Abstract
PTH regulates serum calcium, phosphate, and 1,25-dihydroxyvitamin D (1,25(OH)2D) levels by acting on bone and kidney. In renal proximal tubules (PTs), PTH inhibits reabsorption of phosphate and stimulates the synthesis of 1,25(OH)2D. The PTH receptor couples to multiple G proteins. We here ablated the α-subunit of the stimulatory G protein (Gsα) in mouse PTs by using Cre recombinase driven by the promoter of type-2 sodium-glucose cotransporter (GsαSglt2KO mice). GsαSglt2KO mice were normophosphatemic but displayed, relative to controls, hypocalcemia (1.19 ±0.01 vs 1.23 ±0.01 mmol/L; P < .05), reduced serum 1,25(OH)2D (59.3 ±7.0 vs 102.5 ±12.2 pmol/L; P < .05), and elevated serum PTH (834 ±133 vs 438 ±59 pg/mL; P < .05). PTH-induced elevation in urinary cAMP excretion was blunted in GsαSglt2KO mice (2- vs 4-fold over baseline in controls; P < .05). Relative to baseline in controls, PTH-induced reduction in serum phosphate tended to be blunted in GsαSglt2KO mice (−0.39 ±0.33 vs −1.34 ±0.36 mg/dL; P = .07). GsαSglt2KO mice showed elevated renal vitamin D 24-hydroxylase and bone fibroblast growth factor-23 (FGF23) mRNA abundance (∼3.4- and ∼11-fold over controls, respectively; P < .05) and tended to have elevated serum FGF23 (829 ±76 vs 632 ±60 pg/mL in controls; P = .07). Heterozygous mice having constitutive ablation of the maternal Gsα allele (E1m−/+) (model of pseudohypoparathyroidism type-Ia), in which Gsα levels in PT are reduced, also exhibited elevated serum FGF23 (474 ±20 vs 374 ±27 pg/mL in controls; P < .05). Our findings indicate that Gsα is required in PTs for suppressing renal vitamin D 24-hydroxylase mRNA levels and for maintaining normal serum 1,25(OH)2D.
Serum calcium and phosphate levels are tightly regulated by the actions of several hormones including PTH, 1,25-dihydroxyvitamin D (1,25(OH)2D), and fibroblast growth factor-23 (FGF23) (1–5). PTH acts on the renal proximal tubule (PT) to inhibit reabsorption of phosphate by reducing protein levels of the sodium-phosphate cotransporters on the apical membrane and to increase the circulating levels of 1,25(OH)2D by inducing the expression of 25-hydroxyvitamin D 1α-hydroxylase (Cyp27b1) and by destabilizing the transcript encoding vitamin D 24-hydroxylase (Cyp24a1). PTH also acts on bone cells and renal distal tubule, in which it stimulates bone turnover and enhances calcium reabsorption, respectively. FGF23 is a bone-derived phosphaturic hormone, which also acts on PT to increase phosphate excretion. Unlike PTH, however, FGF23 suppresses Cyp27b1 and increases Cyp24a1 mRNA levels, thereby reducing serum 1,25(OH)2D. Upon binding to its receptor (vitamin D receptor), 1,25(OH)2D stimulates absorption of calcium and phosphate in the gut, reduces PTH synthesis in the parathyroid gland, and exerts negative feedback on its own production by regulating renal levels of Cyp27b1 and Cyp24a1. In addition, 1,25(OH)2D has been shown to stimulate FGF23 production in bone. These hormonal interactions and the individual actions of PTH, 1,25(OH)2D, and FGF23 in different tissues are key to mineral ion homeostasis (1–5).
PTH binds to a cell surface receptor (PTH receptor [PTHR]) that couples to multiple heterotrimeric G proteins including Gs and Gq/11 in kidney cells (6). Biochemical studies have shown that both Gs and Gq/11 signaling are involved in mediating the action of PTH on phosphate reabsorption in PT (7–9). Recent studies with PTH analogs that specifically activate the Gs pathway and knock-in mice expressing a mutant PTHR defective in Gq/11 activation have indicated that, whereas Gs signaling mediates the acute effects of PTH, Gq/11 signaling is required for long-term PTH effects (10, 11). Biochemical and in vitro studies have shown that PTH regulates the synthesis of 1,25(OH)2D mainly via Gs signaling (12–15), although pathways downstream of Gq/11 have also been implicated in this action (16, 17). The role of Gs has also been investigated through the study of patients with pseudohypoparathyroidism (PHP) type-I. This disorder is caused by mutations disrupting the coding exons or the imprinting control elements of the gene encoding the α-subunit of Gs (Gsα) (18–20). Patients with PHP type-I show blunted urinary phosphate excretion in response to PTH injection, as well as a blunted increase in cAMP excretion, consistent with the action of Gsα as a stimulator of cAMP generation (18–20). Patients with PHP type-I display hypocalcemia and hyperphosphatemia in the presence of elevated serum PTH. The hypocalcemia is presumed to result from insufficient serum 1,25(OH)2D.
Although the findings in PHP type-I patients are consistent with the importance of Gs signaling in PTH actions, the genetic mechanisms governing this disorder are complex. The mutations are only on the maternal allele, and the Gsα deficiency also requires the silencing of the paternal Gsα allele in PT, an epigenetic event that takes place in normal individuals (18–20). However, mouse studies have shown that paternal Gsα silencing is incomplete in PT, and that paternal Gsα ablation also leads to a certain degree of Gsα deficiency in this tissue (21–23). Accordingly, several studies have reported biochemical evidence of PTH resistance in some patients who carry paternally inherited Gsα mutations (24–29) and in mice in which paternal Gsα is ablated universally (23, 30). Moreover, Gsα expression is diminished not only in PT but also in certain other tissues, including, in the case of patients carrying coding Gsα mutations (PHP type-Ia), bone and renal distal tubule. Hence, data from patients with PHP type-I and mouse models of this disorder have proven inadequate with respect to understanding of the role of Gs/cAMP signaling in mediating the PT actions of PTH.
In this study, we generated mice with conditional Gsα ablation in this tissue (GsαSglt2KO mice). These mice exhibited PTH resistance, hypocalcemia, and 1,25(OH)2D deficiency. In addition, renal Cyp24a1 mRNA levels were elevated, and serum FGF23 tended to be higher than in controls. These findings were also present in mice heterozygous for universal ablation of maternal Gsα (E1m−/+ mice), ie, a mouse model of PHP type-Ia.
