Abstract
Estrogen receptor alpha (ERα) is a critical player in development and function of the female reproductive system. Perturbations in ERα response can affect wide-ranging aspects of health in humans as well as in livestock and wildlife. Because of its long-known and broad impact, ERα mechanisms of action continue to be the focus on cutting-edge research efforts. Consequently, novel insights have greatly advanced understanding of every aspect of estrogen signaling. In this review, we attempt to briefly outline the current understanding of ERα mediated mechanisms in the context of the female reproductive system.
Estrogen Receptor
The vast majority of estrogen's activities are mediated by the estrogen receptor (ER), a member of the nuclear receptor family of hormone activated transcription factors. Our understanding of the physiological role of estrogen action has been greatly advanced by the generation of experimental mouse and rat models with knockout of receptors or co-activators either globally or in specific tissues and cells, or with knock-in expression of mutated forms of these molecules. These models, used in combination with microarray, RNA next generation sequencing (RNA-seq), and chromatin immunoprecipitation next generation sequencing (ChIP-seq) methods, allow comprehensive mapping of interaction of ERs with the chromatin landscape to impact genomic response. Together, these models and techniques have led to better understanding of the molecular details of estrogen receptor roles in biological processes.
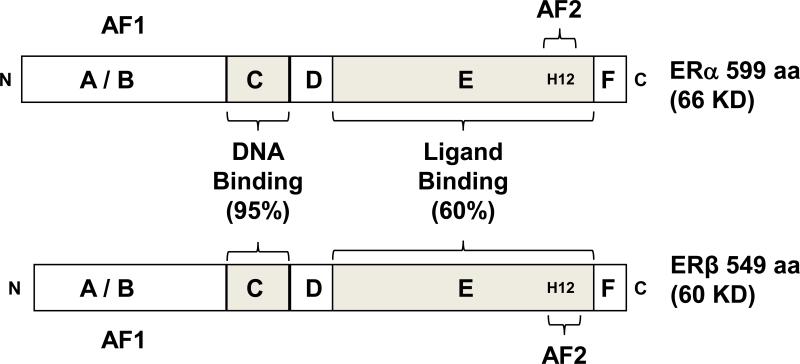
Estrogen receptor α (ERα) cDNA was the first described and cloned estrogen receptor (termed ESR1 ERα) (Walter, et al. 1985). A second ER gene, termed ESR2 (ERβ), was discovered in 1996 (Kuiper, et al. 1996). ERα and ERβ are not isoforms but rather distinct receptors encoded by two separate genes on different chromosomes. ERα is found on chromosome 6 in humans, and chromosome 10 in mice. ERβ is found on chromosome 14 in humans and chromosome 12 in mice. The ERα proteins are 595 and 599 amino acids in length in humans and mice, respectively, with an approximate molecular weight of 66 kDa (Fig. 1) (Gibson and Saunders 2012; Heldring, et al. 2007; Le Romancer, et al. 2011).
Figure 1.
Structures of ERα and ERβ protein with functional domains. Estrogen receptors ERα and ERβ share a conserved domain structure. The A/B domain, at the amino terminus (N) of the protein contains activation function 1 (AF1). The C domain binds to DNA motifs called estrogen responsive elements (EREs). The D domain is called the hinge region, and contributes to DNA binding specificity and nuclear localization of the ERs. The E domain is called the ligand binding domain because it interacts with estrogen, through an arrangement of 11 α helices (H1, and H3 through H12). H12 in this region of the receptor is critical to mediating transcriptional activation via activation function 2 (AF2). At the carboxy terminus (C) is the F domain. The % homology shared between ERα and ERβ in the C and E domains is shown.
The ESR2 encodes a receptor of 549 amino acids in rodents and 530 amino acids in humans, each with an approximate molecular weight of 60–63 kDa (Fig. 1) (Gibson and Saunders 2012). Therefore, ERβ is slightly smaller than ERα, and most of this difference lies within the smaller N-terminus.
Receptor Structure
The estrogen receptors are composed of five functional domains (Fig. 1), an N-terminal domain (NTD) or A/B domain, the DNA-binding (DBD or C) domain, a hinge (D) region, LBD (LBD or E), and a C-terminal F domain (Aagaard, et al. 2011; Brelivet, et al. 2012; Helsen, et al. 2012; Hilser and Thompson 2011; Laudet and Gronemeyer 2001).
