Abstract
Background: Mexican immigrants are disproportionally affected by diet-related risk of metabolic dysfunction. Whether adhering to a traditional Mexican diet or adopting a US diet contributes to metabolic changes associated with future risk of type 2 diabetes and other chronic diseases has not been investigated.
Objective: The purpose of this study was to test in a randomized crossover feeding trial the metabolic responses to a Mexican diet compared with a commonly consumed US diet.
Design: First- and second-generation healthy women of Mexican descent (n = 53) were randomly assigned in a crossover design to consume a Mexican or US diet for 24 d each, separated by a 28-d washout period. Diets were eucaloric and similar in macronutrient composition. The metabolic responses to diets were assessed by measuring fasting serum concentrations of glucose, insulin, insulin-like growth factor 1 (IGF-1), insulin-like growth factor binding protein 3 (IGFBP-3), adiponectin, C-reactive protein (CRP), and interleukin 6 (IL-6), as well as the homeostasis model assessment of insulin resistance (HOMA-IR) at the beginning and end of each period. Linear mixed models tested the intervention effect on the biomarkers, while adjusting for diet sequence, feeding period, baseline and washout biomarker concentrations, age, acculturation, and BMI.
Results: Compared with the US diet, the Mexican diet reduced insulin by 14% [geometric means (95% CIs): 9.3 (8.3, 10.3) compared with 8.0 (7.2, 8.9) μU/mL; P = 0.02], HOMA-IR by 15% [2.0 (1.8, 2.3) compared with 1.7 (1.6, 2.0); P = 0.02], and IGFBP-3 by 6% (mean ± SEM: 2420 ± 29 compared with 2299 ± 29 ng/mL; P < 0.01) and tended to reduce circulating concentrations of IGF-1 by 4% (149 ± 2.6 compared with 144 ± 2.5 ng/mL; P = 0.06). There was no significant intervention effect on serum concentrations of glucose, adiponectin, CRP, or IL-6 in the US compared with the Mexican diet.
Conclusion: Compared with the commonly consumed US diet, the traditional Mexican diet modestly improved insulin sensitivity under conditions of weight stability in healthy women of Mexican descent, while having no impact on biomarkers of inflammation. This trial was registered at clinicaltrials.gov as NCT01369173.
Keywords: inflammatory responses, insulin sensitivity, metabolic responses, Mexican diet, US diet
INTRODUCTION
Mexican immigrants are the largest growing minority group in the United States; compared with non-Hispanic white women, women of Mexican descent are at greater risk of overweight and obesity (1, 2). Obesity greatly increases the risk of low-grade chronic inflammation and insulin resistance, and, therefore, future risk of chronic disease, including type 2 diabetes (T2D),5 cardiovascular disease, and certain types of cancer (3–6). Therefore, Mexican immigrants in the United States with high rates of obesity are at greater risk of insulin resistance and detrimental inflammatory profiles (7).
The ethnic variation in disease risk may be due to genetic predisposition and environmental factors (e.g., diet, socioeconomic status, and/or disparities in access to health care) that may put Mexican descendants at a greater risk of metabolic disease (8, 9). Acculturation, or the process by which immigrants adopt the host-country lifestyle, is suggested as modifying the associations between diet and risk of metabolic disease (10). Among Mexican descendants, greater acculturation has been associated with lower consumption of their mostly healthy traditional Mexican foods, which include plentiful fruit, vegetables, legumes, and whole grains (11, 12). Conversely, greater acculturation has been associated with adherence to US diets, usually low in fruits and vegetables and high in refined grains and added sugars (13, 14).
Diet patterns characterized as Western or typical (standard) of the United States have been associated with a higher risk of T2D, partly through effects on insulin sensitivity, insulin-like growth factors (IGFs), and inflammatory responses (15–18). In contrast, greater adherence to traditional dietary patterns, such as the Mediterranean or Mexican dietary patterns, usually high in fruits, vegetables, and legumes, are associated with reduced inflammation, as measured by serum C-reactive protein (CRP) and serum IL-6 concentrations, and lower risk of T2D (19–22). Observational studies also suggest a link between diet and concentrations of the key anti-inflammatory and insulin-sensitizing adipokine, adiponectin (23, 24), independent of body weight (25, 26). However, it remains largely unclear whether a causal relation underlies these observed associations. It is unknown whether diet composition affects insulin sensitivity and biomarkers of inflammation through a primary effect on body weight and adiposity, or whether the specific types and patterns of food consumed in and of themselves affect these intermediate disease risk biomarkers.
We hypothesized that, compared with a commonly consumed US diet, a traditional Mexican diet would improve insulin sensitivity and lower biomarkers of low-grade chronic inflammation under conditions of weight stability. To test this hypothesis, we measured the metabolic and inflammatory responses to a US compared with a traditional Mexican diet in a randomized crossover feeding trial in first- and second-generation healthy women of Mexican descent.
METHODS
Study population
Study participants were first or second generation healthy women of Mexican descent, aged 18–45 y. Women were eligible if they were premenopausal nonsmokers, and had a BMI (in kg/m2) between 18.5 and 40.0. Women were excluded from the study if they had physician-diagnosed diseases requiring dietary restrictions or certain medications (e.g., anti-diabetics with diabetes, insulin, and statins) that would unduly affect the study outcomes, including those medications directly affecting glucose metabolism. Such diseases included diabetes mellitus, kidney disease, metabolic disorders (e.g., thyroid disease or disorders that require chronic steroid use), cardiovascular disease, or any previous cancer diagnosis or treatment. In addition, women were excluded from eligibility if they had an elevated fasting glucose (≥100 mg/dL) measured at a study screening visit, current or recent pregnancy (within the past year), lactation or plans to become pregnant during the duration of the study, or a daily intake of ≥2 alcoholic drinks. Participants were recruited from the greater Seattle area with the use of in-person and media-based strategies between October 2011 and April 2014. The Institutional Review Board and Clinical Trials Office of the Fred Hutchinson Cancer Research Center (FHCRC) approved the study protocol, and all participants gave written informed consent (clinicaltrials.gov; NCT01369173). Bilingual research staff was available and all study materials were in English and Spanish.
Study design
Enrolled participants provided information on demographic characteristics, physical activity (27), and acculturation through baseline self-administered questionnaires. A 4-item scale, including birthplace, language spoken most of the time, language thought, and ethnic identity, was used to assess acculturation as previously reported by others (28). Study participants were also instructed to record all foods and beverages in a 3-d food record before the feeding trial. Diet records returned by each participant were reviewed for completeness and potential errors before data entry and analyses with the use of the Nutrition Data System for Research (version 2010, Nutrition Coordinating Center, University of Minnesota). Enrolled participants were randomly assigned to consume a US or traditional Mexican diet for 24 d each in a crossover design, separated by a 28-d washout period. On the first day of the feeding trial, weight, height, and body circumferences (waist and hip) were measured with the use of a standardized protocol (29). Weight was monitored 3 times/wk throughout the feeding phase of the trial.
