Abstract
Previous genome-wide association studies have identified common variants in genes associated with bone mineral density (BMD) and risk of fracture. Recently, we identified single nucleotide polymorphisms (SNPs) in Wingless-type mouse mammary tumor virus integration site (WNT)16 that were associated with peak BMD in premenopausal women. To further identify the role of Wnt16 in bone mass regulation, we created transgenic (TG) mice overexpressing human WNT16 in osteoblasts. We compared bone phenotypes, serum biochemistry, gene expression, and dynamic bone histomorphometry between TG and wild-type (WT) mice. Compared with WT mice, WNT16-TG mice exhibited significantly higher whole-body areal BMD and bone mineral content (BMC) at 6 and 12 weeks of age in both male and female. Microcomputer tomography analysis of trabecular bone at distal femur revealed 3-fold (male) and 14-fold (female) higher bone volume/tissue volume (BV/TV), and significantly higher trabecular number and trabecular thickness but lower trabecular separation in TG mice compared with WT littermates in both sexes. The cortical bone at femur midshaft also displayed significantly greater bone area/total area and cortical thickness in the TG mice in both sexes. Serum biochemistry analysis showed that male TG mice had higher serum alkaline phosphatase, osteocalcin, osteoprotegerin (OPG), OPG to receptor activator of NF-kB ligand (tumor necrosis family ligand superfamily, number 11; RANKL) ratio as compared with WT mice. Also, lower carboxy-terminal collagen cross-link (CTX) to tartrate-resistant acid phosphatase 5, isoform b (TRAPc5b) ratio was observed in TG mice compared with WT littermates in both male and female. Histomorphometry data demonstrated that both male and female TG mice had significantly higher cortical and trabecular mineralizing surface/bone surface and bone formation rate compared with sex-matched WT mice. Gene expression analysis demonstrated higher expression of Alp, OC, Opg, and Opg to Rankl ratio in bone tissue in the TG mice compared with WT littermates. Our data indicate that WNT16 is critical for positive regulation of both cortical and trabecular bone mass and structure and that this molecule might be targeted for therapeutic interventions to treat osteoporosis.
The treatments of osteoporosis can be divided into 2 main categories: anabolic and antiresorptive therapies. Anabolic therapies primarily enhance bone formation involving osteoblasts or osteocytes, whereas antiresorptive therapies mainly inhibit bone resorption through interfering with osteoclasts. Most of the current therapies of osteoporosis are targeted to inhibit resorption (1, 2). The only anabolic therapy currently approved by the Food and Drug Administration aimed at enhancing bone formation is teriparatide or recombinant human parathyroid hormone, analog 1–34 (1, 2). Thus, identification of molecules stimulating new bone formation at clinically relevant fracture sites is crucial and will greatly facilitate the prevention and treatment of osteoporosis or other bone-fragility conditions.
The Wingless-type mouse mammary tumor virus integration site (WNT) signaling pathway plays a major role in embryonic development and postnatal health and disease, including bone and mineral homeostasis (3–5). The WNT family is comprised of 19 secreted cysteine-rich glycoproteins. Upon binding to one of the ten different Frizzled receptors and other coreceptors (eg, low density lipoprotein receptor-related protein 5/6) on the cell surface, they activate canonical, noncanonical, or both pathways for transcription of target genes (3–5). Genome-wide association studies (GWAS) have identified common variants in genes in the WNT signaling pathway associated with bone mineral density (BMD) and risk of fracture (6–16). Several recent GWAS studies demonstrated that genetic variations in WNT16 are associated with BMD and risk of fracture in children and adults across multiple populations (9–11, 13–15). Recently, we identified single nucleotide polymorphisms (SNPs) in WNT16 that were associated with peak BMD in premenopausal women (9). Furthermore, global Wnt16 knockout mice exhibit significantly lower cortical bone density and strength as well as increased susceptibility to fracture, and these mice fail to increase periosteal bone formation in response to mechanical loading (13, 14, 17). Recently, osteoblast-specific Wnt16 knockout mice were developed; their bone phenotype was found to be similar to global knockout mice, which underscores the importance of Wnt16 production by osteoblasts per se for skeletal health (18). Together, these results suggest that WNT16 is critical for maintaining bone mass and strength, and that this molecule might be an attractive target for pharmacologic intervention in treating osteoporosis or other low bone mass conditions.
To further understand the role of Wnt16 in bone mass regulation, we engineered transgenic (TG) mice that overexpress human WNT16 (hWNT16) in osteoblasts. We measured bone density, structure and strength, evaluated serum biomarkers of bone metabolism, and performed gene expression analyses, dynamic bone histomorphometry, cellular parameters and cell culture studies using both male and female hWNT16-TG and wild-type (WT) mice. We demonstrate for the first time that WNT16 overexpression influences both trabecular and cortical bone mass and structure in mice.
Materials and Methods
Generation of the Col2.3-hWNT16-TG mice
The cDNA of hWNT16 (IMAGE clone ID 8143948) cloned into the pCR4-TOPO vector, was obtained from Open Biosystem. The WNT16 gene was excised from this vector by EcoR1 digestion and was cloned into the pJ251 plasmid (a kind gift from Dr Ernestina Schipani, University of Michigan), replacing the LacZ sequence that resides between the osteoblast-specific 2.3-kb α1 type 1 collagen promoter and mouse protamine 1 polyA signal (Figure 1A). The transgene expression construct (promoter + WNT16 cDNA + polyA tail sequence) was digested with XhoI and Sph1 and microinjected into pronuclei of B6-fertilized eggs, which were then transferred into B6 foster mothers by the Indiana University Institutional Transgenic Animal Facility. The integration of the transgene(s) into the genome of founder mice was determined by PCR using tail DNA.
Figure 1.
Generation and characterization of the Col2.3-hWNT16-TG mice. The hWNT16 cDNA was cloned into pJ251 plasmid between the osteoblast-specific rat 2.3-kb α1 type 1 collagen promoter and mouse protamine 1 polyA sequences (A). Transgene expression (hWNT16) in various tissues (bone, skin, liver, spleen, heart, and muscle) was measured in both male and female WT and TG mice by real-time PCR. Higher level of hWNT16 was observed in bone tissue compared with other tissues (B). Transgene expression (hWNT16) in bone tissue was compared between WT and TG mice in both male and female. A high level of hWNT16 mRNA was detected only in the TG mice (C). The levels of mWnt16 (endogenous) mRNA expression were determined in both WT and TG male and female mice. The mWnt16 expression level was decreased almost 50% in both male and female TG mice compared with WT mice (D). The expression of hWNT16 in the distal femur (predominantly trabecular bone) was similar to the level of expression in the femur midshaft (cortical bone) in the TG mice (E); *, P < .05.