NTD or A/B Domain
Crystallography of the ER NTD or A/B domain has been largely unsuccessful because this portion of the receptor is unstructured and fluctuates in aqueous solutions. However, evidence suggests that intramolecular interactions between the A/B and other receptor domains are likely to induce a more structured NTD (Aagaard et al. 2011; Hilser and Thompson 2011; McEwan 2004), as evidenced from recent cryogenic Electron Microscopy (cryo-EM) studies (Yi, et al. 2015). Current models of ER signaling incorporate the flexibility of intrinsically disordered (ID) regions of the receptor, including the NTD, into a mechanism of allosteric interaction and co-ordination of ligand, DNA motif and ER domain functions (Aagaard et al. 2011; Hilser and Thompson 2011). The NTD contains the transcriptional activation function-1 (AF-1) domain and provides for cell and promoter-specific activity of the receptor as well as a site for interaction with co-receptor proteins (Table 1). More recent description of full-length ERα structure derived using cryo-EM indicates A/B domain is positioned near the LBD, and facilitates recruitment of the steroid receptor transcriptional co-activator, SRC-3 (Yi et al. 2015). Posttranslational modifications, such as phosphorylation, of the A/B domain can dramatically affect the overall behavior of the receptor and are thought to be an important mechanism for the modulation of AF-1 functions (Le Romancer et al. 2011).
Table 1.
Estrogen Receptor Co-Regulator Complexes.
| Complex | Functions | Comments | References |
|---|---|---|---|
| Src1, Src2, Src3 | interact with Helix12 of agonist bound ER, interact with SWI/SNF, histone modifiers | (Hsia, et al. 2010; Johnson and O'Malley 2012) | |
| Mediator | “bridges” ER and transcriptional “machinery” (RNA Pol II) to control transcription | made up of >20 subunits, MED 1-31, arranged in 3 modules (head, middle, tail) | (Conaway and Conaway 2011; Malik and Roeder 2010) |
| SWI/SNF | regulate access to enhancer sequences via chromatin remodeling, ATPase activity, | Made up of 9+ subunits, examples include BRG1, BRM, BAF subunits | (Roberts and Orkin 2004) |
| Histone Modifiers | Modify histones to increase or decrease transcription | Acetyltransferase (HAT;eg.p300/CBP), deacetyase (HDAC;eg.NCoR), Methyl transferase (eg.PMRT/CARM), demethylase | (Barnes, et al. 2005; Wu and Zhang 2009) |
| 26S Proteasome | “clears” transcriptional modulatory proteins to facilitate subsequent transcription, transcriptional termination | Structure made up of 20S catalytic core particles (CP), 19S regulatory particles (RP) | (Keppler, et al. 2011; Kim, et al. 2011) |
Reproduced, with permission, from Binder AK, Winuthayanon W, Hewitt SC, Couse JF & Korach KS (2015) Steroid receptors in the uterus and ovary. In Knobil and Neill's Physiology of Reproduction, 4th Edn, pp 1099–1193. Eds TM Plant & AJ Zeleznik. Elsevier.
DNA-Binding or C Domain
The C domain of the ER recognizes and binds to the cis-acting enhancer sequences, called estrogen responsive elements (EREs) (Helsen et al. 2012). The C domain contains two zinc fingers, each composed of four cysteine residues that chelate a single Zn2 ion. Crystallography studies indicate a highly conserved structure consisting of dual α-helices positioned perpendicular to each other (Aagaard et al. 2011; Helsen et al. 2012; Hilser and Thompson 2011). Amino acids in the C-terminal “knuckle” of the first zinc finger form the “P-box” (proximal box) of the DNA binding domain and confer DNA sequence recognition specificity to the receptor for binding DNA sequences; hence, the proximal zinc finger is often referred as forming the “recognition helix”. Amino acids at the N-terminal “knuckle” of the second zinc finger form the “D-box” (distal box) and are more specifically involved in differentiating the “spacer” sequence within the ERE as well as providing a secondary interface for receptor dimerization.
The consensus motif (estrogen response element or ERE) that ER binds is composed of a 6-base pair (bp) palindromic sequence arranged as an inverted repeat and separated by a 3-bp spacer, GGTCAnnnTGACC. The inverted-repeat arrangement of the ERE dictates that the ER homodimerizes in a “head-to-head” position when bound to DNA. Structural analysis has revealed the importance of the 10-30 amino acid carboxy terminal extension (CTE) of the DBD in DNA interaction (Aagaard et al. 2011; Helsen et al. 2012; Hilser and Thompson 2011). Although this CTE region is variable between steroid receptors, it is crucial for DNA binding, particularly for sequence selectivity of DNA binding, by extending the interaction surfaces between the receptor and the DNA.
Hinge Region or D Domain
The above described CTE extends into the hinge region, which also contains a nuclear localization signal, and influences cellular compartmentalization of ER, as well as sites of post-translation modifications (Kim, et al. 2006). Current mechanisms suggest this non-conserved and intrinsically disordered (ID) domain is important for intra-molecular allosteric interactions involving the N-terminal and LBD. This type of flexible structural interaction works to allow rapid response to diverse modulators governing changes in biological environments (Kumar and McEwan 2012).
