Abstract
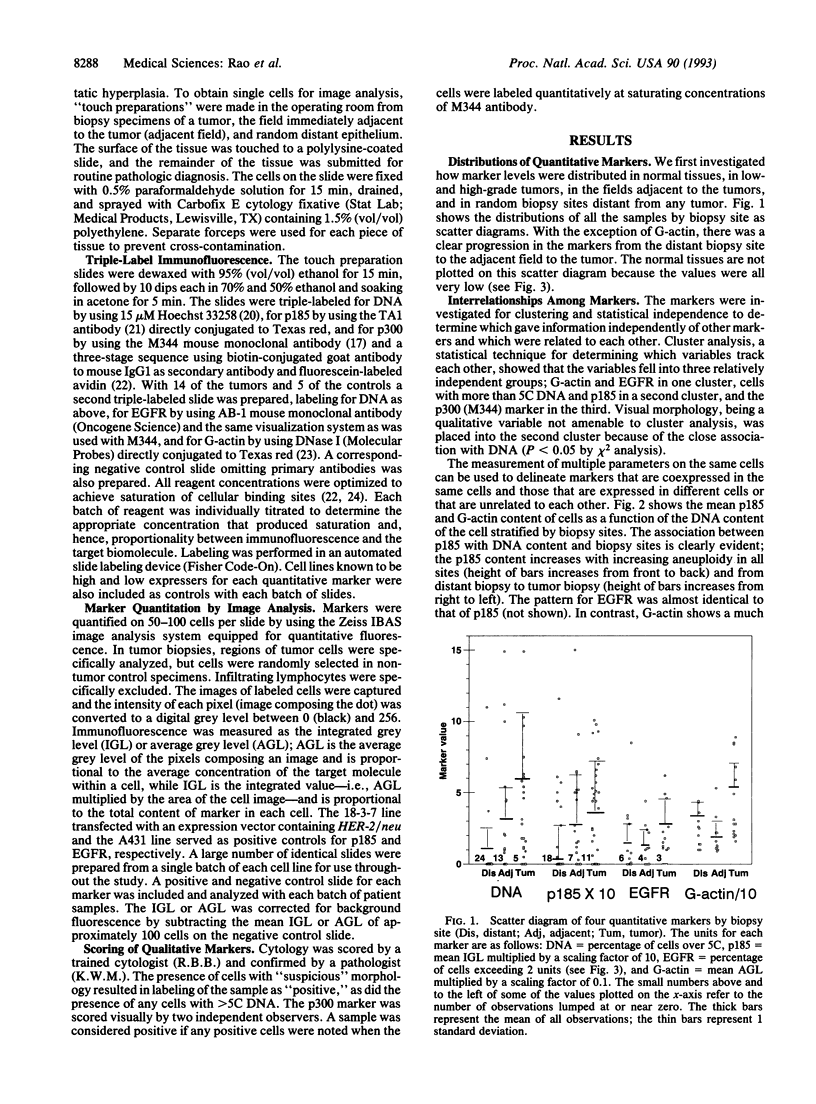
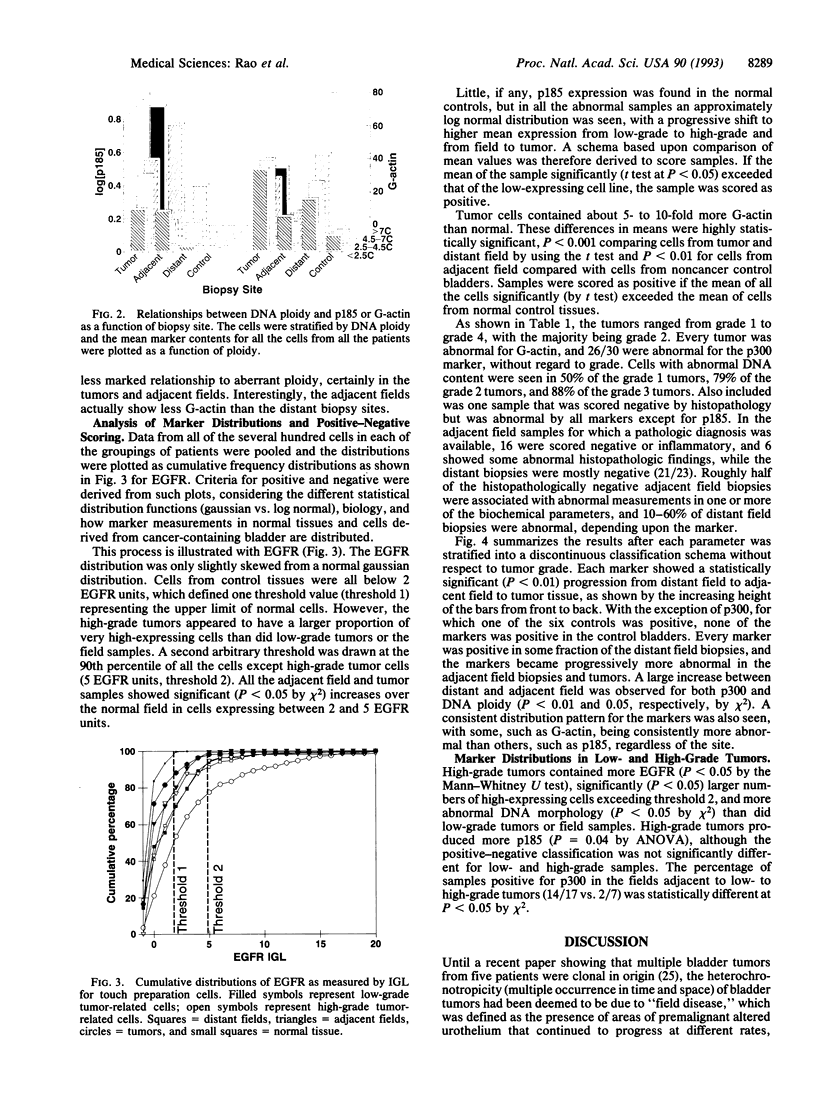
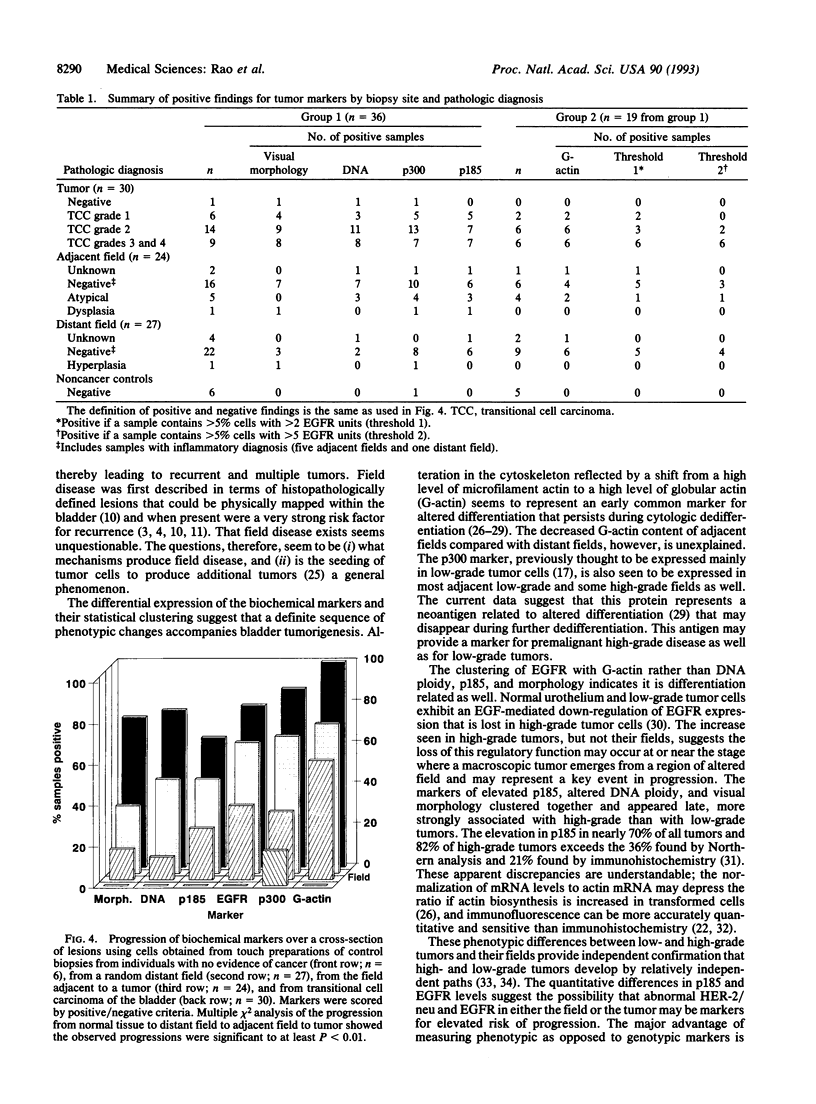
Phenotypic biochemical markers of oncogenesis and differentiation were mapped in bladder biopsies to investigate changes that occur in bladder tumorigenesis and to identify markers for increased bladder cancer risk. Touch preparations from biopsy specimens from 30 patients were obtained from tumors, the adjacent bladder epithelium, and random distant bladder epithelium. Markers, including DNA ploidy, epidermal growth factor receptor (EGFR), and oncoproteins, were quantified in individual cells by using quantitative fluorescence image analysis. Cluster analysis revealed the markers fell into three independent groups: (i) G-actin and EGFR; (ii) ploidy, cytology, and p185 (HER-2/neu oncoprotein) (ERBB2); and (iii) p300, a low-grade tumor antigen. Each marker displayed a gradient of abnormality from distant field to adjacent field to tumor. Different patterns for each marker suggested a developmental sequence of bladder cancer oncogenesis; G-actin was altered in 58% of distant biopsies (vs. 0/6 normals, P < 0.001), ploidy and cytology were altered in < 20% of distant fields and approximately 80% of tumors, and the other markers were intermediate. Patterns of EGFR and p185 suggest low-and high-grade tracks diverge early (P < 0.05 by Mann-Whitney U test for EGFR and ANOVA for p185). In conclusion, this study shows that a sequence of phenotypic changes accompanies development and progression of bladder cancers. Biochemical alterations in cells of the bladder field are often detectable before abnormal pathology, and markers previously thought to be limited to tumors were found in the field. The hierarchy of expression may be useful in identifying high-risk patients, assessing completeness of response to therapy, and monitoring and predicting recurrence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer G., Ono J., Nasiell M., Caspersson T., Kato H., Konaka C., Hayata Y. Reversibility of bronchial cell atypia. Cancer Res. 1982 Oct;42(10):4241–4247. [PubMed] [Google Scholar]