Experimental animals
We used 20 WT and 20 WNT16-TG mice (10 male and 10 female per genotype) in this study. All mice were generated and maintained at Indiana University. Mice were housed in polycarbonate cages in a vivarium maintained on a 12-hour light, 12-hour dark cycle and were fed a regular diet and water ad libitum. The procedures performed throughout the experiment followed the guidelines of the Indiana University Animal Care and Use committee.
Euthanasia and specimen collection
Mice were euthanized at 12 weeks of age, and lower limbs were dissected from these animals. The femora on the right side were immediately stored at [minus 80°C in saline-soaked gauze for subsequent biomechanical testing. The femora on the left side were stripped of muscle, transferred to 70% ethyl alcohol and stored at 4°C for densitometry analyses. In addition, after removing of muscle and periosteum, both ends of the tibia and humeri were cut to flush out the marrow cavity with PBS before transferring them to RNA later stabilization reagent (QIAGEN). We obtained cortical and trabecular bone tissue from femur diaphysis dissected into 2 sections: distal femur which consists mainly of trabecular bone, and proximal femur, containing predominantly cortical bone. We also harvested skin, liver, spleen, heart, and muscle tissues from both WT and TG mice, which were flash frozen immediately and stored at −80°C until use for gene expression analysis.
Gene expression analysis
Measurements of gene expression were performed by real-time PCR using bone and other organs as well as osteoblast and osteoclast developed by cell culture studies from both male and female WT and TG mice. For gene expression analysis, cells or tissues were lysed in TRIzol, and total RNA was isolated using phenol/chloroform method followed by cDNA synthesis using the High Capacity cDNA kit (Life Technologies) using random hexamers. All quantitative polymerase chain reactions were performed using the cDNA equivalent of 20 ng of total RNA using custom-made primer and probe sets from Integrated DNA Technology for hWNT16, endogenous mouse Wnt16 (mWnt16), alkaline phosphatase (Alp), osteocalcin (OC), osteoprotegerin (Opg), and TNF (ligand) superfamily, member 11 (Rankl or Tnfs11). We also investigated 1) Wnt signaling pathway canonical genes, β-catenin (Ctnnb1) and Axin 2 (Axin2); 2) Wnt signaling pathway noncanonical genes, jun protooncogene (c-Jun or Ap1), MAPK 8 (Jnk or Mapk8), protein kinase, cAMP-dependent, catalytic-α (Pka), protein kinase C (Pkc); and 3) osteoclast-specific genes, dendrocyte-expressed 7 transmembrane protein (Dcstamp), nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1 (Nfatc1), acid phosphatase 5, tartrate resistant (Acp5 or Trap), and cathepsin K (Ctsk). Real-time detection of PCR products was accomplished using an ABI PRISM 7900 sequence detector (Applied Biosystem) and normalized to the house-keeping gene β-actin.
Osteoblast and osteoclast culture from bone marrow
Bone marrow was collected from long bones (femur and tibia) of WT and TG WNT16 mice at 6 weeks of age. Osteoblasts were generated from adherent bone marrow stromal cells by culturing them in osteogenic media for 7 days, and osteoclasts were generated by culturing the nonadherent bone marrow cells in macrophage colony stimulating factor (20 ng/mL) and receptor activator of NF-kB ligand (RANKL) (80 ng/mL) for 6 days. For gene expression analysis, osteoblasts and osteoclasts were lysed in TRIzol followed by cDNA synthesis and real-time detection of osteoblast-specific genes (Alp and OC) and osteoclast-specific genes (Dcstamp, Nfatc1, Trap, and Ctsk) as described above.
Dual energy x-ray absorptiometry (DXA)
The whole body and femur of the WT and WNT16-TG mice were scanned using DXA (PIXImus II mouse densitometer; Lunar Corp) with ultrahigh resolution (0.18 × 0.18 mm/pixel). The machine was calibrated each time before DXA scanning using a phantom supplied by the manufacturer. The whole-body scans were performed with the mice in a prone position, with each limb spread on a plastic tray. The global window was defined as the whole-body image minus the calvarium, mandible, and teeth, from which whole-body areal bone mineral density (aBMD) (g/cm2) and bone mineral content (BMC) (g) measurements were obtained. During femur scanning, dissected bones were positioned on a platform supplied by the manufacturer. After completion of the scan of each bone, mutually exclusive region of interest boxes were drawn around the bone, from which femur aBMD (g/cm2) and BMC (g) measurements were obtained.
Microcomputer tomography (μCT) analysis
The femurs of WT and WNT16-TG mice were scanned with a high resolution μCT scanner (vivaCT 40; Scanco Medical AG) with an isotropic voxel size of 10.5 μm3. Before scanning, the CT scanner was calibrated using a phantom according to the manufacturer's recommendation. From the scout-view, the growth plate location was identified, and trabecular bone measurements consisting of 200 slices (2.1 mm) was completed from about 1 mm below the growth plate. The settings for each scan included peak x-ray potential of 55 kVp, x-ray intensity of 72 μA, 1000 projections per 180 degrees, image resolution of 2048 × 2048 pixels, and an integration time of 200 milliseconds. After scanning, contouring was achieved manually a few pixels away from the endocortical surface to isolate the trabecular bone. Image processing of all scans included Gaussian filtering and segmentation by using global thresholds, above which all pixels are considered bone, and below which all pixels are considered nonmineralized tissues (σ = 1; support = 2; threshold density = 170). The same filtering and segmentation values were used for each bone for all trabecular bone measurements. For cortical bone analysis, the midfemur of each bone was determined from the scout-view and a total of 60 slices (30 slices above and 30 slices below the midfemur) were scanned with the same setting. Finally, 3-dimensional and 2-dimensional morphometric evaluations were performed for the cortical and trabecular bone from each scan, and bone volume/tissue volume (BV/TV) and structural parameters (trabecular number [Tb.N], trabecular thickness [Tb.Th], trabecular separation [Tb.Sp], cortical bone area/total area [B.Ar/T.Ar], and cortical thickness [Ct.Th]) were determined.