LBD or E Domain
The LBD or E domain of the ER is a highly structured multifunctional region that primarily serves to specifically bind estrogen and provide for hormone-dependent transcriptional activity through an activation function 2 (AF-2) domain located close to the C-terminus of the E domain. A strong receptor dimerization interface, sites for interaction with heat shock proteins, and nuclear localization signals are also within the E domain (Kumar and McEwan 2012; Laudet and Gronemeyer 2001). Structural studies indicate that the LBD is composed of 11 α-helices (H1, and H3 through H12) arranged in a three-layer α-helical sandwich to create a hydrophobic ligand-binding pocket near the C-terminus of the receptor (Huang, et al. 2010). Receptor binding to an estrogen agonist leads to rearrangement of the LBD such that H11 is repositioned and H12 rotates back toward the core of the domain to form a “lid” over the binding pocket. This agonist-induced repositioning of H12 leads to the formation of a hydrophobic cleft, or “NR box”, by helices 3, 4, and 5 on the receptor surface, constituting the AF2, which serves to recruit coactivators (Table 1) to the receptor complex. In contrast, estrogen antagonists are unable to induce a similar repositioning of H12, leading to a receptor formation that is incompatible with co-activator recruitment and is therefore less likely to activate transcription. The LBDs of ERα and ERβ exhibit approximately 60% homology (Fig. 1) but bind the endogenous estrogen, estradiol (E2), with similar affinity (ERα, 0.1 nM; ERβ, 0.4 nM) (Gibson and Saunders 2012; Le Romancer et al. 2011) indicating only a small portion of the LBD sequence governs the specificity of ligand binding. However, given the divergence in homology, it is not surprising that ERα and ERβ exhibit measurable differences in their affinity for other endogenous steroids and xenoestrogens (Gibson and Saunders 2012; Le Romancer et al. 2011). Natural and synthetic steroidal and non-steroidal ER agonists and antagonists have been described, some of which show specificity or preference for one or the other ER subtype, illustrating differences between the LBDs of ERα and ERβ and provide for conceptual pharmacological tools to discern the overall function of each ER. The most widely used ER sub-type selective ligands currently in use are propylpyrazole (PPT), an ERα selective agonist, and diarylpropionitrate (DPN), an agonist showing preference, but not exclusive selectivity, towards ERβ (Meyers, et al. 2001; Stauffer, et al. 2000).
F Domain
Among the sex steroid receptors, only ERs possess a well-defined F domain (Fig. 1). This region is relatively unstructured with little known function, although some data indicate a role in co-activator recruitment, dimerization and receptor stability (Arao, et al. 2013; Katzenellenbogen, et al. 2000; Koide, et al. 2007; Kumar, et al. 2011; Yang, et al. 2008).
Co-Regulatory Complexes
All steroid receptors interact with co-regulatory molecules, co-activators and co-repressors (George, et al. 2011; Hsia, et al. 2010). The primary co-activator interaction for steroid receptors is with a family of p160/SRC (Steroid Receptor Coactivator) 1, 2 and 3 coactivators (Bulynko and O'Malley 2011; Johnson and O'Malley 2012; Lonard and O'Malley 2005). SRC1 (NCOA1), SRC2 (GRIP1, TIF2) and SRC3 (pCIP, RAC3, ACTR, TRAM, A1B1) interact with helix 12 of ERs via “LXXLL” motifs in their nuclear receptor interacting domain, which are leucine rich regions with “X” designating any amino acid (Johnson and O'Malley 2012). SRCs also contain activation domains that recruit secondary molecules such as p300, and a bHLH-PAS motif within the N-terminal region, which can interact with other transcription factors (Johnson and O'Malley 2012). ERs and SRCs function as a nexus interacting with massive multimeric complexes, including the SWI/SNF chromatin remodeler, mediator complex, or proteasomes (Table 1) (Bulynko and O'Malley 2011). These interactions coordinate the specific functions necessary to allow appropriate gene and cell selective access to chromatin, via modifications of histones or members of co-regulatory complexes (O'Malley, et al. 2012). In this way, co-activators dynamically mediate and coordinate processes necessary to accomplish transcription, including initiation, elongation, termination, and clearing or turnover of the transcriptional modulators.