Diet design
Study intervention diets were designed to be eucaloric (i.e., diets that provided energy content for weight maintenance), and different in foods and beverages, but similar in macronutrient composition (50% energy from carbohydrates, 15% energy from protein, and 35% energy from fat). The standard or typical US diet design was based on the contribution of foods and beverages to the total intake of food items reported by the US population (ages ≥2 y) in the 2003–2004 NHANES (30). These primary foods included refined carbohydrates, vegetable oils, nonfat or low-fat milk, processed foods, processed meats, and sugar-sweetened beverages. It is important to note that the commonly consumed US diet was not designed to meet all of the recommendations for a healthy diet. The traditional Mexican diet design was based on peer-reviewed publications and historical review of food composition of traditional Mexican diets in Mexico and the United States (30–32). Traditional Mexican diets are usually a mixture of Native Mesoamerican foods (pre-Hispanic) and Hispanic foods, which primarily consist of corn-based dishes cooked with chilies, garlic, onions and herbs, beans, squash, citrus fruits, rice, meats, and lard (32). Thus, the Mexican diet was designed to reflect traditional diets in Mexico dating back to pre-Hispanic foods, Hispanic foods introduced by Spaniards, and foods consumed in Mexico up to the 1940s (i.e., before Westernization). Therefore, the traditional Mexican diet in the study included beans, corn tortillas, traditional Mexican soups such as menudo and pozole, traditional Mexican mixed dishes such as tamales, citrus fruits, vegetables [including nopales (cactus pads) and jicama], animal fats, full-fat milk, and “aguas frescas” (a combination of fruits and flowers blended with sugar and water).
A 7-d menu rotation was standardized for the commonly consumed US and traditional Mexican diets with the use of ProNutra (version 3.2, Viocare Technologies), and the Nutrition Data System for Research (version 2010, Nutrition Coordinating Center, University of Minnesota). All foods and most beverages were prepared by the Human Nutrition Laboratory at the FHCRC. The experimental diets were prepared under controlled conditions. Every meal was carefully measured out to meet an individual’s estimated energy requirements, packaged, and distributed to study participants 3 times/wk. Energy intake was regulated to maintain the study participant’s weight within 3% of baseline weight. Individual energy requirements were calculated with the use of the Mifflin equation (33), together with the study participant’s usual intake from the 3-d food record, age, and reported physical activity. Each participant was assigned a specific energy intake amount that ranged between 1800 and 2600 kcal/d, and dietary energy adjustments were made in 200-kcal increments, if necessary.
Participants were “free-living” but were instructed to eat or drink only the foods and beverages provided to them, except for drinking water. They were encouraged to report daily deviations from the prescribed diet, including any nonstudy foods and beverages consumed during the trial. Adherence to the controlled diets was assessed from daily checkoff forms that were customized for each day’s food and beverages and returned food that was weighed and recorded 3 times/wk.
Sample collection and analyses
Trained medical assistants collected blood samples on the first and last day of each feeding period after a 12-h fast, for a total of 4 blood draws. Samples were processed and stored at −80°C until analysis. Glucose was measured at the Northwest Lipid Research Laboratories (University of Washington) on a Roche Module P chemistry autoanalyzer (Roche Diagnostics). The intra-assay CV for glucose was 0.7%. Insulin was measured at the Diabetes Endocrinology Research Center Immunoassay Laboratory (University of Washington) with the use of a Tosoh 2000 autoanalyzer (Tosoh Biosciences). The intra-assay CV for insulin was 7.8%. Total adiponectin (Total Adiponectin EIA; Aplco), IGF-1 (Human IGF-I Quantikine ELISA; R&D Systems), insulin-like growth factor binding protein 3 (IGFBP-3) (Human IGFBP-3 Quantikine ELISA; R&D Systems), and IL-6 (Human IL-6 Quantikine HS ELISA; R&D Systems), were measured according to manufacturers’ instructions with the use of immunoassays. CRP was measured on a Roche Cobas Mira chemistry analyzer with the use of CRP (3) Wide Range reagent (Kamiya Biomedical Company) and a high-sensitivity protocol. These analyses were conducted at the FHCRC Biomarker Core Laboratory. All samples were analyzed in duplicate, and blinded duplicates were included in assay analyses to assess performance. Intra-assay CVs were 1.3%, 1.5%, 1.8%, 2.3%, and 3.3%, and interassay CVs were 8.0%, 6.2%, 6.2%, 7.3%, and 4.8% for adiponectin, IGF-1, IGFBP-3, IL-6, and CRP, respectively.
Statistical analyses
A sample size of 50 participants was determined a priori for a 2-sample z test, assuming no carryover effects from the washout period. The absolute differences between the 2 intervention diets detectable with 80% power (type I error = 0.05) were mean changes of 2.0 for insulin (μU/mL), 2.3 for glucose (mg/mL), 8.5 for IGF-1 (ng/mL), 0.4 for IGFBP-3 (μg/mL), 1.9 for adiponectin (μg/mL), and 0.1 for CRP (mg/L) and IL-6 (pg/mL), respectively. Plots were used to visually examine the distributions of all variables. Shapiro-Wilk and Kolmogorov-Smirnov tests also were used to test the normality of the data. Variables that did not meet the assumption of normality were transformed with the use of the ln function. These included insulin, the calculated HOMA-IR, adiponectin, CRP, and IL-6. HOMA-IR was computed by taking the product of fasting serum insulin (μU/mL) and serum glucose (mg/dL) and dividing this by 405 (34).
The nutrient composition of the experimental diets was compared with the use of t tests. Linear mixed models were used to test the intervention effects, comparing the mean changes in the biomarkers from pre- to postintervention for the US compared with the Mexican diet, while adjusting for diet sequence, feeding period, baseline and washout biomarker concentrations, age, acculturation, and BMI (35, 36). Diet treatment, diet sequence, and feeding period were treated as fixed effects and participant as a random effect in the models. Adjusted least-squares means and 95% CIs for 2-sided tests were calculated, and P values <0.05 were considered to be statistically significant. The adjusted least-squares means for log-transformed variables were presented as back-transformed geometric means. Exploratory subgroup analyses were conducted to assess the metabolic response to the diets stratified by BMI and waist circumference (WC) categories as follows: normal weight (BMI ≥18.2 to <25) compared with overweight or obese (BMI ≥25), and WC <88 cm compared with ≥88 cm, respectively. Because power calculations were performed only for the response to the diets in all study participants, the subgroup analyses were considered to be secondary. For study participants with CRP values ≥10.0 mg/L (n = 4, US diet; and n = 3, Mexican diet), sensitivity analyses were conducted for all biomarkers examined, with the exclusion of these study participants in the main and subgroup analyses. For all endpoints, results remained unchanged; therefore, we did not exclude these participants from the main analyses. All data analyses were conducted with the use of SAS software, version 9.3.