Biomechanical measurements
The right femurs were soaked in a room temperature saline bath overnight before mechanical testing. Measures of whole-bone strength were obtained from each femur positioned posterior side down across the 2 lower supports, spaced 9 mm apart, on a 3-point bending apparatus. The fixtures were mounted in the frame of an electromechanical test machine (TestResources) with a force resolution of 0.05 N. The femurs were held in place by small preload (<1 N) and each bone was loaded to failure in monotonic compression using a crosshead speed of 0.2 mm/s, during which force and displacement measurements were collected every 0.02 seconds. From the force vs displacement curves, yield force (N), stiffness (N/mm), ultimate force (N), and energy to failure (mJ) were calculated using MTestWR software following standard equations (19).
Cortical and trabecular bone dynamic histomorphometry
Mice were given calcein (15 mg/kg) and alizarin (30 mg/kg) ip 10 days and 3 days before euthanasia at 12 weeks of age. The right femurs were used for trabecular bone histomorphometry. The bones were dehydrated in graded series of ethanol and embedded nondecalcified, in methyl-methacrylate. Distal femur sections were cut in the coronal plane with a motorized microtome (Leica Microsystems, Inc) equipped with a tungsten carbide knife. Dynamic bone formation parameters were calculated from the femur midshaft for cortical and distal femur secondary spongiosa for trabecular bone by measuring the unlabeled perimeter, single-labeled perimeter, double-labeled perimeter, and the area between the double labeling using the Bioquant Osteo system (Bioquant Corp). Derived histomorphometric parameters, including mineralizing surface (MS)/bone surface (BS) (%), mineral apposition rate (MAR) (um/y), and bone formation rate (BFR)/BS (um3/um2 per y), were calculated using standard procedures recommended by the American Society of Bone and Mineral Research Histomorphometry Committee (20).
Cellular parameters
We determined B.Ar (in mm2) and bone perimeter (B.Pm) (mm) at distal femur using the same samples used for trabecular bone histomorphometry. In addition, for osteoblast, we compared osteoblast perimeter (Ob.Pm) (mm), osteoblast number (N.Ob), N.Ob over Ob.Pm (N.Ob/Ob.Pm), osteoblast surface over BS (Ob.S/BS) (%), and N.Ob over B.Pm (N.Oc/B.Pm) between WT and TG female mice. For osteoclast, we measured osteoclast perimeter (Oc.Pm) (mm), osteoclast number (N.Oc) over Oc.Pm (N.Oc/Oc.Pm), osteoclast surface over BS (Oc.S/BS) (%), and N.Oc/B.Pm in these mice using standard procedures recommended by the American Society of Bone and Mineral Research Histomorphometry Committee (20).
Serum biomarkers
Serum levels of mouse OPG and RANKL were measured by ELISA kits (R&D Systems) according to the manufacturers' instructions. Serum levels of carboxy-terminal collagen cross-link (CTX), tartrate-resistant acid phosphatase 5, isoform b (TRAcP5b), and OC were measured by ELISA kits (Immunodiagnostics Systems and Biomedical Technologies, Inc) per manufacturers' instructions. Serum calcium, phosphorus, and ALP were measured using the Randox Rx kit (Daytona Analyzer).
Statistical analysis
Quantitative data were expressed as mean ± SEM. Statistical differences between WT and TG groups were tested using the unpaired Student's t test using the statistical software package StatView (Abacus Concepts, Inc). The level of significance was set at P ≤ .05.
Results
WNT16-TG mice had similar body weight as WT mice
TG WNT16 mice were born with expected frequency and appeared healthy with no discernible growth or morphological defects. Compared with WT mice, TG WNT16 mice showed similar body weight (g) in male (21.5 ± 1.0 vs 20.9 ± 0.9; P = .17) and female (17.3 ± 1.1 vs 16.3 ± 1.1; P = .07) at 6 weeks of age, and in male (26.1 ± 1.6 vs 25.0 ± 1.6; P = .11) and female (20.3 ± 1.4 vs 19.4 ± 0.8; P = .09) at 12 weeks of age. The femur length (mm) at 12 weeks of age did not differ between male WT and TG mice (15.07 ± 0.6 vs 15.29 ± 0.4; P = .31). However, female TG mice had slightly shorter femurs compared with WT mice (14.39 ± 0.4 vs 14.92 ± 0.5; P = .03).
WNT16-TG mice expressed high levels of hWNT16 and low levels of mWnt16 in bone tissue
We detected significantly higher levels of transgene (hWNT16) expression in bone tissue compared with other tissues (skin, liver, spleen, heart, and muscle) in the TG mice in both male and female (Figure 1B). In addition, the expression of hWNT16, as expected, was detectable only in the TG mice (Figure 1C). The levels of endogenous mWnt16 mRNA expression in the same samples were significantly lower (40%–50%; P < .05) in the TG male and female mice, compared with WT mice (Figure 1D). Furthermore, we found no difference for the expression of hWNT16 in the distal femur (predominantly trabecular bone) compared with the femur midshaft (cortical bone) in the TG mice (Figure 1E).