Mechanisms of Estrogen Response
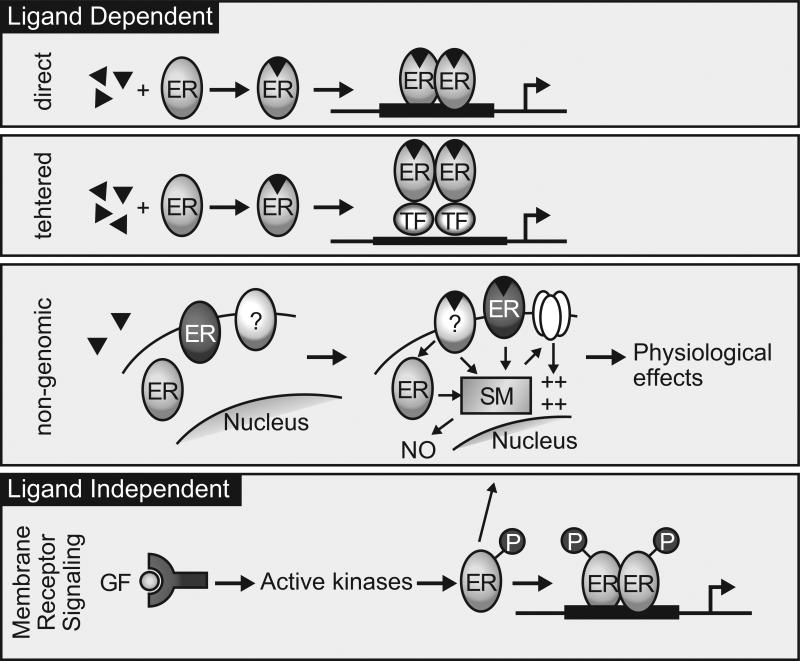
Our understanding of the mechanisms by which estrogens influence cell function and behavior has expanded profoundly since initial models of ligand-dependent activation, which is now referred to as the “classical” or ligand dependent direct DNA binding model of receptor function (Fig. 2). In the years since, numerous discoveries primarily in cell-based systems have been made that illuminate the complexity of ER signaling in cells and tissues. The entrée into the “omics” era has facilitated massive expansion for the study of transcriptional regulation and chromatin remodeling. In addition, several alternative receptor signaling mechanisms that diverge from the classic model have become apparent, including “tethering” of the ER to heterologous DNA-bound transcription factors to provide for regulation of genes that lack ERE sequences (Fig. 2); plasma membrane estrogen signaling, often referred to as “nongenomic” steroid actions and ligand-independent “cross-talk” with intracellular and second messenger systems that provide for ER activation in the absence of the cognate steroid ligand (Fig. 2). These modes of ER responses as currently understood are discussed below.
Figure 2.
Ligand-dependent and ligand-independent nuclear receptor mechanisms. The direct “classic” model of estrogen receptor (ER) action involves direct interaction between ER bound to estrogen (triangles) and ERE; the tethered pathway utilizes indirect “tethering” of ER to genes via interactions with other transcription factors (TF). “Nongenomic” signaling is initiated by membrane-localized receptors modulating extranuclear second messenger (SM) signaling pathways. Ligand-independent responses occur as a result of transduction of membrane receptor signaling, such as growth factors (GF), to nuclear ER. Reproduced, with permission, from Binder AK, Winuthayanon W, Hewitt SC, Couse JF & Korach KS (2015) Steroid receptors in the uterus and ovary. In Knobil and Neill's Physiology of Reproduction, 4th Edn, pp 1099–1193. Eds TM Plant & AJ Zeleznik. Elsevier.
Ligand-Dependent Actions: Direct or Classical
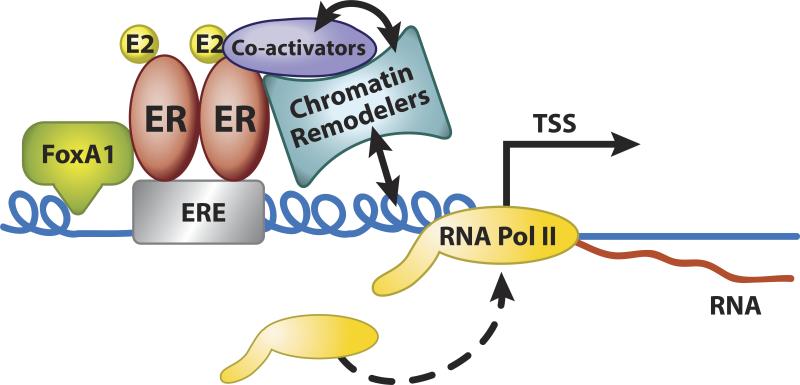
In the classic model of estrogen response (Fig. 2 and 3) estrogen ligands diffuse across the plasma and nuclear membranes to bind ER, primarily localized to the nucleus, resulting in a conformational change in the receptor, transforming it to an “activated” state that interacts with chromatin via ERE motifs and transcriptional mediators. ERs seem to be preferentially recruited to open regions of chromatin (Biddie, et al. 2010). Studies using MCF7 breast cancer cells indicate that FoxA1 acts as a pioneering factor, providing accessible regions in the chromatin that recruit ERα (Fig.3) (Carroll and Brown 2006; Carroll, et al. 2005; Fu, et al. 2011; Zaret and Carroll 2011). The ligand–ERE-bound receptor complex then engages coactivator molecules as described above (Johnson and O'Malley 2012) leading to modulation of transcription rates of responding genes. This classic steroid receptor mechanism is dependent on the functions of both AF-1 and AF-2 domains of the receptor, which synergize via the recruitment of coactivator proteins, most notably the p160 family members (Johnson and O'Malley 2012). Depending on the cell and target gene promoter context, the DNA-bound receptor complex may positively or negatively affect expression of the downstream target gene. Initially, study of ER mediated gene regulation was carried out on a gene-by gene basis using a handful of known hormone regulated transcripts. Now, after numerous comprehensive analyses of hormonally regulated transcriptional profiles, using microarray and more recently RNA-seq, thousands of ER targets have been found in various cell lines and tissues.