Materials and Methods
Generation and maintenance of mice
To generate PT specific Gsα null mice, Gsα exon 1 floxed mice (31) were intercrossed to transgenic mice expressing Cre recombinase driven by the promoter of sodium-glucose cotransporter type-2 (Sglt2) (Sglt2-Cre) (32). Cre-negative, Gsα exon 1 floxed littermates were used as controls. For locating Sglt2-Cre activity in kidney RosamT/mG reporter strain (JaxMice; stock number 007576) was intercrossed with Sglt2-Cre mice, and for determining the reduction in Gsα mRNA, RosatdTomato reporter strain (JaxMice; stock number 007914) was intercrossed with Gsα exon 1 floxed and Sglt2-Cre mice. E1m−/+ mice have been described previously (31). GsαSglt2KO and E1m−/+ mice were maintained in cluster of differentiation 1 and Friend Virus B backgrounds, respectively. The study and control mice were fed a diet containing 1.11% calcium, 0.80% phosphorus, 2.5-IU/g vitamin D3 (Prolab Isopro RMH 3000; Labdiet). All analyses were performed in 2- to 4-month-old adult mice, except for the fluorescence imaging of kidneys from Sglt2-Cre;RosamT/mG double mutants, which were collected at age 4 weeks. All mice in this study were housed in the Center for Comparative Medicine at the Massachusetts General Hospital, and all experiments were approved by the Institutional Subcommittee on Research Animal Care.
Laser cut microdissection (LCM), RNA preparation, and quantitative real-time RT-PCR (qRT-PCR)
Tissues from both genders were used without regard to sex. Kidneys were snap frozen in Optimal Cutting Temperature compound embedding medium (Tissue-Tek). Frozen samples were cut into 8- to 10-μm-thick sections on MMI membrane slides (MMI Molecular Machines & Industries). The tubules were isolated by LCM, as described previously (33). In experiments using RosatdTomato reporter, tubules expressing tdTomato were isolated by LCM. Total RNA was extracted using the absolutely RNA Nanoprep kit (Agilent Technologies) according to the manufacturer's instructions. qRT-PCR was performed by using one-step Quanti-Tect RT-PCR kit (QIAGEN) with TaqMan probes using β-actin as control (Mm00530548_m1 for Gαs and Mm00607939_s1 for β-actin from Applied Biosystems). For PTH-induced alteration of Cyp27b1 mRNA abundance, kidneys were removed 2 hours after injection, snap-frozen in liquid nitrogen, and stored at −80°C until total RNA isolation. Kidneys for baseline measurements were also snap-frozen immediately after removal and kept at −80°C until total RNA isolation. Femurs were snap frozen after the removal of distal and proximal heads and subsequent flushing of the bone marrow with PBS. Whole kidney total RNA was isolated by using the RNeasy Plus Mini kit (QIAGEN), and bone total RNA by using TRIzol reagent (Invitrogen). cDNA was synthesized by using the ProtoScript II First stand cDNA synthesis kit (New England Biolabs). SYBR green (Roche) was employed for quantitative PCR, using β-actin as control in separate reactions. Primer sequences for amplification of Cyp27b1 and Cyp24a1 transcripts were previously described (34). Sequences of primers used for the amplification of Fgf23, Tnfsf11, Tnfrsf11b, Pth1r, Vdr, Slc34a1, and Slc34a3 transcripts are presented in Supplemental Table 1.
Proximal tubule protein isolation, Western blotting, and fluorescence microscopy
Proximal tubule enriched cortices were prepared by the Percoll density gradient method, as described previously (35). Kidneys were isolated from both genders without regard to sex. Isolated proximal tubule proteins were lysed in a Tris-buffered solution containing 150mM NaCl and 1% Triton X-100 with a protease inhibitor cocktail (Roche). Protein lysates were subsequently separated on 10% SDS-PAGE and analyzed by Western blotting using a polyclonal antibody against the C-terminal decapeptide of Gsα (Millipore) (please see Table 1). Densitometry was employed for quantitation of Gsα protein relative to β-actin by using ImageJ (36). For determining the renal expression of Sglt2-driven Cre recombinase, kidneys of 4-week-old double mutant offspring from RosamT/mG and Sglt2-Cre intercrosses were removed and fixed overnight with 4% paraformaldehyde/PBS; 10-μm sections were obtained and visualized under epifluorescence by using Nikon Eclipse E800 microscope. S1/2 and S3 PT segments were recognized by their anatomical location and morphological appearance.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Gsα | C-terminal decapeptide | Anti-Gsα | Millipore, 06-237 | Rabbit; polyclonal | 1:2000 |
| β-Actin | Between amino acids 350–375 at the C terminus of actin of human origin | Antiactin | Santa Cruz Biotechnology, Inc, sc-8432 | Mouse; monoclonal | 1:500 |
Serum biochemistry measurements
Analyses were performed in both sexes. Blood samples were collected after overnight fasting (with access to water). The heparinized blood sample for ionized calcium measurement, performed by using a Siemens RapidLab 348 Ca2+/pH analyzer, was collected from tail vein, and other samples collected by submandibular bleeding. Serum and urinary phosphate was determined by colorimetric measurements (Wako). Mouse PTH (PTH1–84) and FGF23 (detecting both the intact peptide and the C-terminal fragment) measurements were conducted with commercial kits (both from Immutopics, Inc). Serum 1,25(OH)2D was measured by using a commercial kit (IDS Ltd). The index of fractional phosphate excretion was calculated as previously described (11). Urinary creatinine was measured by using a commercial kit (Stanbio).
PTH-induced urinary excretion of cAMP and reduction in serum phosphate
Mice were injected sc with a single dose of 50 nmol/kg body weight of human PTH(1–34) synthesized by the Peptide/Protein Core Facility at Massachusetts General Hospital. For urinary cAMP measurements, the urine samples were collected at single time points at baseline and at 30, 60, and 120 minutes after injection. Urinary cAMP was measured by RIA, as described (22), and results were normalized by urinary creatinine (Stanbio). For analyzing PTH-induced changes in plasma phosphate, blood samples were collected before and 120 minutes after injection, by either tail vein or submandibular bleeding.
Statistical analysis
All data are presented as mean ± SEM. Statistical significance for differences between groups was determined by 2-sided Student's t test, after identification and removal of outliers according to the Grubs test and the Robust regression and Outlier removal method. The densitometry analysis of Western blottings for Gsα protein was analyzed by using 1-sample t test. Statistical analyses were performed by using GraphPad Prism. P < .05 was considered statistically significant, and P ≤ .10 and P ≥ .05 were considered a tendency for the groups to be different.