WNT16-TG mice exhibited higher whole-body, femur, and spine aBMD and BMC measured by DXA
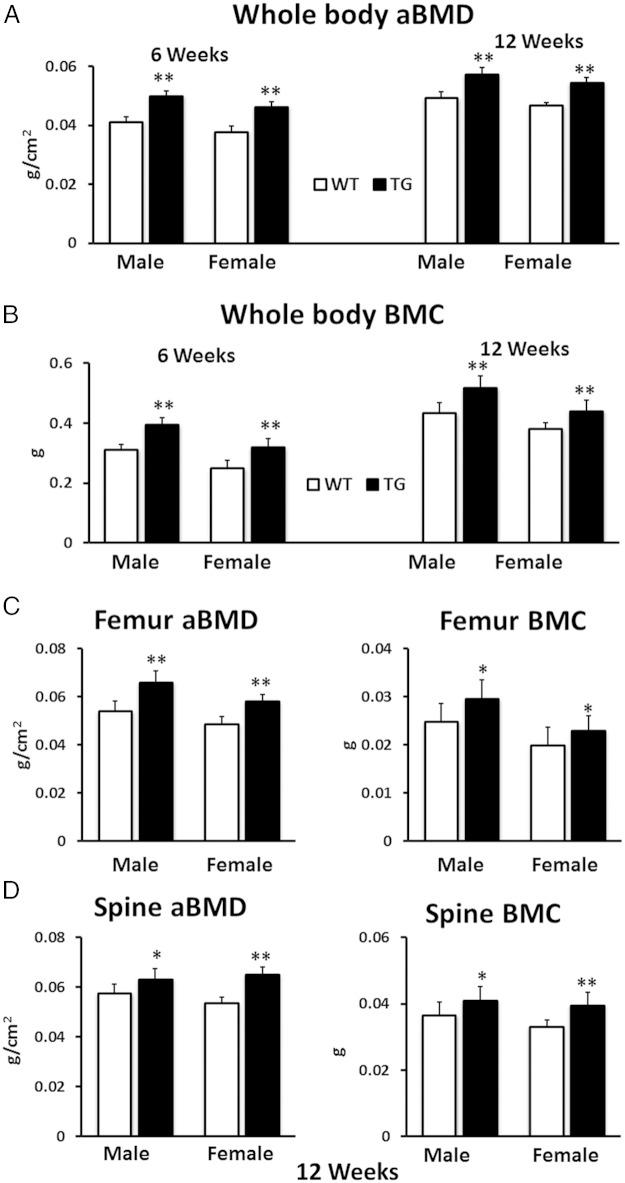
Male TG WNT16 mice had significantly higher whole-body aBMD and BMC (21% and 26%, respectively; P < .0001) compared with WT littermates at 6 weeks of age (Figure 2, A and B). Similarly, female TG mice showed 21% higher whole-body aBMD and 28% higher BMC (P < .0001) compared with female WT mice at this age. At 12 weeks of age, both male and female TG WNT16 mice had significantly higher whole-body aBMD (16% and 17%, respectively; P < .0001) and BMC (19% and 16%, respectively; P < .0005) than WT littermates (Figure 2, A and B). In addition, femoral aBMD measured at 12 weeks of age showed significantly higher values for both TG male and female mice (22% and 19%, respectively; P < .0001) as compared with WT mice (Figure 2C). Similarly, femur BMC was 19% higher in male (P = .007) and 16% higher in female (P = .03) TG mice (Figure 2C). Spine DXA was measured for lumbar 1 through 5 at 12 weeks of age. The aBMD in TG mice was 10% higher (P < .05) in male and 22% higher (P < .0001) in female compared with WT mice (Figure 2D). Similarly, spine BMC was 12% higher in male (P < .05) and 20% higher in female (P < .0005) TG mice (Figure 2D).
Figure 2.
Whole-body, femur, and spine aBMD and BMC measured by DXA. Both male and female TG WNT16 mice exhibited significantly higher whole-body aBMD (A) and BMC (B) at 6 and 12 weeks of age compared with WT mice. TG WNT16 mice also had significantly higher femur and spine (lumbar 1–5) aBMD and BMC (C and D) at 12 weeks of age in both male and female; *, P < .05; **, P < .0005.
WNT16-TG mice displayed higher bone mass and improved microarchitecture in cancellous and cortical bone measured by μCT
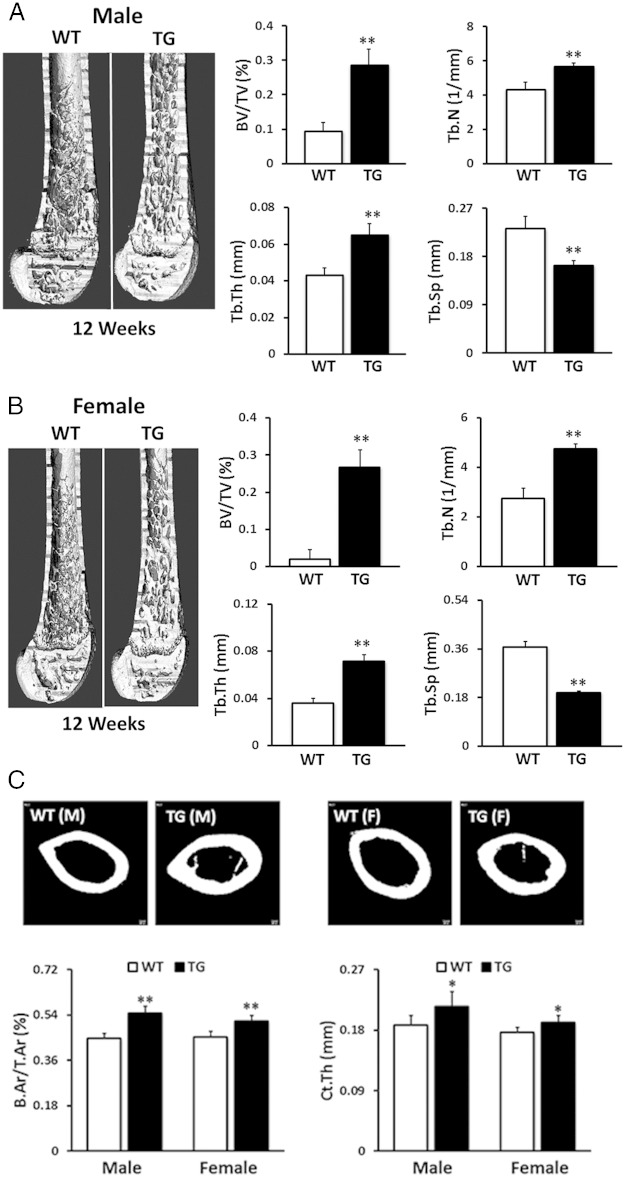
To further identify the effects of the hWNT16 transgene on skeletal properties in greater detail, we measured bone mass and microarchitectural properties in the distal femur for cancellous bone and at the femoral midshaft for the cortical bone in 12-week-old mice using μCT. Male TG WNT16 mice displayed 3-fold higher (P < .0001) trabecular BV/TV, whereas female WNT16-TG mice exhibited 13-fold increase (P < .0001) in BV/TV at distal femur compared with their WT littermates (Figure 3, A and B). Male WNT16-TG mice also showed 31% higher Tb.N and 52% higher Tb.Th and 29% lower Tb.Sp (P < .0001), whereas female WNT16-TG mice exhibited 74% higher Tb.N and 99% higher Tb.Th and 46% lower Tb.Sp (P < .0001) at the same site (Figure 3, A and B). Cortical bone morphometry at midfemur revealed that male WNT16-TG mice had 22% higher B.Ar/T.Ar and 14% higher Ct.Th (P < .0001), whereas female WNT16-TG mice exhibited 14% higher B.Ar/T.Ar and 8% higher Ct.Th (P < .0001) compared with sex-matched WT mice (Figure 3C). Two-way ANOVA (gender and genotype interaction) analysis showed sex-specific differences for trabecular (P < .005) and cortical (P < .05) bone phenotypes in these mice. Overall, these data indicate that male TG mice exhibited a more dramatic effect of the transgene than did female mice in the cortical compartment, whereas female mice displayed a more pronounced effect of the transgene than did male mice in the cancellous compartment.