Figure 3.
Model of chromatin dynamics in ER mediated transcription. FoxA1 interacts with chromatin, providing access for ER to nearby EREs. ER then interacts with transcriptional co-activators and chromatin modifying enzymes to open up transcription start sites (TSS) for RNA polymerase II (PolII), allowing initiation of transcription. Reproduced, with permission, from Wall EH, Hewitt SC, Case LK, Lin CY, Korach KS & Teuscher C (2014) The role of genetics in estrogen responses: a critical piece of an intricate puzzle. FASEB Journal 28 5042–5054.
Indirect/Tethered Actions (ERE Independent)
In in vitro reporter gene systems, ligand-activated ER can modulate the expression of genes that lack a conspicuous ERE within their promoter (Kushner, et al. 2000; Safe and Kim 2004, 2008). This mechanism of ERE-independent steroid receptor activation is postulated to involve a “tethering” of the ligand-activated receptor to transcription factors that are directly bound to DNA via their respective response elements (Fig. 2). However, the ERαEAAE/EAAE mouse, which is mutated in the ERα DBD and lacks ERE binding, does not exhibit estrogen response in vivo, indicating the tethering mechanism, at least on its own, is unable to mediate hormonal responses (Ahlbory-Dieker, et al. 2009; Hewitt, et al. 2014) and is likely complimentary to the direct DNA stimulated responses.
Non-Genomic Actions
Rapid effects of E2 have been described, including a rapid activation of endothelial nitric oxide synthase in endothelial cells (Levin 2011) and potentiation of nerve conductance (Kim, et al. 2011; Takeo and Sakuma 1995). Because these estrogen effects occur within minutes, they have been thought to not involve direct estrogen receptor activation of gene transcription, they are often collectively referred to as representing “non-genomic” pathways of estrogen action. Questions remain concerning whether the membrane-associated receptors mediating these events are identical or variant forms of the ER or instead distinct receptors altogether.
One potential mediator of rapid membrane localized hormone response is the G protein coupled estrogen receptor (GPER; originally referred to as GPR30), which is activated by E2 (Prossnitz and Barton 2011). Gper null mice lack reproductive phenotypes (Langer, et al. 2010), although effects on the degrees of uterine responses elicited by E2 have been observed with G15, a GPER selective antagonist, suggesting a potential role for GPER in modulating ERα mediated responsiveness (Gao, et al. 2011).
Ligand Independent Actions: Membrane Receptor Cross-Talk
Peptide growth factors are able to activate ERα–mediated gene expression via mitogen-activated protein kinase activation of ERα in the absence of E2 (Fig. 2). Likewise, growth factors are able to mimic the effects of E2 in the rodent uterus via E2 independent activation of ERα (Curtis and Korach 1999; Fox, et al. 2009). In some cases, the MAP kinase protein ERK is co-recruited to chromatin with ERα (Madak-Erdogan, et al. 2011). Ligand-independent activation of estrogen receptors is believed to rely largely on cellular kinase pathways that alter the phosphorylation state of the receptor and/or its associated proteins (e.g., coactivators, heat shock proteins) (Fig. 2).
Uterine Response to Estradiol
Utilizing animal models to follow and manipulate estrogen responsiveness is one way to understand and describe mechanisms of estrogen responses. The reproductive function of the mouse has been especially well studied and characterized in this manner. Treatment of ovariectomized mice with estrogens (e.g., E2 or diethylstilbestrol - DES) has long served as an experimental model to mimic the uterine events that occur during the estrous phase of the rodent cycle or immediately after the preovulatory E2 surge. Morphological and biochemical changes occur in the rodent uterus after estrogen stimulation following an established biphasic temporal pattern (Hewitt, et al. 2003). Estrogen-stimulated changes in the rodent uterus that occur early, within the first 6 hours after treatment, include increases in nuclear ER occupancy, water imbibition, vascular permeability and hyperemia, prostaglandin release, glucose metabolism, eosinophil infiltration, gene expression (e.g., c-fos), lipid and protein synthesis. ERα ChIP-Seq profiles from in vivo studies of uterine tissues show that in the unstimulated state the receptor pre-occupies chromatin sites in the absence of hormone and that E2 treatment increases ERα recruitment (Hewitt, et al. 2012). The above processes are followed by responses that peak after 24–72 hours and include dramatic increases in RNA and DNA synthesis, epithelial proliferation, and differentiation of epithelial cells toward a more columnar secretory phenotype, dramatic increases in uterine weight, and continued gene expression.