- Bass R. A., Hemstreet G. P., 3rd, Honker N. A., Hurst R. E., Doggett R. S. DNA cytometry and cytology by quantitative fluorescence image analysis in symptomatic bladder cancer patients. Int J Cancer. 1987 Nov 15;40(5):698–705. doi: 10.1002/ijc.2910400522. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Bergui L., Tesio L., Corbascio G., Tousco F., Marchisio P. C. Cytoskeleton organization is aberrantly rearranged in the cells of B chronic lymphocytic leukemia and hairy cell leukemia. Blood. 1986 Jan;67(1):233–239. [PubMed] [Google Scholar]
- Cohen S. M., Ellwein L. B. Genetic errors, cell proliferation, and carcinogenesis. Cancer Res. 1991 Dec 15;51(24):6493–6505. [PubMed] [Google Scholar]
- Fradet Y., Islam N., Boucher L., Parent-Vaugeois C., Tardif M. Polymorphic expression of a human superficial bladder tumor antigen defined by mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7227–7231. doi: 10.1073/pnas.84.20.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frang D., Somogyi L., Jilling A. Current diagnostic and therapeutic methods in the treatment of vesical tumours. Int Urol Nephrol. 1988;20(6):597–609. doi: 10.1007/BF02549492. [DOI] [PubMed] [Google Scholar]
- Friedman E., Verderame M., Lipkin M., Pollack R. Altered actin cytoskeletal patterns in two premalignant stages in human colon carcinoma development. Cancer Res. 1985 Jul;45(7):3236–3242. [PubMed] [Google Scholar]
- Hemstreet G. P., 3rd, Hurst R. E., Bass R. A., Rao J. Y. Quantitative fluorescence image analysis in bladder cancer screening. J Occup Med. 1990 Sep;32(9):822–828. doi: 10.1097/00043764-199009000-00013. [DOI] [PubMed] [Google Scholar]