Results
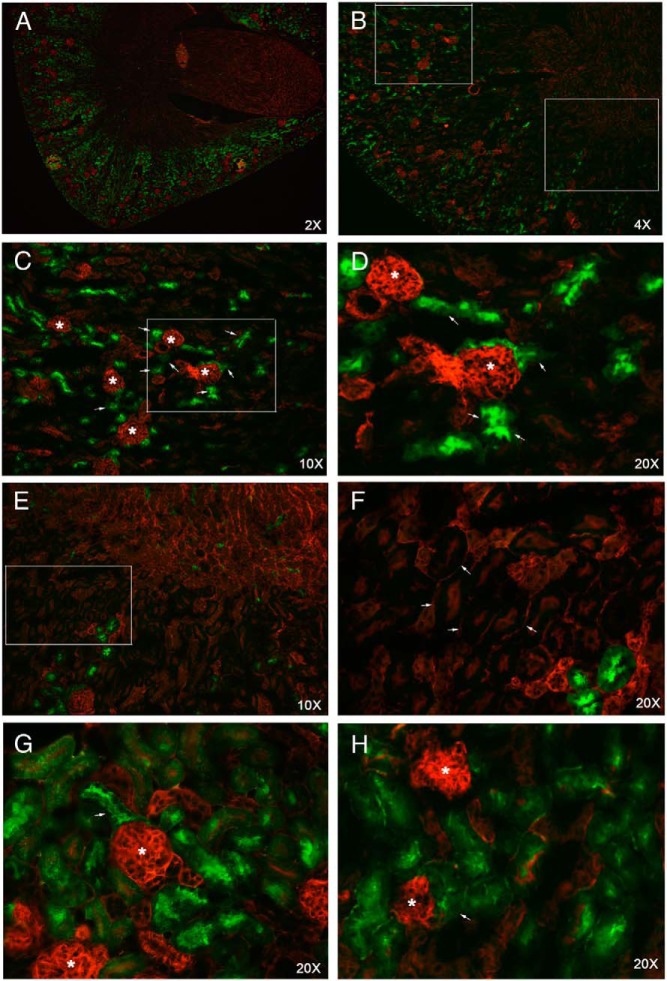
To investigate the role of Gsα in mediating the action of PTH in PT in vivo, we generated mice in which Gsα was conditionally ablated in this tissue. We took advantage of the Sglt2-Cre transgenic mice, which had been successfully employed for gene ablation in PT (37–39). To determine the expression pattern of Cre in kidney, we crossed the Sglt2-Cre mice to the RosamT/mG reporter strain (40). Cells in these mice express a membrane-localized form of red fluorescent protein, unless Cre is expressed, in which case they express a membrane-localized form of green fluorescent protein (GFP). The GFP expression, indicating the activity of Cre, was present mostly in the juxtaglomerular tubules, which comprise the convoluted portion of PT (Figure 1); however, GFP expression was detected in few PTs located in the outer stripe of renal medulla, which comprise the straight portion of PT (Figure 1).
Figure 1.
Sglt2-Cre recombinase activity is demonstrated in the renal cortex by using the RosamT/mG Cre reporter mice (RosamT/mG;Sglt2-Cre mice). A and B, Low-magnification images of kidney transverse sections from 4-week-old RosamT/mG Cre reporter mice. In the absence of Cre, cells express red fluorescent protein, whereas the presence of Cre activity results in the expression of GFP. Boxes indicate the areas magnified in C and E. C, ×10 magnification of the cortical area marked in B. D, ×20 magnification of the area marked in C; asterisks indicate glomeruli; arrows indicate the Cre-positive S1 segments surrounding the glomeruli. E, ×10 magnification of the outer stripe area marked in B. F, ×20 magnification of the area marked in E; arrows point to S3 segments that lack Cre activity. Note that the red fluorescent protein is expressed in the basal and the apical membranes of an S3 tubule if Cre is not expressed. G and H, ×20 magnification of areas in the cortex; asterisks indicate the glomeruli; arrows point to the earliest portions of the S1 segments.
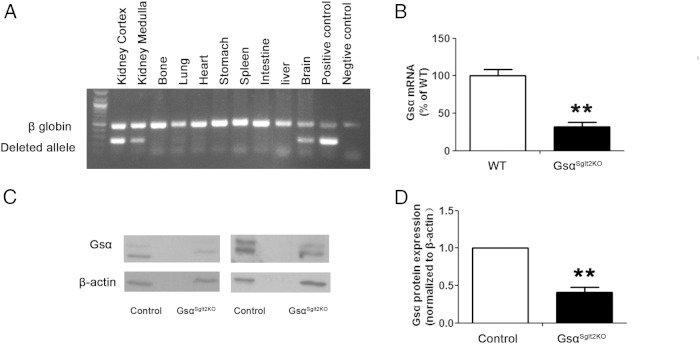
To ablate Gsα in PT, we crossed the Sglt2-Cre mice to mice homozygous for the floxed Gsα exon 1 allele and obtained double mutant offspring (GsαSglt2KO mice). PCR using DNA from different tissues of GsαSglt2KO mice and primers flanking the loxP sites generated an amplicon from the deleted allele in renal cortex, as well as renal medulla, but not in bone or a number of other tissues investigated; however, a faint PCR band was visible in DNA from brain (Figure 2A). To determine the extent of Gsα ablation in PT we fluorescently labeled these tubules by utilizing the RosatdTomato reporter strain, in which Cre activity results in the expression of the red fluorescent protein variant tdTomato (41). We measured Gsα mRNA levels in PT isolated by LCM of kidney sections from littermates expressing Sglt2-Cre and RosatdTomato in the absence (wild-type) or the presence (GsαSglt2KO) of floxed Gsα exon 1 alleles. qRT-PCR analysis using total RNA obtained from these tubules showed that the abundance of Gsα mRNA in GsαSglt2KO mice was approximately 31% of that in control littermates (Figure 2B). Western blot analysis of proximal tubule enriched renal cortices showed that Gsα protein abundance in GsαSglt2KO mice was approximately 42% of that in control littermates (Figure 2, C and D). Note that proximal tubule enriched cortices contain all PT segments, including S3, as well as contaminating endothelial cells (42).
Figure 2.
GsαSglt2KO show significantly reduced Gsα expression in PT. A, A duplex PCR was used to amplify the deleted Gnas exon 1 allele (deleted allele) together with a portion of the gene encoding β-globin (β-globin) in genomic DNA (35 cycles of amplification was used). The positive control was from the tail of a mouse in which the paternal Gnas exon 1 was deleted universally through the same Cre/lox approach as in GsαSglt2KO. The other lanes were from different tissues of GsαSglt2KO mice (representative of 2 independent experiments). B, Gsα mRNA was analyzed by qRT-PCR using RNA isolated from proximal tubules by LCM. For LCM, the tubules were marked in the double mutant offspring of Sglt2-Cre and RosatdTomato mice, both of which were also heterozygous for the floxed exon 1 allele. Double transgenic offspring carrying homozygous floxed alleles (KO) was compared with double transgenic offspring carrying no floxed alleles (WT). n = 3 per group. **, P < .01. C, Gsα protein level was examined in proximal tubule enriched renal cortices by Western blotting (representative data from 3 independent experiments). D, Densitometry analysis showing the reduction in Gsα protein level in GsαSglt2KO mice compared with control littermates (results from 3 independent experiments). n = 4 per genotype; **, P < .01.