Changes in Uterine Gene Expression
The dramatic physiological changes that occur in the uterus in response to steroid hormones are presumably the ultimate effects of equally dramatic changes in gene expression among the uterine cells. It is unlikely that the E2–ER complex is directly involved in mediating the whole genomic response in the uterus but more plausibly serves to stimulate a cascade of downstream signaling pathways that act to amplify the estrogen action. However, early investigations of the genomic response to estrogens in the rodent uterus discovered a handful of genes that are directly regulated via the classic ER mode of action, including progesterone receptor (Pgr) and lactoferrin or lactotransferrin (Ltf). Microarray analysis has significantly advanced understanding of genomic response of the rodent uterus to E2. Numerous studies have used microarray techniques to map the global gene expression patterns after estrogen exposure in the uterus and largely demonstrate that the biphasic uterine response to estrogens, so well characterized by physiological indicators above, is mirrored by the global changes in gene expression (Andrade, et al. 2002; Fertuck, et al. 2003; Hewitt, et al. 2005; Hewitt et al. 2003; Ho Hong, et al. 2004; Hong, et al. 2006; Moggs, et al. 2004; Watanabe, et al. 2003). The clearly defined patterns of early and late response genes found in mouse uterine tissues are completely lacking in ERα–null (αERKO, Ex3αERKO) uteri (Hewitt et al. 2003; Hewitt, et al. 2010a). The identified genes fall into functional groups, including signal transduction, gene transcription, metabolism, protein synthesis and processing, immune function, and cell cycle. The expression levels of a striking number of genes are actively repressed by estrogen in the mouse uterus, and these effects were absent in ERα–null uteri or are relieved by co-treatment with ER antagonists in the presence of ERα, indicating that ERα is also actively involved in transcriptional repression as part of mediating the physiological responses (Hewitt et al. 2003; Hewitt et al. 2010a).
Whole transcriptome analyses are now routinely incorporated into studies of disruptions in signaling pathways underlying uterine phenotypes of mouse models such as those described in Table 2. Thus, microarray comparisons have now become just one of many tools employed for investigation of uterine functions.
Table 2.
Uterine Phenotypes in Mice Null or Mutated for Estrogen Receptors or Estrogen Signaling.
| Mutated or null for sex steroid receptors and signaling | Uterine phenotypes | References |
|---|---|---|
|
Esr1−/− (Homozygous null alleles for ERα: αERKO and Ex3αERKO) |
Normal uterine development but exhibits hypoplastic uteri. Insensitive to the proliferative and differentiating effects of endogenous, growth factors and exogenous E2. Implantation defect. *lack decidualization. Infertile. |
(Antonson, et al. 2012; Curtis Hewitt, et al. 2002; Curtis, et al. 1999; Dupont, et al. 2000; Hewitt, et al. 2010a; Lubahn, et al. 1993) |
|
NERKI+/− (One mutated allele of two-point mutation in ERα DBD and one WT allele) |
Normal uterine development but exhibits hyperplastic uteri. Hypersensitive to estrogen. Infertile. |
(Jakacka, et al. 2002) |
|
KIKO (ERAA/−) (One mutated allele of two-point mutation in DNA binding domain of ERα and one ERαKO allele) |
Normal uterine development. Insensitive to the proliferative effects of exogenous E2 treatment. ERAA binds HRE and induces genes that are normally progesterone responsive Infertile. |
(Hewitt, et al. 2010b; O'Brien, et al. 2006) |
|
ERαEAAE/EAAE (Homozygous animal of 4-point mutation of DBD ERα) |
Normal uterine development but exhibits hypoplastic uteri. Loss of E2-induced uterine transcripts. Infertile. |
(Ahlbory-Dieker, et al. 2009) |
|
ERαAF-10 (Deletion of amino acids 2-128 on ERα) |
Normal uterine development and architecture. Blunted E2 response. Infertile. |
(Abot, et al. 2013; Billon-Gales, et al. 2009) |
|
ERαAF-20 (Deletion of amino acids 543-549 on ERα) |
Normal uterine development but exhibits hypoplastic uteri. Insensitive to E2 treatment. Infertile. |
(Billon-Gales, et al. 2011) |
|
ENERKI (ERαG525L) (Homozygous animal of one point mutation in LBD of ERα) |
Normal uterine development but exhibits hypoplastic uteri. Insensitive to E2 treatment. IGF-1 induced slight uterine epithelial proliferation compared to control littermates (nonhomogenous pattern). Infertile. |