RESULTS
Baseline characteristics
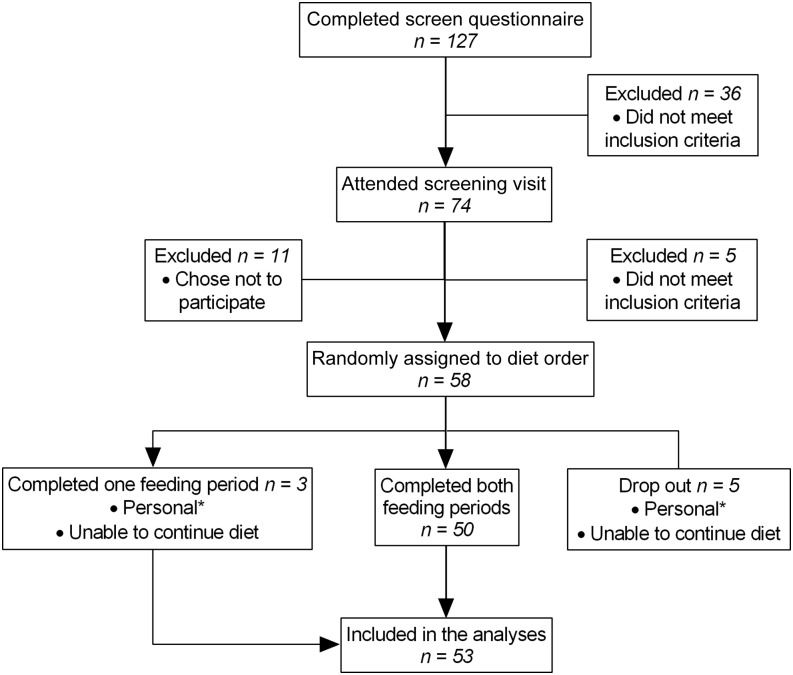
First- and second-generation healthy women of Mexican descent aged 18–45 y were enrolled in the feeding trial. Of the 58 participants enrolled, 50 completed both feeding periods, whereas 3 completed only 1 feeding period (Figure 1). There was no difference in the distribution of age, BMI, education, or acculturation status between the 50 women who completed both feeding periods compared with the 3 women who completed 1 feeding period (data not shown). Of these 53 women who completed at least 1 feeding period and were included in the final analyses, the majority were born in Mexico (62%), and 49% were overweight or obese (BMI ≥25) (Table 1). Adherence to the controlled diets was excellent, with participants consuming 97% of the provided foods and beverages from both diet arms. Consumption of any nonstudy foods was reported only by one-third of study participants, and contributed to less than 3% of participants’ total energy intake on both diet arms. Participants’ body weight remained stable for both diet arms as evaluated by linear mixed models adjusted for diet sequence and feeding period (mean ± SD: Mexican diet: 67.3 ± 0.7 kg; US diet: 67.4 ± 0.7 kg; P = 0.5) (data not shown).
FIGURE 1 .
Flow diagram of study enrollment. *Included time constraints (n = 3), illness (n = 1), and unstable housing (n = 1).
TABLE 1.
Baseline and demographic characteristics and BMI category of 53 first- and second-generation healthy women of Mexican descent1
| Baseline characteristics | All study participants (n = 53) | Normal weight (n = 27) | Overweight or obese (n = 26) |
| Age, y | 27 ± 8 | 25 ± 7 | 30 ± 9* |
| BMI, kg/m2 | 26 ± 5 | 22 ± 1 | 30 ± 4 |
| Waist circumference, cm | 84 ± 12 | 75 ± 5 | 94 ± 9 |
| Hip circumference, cm | 101 ± 10 | 94 ± 6 | 109 ± 7 |
| Waist-to-hip ratio | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 |
| Education | |||
| High school diploma | 11 (23) | 4 (15) | 7 (33) |
| Some college | 19 (40) | 12 (44) | 7 (33) |
| College degree or higher | 18 (37) | 11 (41) | 7 (34) |
| Marital status | |||
| Married | 17 (33) | 5 (19) | 12 (50)* |
| Single | 34 (67) | 22 (81) | 12 (50)* |
| Employment status | |||
| Employed (full- or part-time) | 26 (51) | 12 (44) | 14 (58) |
| Full-time student | 20 (39) | 12 (44) | 8 (33) |
| Unemployed | 5 (10) | 3 (12) | 2 (9) |
| Place of birth | |||
| Mexico | 33 (62) | 13 (48) | 20 (77)* |
| United States | 20 (38) | 14 (52) | 6 (23)* |
| Language spoken | |||
| Spanish | 24 (46) | 9 (35) | 15 (58) |
| English | 28 (54) | 17 (65) | 11 (42) |
| Language thought | |||
| Spanish | 33 (62) | 15 (56) | 18 (69) |
| English | 20 (38) | 12 (44) | 8 (31) |
| Ethnic identity | |||
| Mexican | 34 (64) | 13 (48) | 21 (81)* |
| Mexican-American | 19 (36) | 14 (52) | 5 (19)* |
| Acculturation score2 | 1.7 ± 1.6 | 2.2 ± 1.6 | 1.1 ± 1.4* |
Values are means ± SDs or n (%). *Significantly different from normal-weight women, P < 0.05. t tests were used to compare continuous variables by BMI category (normal weight compared with overweight or obese), and χ2 tests were used to compare categorical variables by BMI category.
Calculated with the use of place of birth, language spoken, language thought, and ethnic identification; ranges from 0 to 4.
Nutrient content of the commonly consumed US and traditional Mexican diets
Compared with the US diet, the Mexican diet was higher in total dietary fiber (36.3 compared with 15.0 g/d), soluble fiber (10.2 compared with 5.1 g/d), insoluble fiber (25.7 compared with 9.9 g/d), vegetable protein (39.3 compared with 25.7 g/d), and saturated fat (36.4 compared with 26.2 g/d) (Table 2). The US diet was higher than the Mexican diet in fructose (31.4 compared with 12.8 g/d), added sugars (92.3 compared with 38.4 g/d), glycemic index (64 compared with 57), and glycemic load (174 compared with 139). In terms of food groups, and compared with the US diet, the traditional Mexican diet was higher in fruits, vegetables, legumes, whole grains (including corn tortillas), animal fats, and full-fat milk. The US diet was higher in refined grains, processed meats, vegetable oils, grain-based desserts, nonfat or low-fat milk, and sugar-sweetened beverages compared with the Mexican diet. Examples of study menus are in Supplemental Table 1.
TABLE 2.