The ability of PTH to activate receptor signaling in PT can be assessed by measuring PTH-induced increase in urinary cAMP excretion (43). The baseline urinary cAMP levels in GsαSglt2KO and control littermates appeared comparable (179.7 vs 157.5 nmol/mg creatinine, respectively). In control mice, PTH injection resulted in an approximately 4-fold increase in urinary cAMP level in 30 minutes, which declined to baseline by 60 minutes. In GsαSglt2KO mice, however, the PTH-induced urinary cAMP excretion was significantly blunted, as the PTH injection resulted only in an approximately 2-fold increase in urinary cAMP excretion in 30 minutes; the difference between the GsαSglt2KO and control littermates was statistically significant (Figure 3A). Reflecting the action of PTH in PT, PTH injection results in reduced plasma phosphate in mice (44). The reduction of plasma phosphate 2 hours after PTH injection in GsαSglt2KO mice was approximately 30% of that in control mice (−0.39 ± 0.33 vs −1.34 ± 0.36 mg/dL, respectively); however, the difference did not reach statistical significance, P = .07) (Figure 3B). PTH stimulates the promoter of Cyp27b1 via a mechanism involving cAMP generation (45) and, possibly, additional signaling pathways (16, 17). However, GsαSglt2KO mice showed intact PTH-stimulated Cyp27b1 expression, as PTH injection increased the abundance of renal Cyp27b1 mRNA markedly and significantly over the baseline in both control (∼8-fold) and GsαSglt2KO littermates (∼6-fold) (Figure 3C). The levels attained after PTH injection in controls were not significantly different from those in GsαSglt2KO mice.
Figure 3.
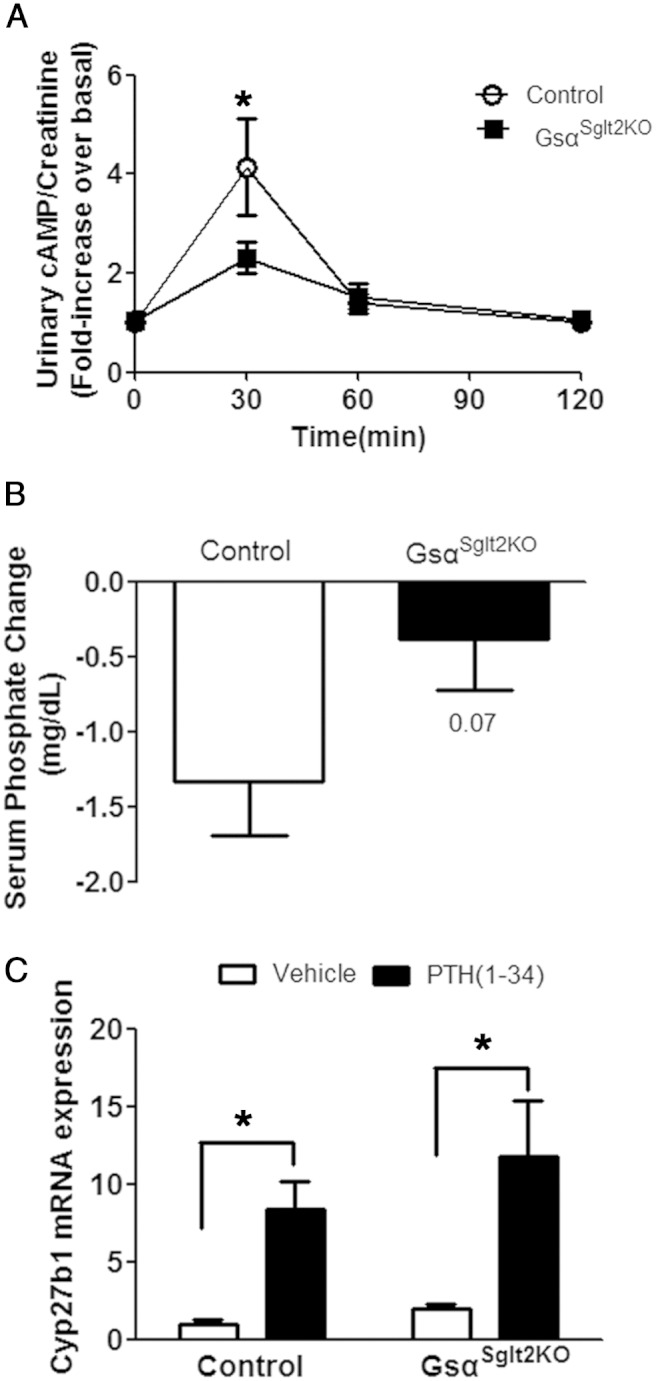
PTH-induced urinary cAMP excretion and PTH-induced serum phosphate reduction in GsαSglt2KO are blunted. A, Urine cAMP levels in GsαSglt2KO and control littermates after sc PTH(1–34) injection (50 nmol/kg). Urine was collected at each time point. The results were normalized by the baseline value in each experiment (control; n = 10, 11, 10, 17; GsαSglt2KO; n = 13, 21, 17, 19 at each time point, *, P < .05). B, Serum phosphate level changes in control and GsαSglt2KO littermates 2 hours after sc PTH(1–34) (50 nmol/kg) (n = 11 for control, n = 17 for GsαSglt2KO); P value, below the error bar. C, Renal Cyp27b1 mRNA abundance in control (n = 3, vehicle; n = 6, PTH) and GsαSglt2KO (n = 5, vehicle; n = 5, PTH) littermates 2 hours after sc PTH(1–34) (50 nmol/kg) injection; *, P < .05.