- Hemstreet G. P., 3rd, Rao J. Y., Hurst R. E., Bonner R. B., Jones P. L., Vaidya A. M., Fradet Y., Moon R. C., Kelloff G. J. Intermediate endpoint biomarkers for chemoprevention. J Cell Biochem Suppl. 1992;16I:93–110. doi: 10.1002/jcb.240501320. [DOI] [PubMed] [Google Scholar]
- Hemstreet G. P., 3rd, Rollins S., Jones P., Rao J. Y., Hurst R. E., Bonner R. B., Hewett T., Smith B. G. Identification of a high risk subgroup of grade 1 transitional cell carcinoma using image analysis based deoxyribonucleic acid ploidy analysis of tumor tissue. J Urol. 1991 Dec;146(6):1525–1529. doi: 10.1016/s0022-5347(17)38157-0. [DOI] [PubMed] [Google Scholar]
- Hemstreet G. P., Schulte P. A., Ringen K., Stringer W., Altekruse E. B. DNA hyperploidy as a marker for biological response to bladder carcinogen exposure. Int J Cancer. 1988 Dec 15;42(6):817–820. doi: 10.1002/ijc.2910420602. [DOI] [PubMed] [Google Scholar]
- Heney N. M., Ahmed S., Flanagan M. J., Frable W., Corder M. P., Hafermann M. D., Hawkins I. R. Superficial bladder cancer: progression and recurrence. J Urol. 1983 Dec;130(6):1083–1086. doi: 10.1016/s0022-5347(17)51695-x. [DOI] [PubMed] [Google Scholar]
- Hill R. P. Tumor progression: potential role of unstable genomic changes. Cancer Metastasis Rev. 1990 Sep;9(2):137–147. doi: 10.1007/BF00046340. [DOI] [PubMed] [Google Scholar]
- Ikkai T., Mihashi K., Kouyama T. Pulse fluorimetric study of labelled actin--DNase I complex. FEBS Lett. 1980 Jan 14;109(2):216–218. doi: 10.1016/0014-5793(80)81090-8. [DOI] [PubMed] [Google Scholar]
- Jones P. L., O'Hare C. M., Bass R. A., Rao J. Y., Hemstreet G. P., Hurst R. E. Quantitative immunofluorescence, anti-ras p21 antibody specificity, and cellular oncoprotein levels. Biochem Biophys Res Commun. 1990 Mar 16;167(2):464–470. doi: 10.1016/0006-291x(90)92046-3. [DOI] [PubMed] [Google Scholar]
