):741–5. doi: 10.1093/ajcn/63.5.741. [DOI] [PubMed] [Google Scholar]
- 39.Morita N, Minoda T, Munekiyo M, et al. Case-control study of ulcerative colitis in Japan [Abstract in English] In: Ohno Y, editor. Annual report of the Research Committee on Epidemiology of Intractable Diseases, the Ministry of Health and Welfare of Japan. Nagoya, Japan: The Department of Preventive Medicine, School of Medicine, Nagoya University; 1996. Mar, pp. 153–8. [Google Scholar]
- 40.Morita N, Ohnaka O, Ando S, et al. Case-control study of Crohn’s disease in Japan. In: Ohno Y, editor. Annual report of the Research Committee on Epidemiology of Intractable Diseases, the Ministry of Health and Welfare of Japan. Nagoya, Japan: The Department of Preventive Medicine, School of Medicine, Nagoya University; 1996. Mar, pp. 58–64. [Google Scholar]
- 41.Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: the E3N prospective study. Am J Gastroenterol. 2010 Oct;105(10):2195–201. doi: 10.1038/ajg.2010.192. DOI: http://dx.doi.org/10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- 42.Maconi G, Ardizzone S, Cucino C, Bezzio C, Russo AG, Bianchi Porro G. Pre-illness changes in dietary habits and diet as a risk factor for inflammatory bowel disease: a case-control study. World J Gastroenterol. 2010 Sep 14;16(34):4297–304. doi: 10.3748/wjg.v16.i34.4297. DOI: http://dx.doi.org/10.3748/wjg.v16.i34.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietary and other risk factors of ulcerative colitis A case-control study in Japan. Epidemiology Group of the Research Committee of Inflammatory Bowel Disease in Japan. J Clin Gastroenterol. 1994 Sep;19(2):166–71. [PubMed] [Google Scholar]
- 44.Russel MG, Engels LG, Muris JW, et al. Modern life in the epidemiology of inflammatory bowel disease: a case-control study with special emphasis on nutritional factors. Eur J Gastroenterol Hepatol. 1998 Mar;10(3):243–9. doi: 10.1097/00042737-199803000-00010. DOI: http://dx.doi.org/10.1097/00042737-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto N, Kono S, Wakai K, et al. Epidemiology Group of the Research Committee on Inflammatory Bowel Disease in Japan Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis. 2005 Feb;11(2):154–63. doi: 10.1097/00054725-200502000-00009. DOI: http://dx.doi.org/10.1097/00054725-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Washoku, traditional dietary cultures of the Japanese, notably for the celebration of New Year [Internet] Paris, France: United Nations Educational, Scientific and Cultural Organization; 2013. [cited 2015 Jun 23]. Available from: www.unesco.org/culture/ich/RL/00869. [Google Scholar]
- 47.Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Prev Med. 1980 Jul;9(4):469–83. doi: 10.1016/0091-7435(80)90042-0. DOI: http://dx.doi.org/10.1016/0091-7435(80)90042-0. [DOI] [PubMed] [Google Scholar]
- 48.Vanhoutvin SA, Troost FJ, Hamer HM, et al. Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One. 2009 Aug 25;4(8):e6759. doi: 10.1371/journal.pone.0006759. DOI: http://dx.doi.org/10.1371/journal.pone.0006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan SH, Louis P, Thomson JM, Flint HJ. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol. 2009 Aug;11(8):2112–22. doi: 10.1111/j.1462-2920.2009.01931.x. DOI: http://dx.doi.org/10.1111/j.1462-2920.2009.01931.x. [DOI] [PubMed] [Google Scholar]