Figure 3.
Trabecular and cortical bone morphometry measured by μCT. Compared with WT mice, male (A) and female (B) TG WNT16 mice showed significantly higher trabecular bone volume (BV/TV), Tb.N, and Tb.Th but significantly lower Tb.Sp at distal femur at 12 weeks of age. Cortical bone morphometry at the femur midshaft revealed that both male and female TG WNT16 mice had higher cortical B.Ar/T.Ar and Ct.Th, compared with their WT littermates at 12 weeks of age (C); *, P < .005; **, P < .0001.
Serum markers of bone formation and resorption are influenced in WNT16-TG mice
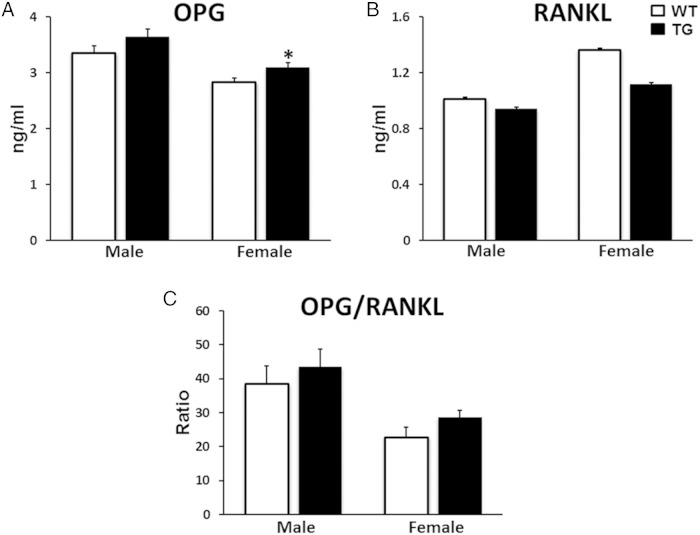
To understand potential changes in serum biochemistry induced by TG overexpression of WNT16 in bone, we measured biomarkers related to skeletal metabolism in serum samples collected from 12-week-old mice. WNT16-TG mice showed similar levels of calcium and phosphorus compared with their WT littermates in both male and female (Table 1). Male WNT16-TG mice showed elevated levels of ALP (21%; P = .03) and OC (41%; P = .04), 2 serum markers of bone formation, compared with the sex-matched WT littermates (Table 1). In addition, we identified a trend of higher levels of serum OPG (9%; P = .1) in male and significantly higher level of serum OPG (9%; P = .05) in female TG mice compared with sex-matched WT mice (see figure 9 below). Also, we observed a trend of higher level of serum OPG/RANKL in the TG mice in female (26%; P = .1) (see figure 9 below). Serum bone resorption marker CTX level did not differ significantly between the 2 genotypes in either sex (Table 1), whereas the osteoclast marker TRAPc5b level was elevated (22%; P = .02) in female TG mice compared with their WT littermates (Table 1). In addition, a trend of lower CTX to TRAPc5b ratio, an indication of resorption per osteoclast, was observed in both male and female WNT16-TG mice (14% and 22%, respectively) compared with sex-matched WT animals (Table 1).
Table 1.
Comparison of Serum Biochemistry and Biomarkers of Bone Formation and Resorption in Male and Female WT and TG Col2.3-WNT16 Mice
| Calcium (mg/dL) | Phosphorus (mg/dL) | ALP (IU/L) | OC (ng/mL) | CTX (ng/mL) | TRAP5b (IU/L) | CTX/TRAP5b | |
|---|---|---|---|---|---|---|---|
| Male | |||||||
| WT | 9.19 ± 0.10 | 10.29 ± 0.44 | 83.5 ± 4.59 | 55.2 ± 5.07 | 34.8 ± 4.80 | 7.00 ± 0.36 | 4.98 ± 0.67 |
| TG | 8.82 ± 0.14 | 9.15 ± 0.54 | 100.8 ± 4.33 | 78.1 ± 6.89 | 30.1 ± 2.85 | 7.03 ± 0.29 | 4.29 ± 0.36 |
| P value | 0.07 | 0.16 | 0.03 | 0.04 | 0.47 | 0.95 | 0.43 |
| Female | |||||||
| WT | 9.18 ± 0.13 | 9.54 ± 0.27 | 116.8 ± 2.55 | 71.8 ± 8.50 | 44.9 ± 7.44 | 8.21 ± 0.32 | 5.53 ± 0.97 |
| TG | 9.17 ± 0.11 | 10.40 ± 0.28 | 121.3 ± 6.40 | 72.0 ± 9.01 | 43.5 ± 9.70 | 10.05 ± 0.51 | 4.29 ± 0.93 |
| P value | 0.98 | 0.08 | 0.59 | 0.98 | 0.91 | 0.02 | 0.37 |
Bold represents significant difference.
Biomechanical properties of femoral diaphysis are improved in male WNT16-TG mice
To identify whether the higher bone mass and better microarchitecture in TG mice were associated with improvements in mechanical properties of the skeleton, we tested femurs from WNT16-TG and WT mice using monotonic 3-point bending tests. Male WNT16-TG mice had significantly higher yield force (10%; P = .02) and higher ultimate force (19%; P = .04) as compared with male WT mice (Figure 4). In addition, male TG mice showed a trend of higher stiffness, energy to yield and energy to failure (12%, 9%, and 13%, respectively), although the differences failed to reach statistical significance. In contrast, male TG mice exhibited significantly lower (30%; P = .002) postyield displacement, which contributed to a similar measure of energy to failure between TG and WT mice. Also, we found no differences in biomechanical properties between female WT and TG mice (Figure 4).
Figure 4.
Measurement of bone strength by femur biomechanical test. Three-point bending test of femora from 12-week-old mice demonstrated that male TG WNT16 mice had a trend of higher stiffness (A) and had significantly higher yield force and ultimate force compared with WT mice (B and C). In contrast, male TG mice showed significantly lower postyield displacement compared with WT mice (D). On the other hand, all femur biomechanical parameters did not differ between female WT and TG mice (A–D); *, P < .05.