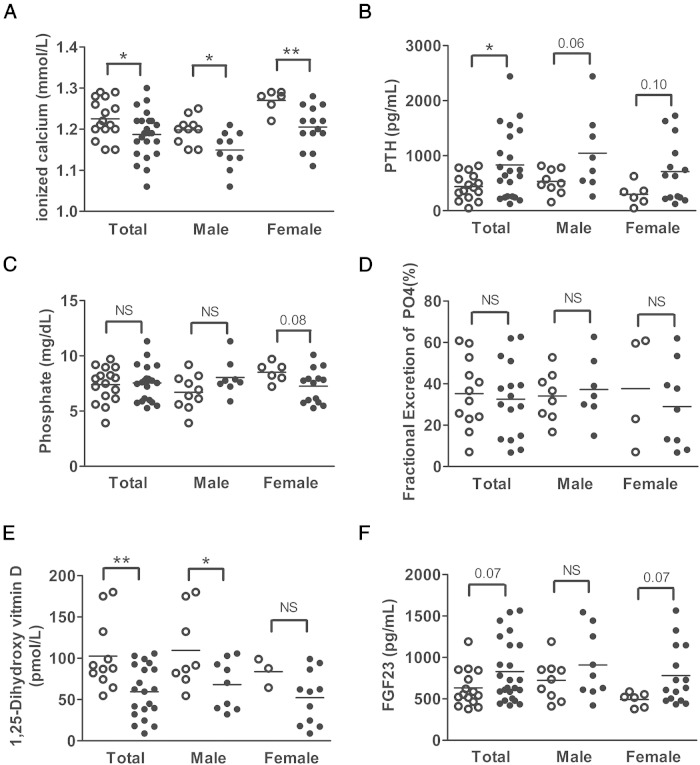
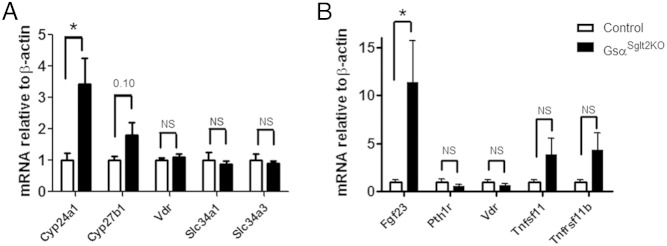
Blood ionized calcium was significantly reduced and serum PTH was significantly increased in GsαSglt2KO mice compared with controls; however, serum phosphate, as well as the index of fractional phosphate excretion, was comparable between GsαSglt2KO and control littermates (Figure 4, A–D). GsαSglt2KO mice also showed significantly reduced serum 1,25(OH)2D compared with control littermates (Figure 4E). We also measured the level of FGF23 to determine whether this phosphaturic agent, which also suppresses 1,25(OH)2D synthesis, may be involved in the development of these biochemical alterations. Serum FGF23 was higher in GsαSglt2KO mice than in control littermates, although statistical significance could not be reached (P = .07) (Figure 4F). By and large, these findings were also true when males and females were analyzed separately (Figure 4, A–F); however, the difference in mean 1,25(OH)2D values was not statistically significant between female GsαSglt2KO and control mice (Figure 4E). We then quantitated the levels of transcripts regulated by PTH, 1,25(OH)2D, and FGF23 in PT. The abundance of Cyp24a1 mRNA (∼3.4-fold) was significantly elevated in GsαSglt2KO kidneys compared with controls, and the abundance of Cyp27b1 mRNA tended to be mildly elevated (∼1.8-fold; P = .10). No significant differences were detected in the levels of Vdr, Slc34a1 (sodium-phosphate cotransporter type 2a [Npt2a]), and Slc34a3 (Npt2c) mRNA (Figure 5A). In addition, Fgf23 mRNA abundance in bone was significantly higher in GsαSglt2KO than control littermates (∼11-fold), whereas the levels of Pth1r, Vdr, Tnfsf11 (receptor activator of nuclear factor κ-B ligand), and Tnfrsf11b (osteoprotegerin) mRNA were not significantly different (Figure 5B).
Figure 4.
Serum biochemistries in control (open circles) and GsαSglt2KO (filled circles) littermates. Blood ionized calcium (A), serum PTH (B), serum phosphate (C), index of fractional phosphate (PO4) excretion (D), serum 1,25(OH)2D (E), and serum FGF23 (F) were measured in adult mice. Individual values and mean (horizontal line) are shown; *, P < .05; **, P < .01; NS, not statistically significant; P ≤ .10 and P ≥ .05 are shown above the datasets.
Figure 5.
Levels of renal Cyp24a1 mRNA and bone Fgf23 mRNA are elevated in GsαSglt2KO mice. A, Whole kidney levels of Cyp24a1, Cyp27b1, Vdr, Slc34a1, and Slc34a3 mRNA in control (n = 7–8 for Cyp24a1 and Cyp27b1; n = 4 for Vdr, Slc34a1, and Slc34a3) and GsαSglt2KO (n = 9–10 for Cyp24a1 and Cyp27b1; n = 5 for Vdr, Slc34a1, and Slc34a3) littermates. B, Bone levels of Fgf23, Pth1r, Vdr, Tnfsf11, and Tnfrsf11b mRNA in control (n = 8 for Fgf23; n = 7 for the others) and GsαSglt2KO (n = 8 for Fgf23; n = 11 for the others) mice. Measurements were done by using qRT-PCR relative to β-actin and normalized to values obtained from control littermates; *, P < .05; NS, not statistically significant; P ≤ .10 and P ≥ .05 are shown above the datasets.
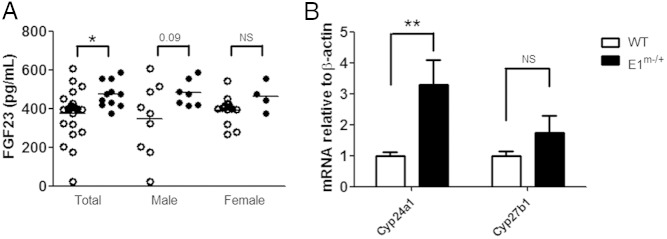
It has been reported that, similar to the findings in GsαSglt2KO mice, serum 1,25(OH)2D tends be reduced in E1m−/+ mice (ie, PHP type-Ia mouse model) (33). The E1m−/+ mice, like GsαSglt2KO mice, exhibit significantly diminished Gsα levels in PT at age 2 months (23). We thus further investigated these mice and revealed that E1m−/+ also showed significantly higher levels of serum FGF23 and renal Cyp24a1 mRNA (∼3.3-fold) than wild-type littermates (Figure 6, A and B). The abundance of Cyp27b1 mRNA was not significantly increased in E1m−/+ kidneys (Figure 6B).
Figure 6.
Serum FGF23 and renal Cyp24a1 mRNA abundance are increased in E1m−/+ mice. A, Serum FGF23 in wild-type (open circles) and E1m−/+ (filled circles) littermates. B, Whole kidney levels of Cyp24a1 and Cyp27b1 mRNA in wild-type and E1m−/+ littermates; n = 7 and 5 for Cyp24a1; n = 7 and 7 for Cyp27b1, respectively. Measurements were done by using qRT-PCR relative to β-actin and normalized to values obtained from control littermates; *, P < .05; **, P < .01; NS, not statistically significant.
Discussion
In this study we ablated Gsα conditionally in the mouse PT to examine its role in mediating the action of PTH in this tissue. Our findings indicate that the loss of Gsα in PT results in PTH resistance in this tissue, leading to hypocalcemia and diminished serum 1,25(OH)2D with elevated serum PTH. The GsαSglt2KO mice also demonstrated significantly elevated renal Cyp24a1 mRNA abundance and tended to have increased serum FGF23 levels, phenotypic features that we also found in a mouse model of PHP type-Ia.