Daily planned mean energy, macronutrient content, and food group servings of 2200-kcal/d traditional Mexican compared with US diets as commonly consumed1
| Nutrients | Traditional Mexican diet (7 d) | US diet (7 d) | P2 |
| Energy intake,3 kcal | 2259 ± 166 | 2225 ± 35.6 | 0.61 |
| Total carbohydrate, %E | 49.4 ± 6.10 | 51.7 ± 1.61 | 0.37 |
| Total sugars, g/d | 94.7 ± 29.2 | 127 ± 37.1 | 0.09 |
| Fructose4 | 12.8 ± 2.74 | 31.4 ± 10.4 | <0.01 |
| Lactose4 | 15.7 ± 8.74 | 10.7 ± 7.28 | 0.27 |
| Sucrose4 | 54.7 ± 20.0 | 52.9 ± 22.9 | 0.87 |
| Added sugars | 38.4 ± 19.5 | 92.3 ± 37.9 | <0.01 |
| Glycemic index5 | 56.7 ± 4.90 | 63.9 ± 5.11 | 0.01 |
| Glycemic load5 | 139 ± 30.3 | 174 ± 16.6 | 0.02 |
| Dietary total fiber, g/d | 36.3 ± 7.80 | 15.0 ± 2.74 | <0.01 |
| Soluble fiber | 10.2 ± 3.34 | 5.11 ± 1.69 | <0.01 |
| Insoluble fiber | 25.7 ± 5.30 | 9.88 ± 1.58 | <0.01 |
| Total protein, %E | 16.1 ± 2.31 | 15.1 ± 1.22 | 0.32 |
| Animal protein, g/d | 51.4 ± 11.4 | 58.4 ± 5.74 | 0.17 |
| Vegetable protein, g/d | 39.3 ± 9.75 | 25.7 ± 5.08 | <0.01 |
| Total fat, %E | 36.7 ± 5.96 | 34.2 ± 1.61 | 0.31 |
| Monounsaturated fat, g/d | 36.0 ± 8.92 | 30.0 ± 3.90 | 0.13 |
| Polyunsaturated fat, g/d | 11.8 ± 2.42 | 21.5 ± 4.98 | <0.01 |
| Saturated fat, g/d | 36.4 ± 8.36 | 26.2 ± 5.92 | 0.02 |
| Foods, servings/d | |||
| Fruits (including avocados) | 3.5 ± 1.1 | 0.9 ± 1.0 | <0.01 |
| Vegetables | 4.1 ± 1.7 | 2.1 ± 1.5 | 0.04 |
| Legumes | 1.5 ± 0.9 | 0.0 ± 0.0 | — |
| Whole grains (including corn tortillas) | 6.1 ± 1.3 | 0.0 ± 0.0 | — |
| Refined grains | 2.3 ± 1.6 | 4.1 ± 1.3 | 0.03 |
| Processed meats | 0.0 ± 0.0 | 2.3 ± 2.0 | — |
| Animal fats (including lard) | 5.8 ± 2.9 | 0.8 ± 1.0 | <0.01 |
| Vegetable oils | 0.1 ± 0.2 | 3.1 ± 2.3 | <0.01 |
| Grain-based desserts | 0.0 ± 0.0 | 1.5 ± 0.6 | — |
| Full-fat milk | 1.1 ± 0.7 | 0.0 ± 0.0 | — |
| Nonfat or low-fat milk | 0.0 ± 0.0 | 0.5 ± 0.5 | — |
| Sugar-sweetened beverages | 0.0 ± 0.0 | 1.6 ± 0.7 | — |
Values are means ± SDs. Analyses were conducted with the use of Nutrition Data System for Research software, version 2010 (Nutrition Coordinating Center, University of Minnesota). %E, percentage of energy.
t tests were conducted to compare the contents of the traditional Mexican and US diets.
Calculated as sums of 4 kcal/g carbohydrate, 9 kcal/g fat, and 4 kcal/g total protein.
Values correspond to naturally occurring sugars in foods and beverages provided.
Values correspond to glucose reference.
Intervention effect of the diets on serum biomarkers of metabolic response
The intervention effect of the diets was tested by comparing the mean changes in the biomarkers from pre- to postintervention for the US compared with the Mexican diet in all participants and by BMI category (normal weight compared with overweight or obese). Fasting blood glucose concentrations were similar between diet treatments, and intervention effects did not differ by BMI category (Table 3). Fasting serum insulin was reduced by 14% (P = 0.02) and HOMA-IR by 15% (P = 0.02) for the Mexican compared with the US diet in all study participants. Compared with the US diet, the Mexican diet reduced circulating concentrations of IGFBP-3 by 6% (P < 0.01) and tended toward a reduction in circulating concentrations of IGF-1 by 4% (P = 0.06) in all study participants. There was no statistically significant intervention effect on the IGF1:IGFBP3 ratio, adiponectin, CRP, or IL-6 in all study participants or by BMI category. The only endpoint for which the response to diets differed significantly between normal weight and overweight or obese women was circulating concentrations of IGFBP-3 (P = 0.03).
TABLE 3.