Cortical and trabecular BFR and MS are significantly increased by hWNT16 overexpression
To determine whether greater cortical and trabecular bone volume in WNT16-TG mice was driven by enhanced bone formation, we measured dynamic bone formation parameters in the femur midshaft and distal femur secondary spongiosa using fluorochrome labels embedded in the bone before sacrifice at 12 weeks of age. We compared MS (MS/BS), MAR, and BFR (BFR/BS) between sex-matched WT and TG mice. Both male and female TG mice had significantly higher MS/BS (31% and 135%, respectively; P < .005) than their sex-matched WT littermates (Figure 5B). Male TG mice also had 61% higher MAR (P < .005) compared with WT male littermates, but the observed 18% increase in MAR in female TG mice failed to reach statistical significance (P = .2). In addition, BFR/BS in both male and female TG mice were significantly higher (95% and 177%, respectively; P < .0005) than in sex-matched control WT mice (Figure 5B). Female TG mice also displayed significantly higher periosteal MS/BS (20%; P < .05) and a trend of higher BFR/BS (19%; P = .06) at femur midshaft compared with sex-matched WT mice (Figure 5C).
Figure 5.
Dynamic cortical and trabecular bone histomorphometric measurements. Fluorochrome labeling of trabecular bone from 12-week-old mice (A) revealed that both male and female TG mice had significantly higher distal femur MS/BS compared with WT mice (B). Male TG mice also showed significantly higher MAR and female TG mice had a trend of higher MAR compared with WT littermates (B). In addition, BFR in both male and female TG mice were significantly higher compared with WT mice (B). Female TG mice also displayed significantly higher periosteal MS/BS and a trend of higher BFR/BS at femur midshaft compared with sex-matched WT mice (C); *, P < .005; **, P < .0005.
N.Oc and surface area are unchanged, whereas Oc.S area is significantly decreased, by hWNT16 overexpression
Although the B.Ar (2.4-fold; P < .0001), B.Pm (53%; P < .0005), and Ob.Pm (49%; P < .05) were significantly higher in the TG female mice compared with WT mice (Figure 6, A and B), N.Ob, N.Ob/Ob.Pm, and Ob.S/BS did not differ between sex-matched WT and TG mice (Figure 6, C and E). In addition, Oc.Pm and N.Oc/Oc.Pm were similar between the 2 genotypes (Figure 6, B and D). In contrast, Oc.S/BS and N.Oc/B.Pm were significantly lower (35%, P < .0005 and 34%, P < .0001, respectively) in the TG mice compared with sex-matched WT littermates (Figure 6F).
Figure 6.
Measurements of osteoblast and N.Ocs and surfaces. TG female mice showed significantly higher B.Ar, B.Pm, and Ob.Pm compared with WT mice (A and B). The N.Ob, N.Ob/Ob.Pm, and Ob.S/BS did not differ between sex-matched WT and TG mice (C and E). In addition, Oc.Pm and N.Oc/Oc.Pm were similar between the 2 genotypes (6B and 6D). In contrast, Oc.S/BS and N.Oc/B.Pm were significantly decreased in the TG mice compared with sex-matched WT littermates (F); *, P < .05; **, P < .005.
Gene expression analysis
Higher mRNA levels of Alp (118%; P < .001 in male and 59%; P = .1 in female) and OC (30%; P = .07 in male and 68%; P < .001 in female) were observed in the long bone of the TG mice compared with their WT littermates (Figure 7, A and B). In addition, expression of Opg was up-regulated in the same tissue (88%; P < .001), whereas Rankl expression did not change resulting in higher Opg to Rankl ratio (81%; P < .005) in the TG male mice compared with WT mice (Figure 7, C–E). Comparing canonical- and noncanonical Wnt signaling pathway genes in bone tissue between WT and TG mice, we observed a trend of higher mRNA levels of Ctnnb1 (106% in male, P = .1; and 67% in female, P = .2) and Axin2 (45% in male and 76% in female; P = .2) (Figure 8, A and B) but similar levels of c-Jun/Ap1, Jnk/Mapk8, Pka, and Pkc in the TG mice (Figure 8, C–F).
Figure 7.
Analyses of gene expression in the bone tissue. The mRNA levels of Alp and OC were higher in the bone tissue of both male and female TG WNT16 mice compared with the sex-matched WT littermates (A and B). In addition, expression of Opg was up-regulated in the same tissue, whereas Rankl expression did not change resulting in higher Opg to Rankl ratio in the TG male mice compared with the WT mice (C–E); *, P < .05.
Figure 8.
Canonical vs noncanonical gene expression analyses. The mRNA levels of Wnt canonical signaling pathway genes (Ctnnb1 and Axin2) were higher in the TG WNT16 mice (A and B), whereas the expression levels of noncanonical pathway genes (c-Jun/Ap1, Jnk/Mapk8, Pka, and Pkc) were similar in the TG mice (C–F) compared with their WT littermates in both male and female.
Osteoblast and osteoclast culture
Osteoblasts differentiated from bone marrow from WT and TG mice showed a trend of higher level of Alp (57%; P = .1) in female and significantly higher level of OC expression in both male (5-fold; P < .05) and female (4-fold; P < .05) TG mice compared with their WT littermates (Figure 10, A and B). The ratio of Opg to Rankl expression was similar between the genotypes (Figure 10C). Bone marrow-derived osteoclast gene expression analysis demonstrated no difference of Dcstamp, Nfatc1, Trap, and Ctsk between WT and TG mice in male (Figure 10, D–G). However, we observed a trend of lower expression of Ctsk (26%) and significantly lower expression of Dcstamp (20%; P < .05) in female TG osteoclasts compared with the WT cells (Figure 10, D and G). To compare the differentiation capacity of the mesenchymal and hematopoietic stem cells between WT and TG mice, we cultured these cells isolated from bone marrow for different time periods and examined their proliferation and differentiation towards osteoblast and osteoclast, respectively. We observed lower number of osteoclasts on day 2, 4, and 6 (47%, 25%, and 27%, respectively) in the TG mice compared with WT mice (Supplemental Figure 1B). In contrast, we detected approximately 25% higher N.Obs on each of day 4, 11, and 18 in the TG mice compared with WT littermates (Supplemental Figure 1A).
Figure 10.
Bone marrow-derived osteoblast and osteoclast gene expression analysis. Osteoblasts differentiated from bone marrow from WT and TG mice showed a trend of higher level of Alp in female and significantly higher level of OC expression in both male and female TG mice compared with their WT littermates (A and B). The ratio of Opg to Rankl expression was similar between the genotypes (C). Osteoclasts generated from bone marrow of WT and TG WNT16 mice demonstrated no difference of Dcstamp, Nfatc1, Trap, and Ctsk (D–G) between WT and TG mice in male; however, we observed a trend of lower expression of Ctsk (G) and significantly lower expression of Dcstamp (D) in female TG osteoclasts compared with WT cells; *, P < .05.