Sglt2 is expressed much more abundantly in the convoluted portion of PT including S1/S2 segments than in the straight portion including S3 segments (46–48), consistent with the expression profile of the Sglt2-driven Cre demonstrated in our study. Our PCR analyses showed some Gsα ablation in renal medulla. This finding likely reflects, based on our data obtained from RosamT/mG and Sglt2-Cre mice intercrosses, the activity of the Sglt2-Cre in few tubules in the straight portion of PT, as well as in some cells located in the inner stripe of renal medulla (see Figure 1, E and F). Previous studies have shown that most molecules downstream of PTHR are located mainly in the convoluted PT. For example, PTH-induced adenylyl cyclase activity was found to be approximately 7-fold higher in the convoluted portion than in the straight portion (49). Also, PTH-induced internalization of Npt2a is mostly detected in superficial S1 segments (50). Consistent with these findings, our mouse model showed a blunted PTH-induced urinary cAMP excretion and a blunted PTH-induced reduction of plasma phosphate. Moreover, GsαSglt2KO mice displayed increased serum PTH but unaltered levels of renal Slc34a1 mRNA, the transcript encoding the Npt2a. This finding is also consistent with PTH resistance in PT, given that chronic exposure to PTH leads to diminished levels of the Npt2a transcript in kidney (11, 51). In contrast, PTH-induced elevation of Cyp27b1 mRNA was intact in GsαSglt2KO mice, suggesting perhaps that the amount of Gsα remaining in PT is sufficient to mediate this action of PTH. It is also possible, however, that the induction of Cyp27b1 expression by PTH entails Gsα-independent signaling mechanisms, as suggested previously (16, 17). Alternatively, the acute action of PTH in this regard may take place in other portions of PT, such as in the straight tubule (S3 segment), or in the distal nephron.
We found that 1,25(OH)2D levels are significantly reduced in GsαSglt2KO mice. Because these mice showed significantly elevated Cyp24a1 mRNA levels and tended to have mildly elevated Cyp27b1 mRNA levels, it appears likely that elevated Cyp24a1 expression is responsible for the reduced serum 1,25(OH)2D. PTH destabilizes Cyp24a1 mRNA via a Gsα/cAMP-mediated mechanism (15). Therefore, the elevation of renal Cyp24a1 mRNA in the presence of increased serum PTH is consistent with PTH resistance in PT of GsαSglt2KO mice. A study, however, has shown that PTH/cAMP signaling can stimulate Cyp24a1 mRNA expression in renal distal tubular cells through up-regulation of vitamin D receptor (52), making it possible that elevated Cyp24a1 mRNA levels reflect the unimpaired action of PTH in the distal tubule. However, this possibility seems unlikely, because we found that GsαSglt2KO mice not only have reduced serum 1,25(OH)2D concentration but also show unaltered levels of Vdr mRNA in kidney.
The rise in bone Fgf23 mRNA in GsαSglt2KO mice is likely to be secondary to the inability of PTH to promote adequate phosphate excretion, although other mechanisms are also conceivable, including a direct effect of PTH on bone, as suggested by some studies (53, 54). We do not have a clear explanation, however, for our finding that serum FGF23 is mildly and not statistically significantly elevated despite the marked increase in bone Fgf23 mRNA. This discrepancy may perhaps reflect the differences in sample collection. The mice were fasted overnight before blood draw for serum isolation, whereas food was available ad libitum until sacrifice for the removal of femurs. Nonetheless, the trend toward elevated serum FGF23 and the increased bone Fgf23 mRNA in GsαSglt2KO mice may suggest that FGF23 plays a role in the development of 1,25(OH)2D deficiency. For example, it is possible that an additional factor contributing to the increased renal Cyp24a1 mRNA abundance is FGF23, given that this hormone is a strong stimulator of Cyp24a1 expression (55). Moreover, we found that renal Cyp27b1 mRNA levels in GsαSglt2KO mice are not significantly elevated, even though 1,25(OH)2D deficiency, like increased serum PTH, is a strong inducer of renal Cyp27b1 mRNA abundance (56–58). Given that FGF23 is also an inhibitor of Cyp27b1 transcription (55), it is tempting to speculate that this finding also reflects increased FGF23 action in the kidney combined with reduced 1,25(OH)2D and increased PTH actions. Further studies are needed to elucidate the role of FGF23 in this context.
PHP type-Ib is caused by imprinting mutations of GNAS, the gene encoding Gsα and different splice variants, leading to Gsα deficiency solely in those tissues in which expression from the paternal Gsα allele is normally silenced (18–20). Among PTH responsive tissues, paternal Gsα silencing has been shown only in PT (59, 60), and the primary defect in patients with PHP type-Ib is PTH resistance in this tissue. Hence, the GsαSglt2KO mice can be considered a model of PHP type-Ib, although patients with this disorder also demonstrate mild TSH resistance and, occasionally, AHO features, reflecting the existence of paternal Gsα silencing in certain other tissues (18–20). A mouse model of PHP type-Ib has been generated by introducing one of the previously described causative GNAS mutations into the mouse genome (22, 61). Those mice also show hypocalcemia with elevated serum PTH, whereas their serum phosphate levels are modestly elevated (22).
The biochemical phenotype of GsαSglt2KO mice, including normophosphatemia, is similar to that of PHP type-Ia mice studied in this and other studies (30, 31, 33), but some minor differences exist. The differences could be due, at least partly, to the genetic backgrounds in which these mouse models are maintained. In addition, Gsα haploinsufficiency may exist in bone and renal distal tubules of E1m−/+ mice, contributing to the disruption in mineral ion metabolism. Consistent with a role for Gsα haploinsufficiency in the biochemical phenotype, mildly elevated serum PTH values were detected in mice heterozygous for paternal Gsα ablation (23, 30). Furthermore, it is possible that the temporospatial pattern of Gsα ablation in PT is different in GsαSglt2KO mice from that in E1m−/+ mice, in which the ablation relies on the normal silencing of the paternal allele. Note that the PT segments in which this epigenetic event takes place have yet to be identified.
The mechanisms leading to the biochemical defects seen in patients with PHP type-I have remained incompletely understood. Many, but not all, reports describe reduced 1,25(OH)2D values in these patients, and accordingly, we revealed significantly lower serum 1,25(OH)2D in GsαSglt2KO mice than in controls. Likewise, mean 1,25(OH)2D value was shown to be reduced in E1m−/+ mice, although the difference between E1m−/+ and wild-type littermates was not statistically significant (33). Our findings obtained from GsαSglt2KO and E1m−/+ mice suggest that reduced 1,25(OH)2D levels results from increased renal Cyp24a1 mRNA levels. The trigger for the latter is likely to be the PTH resistance in PT, but other mechanisms may also play a role, such as elevated serum FGF23 (statistically significant in E1m−/+ mice). Not much is known about serum FGF23 levels in PHP type-I patients, but a single case affected by PHP type-Ib has been reported to have mildly increased serum FGF23 (62). Further investigations are needed to determine whether serum FGF23 is indeed elevated in PHP type-I patients and whether it contributes to the abnormal vitamin D metabolism in this disorder.