Intervention effect of the commonly consumed US diet compared with the traditional Mexican diet on serum biomarkers of disease risk for 53 first- and second-generation healthy women of Mexican descent and by BMI category1
| US diet |
Traditional Mexican diet |
||||||
| Biomarkers | n | Value | n | Value | Mean difference | P | P4 |
| Glucose, mg/dL | |||||||
| All study participants | 51 | 89.4 ± 0.87 | 52 | 88.6 ± 0.87 | 0.82 | 0.422 | |
| Normal weight | 26 | 86.6 ± 1.11 | 26 | 85.6 ± 1.13 | 1.02 | 0.92 | |
| Overweight or obese | 25 | 92.2 ± 1.37 | 26 | 91.8 ± 1.34 | 0.36 | ||
| Insulin, μU/mL | |||||||
| All study participants | 51 | 9.29 (8.35, 10.3) | 52 | 8.03 (7.22, 8.93) | 1.26 | 0.022 | |
| Normal weight | 26 | 7.73 (6.43, 9.29) | 26 | 6.24 (5.18, 7.52) | 1.49 | 0.36 | |
| Overweight or obese | 25 | 11.2 (9.90, 12.7) | 26 | 10.3 (9.09, 11.6) | 0.95 | ||
| HOMA-IR | |||||||
| All study participants | 51 | 2.05 (1.82, 2.30) | 52 | 1.75 (1.56, 1.96) | 0.30 | 0.022 | |
| Normal weight | 26 | 1.65 (1.36, 2.01) | 26 | 1.31 (1.08, 1.60) | 0.34 | 0.39 | |
| Overweight or obese | 25 | 2.55 (2.22, 2.94) | 26 | 2.31 (2.01, 2.65) | 0.24 | ||
| IGF-1, ng/mL | |||||||
| All study participants | 51 | 149 ± 2.52 | 52 | 144 ± 2.51 | 5.72 | 0.062 | |
| Normal weight | 26 | 176 ± 3.91 | 26 | 166 ± 3.97 | 10.4 | 0.10 | |
| Overweight or obese | 25 | 123 ± 3.19 | 26 | 122 ± 3.11 | 1.60 | ||
| IGFBP-3, ng/mL | |||||||
| All study participants | 51 | 2420 ± 29.4 | 52 | 2299 ± 29.4 | 121 | <0.012 | |
| Normal weight | 26 | 2696 ± 44.0 | 26 | 2484 ± 44.9 | 212 | 0.013 | 0.03 |
| Overweight or obese | 25 | 2145 ± 35.7 | 26 | 2113 ± 34.8 | 31.1 | 0.493 | |
| IGF-1:IGFBP-3 | |||||||
| All study participants | 51 | 6.13 ± 0.10 | 52 | 6.22 ± 0.10 | −0.09 | 0.382 | |
| Normal weight | 26 | 6.55 ± 0.15 | 26 | 6.68 ± 0.15 | −0.13 | 0.77 | |
| Overweight or obese | 25 | 5.72 ± 0.13 | 26 | 5.74 ± 0.13 | −0.03 | ||
| Total adiponectin, μg/mL | |||||||
| All study participants | 51 | 6.57 (6.28, 6.88) | 51 | 6.63 (6.33, 6.93) | −0.05 | 0.772 | |
| Normal weight | 26 | 7.21 (6.72, 7.73) | 26 | 7.29 (6.79, 7.83) | −0.09 | 0.82 | |
| Overweight or obese | 25 | 5.97 (5.65, 6.32) | 26 | 6.03 (5.71, 6.37) | −0.06 | ||
| CRP, mg/L | |||||||
| All study participants | 51 | 0.94 (0.70, 1.28) | 51 | 0.93 (0.68, 1.25) | 0.02 | 0.912 | |
| Normal weight | 26 | 0.54 (0.33, 0.86) | 26 | 0.62 (0.39, 0.98) | −0.08 | 0.44 | |
| Overweight or obese | 25 | 1.68 (1.11, 2.54) | 26 | 1.36 (0.91, 2.05) | 0.31 | ||
| IL-6, pg/mL | |||||||
| All study participants | 51 | 1.36 (1.16, 1.58) | 51 | 1.32 (1.13, 1.54) | 0.04 | 0.782 | |
| Normal weight | 26 | 1.00 (0.78, 1.28) | 26 | 1.03 (0.80, 1.33) | −0.03 | 0.68 | |
| Overweight or obese | 25 | 1.85 (1.55, 2.20) | 26 | 1.68 (1.41, 1.99) | 0.17 | ||
Values are means ± SEMs or geometric means with 95% CIs in parentheses. Normal weight: BMI (in kg/m2) <25; overweight or obese: BMI ≥25. CRP, C-reactive protein; IGF-1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein 3.
From linear mixed models testing the intervention effect of the Mexican compared with the US diet while adjusting for diet sequence, feeding period, baseline and washout biomarker concentrations, age, acculturation composite score, and BMI.
For diet effect within BMI category (normal weight compared with overweight or obese).
For diet effect difference between BMI category (normal weight compared with overweight or obese).
We further examined whether the intervention effect of the diets on biomarkers of metabolic response differed by WC category (Table 4) in the secondary analyses. There was no intervention effect on the IGF1:IGFBP3 ratio by WC category. Finally, similar to the results for adiposity categories based on BMI, there was a statistically significant difference in the response to the diets on circulating concentrations of IGF-1 and IGFBP-3 by WC category (P < 0.05), whereas no difference in the inflammatory response to the diets was found by WC category, as measured by adiponectin, CRP, or IL-6 concentrations.
TABLE 4.
Intervention effect of the commonly consumed US diet compared with the traditional Mexican diet on serum biomarkers of disease risk for 53 first- and second-generation healthy women of Mexican descent by waist circumference category1
| US diet |
Traditional Mexican diet |
||||||
| Biomarkers | n | Value | n | Value | Mean difference | P2 | P3 |
| Glucose, mg/dL | |||||||
| Waist circumference <88 cm | 34 | 87.2 ± 1.12 | 35 | 86.2 ± 1.13 | 0.99 | 0.78 | |
| Waist circumference ≥88 cm | 17 | 93.7 ± 1.18 | 17 | 93.4 ± 1.18 | 0.29 | ||
| Insulin, μU/mL | |||||||
| Waist circumference <88 cm | 34 | 8.12 (7.05, 9.47) | 35 | 6.71 (5.79, 7.78) | 1.46 | 0.26 | |
| Waist circumference ≥88 cm | 17 | 12.0 (10.4, 14.0) | 17 | 11.6 (10.0, 13.4) | 0.47 | ||
| HOMA-IR | |||||||
| Waist circumference <88 cm | 34 | 1.76 (1.50, 2.06) | 35 | 1.42 (1.21, 1.67) | 0.34 | 0.26 | |
| Waist circumference ≥88 cm | 17 | 2.78 (2.36, 3.27) | 17 | 2.66 (2.26, 3.13) | 0.12 | ||
| IGF-1, ng/mL | |||||||
| Waist circumference <88 cm | 34 | 165 ± 3.24 | 35 | 154 ± 3.25 | 10.6 | <0.01 | <0.01 |
| Waist circumference ≥88 cm | 17 | 119 ± 4.18 | 17 | 124 ± 4.18 | −5.43 | 0.33 | |
| IGFBP-3, ng/mL | |||||||
| Waist circumference <88 cm | 34 | 2579 ± 36.5 | 35 | 2392 ± 36.6 | 186 | <0.01 | 0.03 |
| Waist circumference ≥88 cm | 17 | 2101 ± 56.2 | 17 | 2116 ± 56.3 | −15.3 | 0.76 | |
| IGF-1:IGFBP-3 | |||||||
| Waist circumference <88 cm | 34 | 6.37 ± 0.12 | 35 | 6.40 ± 0.12 | −0.03 | 0.38 | |
| Waist circumference ≥88 cm | 17 | 5.63 ± 0.21 | 17 | 5.86 ± 0.21 | −0.24 | ||
| Total adiponectin, μg/mL | |||||||
| Waist circumference <88 cm | 34 | 7.07 (6.65, 7.51) | 35 | 7.05 (6.63, 7.48) | 0.02 | 0.64 | |
| Waist circumference ≥88 cm | 17 | 5.70 (5.33, 6.08) | 17 | 5.89 (5.51, 6.29) | −0.19 | ||
| CRP, mg/L | |||||||
| Waist circumference <88 cm | 34 | 0.61 (0.41, 0.93) | 35 | 0.71 (0.47, 1.07) | −0.09 | 0.38 | |
| Waist circumference ≥88 cm | 17 | 2.3 (1.6, 3.3) | 17 | 1.7 (1.1, 2.5) | 0.61 | ||
| IL-6, pg/mL | |||||||
| Waist circumference <88 cm | 34 | 1.08 (0.88, 1.32) | 35 | 1.11 (1.91, 1.36) | −0.03 | 0.59 | |
| Waist circumference ≥88 cm | 17 | 2.14 (1.75, 2.61) | 17 | 1.88 (1.54, 2.29) | 0.26 | ||
Values are means ± SEMs or geometric means with 95% CIs in parentheses. CRP, C-reactive protein; IGF-1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein 3.