Discussion
Our goal in this study was to understand the skeletal consequences of increased WNT16 expression in bone tissue. We found that overexpression of hWNT16 in Col2.3-expressing cells significantly increased whole-body and site-specific (femur and spine) aBMD and BMC and significantly improved cancellous and cortical microarchitectural properties in the femur in both male and female TG mice. We also observed that the same transgene produced a stronger cortical bone phenotype in male mice and a superb cancellous bone phenotype in female mice. Not surprisingly, the strong cortical phenotype in male TG mice was also associated with significantly improved bone biomechanical properties, whereas the mild cortical phenotype in female TG mice failed to produce a measurable change in bone strength. Female mice also exhibited a much more pronounced effect of the transgene in the cancellous compartment (eg, BV/TV) than males, which was due to the greater increase in MS and BFR among female TG mice compared with males. Furthermore, serum bone formation markers (ALP and OC) were up-regulated, whereas a trend of lower resorption (CTX to TRAP ratio) was observed in the TG mice. Gene expression analysis demonstrated higher expression of Alp, OC, Opg, and Opg to Rankl ratio in bone tissue in the TG mice compared with WT littermates. Together, these results suggest that WNT16 is critical for positive regulation of both cortical and trabecular bone mass and structure.
Several studies have demonstrated that global deletion of Wnt16 in mice decreases cortical bone volume and thickness, increases cortical porosity, and results in spontaneous fractures at multiple skeletal sites (13, 14, 17). However, despite the strong cortical phenotype observed in Wnt16 knockout mice, these mice do not appear to manifest a significant trabecular bone phenotype. Recently, conditional deletion of Wnt16 from osteoblast-lineage cells also revealed that Wnt16 is a critical regulator for maintenance of cortical bone mass and fracture susceptibility (18). In contrast, we found a robust trabecular bone phenotype in the osteoblast-specific TG Wnt16 mice, in both males and females. TG mice had 3-fold (male) and 14-fold (female) higher trabecular bone volume at the distal femur at 12 weeks of age, compared with WT mice. Our data is consistent with reported association between WNT16 and spine and heel BMD (predominantly trabecular bone) in several human GWAS studies performed by different groups (7, 9, 16). On the other hand, the improvement of cortical bone volume (22% and 14%, respectively) and thickness (14% and 8%, respectively) in both male and female TG mice was driven by cellular activity occurring mostly to the endocortical surface. These phenotypic differences observed between TG and knockout models might be due to the variations of complex signaling among several Wnts as well as to cross talk among different signaling pathways involved in overall bone homeostasis (21). Importantly, these data indicate that Wnt16 plays a significant role for the acquisition of both cortical and trabecular bone mass, structure, and strength.
The 3-point bending test of femur showed that male TG WNT16 mice had significantly higher yield force and ultimate force but a trend of higher energy to yield and stiffness compared with WT mice. However, similar energy to failure was observed between WT and TG mice due to significantly lower postyield displacement (24% and 22%, respectively) exhibited by the male and female TG mice (Figure 4). On the other hand, all femur biomechanical parameters did not differ between female WT and TG mice. These data indicate that the biomechanical properties we measured are not influenced significantly by trabecular bone mass and structure gain in the TG mice. Moreover, although cortical bone volume and thickness were improved in male and female TG mice, new cortical bone gain was distributed mostly on the endocortical surface, which has very little influence on biomechanical parameters of the whole bone.
Cortical and trabecular bone histomorphometric measurements indicate that male TG mice not only had more active trabecular BS (MS/BS) but also had increased osteoblast activity (MAR) (Figure 5B). Together, this leads to 95% higher BFR (BFR/BS) in the male TG mice compared with sex-matched WT mice. Female TG mice also showed 135% higher MS and a modest increase (18%) in MAR, leading to 177% increase of BFR/BS compared with WT littermates (Figure 5B). Female TG mice also displayed 20% higher periosteal MS/BS and 19% higher BFR/BS at femur midshaft compared with sex-matched WT mice (Figure 5C). In a previous study, Wergedal et al (17) also demonstrated that the periosteal BFR and MAR were significantly reduced in female Wnt16 knockout mice compared with WT mice at 12 weeks of age. These results suggest that WNT16 influences cortical and trabecular bone metabolism by modifying both N.Oc as well as their activity.
We detected higher levels of Alp and OC mRNA in the bone tissue of the TG mice compared with their WT littermates (Figure 7, A and B), which align with our finding of higher serum ALP and OC levels in the TG mice (Table 1). Previous study showed that deletion of Wnt16 in mice significantly lowered the expression levels of Alp and Runx2, genes essential for osteoblast differentiation (17). These data suggest that Wnt16 mediates its anabolic effect by influencing osteoblast development and function. In addition, we found that expression of Opg mRNA was up-regulated, whereas Rankl expression did not change resulting in higher Opg to Rankl ratio in the TG mice compared with WT mice (Figure 7, C and D). In consistent with these results, we identified a trend of higher levels of serum OPG and OPG to RANKL ratio in the TG mice (Figure 9C). Similarly, expression of Opg was lower, whereas Rankl to Opg ratio was higher in the Wnt16 knockout mice compared with the WT littermates (18). These results suggest that, in addition to direct effect on osteoblast, Wnt16 might indirectly suppress osteoclast formation and differentiation for the development and maintenance of bone mass and strength.
Figure 9.
Measurements of serum levels of OPG and RANKL. A trend of higher level of serum OPG in male and significantly higher level of serum OPG in female TG WNT16 mice were observed compared with WT mice (A). Similar serum RANKL level was observed in male WT and TG WNT16 mice whereas a trend of lower level of RANKL was observed in female TG mice compared with sex-matched WT mice (B). Both male and female TG mice displayed a trend of higher level of serum OPG to RANKL ratio (C); *, P < .05.