(Sinkevicius, et al. 2008) |
| AF2ERKI/KI (Homozygous knock-in of two-point mutation in LBD of ERα) | Normal uterine development but exhibits hypoplastic uteri. Insensitive to E2 treatment. ER antagonists and partial agonist (ICI 182,780 and TAM) induced uterine epithelial proliferation. Growth factor did not induce the uterine epithelial cell proliferation. Infertile. |
(Arao, et al. 2011) |
| ERα Epi-cKO (epithelial cell specific deletion of ERα using Wnt7aCre+;Esr1f/f mouse model) | Normal uterine development. Sensitive to E2- and growth factor-induced epithelial cell proliferation. Lack full uterine growth response to E2. Selective loss of E2-target gene response. Implantation and decidualization defects. Infertile. |
(Pawar, et al. 2015; Winuthayanon, et al. 2014; Winuthayanon, et al. 2010) |
| Esr1d/d(Uterine deletion of ERα using PgrCre+;Esr1f/f mouse model) | Normal uterine development. Hypoplastic uteri. Defective decidual response. |
(Pawar et al. 2015) |
| Esr2−/− (Homozygous null alleles for ERβ: βERKO, Ex3βERKO, and **ERβSTL-/L-) | Exhibit grossly normal uterine development and function. Sensitive to E2 treatment. Some Esr2−/− lines reported elevated uterine epithelial proliferation after E treatment compared to WT Some are complete sterile (due to ovarian phenotype). |
(Antal, et al. 2008; Dupont et al. 2000; Krege, et al. 1998; Wada-Hiraike, et al. 2006) |
| αβERKO (Homozygous null for both ERα and ERβ | Normal uterine development but exhibit hypoplastic uteri, similar to those of Esr1−/−. Insensitive to E2, infertile | (Couse, et al. 1999; Dupont et al. 2000) |
| Cyp19a1−/− (Homozygous null aromatase: ArKO) | Normal uterine development but exhibits hypoplastic uteri. Sensitive to E2-induced epithelial cell proliferation. Infertile. |
(Fisher, et al. 1998; Toda, et al. 2001) |
| Esr1C541A palmitoylation deficient mutants | C451A-ERα normal uterine development, E2 growth response Nuclear-only ERα [NOER] hypoplastic ERα-null like uterus |
(Adlanmerini, et al. 2014) (Pedram, et al. 2014) |
Reproduced, with permission, from Binder AK, Winuthayanon W, Hewitt SC, Couse JF & Korach KS (2015) Steroid receptors in the uterus and ovary. In Knobil and Neill's Physiology of Reproduction, 4th Edn, pp 1099–1193. Eds TM Plant & AJ Zeleznik. Elsevier.
αERKO females have a similar uterine phenotype to the newer Ex3αERKO except for maintaining decidualization response, which may due to the splice variants in the original αERKO that retains ER activities.
ERβSTL-/L- females are the only line of ERβ knockout animals that reported to be completely sterile.
Chip-seq
Evaluation of sites of transcription factor interaction with chromatin, by enriching a DNA binding protein, such as ERα, that has been crosslinked in situ to chromatin, with immunoprecipitation (Chromatin Immunoprecipitation or ChIP), followed by hybridizing the associated DNA to a chip tiled with promoter region sequences (ChIP-Chip) or by “next generation” massively parallel sequencing (ChIP-seq), have been developed and widely utilized to study sites of ER interaction (Biddie et al. 2010; Farnham 2009; Green and Han 2011; Martens, et al. 2011; Meyer, et al. 2012; Park 2009). Initial studies focused on ERα binding in MCF7 breast cancer cells, and several similar studies followed, which are summarized and compared in several review articles (Cheung and Kraus 2010; Deblois and Giguere 2008; Gao and Dahlman-Wright 2011; Gilfillan, et al. 2012; Tang, et al. 2011). These reported that most sites were distal from transcriptional start sites (TSS), or were in intronic regions, rather than adjacent to TSS, as models of ER regulation of target transcripts had hypothesized. These comprehensive maps of cis-acting transcriptional regulators have been dubbed “Cistromes”. The initial ERα cistrome-associated sequences were evaluated for enrichment of transcription factor motifs, and confirmed binding to the experimentally defined “ERE” sequence. In the case of the MCF7 tumor cells, enrichment of motifs for forkhead binding factors (Fox) was apparent as mentioned in the earlier section. Owing to the abundant expression of the FoxA1 member of the Fox family, a potential role for FoxA1 in estrogen response was pursued with an arsenal of bioinformatic, Next Gen sequencing and biological studies that demonstrated FoxA1's role as “pioneer”, creating accessible regions of the chromatin that were subsequently targeted by ERα (Lupien, et al. 2009) (Zaret and Carroll 2011).