From linear mixed models testing the intervention effect of the Mexican compared with the US diet while adjusting for diet sequence, feeding period, baseline and washout biomarker concentrations, age, and acculturation composite score for diet effect within waist circumference category (<88 compared with ≥88 cm).
For diet effect difference between waist circumference category (<88 compared with ≥88 cm).
DISCUSSION
This controlled feeding trial tested metabolic responses to a traditional Mexican compared with a commonly consumed US diet in first- and second-generation healthy women of Mexican descent. Under eucaloric conditions, and compared with the US diet, the traditional Mexican diet improved insulin sensitivity (HOMA-IR) by 15%, reduced circulating concentrations of IGFBP‑3 by 6%, and tended to reduce IGF-1 circulating concentrations by 4%. However, the traditional Mexican compared with the US diet had no effect on serum concentrations of adiponectin, CRP, or IL-6. Nonetheless, these findings support the proposed benefits of following a traditional Mexican diet high in fruits, vegetables, beans, corn tortillas, soups, full-fat milk, and Mexican cheeses to help reduce diet-related insulin resistance and potential risk of metabolic dysfunction–related diseases.
Our finding of significantly lower fasting insulin and HOMA-IR after the Mexican compared with the US diet in healthy women is notable. It should be emphasized that these diets were consumed under eucaloric conditions (i.e., body weight was kept experimentally at the baseline level). We expected a larger diet effect in the overweight or obese women, assuming that a possibly baseline insulin resistance may make them more likely to improve their biomarker profiles on a healthier diet. However, we saw a trend toward a greater difference in HOMA-IR in the normal-weight women in exploratory subgroup analyses by BMI category. One potential explanation for this is that, in our study population, body weight was inversely associated with age and acculturation status, such that the normal-weight women were younger and more acculturated. It is possible that they benefitted more from the traditional Mexican diet than the less acculturated overweight or obese women because of their otherwise more Westernized habitual diet. Another possible explanation is that the overweight or obese women may have benefitted less from the traditional Mexican diet because of a dominant effect of excess fat mass on processes determining insulin sensitivity. It could be speculated that a beneficial impact from diet composition on metabolic health may take longer to manifest in this group, or it may become apparent only when combined with weight loss.
The traditional Mexican diet differed in many ways from the commonly consumed US diet, which may explain the differential effect on insulin sensitivity. These include 1) carbohydrate quality (glycemic load, fiber content and type of fiber, and added sugar content); 2) dietary sources of protein (predominantly plant compared with animal); 3) fatty acid composition (full-fat dairy and lard compared with low-fat milk and vegetable oil); and 4) micronutrient density. The traditional Mexican diet was higher in dietary fiber and lower in added sugars and glycemic index, all factors that previous trials suggest could affect insulin sensitivity (37), although the data are not fully consistent in this regard (37, 38). Although only a few other randomized controlled feeding trials exist and findings are inconsistent, observational studies suggest that traditional diets high in fruits, vegetables, and legumes, and low in glycemic index, reduce the risk of metabolic disease (17, 39–42).
Compared with those on the commonly consumed US diet, those on the traditional Mexican diet showed a modest but meaningful reduction in circulating concentrations of IGFBP-3 and a trending decrease in IGF-1. In exploratory stratified analyses by WC category, we found a differential diet effect in the reductions of IGFs in women with WC <88 compared with ≥88cm. This differential effect could be explained by the diet-induced hyperinsulinemia in the US compared with the Mexican diet that may contribute to higher bioavailability of IGFs (43). The lack of a substantial reduction in IGFs in women with a higher WC may indicate that a longer and more intense intervention or weight loss, along with dietary modification, may be necessary to elicit a change in serum IGFs in Latinas with higher body weight (44).Whether the overall reduction in IGFs can be attributed to any of the specific dietary components of the traditional Mexican diet (i.e., nutrients, specific foods, or bioactive compounds) or a combined effect of these dietary factors cannot be determined from our study, because we tested the whole diet (45–48). Nonetheless, our findings are in agreement with a randomized crossover feeding trial of low– compared with high–glycemic load diets in healthy individuals, which reported a reduction in IGF-1 by 4% (49). Furthermore, in a case-control study of individuals with colorectal adenomas, a diet pattern low in fat and high in dietary fiber, fruits, and vegetables was associated with reduced IGF-1 in both the case and control groups (50), although others have found null or opposite findings (51, 52). The discrepancy between studies may be explained in part by differences in study designs, as well as the nutritional content of the diets and the ethnic-specific heterogeneity in endogenous concentrations of IGFs (43, 44).
Although the traditional Mexican compared with the US diet did not contribute to an intervention effect on the inflammatory biomarkers examined, it is unlikely that the null results are due to lower adherence to the diets, because study participants consumed at least 97% of all foods provided. It is possible that diet-related changes on inflammatory biomarkers are most effective when coupled with weight loss. In agreement with this possibility, in a randomized crossover feeding trial in healthy individuals, a low– compared with high–glycemic load eucaloric diet significantly reduced CRP and increased adiponectin concentrations, respectively, only in individuals with high compared with low body-fat mass (53). Similarly, in another controlled feeding trial in obese individuals, a diet rich in ω-3 fatty acids did not increase adiponectin concentrations under eucaloric conditions, but only during the study’s ad libitum period, when study participants’ body weights were reduced (54). This evidence suggests that diet-related changes in inflammatory profiles may be greater when coupled with weight loss.
The strengths of the study include being the first controlled feeding trial, to our knowledge, to test the effects of a US compared with a Mexican diet in an ethnic group disproportionally affected by diet-related risk of metabolic disease. This study is also one of few feeding trials in healthy and normoglycemic participants. As for the Mexican diet, we acknowledge the nutritional transition to Western-like dietary patterns in Mexico (55). However, the Mexican diet in the study was designed to reflect diets before the widespread presence of US-based industries in Mexico (i.e., up to the 1940s) (56). Limitations of the study include a modest sample size and the limited generalization of our findings; moreover, although adherence to the diets appeared to be high, there is always the possibility that some participants may not have been fully compliant. Finally, HOMA-IR is largely a measure of hepatic rather than insulin resistance. Dynamic tests may be better to capture changes in systemic insulin (57).
In conclusion, compared with the commonly consumed US diet, the traditional Mexican diet improved insulin sensitivity, reduced circulating concentrations of IGFBP-3, and tended to reduce circulating concentrations of IGF-1 under conditions of weight stability. A novel contribution of this study is a better understanding of the effect of this particular dietary pattern that may be retained or adopted by Mexican immigrants as they acculturate to the US lifestyle. Our findings can inform future dietary interventions in women of Mexican descent who would benefit from maintaining their traditional Mexican diets.