- McGowan P. F., Hurst R. E., Bass R. A., Wilcox L. J., Hemstreet G. P., Postier R. G. Equilibrium binding of Hoechst 33258 and Hoechst 33342 fluorochromes with rat colorectal cells. J Histochem Cytochem. 1988 Jul;36(7):757–762. doi: 10.1177/36.7.2454985. [DOI] [PubMed] [Google Scholar]
- McKenzie S. J., Marks P. J., Lam T., Morgan J., Panicali D. L., Trimpe K. L., Carney W. P. Generation and characterization of monoclonal antibodies specific for the human neu oncogene product, p185. Oncogene. 1989 May;4(5):543–548. [PubMed] [Google Scholar]
- Messing E. M., Hanson P., Ulrich P., Erturk E. Epidermal growth factor--interactions with normal and malignant urothelium: in vivo and in situ studies. J Urol. 1987 Nov;138(5):1329–1335. doi: 10.1016/s0022-5347(17)43593-2. [DOI] [PubMed] [Google Scholar]
- Mostofi F. K., Davis C. J., Jr, Sesterhenn I. A. Current understanding of pathology of bladder cancer and attendant problems. J Occup Med. 1990 Sep;32(9):793–796. doi: 10.1097/00043764-199009000-00008. [DOI] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Gene expression, cellular diversification and tumor progression to the metastatic phenotype. Bioessays. 1991 Jul;13(7):337–342. doi: 10.1002/bies.950130706. [DOI] [PubMed] [Google Scholar]
- Norming U., Nyman C. R., Tribukait B. Comparative flow cytometric deoxyribonucleic acid studies on exophytic tumor and random mucosal biopsies in untreated carcinoma of the bladder. J Urol. 1989 Dec;142(6):1442–1447. doi: 10.1016/s0022-5347(17)39121-8. [DOI] [PubMed] [Google Scholar]
- Paraskeva C., Williams A. C. Promotability and tissue specificity of hereditary cancer genes: do hereditary cancer patients have a reduced requirement for tumor promotion because all their somatic cells are heterozygous at the predisposing locus? Mol Carcinog. 1992;5(1):4–8. doi: 10.1002/mc.2940050104. [DOI] [PubMed] [Google Scholar]
