Abstract
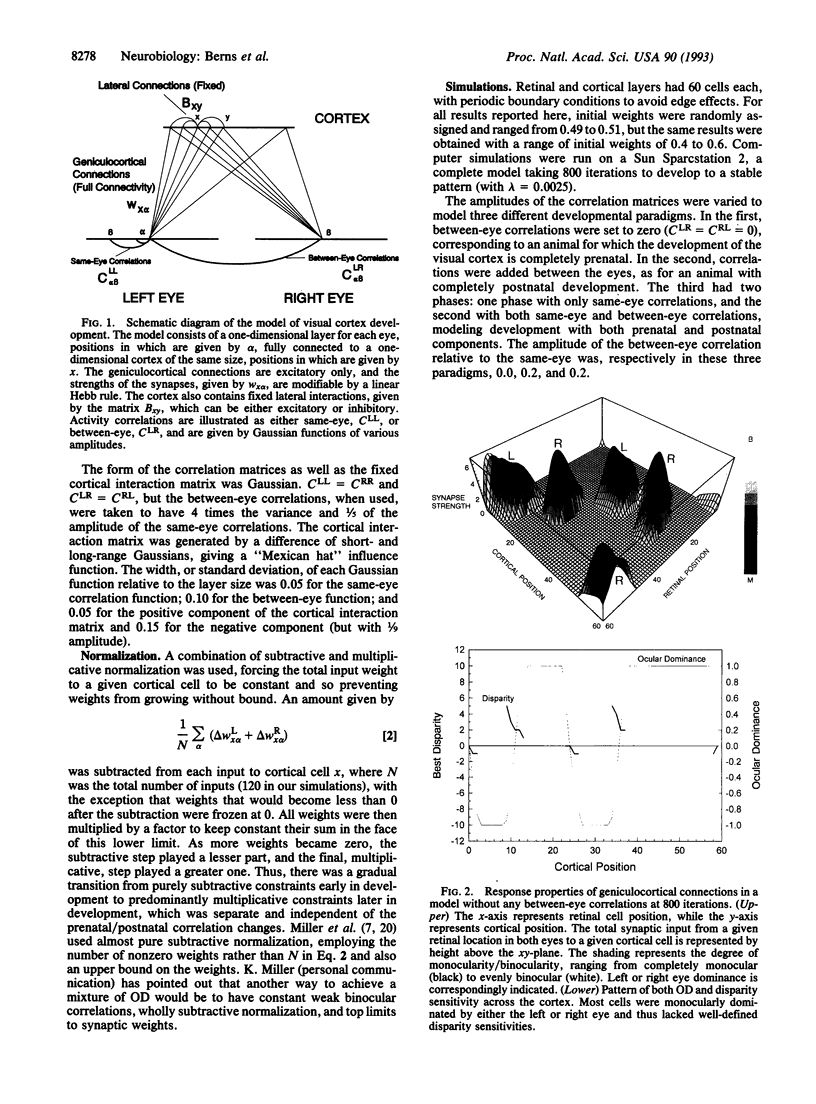
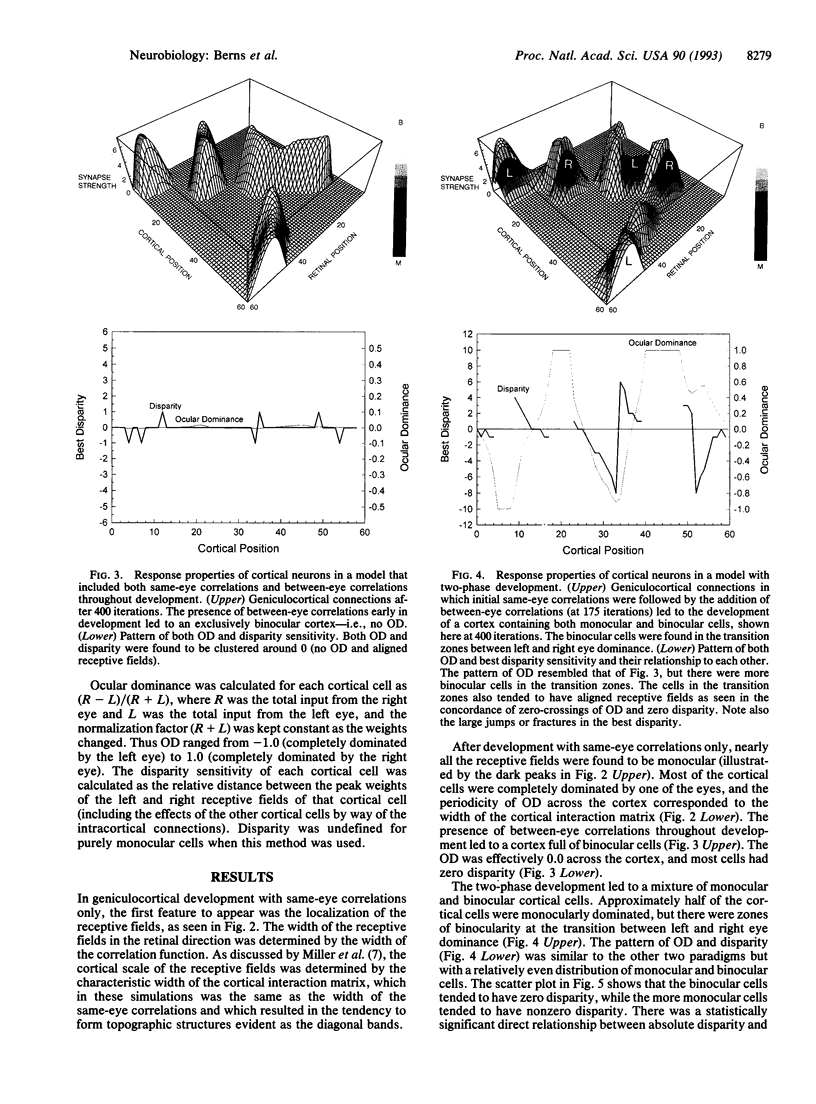
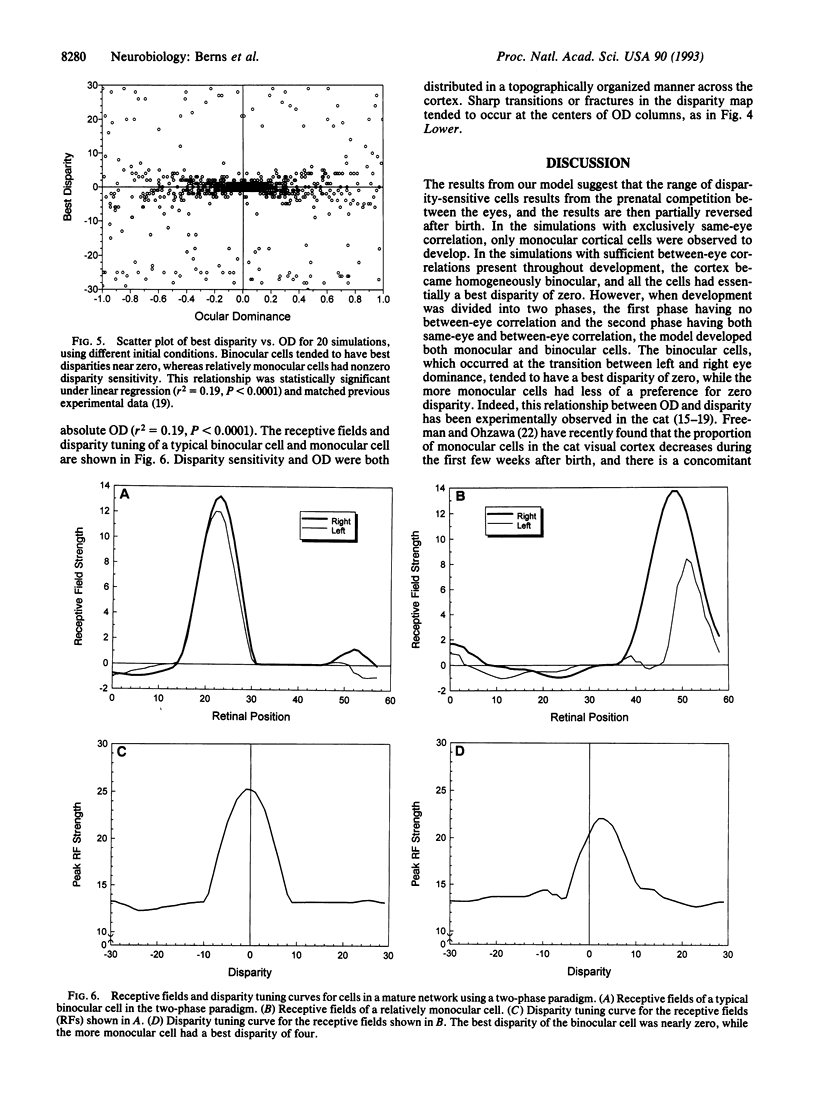
Neurons in the visual cortex require correlated binocular activity during a critical period early in life to develop normal response properties. We present a model for how the disparity selectivity of cortical neurons might arise during development. The model is based on Hebbian mechanisms for plasticity at synapses between geniculocortical neurons and cortical cells. The model is driven by correlated activity in retinal ganglion cells within each eye before birth and additionally between eyes after birth. With no correlations present between the eyes, the cortical model developed only monocular cells. Adding a small amount of correlation between eyes at the beginning of development produced cortical neurons that were entirely binocular and tuned to zero disparity. However, if an initial phase of purely same-eye correlations was followed by a second phase of development that included correlations between eyes, the cortical model became populated with both monocular and binocular cells. Moreover, in the two-phase model, binocular cells tended to be selective for zero disparity, whereas the more monocular cells tended to have nonzero disparity. This relationship between ocular dominance and disparity has been observed in the visual cortex of the cat by other workers. Differences in the relative timing of the two developmental phases could account for the higher proportion of monocular cells found in the visual cortices of other animals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfeld E., Grinvald A. Relationships between orientation-preference pinwheels, cytochrome oxidase blobs, and ocular-dominance columns in primate striate cortex. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11905–11909. doi: 10.1073/pnas.89.24.11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G. G. Differential imaging of ocular dominance and orientation selectivity in monkey striate cortex. J Neurosci. 1992 Aug;12(8):3115–3138. doi: 10.1523/JNEUROSCI.12-08-03115.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer T., Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991 Oct 3;353(6343):429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- Ferster D. A comparison of binocular depth mechanisms in areas 17 and 18 of the cat visual cortex. J Physiol. 1981 Feb;311:623–655. doi: 10.1113/jphysiol.1981.sp013608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., Krüger J. Disparity tuning and binocularity of single neurons in cat visual cortex. Exp Brain Res. 1979 Mar 9;35(1):1–8. doi: 10.1007/BF00236780. [DOI] [PubMed] [Google Scholar]
- Freeman R. D., Ohzawa I. Development of binocular vision in the kitten's striate cortex. J Neurosci. 1992 Dec;12(12):4721–4736. doi: 10.1523/JNEUROSCI.12-12-04721.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. C., Raiten E. J. Ocular dominance and disparity-sensitivity: why there are cells in the visual cortex driven unequally by the two eyes. Exp Brain Res. 1986;64(3):505–514. doi: 10.1007/BF00340488. [DOI] [PubMed] [Google Scholar]
- LeVay S., Voigt T. Ocular dominance and disparity coding in cat visual cortex. Vis Neurosci. 1988;1(4):395–414. doi: 10.1017/s0952523800004168. [DOI] [PubMed] [Google Scholar]
- Linsker R. From basic network principles to neural architecture: emergence of spatial-opponent cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7508–7512. doi: 10.1073/pnas.83.19.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. D. Development of orientation columns via competition between ON- and OFF-center inputs. Neuroreport. 1992 Jan;3(1):73–76. doi: 10.1097/00001756-199201000-00019. [DOI] [PubMed] [Google Scholar]