Acknowledgments
The authors’ responsibilities were as follows—MS-T, JDDT, and C-YW: analyzed the data; MK, JWL, and MLN: designed the research; KLB, LL, and AV: designed the experimental diets; KLB, LL, and XS: implemented the study protocol; MS-T: wrote the manuscript; MS-T, MK, JWL, and MLN: had primary responsibility for the final content; and all authors: critically reviewed and revised the manuscript and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CRP, C-reactive protein; FHCRC, Fred Hutchinson Cancer Research Center; IGF, insulin-like growth factor; IGFBP-3, insulin-like growth factor binding protein 3; T2D, type 2 diabetes; WC, waist circumference.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 2012;307:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamtani M, Kulkarni H, Dyer TD, Almasy L, Mahaney MC, Duggirala R, Comuzzie AG, Blangero J, Curran JE. Waist circumference independently associates with the risk of insulin resistance and type 2 diabetes in Mexican American families. PLoS One 2013;8:e59153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 4.Song Y, Manson JE, Tinker L, Howard BV, Kuller LH, Nathan L, Rifai N, Liu SM. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women—the Women’s Health Initiative Observational Study. Diabetes Care 2007;30:1747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat 2013;137:307–14. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Hebert JR, Manson JE, Balasubramanian R, Liu SM, Lamonte MJ, Bird CE, Ockene JK, Qiao YX, Olendzki B, et al. Determinants of racial/ethnic disparities in incidence of diabetes in postmenopausal women in the U.S.—the Women’s Health Initiative 1993-2009. Diabetes Care 2012;35:2226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aponte J. Diabetes-related risk factors across Hispanic subgroups in the Hispanic Health and Nutritional Examination Survey (1982–1984). Public Health Nurs 2009;26:23–38. [DOI] [PubMed] [Google Scholar]
- 8.Katzmarzyk PT, Staiano AE. New race and ethnicity standards: elucidating health disparities in diabetes. BMC Med 2012;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowie JV, Juon HS, Cho J, Rodriguez EM. Factors associated with overweight and obesity among Mexican Americans and Central Americans: results from the 2001 California Health Interview Survey. Prev Chronic Dis 2007;4:A10. [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia L, Gold EB, Wang L, Yang XW, Mao M, Schwartz AV. The relation of acculturation to overweight, obesity, pre-diabetes and diabetes among Us Mexican-American women and men. Ethn Dis 2012;22:58–64. [PMC free article] [PubMed] [Google Scholar]
- 11.Montez JK, Eschbach K. Country of birth and language are uniquely associated with intakes of fat, fiber, and fruits and vegetables among Mexican-American women in the United States. J Am Diet Assoc 2008;108:473–80. [DOI] [PubMed] [Google Scholar]
- 12.Batis C, Hernandez-Barrera L, Barquera S, Rivera JA, Popkin BM. Food acculturation drives dietary differences among Mexicans, Mexican Americans, and non-Hispanic whites. J Nutr 2011;141:1898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffey KJ, Gordon-Larsen P, Ayala GX, Popkin BM. Birthplace is associated with more adverse dietary profiles for US-born than for foreign-born Latino adults. J Nutr 2008;138:2428–35. [DOI] [PubMed] [Google Scholar]
- 14.Sofianou A, Fung TT, Tucker KL. Differences in diet pattern adherence by nativity and duration of US residence in the Mexican-American population. J Am Diet Assoc 2011;111(10):1563-9 e2. [DOI] [PubMed] [Google Scholar]
- 15.Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, Heidemann C, Colditz GA, Hu FB. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005;82:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albuquerque RC, Baltar VT, Marchioni DM. Breast cancer and dietary patterns: a systematic review. Nutr Rev 2014;72:1–17. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Morán M, Guerrero-Romero F, Rascon-Pacheco RA, Social IMS. Dietary factors related to the increase of cardiovascular risk factors in traditional Tepehuanos communities from Mexico. A 10 year follow-up study. Nutr Metab Cardiovasc Dis 2009;19:409–16. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2004;80:1029–35. [DOI] [PubMed] [Google Scholar]
- 19.Qiao Y, Tinker L, Olendzki BC, Hebert JR, Balasubramanian R, Rosal MC, Hingle M, Song YQ, Schneider KL, Liu SM, et al. Racial/ethnic disparities in association between dietary quality and incident diabetes in postmenopausal women in the United States: the Women’s Health Initiative 1993-2005. Ethn Health 2014;19:328–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montonen J, Knekt P, Jarvinen R, Aromaa A, Reunanen A. Whole-grain and fiber intake and the incidence of type 2 diabetes. Am J Clin Nutr 2003;77:622–9. [DOI] [PubMed] [Google Scholar]
- 21.Meyer KA, Kushi LH, Jacobs DR, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. [DOI] [PubMed] [Google Scholar]
- 22.Buckland G, Travier N, Cottet V, Gonzalez CA, Lujan-Barroso L, Agudo A, Trichopoulou A, Lagiou P, Trichopoulos D, Peeters PH, et al. Adherence to the Mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer 2013;132:2918–27. [DOI] [PubMed] [Google Scholar]
- 23.Robinson K, Prins J, Venkatesh B. Clinical review: adiponectin biology and its role in inflammation and critical illness. Crit Care 2011;15:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidenced. Am J Clin Nutr 2007;86:s858–s66. [DOI] [PubMed] [Google Scholar]
- 25.Fargnoli JL, Fung TT, Olenczuk DM, Chamberland JP, Hu FB, Mantzoros CS. Adherence to healthy eating patterns is associated with higher circulating total and high-molecular-weight adiponectin and lower resistin concentrations in women from the Nurses’ Health Study. Am J Clin Nutr 2008;88:1213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantzoros CS, Williams CJ, Manson JE, Meigs JB, Hu FB. Adherence to the Mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. Am J Clin Nutr 2006;84:328–35. [DOI] [PubMed] [Google Scholar]
- 27.Staten LK, Taren DL, Howell WH, Tobar M, Poehlman ET, Hill A, Reid PM, Ritenbaugh C. Validation of the Arizona Activity Frequency Questionnaire using doubly labeled water. Med Sci Sports Exerc 2001;33:1959–67. [DOI] [PubMed] [Google Scholar]
- 28.Coronado GD, Thompson B, McLerran D, Schwartz SM, Koepsell TD. A short acculturation scale for Mexican-American populations. Ethn Dis 2005;15:53–62. [PubMed] [Google Scholar]
- 29.Norgan NG. Anthropometric standardization reference manual. Lohman TG, Roche AF, Martorell R, editors. Human Kinetics Books 1988;31:1493–4. [Google Scholar]
- 30.Usual dietary intakes. Food intakes, US Population, 2007–10. Applied Research Program Web site. National Cancer Institute [Internet]. [Updated 2014 May 22; cited 2015 Mar 10.] Available from: http://appliedresearch.cancer.gov/diet/foodsources/food_groups/table3.html.