In summary, our study shows that Gsα is essential for the proximal tubular actions of PTH and that the loss of this signaling protein results in reduced serum 1,25(OH)2D with increased renal Cyp24a1 mRNA abundance.
Acknowledgments
Present address for V.M.: Kadmon Corporation, Molecular Signaling, Alexandria Center for Life Science, New York, NY 10016.
This work was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK073911 (to M.B.) and R01DK46718 (to H.J.) and the Intramural Research of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Cyp24a1
- vitamin D 24-hydroxylase
- Cyp27b1
- 25-hydroxyvitamin D 1α-hydroxylase
- FGF23
- fibroblast growth factor-23
- GFP
- green fluorescent protein
- Gsα
- α-subunit of Gs
- LCM
- laser cut microdissection
- Npt2a
- sodium-phosphate cotransporter type 2a
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- PHP
- pseudohypoparathyroidism
- PT
- renal proximal tubule
- PTHR
- PTH receptor
- qRT-PCR
- quantitative real-time RT-PCR
- Sglt2
- sodium-glucose cotransporter type-2.
References
- 1. Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quarles LD. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol. 2012;8:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lanske B, Razzaque MS. Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int. 2014;86:1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farrow EG, White KE. Recent advances in renal phosphate handling. Na Rev Nephrol. 2010;6:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bikle D, Adams JS, Christakos S. Vitamin D: production, metabolism, mechanism of action, and clinical requirements. In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th ed Ames, IA: Wiley-Blackwell; 2013. [Google Scholar]
- 6. Bringhurst FR, Jüppner H, Guo J, et al. Cloned, stably expressed parathyroid hormone (PTH)/PTH-related peptide receptors activate multiple messenger signals and biological responses in LLC-PK1 kidney cells. Endocrinology. 1993;132:2090–2098. [DOI] [PubMed] [Google Scholar]
- 7. Pfister MF, Forgo J, Ziegler U, Biber J, Murer H. cAMP-dependent and -independent downregulation of type II Na-Pi cotransporters by PTH. Am J Physiol. 1999;276:F720–F725. [DOI] [PubMed] [Google Scholar]
- 8. Traebert M, Völkl H, Biber J, Murer H, Kaissling B. Luminal and contraluminal action of 1–34 and 3–34 PTH peptides on renal type IIa Na-P(i) cotransporter. Am J Physiol Renal Physiol. 2000;278:F792–F798. [DOI] [PubMed] [Google Scholar]
- 9. Capuano P, Bacic D, Roos M, et al. Defective coupling of apical PTH receptors to phospholipase C prevents internalization of the Na+-phosphate cotransporter NaPi-IIa in Nherf1-deficient mice. Am J Physiol Cell Physiol. 2007;292:C927–C934. [DOI] [PubMed] [Google Scholar]
- 10. Nagai S, Okazaki M, Segawa H, et al. Acute down-regulation of sodium-dependent phosphate transporter NPT2a involves predominantly the cAMP/PKA pathway as revealed by signaling-selective parathyroid hormone analogs. J Biol Chem. 2011;286:1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo J, Song L, Liu M, et al. Activation of a non-cAMP/PKA signaling pathway downstream of the PTH/PTHrP receptor is essential for a sustained hypophosphatemic response to PTH infusion in male mice. Endocrinology. 2013;154:1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rasmussen H, Wong M, Bikle D, Goodman DB. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972;51:2502–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larkins RG, MacAuley SJ, Rapoport A, et al. Effects of nucleotides, hormones, ions, and 1,25-dihydroxycholecalciferon on 1,25-dihydroxycholecalciferol production in isolated chick renal tubules. Clin Sci Mol Med. 1974;46:569–582. [DOI] [PubMed] [Google Scholar]
- 14. Horiuchi N, Suda T, Takahashi H, Shimazawa E, Ogata E. In vivo evidence for the intermediary role of 3′,5′-cyclic AMP in parathyroid hormone-induced stimulation of 1α,25-dihydroxyvitamin D3 synthesis in rats. Endocrinology. 1977;101:969–974. [DOI] [PubMed] [Google Scholar]
- 15. Zierold C, Mings JA, DeLuca HF. Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc Natl Acad Sci USA. 2001;98:13572–13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ro HK, Tembe V, Favus MJ. Evidence that activation of protein kinase-C can stimulate 1,25-dihydroxyvitamin D3 secretion by rat proximal tubules. Endocrinology. 1992;131:1424–1428. [DOI] [PubMed] [Google Scholar]
- 17. Janulis M, Tembe V, Favus MJ. Role of protein kinase C in parathyroid hormone stimulation of renal 1,25-dihydroxyvitamin D3 secretion. J Clin Invest. 1992;90:2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein α-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. [DOI] [PubMed] [Google Scholar]
- 19. Levine MA. An update on the clinical and molecular characteristics of pseudohypoparathyroidism. Curr Opin Endocrinol Diabetes Obes. 2012;19:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bastepe M. Genetics and epigenetics of parathyroid hormone resistance. Endocr Dev. 2013;24:11–24. [DOI] [PubMed] [Google Scholar]
- 21. Yu S, Yu D, Lee E, et al. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein a-subunit (Gsa) knockout mice is due to tissue-specific imprinting of the Gsa gene. Proc Natl Acad Sci USA. 1998;95:8715–8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernández-Rebollo E, Maeda A, Reyes M, et al. Loss of XLαs (extra-large αs) imprinting results in early postnatal hypoglycemia and lethality in a mouse model of pseudohypoparathyroidism Ib. Proc Natl Acad Sci USA. 2012;109:6638–6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turan S, Fernandez-Rebollo E, Aydin C, et al. Postnatal establishment of allelic Gαs silencing as a plausible explanation for delayed onset of parathyroid hormone resistance owing to heterozygous Gαs disruption. J Bone Miner Res. 2014;29:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schuster V, Kress W, Kruse K. Paternal and maternal transmission of pseudohypoparathyroidism type Ia in a family with Albright hereditary osteodystrophy: no evidence of genomic imprinting. J Med Genet. 1994;31:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aldred MA, Aftimos S, Hall C, et al. Constitutional deletion of chromosome 20q in two patients affected with albright hereditary osteodystrophy. Am J Med Genet. 2002;113:167–172. [DOI] [PubMed] [Google Scholar]
- 26. Lebrun M, Richard N, Abeguilé G, et al. Progressive osseous heteroplasia: a model for the imprinting effects of GNAS inactivating mutations in humans. J Clin Endocrinol Metab. 2010;95:3028–3038. [DOI] [PubMed] [Google Scholar]