Comparing canonical and noncanonical Wnt signaling pathway related genes in bone tissue between WT and TG mice, we observed higher mRNA levels of canonical genes (Ctnnb1 and Axin2) (Figure 8, A and B) but similar levels of noncanonical genes (c-Jun/Ap1, Jnk/Mapk8, Pka, and Pkc) in the TG mice (Figure 8C). We also observed higher β-catenin protein levels in bone tissue in the TG mice compared with WT littermates (data not shown). Similar to bone tissue, osteoblasts differentiated from bone marrow from WT and TG mice showed a trend of higher level of Alp in female and significantly higher level of OC expression in both male and female TG mice compared with their WT littermates (Figure 10, A and B). When we compared the differentiation and proliferation capacity bone marrow mesenchymal stem cells towards osteoblasts, we detected higher N.Obs on day 4, 11, and 18 in the TG mice compared with WT mice (Supplemental Figure 1A). Recently, it was demonstrated that Wnt16 inhibit osteoclastogenesis through direct effects on osteoclast progenitors, in addition to its indirect effects on osteoblast (18). In this study, bone marrow-derived osteoclast gene expression analysis demonstrated a trend of lower expression of Ctsk and significantly lower expression of Dcstamp in female TG osteoclasts compared with WT cells (Figure 10, D and G). Also, comparing the capacity of bone marrow hematopoietic cells towards osteoclasts differentiation between the 2 genotypes on different days we detected lower number of osteoclasts on day 2, 4, and 6 in the TG mice (47%, 25%, and 27%, respectively) compared with WT mice (Supplemental Figure 1B). In addition, CTX and CTX to Trap ratio derived from the conditioned media form osteoclast culture were lower (21% and 48%, respectively) in the TG mice compared with WT littermates (data not shown). Together, these data suggest that bone mass phenotype we observed in the TG mice is due to osteoblast mediated anabolic effect of WNT16 involving canonical Wnt signaling pathway as well as suppression of osteoclast formation and differentiation for the maintenance of overall bone homeostasis.
In conclusion, we demonstrated that WNT16-TG mice exhibited significantly higher mineralization surface, BFR and BMD in the trabecular bone in both male and female. Cortical B.Ar and Ct.Th were also improved in these TG mice, particularly in males. In addition, serum bone formation markers were up-regulated, whereas a trend of lower bone resorption was observed. Our data indicate that WNT16 is critical for positive regulation of both cortical and trabecular bone mass and structure, and that this molecule might be targeted for therapeutic interventions to treat osteoporosis or other low bone mass and high bone-fragility conditions.
Acknowledgments
This work was supported by National Institutes of Health Grants AG041517 and AR053237 and the Veteran's Administration Grant BX001478.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aBMD
- areal bone mineral density
- Alp
- alkaline phosphatase
- Axin2
- Axin 2
- B.Ar
- bone area
- BFR
- bone formation rate
- BMC
- bone mineral content
- BMD
- bone mineral density
- B.Pm
- bone perimeter
- BS
- bone surface
- BV/TV
- bone volume/tissue volume
- μCT
- microcomputer tomography
- Ctnnb1
- β-catenin
- Ctsk
- cathepsin K
- Ct.Th
- cortical thickness
- CTX
- carboxy-terminal collagen cross-link
- Dcstamp
- dendrocyte-expressed 7 transmembrane protein
- DXA
- dual energy x-ray absorptiometry
- GWAS
- genome-wide association studies
- hWNT16
- human WNT16
- MAR
- mineral apposition rate
- MS
- mineralizing surface
- mWnt16
- mouse Wnt16
- Nfatc1
- nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1
- N.Ob
- osteoblast number
- N.Ob/Ob.Pm
- N.Ob over Ob.Pm
- N.Oc
- osteoclast number
- N.Oc/B.Pm
- N.Ob over B.Pm
- N.Oc/Oc.Pm
- N.Oc over Oc.Pm
- Oc.Pm
- osteoclast perimeter
- Ob.S/BS
- osteoblast surface over BS
- OC
- osteocalcin
- Oc.S/BS
- osteoclast surface over BS
- Opg
- osteoprotegerin
- Pka
- protein kinase, cAMP-dependent, catalytic-α
- Pkc
- protein kinase C
- RANKL
- receptor activator of NF-kB ligand
- T.Ar
- Total area
- Tb.N
- trabecular number
- Tb.Sp
- trabecular separation
- Tb.Th
- trabecular thickness
- TG
- transgenic
- TRAPc5b
- tartrate-resistant acid phosphatase 5, isoform b
- WNT
- Wingless-type mouse mammary tumor virus integration site
- WT
- wild type.
References
- 1. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewiecki EM. New targets for intervention in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol. 2011;7(11):631–638. [DOI] [PubMed] [Google Scholar]
- 3. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. [DOI] [PubMed] [Google Scholar]
- 4. Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol. 2009;1(2):a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. [DOI] [PubMed] [Google Scholar]
- 6. Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moayyeri A, Hsu YH, Karasik D, et al. Genetic determinants of heel bone properties: genome-wide association meta-analysis and replication in the GEFOS/GENOMOS consortium. Hum Mol Genet. 2014;23(11):3054–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kemp JP, Medina-Gomez C, Estrada K, et al. Phenotypic dissection of bone mineral density reveals skeletal site specificity and facilitates the identification of novel loci in the genetic regulation of bone mass attainment. PLoS Genet. 2014;10(6):e1004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koller DL, Zheng HF, Karasik D, et al. Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs associated with bone mineral density in premenopausal women. J Bone Miner Res. 2013;28(3):547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Medina-Gomez C, Kemp JP, Estrada K, et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 2012;8(7):e1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng HF, Tobias JH, Duncan E, et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012;8(7):e1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berndt SI, Gustafsson S, Mägi R, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013;45(5):501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medina-Gomez C, Kemp JP, Estrada K, et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 2012;8(7):e1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng HF, Tobias JH, Duncan E, et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012; 8(7):e1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. García-Ibarbia C, Pérez-Núñez MI, Olmos JM, et al. Missense polymorphisms of the WNT16 gene are associated with bone mass, hip geometry and fractures. Osteoporos Int. 2013;24(9):2449–2454. [DOI] [PubMed] [Google Scholar]
- 16. Hendrickx G, Boudin E, Fijałkowski I, et al. Variation in the Kozak sequence of WNT16 results in an increased translation and is associated with osteoporosis related parameters. Bone. 2014;59:57–65. [DOI] [PubMed] [Google Scholar]
- 17. Wergedal JE, Kesavan C, Brommage R, Das S, Mohan S. Role of WNT16 in the regulation of periosteal bone formation in female mice. Endocrinology. 2015;156(3):1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Movérare-Skrtic S, Henning P, Liu X, et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med. 2014;20(11):1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14(4):595–608. [DOI] [PubMed] [Google Scholar]
- 20. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–192. [DOI] [PubMed] [Google Scholar]