- 31.Rivera JA, Barquera S, Campirano F, Campos I, Safdie M, Tovar V. Epidemiological and nutritional transition in Mexico: rapid increase of non-communicable chronic diseases and obesity. Public Health Nutr 2002;5(1A):113–22. [DOI] [PubMed] [Google Scholar]
- 32.Avial CA, Shamah LT, Chavez A, Gomez C. ENURBAL 2002 Encuesta Urbana de Alimentación y Nutrición en la Zona Metropolitana de la Ciudad de México: Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Instituto Nacional de Salud Pública. [Urban Food and Nutrition Survey in the metropolitan area of Mexico city: National Institute of Medical Sciences and Nutrition Salvador Zubirán. National Institute of Public Health]. 2002 (in Spanish).
- 33.Mifflin MD, Stjeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy-expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 35.Maringwa JT, Geys H, Shkedy Z, Faes C, Molenberghs G, Aerts M, Van Ammel K, Teisman A, Bijnens L. Analysis of cross-over designs with serial correlation within periods using semi-parametric mixed models. Stat Med 2008;27:6009–33. [DOI] [PubMed] [Google Scholar]
- 36.Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials 2009;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gower BA, Goree LL, Chandler-Laney PC, Ellis AC, Casazza K, Granger WM. A higher-carbohydrate, lower-fat diet reduces fasting glucose concentration and improves beta-cell function in individuals with impaired fasting glucose. Metabolism 2012;61:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sacks FM, Carey VJ, Anderson CAM, Miller ER, Copeland T, Charleston J, Harshfield BJ, Laranjo N, McCarron P, Swain J, et al. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb Randomized Clinical Trial. JAMA 2014;312(23):2531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denova-Gutiérrez E, Castanon S, Talavera JO, Flores M, Macias N, Rodriguez-Ramirez S, Flores YN, Salmeron J. Dietary patterns are associated with different indexes of adiposity and obesity in an urban Mexican population. J Nutr 2011;141:921–7. [DOI] [PubMed] [Google Scholar]
- 40.Murtaugh MA, Herrick JS, Sweeney C, Baumgartner KB, Guiliano AR, Byers T, Slattery ML. Diet composition and risk of overweight and obesity in women living in the southwestern United States. J Am Diet Assoc 2007;107:1311–21. [DOI] [PubMed] [Google Scholar]
- 41.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2006;83:1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome—a randomized trial. JAMA 2004;292:1440–6. [DOI] [PubMed] [Google Scholar]
- 43.Rajpathak SN, Gunter MJ, Wylie-Rosett J, Ho GYF, Kaplan RC, Mazumdar R, Rohan TE, Strickler HD. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab Res Rev 2009;25:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henderson KD, Goran MI, Kolonel LN, Henderson BE, Le Marchand L. Ethnic disparity in the relationship between obesity and plasma insulin-like growth factors: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 2006;15:2298–302. [DOI] [PubMed] [Google Scholar]
- 45.Pinent M, Castell A, Baiges I, Montagut G, Arola L, Ardevol A. Bioactivity of flavonoids on insulin-secreting cells. Compr Rev Food Sci F 2008;7:299–308. [DOI] [PubMed] [Google Scholar]
- 46.Xu N, Zhang L, Dong J, Zhang X, Chen YG, Bao B, Liu J. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol Nutr Food Res 2014;58:1258–68. [DOI] [PubMed] [Google Scholar]
- 47.Beasley JM, Wedick NM, Rajpathak SN, Xue X, Holmes MD, Gunter MJ, Wylie-Rosett J, Rohan TE, Pollak M, Kaplan RC, et al. Circulating IGF-axis protein levels and their relation with levels of plasma adipocytokines and macronutrient consumption in women. Growth Horm IGF Res 2014;24:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brand-Miller JC, Liu V, Petocz P, Baxter RC. The glycemic index of foods influences postprandial insulin-like growth factor-binding protein responses in lean young subjects. Am J Clin Nutr 2005;82:350–4. [DOI] [PubMed] [Google Scholar]
- 49.Runchey SS, Pollak MN, Valsta LM, Coronado GD, Schwarz Y, Breymeyer KL, Wang C, Wang CY, Lampe JW, Neuhouser ML. Glycemic load effect on fasting and post-prandial serum glucose, insulin, IGF-1 and IGFBP-3 in a randomized, controlled feeding study. Eur J Clin Nutr 2012;66:1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flood A, Mai V, Pfeiffer R, Kahle L, Remaley AT, Rosen CJ, Lanza E, Schatzkin A. The effects of a high-fruit and -vegetable, high-fiber, low-fat dietary intervention on serum concentrations of insulin, glucose, IGF-1 and IGFBP-3. Eur J Clin Nutr 2008;62:186–96. [DOI] [PubMed] [Google Scholar]
- 51.Gann PH, Kazer R, Chatterton R, Gapstur S, Thedford K, Helenowski I, Giovanazzi S, Van Horn L. Sequential, randomized trial of a low-fat, high-fiber diet and soy supplementation: effects on circulating IGF-I and its binding proteins in premenopausal women. Int J Cancer 2005;116:297–303. [DOI] [PubMed] [Google Scholar]
- 52.Young LR, Kurzer MS, Thomas W, Redmon JB, Raatz SK. Low-fat diet with omega-3 fatty acids increases plasma insulin-like growth factor concentration in healthy postmenopausal women. Nutr Res 2013;33:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neuhouser ML, Schwarz Y, Wang CC, Breymeyer K, Coronado G, Wang CY, Noar K, Song XL, Lampe JW. A low-glycemic load diet reduces serum c-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr 2012;142:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kratz M, Swarbrick MM, Callahan HS, Matthys CC, Havel PJ, Weigle DS. Effect of dietary n-3 polyunsaturated fatty acids on plasma total and high-molecular-weight adiponectin concentrations in overweight to moderately obese men and women. Am J Clin Nutr 2008;87:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng M, Fleming T, Robinson M. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivera JA, Barquera S, Gonzalez-Cossio T, Olaiz G, Sepulveda J. Nutrition transition in Mexico and in other Latin American countries. Nutr Rev 2004;62:S149–57. [DOI] [PubMed] [Google Scholar]
- 57.Venkataraman K, Khoo CM, Leow MKS, Khoo EYH, Isaac AV, Zagorodnov V, Sadananthan SA, Velan SS, Chong YS, Gluckman P, et al. New measure of insulin sensitivity predicts cardiovascular disease better than HOMA estimated insulin resistance. PLoS One 2013;8:e74410. [DOI] [PMC free article] [PubMed] [Google Scholar]