- Pienta K. J., Partin A. W., Coffey D. S. Cancer as a disease of DNA organization and dynamic cell structure. Cancer Res. 1989 May 15;49(10):2525–2532. [PubMed] [Google Scholar]
- Presti J. C., Jr, Reuter V. E., Galan T., Fair W. R., Cordon-Cardo C. Molecular genetic alterations in superficial and locally advanced human bladder cancer. Cancer Res. 1991 Oct 1;51(19):5405–5409. [PubMed] [Google Scholar]
- Rao J. Y., Hemstreet G. P., 3rd, Hurst R. E., Bonner R. B., Min K. W., Jones P. L. Cellular F-actin levels as a marker for cellular transformation: correlation with bladder cancer risk. Cancer Res. 1991 Jun 1;51(11):2762–2767. [PubMed] [Google Scholar]
- Rao J. Y., Hurst R. E., Bales W. D., Jones P. L., Bass R. A., Archer L. T., Bell P. B., Hemstreet G. P., 3rd Cellular F-actin levels as a marker for cellular transformation: relationship to cell division and differentiation. Cancer Res. 1990 Apr 15;50(8):2215–2220. [PubMed] [Google Scholar]
- SLAUGHTER D. P., SOUTHWICK H. W., SMEJKAL W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953 Sep;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sidransky D., Frost P., Von Eschenbach A., Oyasu R., Preisinger A. C., Vogelstein B. Clonal origin bladder cancer. N Engl J Med. 1992 Mar 12;326(11):737–740. doi: 10.1056/NEJM199203123261104. [DOI] [PubMed] [Google Scholar]
- Sporn M. B. Carcinogenesis and cancer: different perspectives on the same disease. Cancer Res. 1991 Dec 1;51(23 Pt 1):6215–6218. [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Wood D. P., Jr, Wartinger D. D., Reuter V., Cordon-Cardo C., Fair W. R., Chaganti R. S. DNA, RNA and immunohistochemical characterization of the HER-2/neu oncogene in transitional cell carcinoma of the bladder. J Urol. 1991 Nov;146(5):1398–1401. doi: 10.1016/s0022-5347(17)38123-5. [DOI] [PubMed] [Google Scholar]