- Miller K. D., Keller J. B., Stryker M. P. Ocular dominance column development: analysis and simulation. Science. 1989 Aug 11;245(4918):605–615. doi: 10.1126/science.2762813. [DOI] [PubMed] [Google Scholar]
- Montague P. R., Gally J. A., Edelman G. M. Spatial signaling in the development and function of neural connections. Cereb Cortex. 1991 May-Jun;1(3):199–220. doi: 10.1093/cercor/1.3.199. [DOI] [PubMed] [Google Scholar]
- Ohzawa I., DeAngelis G. C., Freeman R. D. Stereoscopic depth discrimination in the visual cortex: neurons ideally suited as disparity detectors. Science. 1990 Aug 31;249(4972):1037–1041. doi: 10.1126/science.2396096. [DOI] [PubMed] [Google Scholar]
- Poggio G. F., Fischer B. Binocular interaction and depth sensitivity in striate and prestriate cortex of behaving rhesus monkey. J Neurophysiol. 1977 Nov;40(6):1392–1405. doi: 10.1152/jn.1977.40.6.1392. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. Impulse activity and the patterning of connections during CNS development. Neuron. 1990 Dec;5(6):745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Harris W. A. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986 Aug;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindale N. V. A model for the formation of ocular dominance stripes. Proc R Soc Lond B Biol Sci. 1980 Jun 24;208(1171):243–264. doi: 10.1098/rspb.1980.0051. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Willshaw D. J., von der Malsburg C. How patterned neural connections can be set up by self-organization. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):431–445. doi: 10.1098/rspb.1976.0087. [DOI] [PubMed] [Google Scholar]