- 27. Ward S, Sugo E, Verge CF, Wargon O. Three cases of osteoma cutis occurring in infancy. A brief overview of osteoma cutis and its association with pseudo-pseudohypoparathyroidism. Australas J Dermatol. 2011;52:127–131. [DOI] [PubMed] [Google Scholar]
- 28. Lau K, Willig RP, Hiort O, Hoeger PH. Linear skin atrophy preceding calcinosis cutis in pseudo-pseudohypoparathyroidism. Clin Exp Dermatol. 2012;37:646–648. [DOI] [PubMed] [Google Scholar]
- 29. Turan S, Thiele S, Tafaj O, et al. Evidence of hormone resistance in a pseudo-pseudohypoparathyroidism patient with a novel paternal mutation in GNAS. Bone. 2015;71:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Germain-Lee EL, Schwindinger W, Crane JL, et al. A mouse model of Albright hereditary osteodystrophy generated by targeted disruption of exon 1 of the Gnas gene. Endocrinology. 2005;146:4697–4709. [DOI] [PubMed] [Google Scholar]
- 31. Chen M, Gavrilova O, Liu J, et al. Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci USA. 2005;102:7386–7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rubera I, Poujeol C, Bertin G, et al. Specific Cre/Lox recombination in the mouse proximal tubule. J Am Soc Nephrol. 2004;15:2050–2056. [DOI] [PubMed] [Google Scholar]
- 33. Liu Z, Segawa H, Aydin C, et al. Transgenic overexpression of the extra-large Gsα variant XLαs enhances Gsα-mediated responses in the mouse renal proximal tubule in vivo. Endocrinology. 2011;152:1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turan S, Bastepe M. The GNAS complex locus and human diseases associated with loss-of-function mutations or epimutations within this imprinted gene. Horm Res Paediatr. 2013;80:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doctor RB, Chen J, Peters LL, Lux SE, Mandel LJ. Distribution of epithelial ankyrin (Ank3) spliceoforms in renal proximal and distal tubules. Am J Physiol. 1998;274:F129–F138. [DOI] [PubMed] [Google Scholar]
- 36. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xia S, Li X, Johnson T, Seidel C, Wallace DP, Li R. Polycystin-dependent fluid flow sensing targets histone deacetylase 5 to prevent the development of renal cysts. Development. 2010;137:1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tran M, Tam D, Bardia A, et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li HC, Du Z, Barone S, et al. Proximal tubule specific knockout of the Na(+)/H(+) exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. J Mol Med. 2013;91:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. [DOI] [PubMed] [Google Scholar]
- 41. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhuang Z, Marshansky V, Breton S, Brown D. Is caveolin involved in normal proximal tubule function? Presence in model PT systems but absence in situ. Am J Physiol Renal Physiol. 2011;300:F199–F206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chase LR, Melson GL, Aurbach GD. Pseudohypoparathyroidism: defective excretion of 3′,5′-AMP in response to parathyroid hormone. J Clin Invest. 1969;48:1832–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maeda A, Okazaki M, Baron DM, et al. Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc Natl Acad Sci USA. 2013;110:5864–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brenza HL, Kimmel-Jehan C, Jehan F, et al. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1a-hydroxylase gene promoter. Proc Natl Acad Sci USA. 1998;95:1387–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. You G, Lee WS, Barros EJ, et al. Molecular characteristics of Na(+)-coupled glucose transporters in adult and embryonic rat kidney. J Biol Chem. 1995;270:29365–29371. [DOI] [PubMed] [Google Scholar]
- 48. Vrhovac I, Balen Eror D, Klessen D, et al. Localizations of Na-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467:1881–1898. [DOI] [PubMed] [Google Scholar]
- 49. Kawashima H, Torikai S, Kurokawa K. Localization of 25-hydroxyvitamin D3 1 α-hydroxylase and 24-hydroxylase along the rat nephron. Proc Natl Acad Sci USA. 1981;78:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Picard N, Capuano P, Stange G, et al. Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflugers Arch. 2010;460:677–687. [DOI] [PubMed] [Google Scholar]
- 51. Murray RD, Holthouser K, Clark BJ, et al. Parathyroid hormone (PTH) decreases sodium-phosphate cotransporter type IIa (NpT2a) mRNA stability. Am J Physiol Renal Physiol. 2013;304:F1076–F1085. [DOI] [PubMed] [Google Scholar]
- 52. Yang W, Friedman PA, Kumar R, et al. Expression of 25(OH)D3 24-hydroxylase in distal nephron: coordinate regulation by 1,25(OH)2D3 and cAMP or PTH. Am J Physiol. 1999;276:E793–E805. [DOI] [PubMed] [Google Scholar]
- 53. Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889. [DOI] [PubMed] [Google Scholar]
- 54. Rhee Y, Bivi N, Farrow E, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. [DOI] [PubMed] [Google Scholar]
- 56. Murayama A, Takeyama K, Kitanaka S, et al. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1α-hydroxylase gene by parathyroid hormone, calcitonin, and 1α,25(OH)2D3 in intact animals. Endocrinology. 1999;140:2224–2231. [DOI] [PubMed] [Google Scholar]
- 57. Healy KD, Zella JB, Prahl JM, DeLuca HF. Regulation of the murine renal vitamin D receptor by 1,25-dihydroxyvitamin D3 and calcium. Proc Natl Acad Sci USA. 2003;100:9733–9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Iida K, Shinki T, Yamaguchi A, DeLuca HF, Kurokawa K, Suda T. A possible role of vitamin D receptors in regulating vitamin D activation in the kidney. Proc Natl Acad Sci USA. 1995;92:6112–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weinstein LS, Yu S, Ecelbarger CA. Variable imprinting of the heterotrimeric G protein G(s) α-subunit within different segments of the nephron. Am J Physiol Renal Physiol. 2000;278:F507–F514. [DOI] [PubMed] [Google Scholar]
- 60. Mantovani G, Bondioni S, Locatelli M, et al. Biallelic expression of the Gsα gene in human bone and adipose tissue. J Clin Endocrinol Metab. 2004;89:6316–6319. [DOI] [PubMed] [Google Scholar]
- 61. Fröhlich LF, Mrakovcic M, Steinborn R, Chung UI, Bastepe M, Jüppner H. Targeted deletion of the Nesp55 DMR defines another Gnas imprinting control region and provides a mouse model of autosomal dominant PHP-Ib. Proc Natl Acad Sci USA. 2010;107:9275–9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Collins MT, Lindsay JR, Jain A, et al. Fibroblast growth factor-23 is regulated by 1α,25-dihydroxyvitamin D. J Bone Miner Res. 2005;20:1944–1950. [DOI] [PubMed] [Google Scholar]