ChIP-seq analysis examining the ERα binding sites in mouse uterine tissue indicated that, much like the MCF7 breast cancer study, most ERα sites were not proximal to TSS (Hewitt et al. 2012). ERs bind to thousands of sites within the cellular chromatin, and not all potential EREs in every cell bind ER. Rather, it is apparent that chromatin exhibits “pre-opened” regions destined to recruit ER (Grontved and Hager 2012). For ER in MCF7, FoxA1 can establish ER accessible regions. The accessible chromatin regions are co-localized within nuclear “hubs”, which seem to optimize frequency of interaction with ER (Grontved and Hager 2012). ChIP seq is also used to locate other molecules involved in chromatin remodeling and transcriptional regulation, and to examine activating or repressive histone modifications or “marks”. These maps of relative locations and dynamics of ER and chromatin components greatly enhance our understanding of hormone response mechanisms (Deblois and Giguere 2008; Gilfillan et al. 2012; Green and Han 2011; Martens et al. 2011; Meyer et al. 2012).
Uterine Phenotypes in Mouse Models of Disrupted Estrogen Signaling
Mouse models of disrupted estrogen receptor signaling have proven invaluable to experimental investigation of estrogen actions and the contribution of each ER form to these functions (Table 2). In addition to the ER-null models are lines of mice that lack the capacity to synthesize E2 due to disruption of the Cyp19 gene (Fisher, et al. 1998; Toda, et al. 2001). Below we will describe how these different mouse models have helped to delineate the biological role of ER mechanisms in estrogen hormone action.
ERα null patients and mice
Only one male patient and one female patient with ERα mutation have been described (Quaynor, et al. 2013; Smith, et al. 1994). The male patient's mutation is a true null since no ERα protein is expressed due to the mutation generating a premature stop codon in the A/B domain. The female patient has a single point mutation in her ERα LBD that results in decreased activity by reducing the receptor's affinity for coactivator proteins more than 200 fold.
There are currently numerous reported lines of ERα-null mice and additional lines of mice with mutations in functional domains of ERα. Three separate lines of ERα-null mice were generated: the αERKO, first described by Lubahn et al. in 1993 (Lubahn, et al. 1993), the ERαKO (or Ex3αERKO), described by Dupont et al. in 2000 (Dupont, et al. 2000) and by Hewitt in 2010 (Hewitt et al. 2010a), and ERα−/− described by Antonson et al. in 2012 (Antonson, et al. 2012). Homologous recombination was employed to disrupt ERα (αERKO), or cre-mediated recombination was used to completely excise exon 3, which encodes the ER DNA binding domain (Antonson et al. 2012; Dupont et al. 2000; Hewitt et al. 2010a) of the murine Esr1 (ERα) gene (ERαKO, Ex3αERKO and ERα−/−). The uterine estrogenic response in αERKO females differs from the latter two lines, but the overall spectrum of phenotypes are the same, as αERKO animals have minimal level of truncated ERα protein produced from a splice variant, which preserves some residual biological functions (Couse, et al. 1995), but all ERα null female mice are infertile. Recently, an ERα null rat has been derived using zinc finger nuclease (ZFN) genome editing. All phenotypes in the ERα null rats examined thus far were previously seen in the ERα null mice, including infertility due to hypoplastic uteri, polycystic ovaries, and ovulation defects (Rumi, et al. 2014). The female patient with homozygous ERα mutation also has cystic ovaries and a small uterus despite elevated circulating serum E2 (Quaynor et al. 2013).
The essential role of ERα in uterine response to estrogen is indicated by the loss of early phase effects of water imbibition and hyperemia as well as the late-phase effects of increased DNA synthesis and epithelial proliferation in ERα-null uteri (Couse et al. 1995; Hewitt et al. 2010a; Korach, et al. 1996). The αERKO model was the first test of a prevailing hypothesis that early uterine effects were non-receptor mediated (Lubahn et al. 1993). Lack of these early responses of water imbibition, hyperemia and eosinophil infiltration in αERKO indicated that ERα was involved in some manner and these responses clearly require the estrogen receptor. Additionally, ovariectomized mice normally exhibit a three- to four-fold increase in uterine weight after three daily treatments with E2 or DES, whereas no such response is observed in the uteri of ERα-null females (Hewitt et al. 2010a; Korach 1994; Lubahn et al. 1993). Uteri of mice that lack ERα just in uterine epithelial cells (Wnt7aCre+;Esr1f/f, called ERα Epi-cKO) have an initial proliferative response to estrogen, but full uterine response is impaired, as the growth after 3 days of estrogen treatment is significantly less than expected (Winuthayanon, et al. 2010). The total lack of response to estrogens in ERα-null uteri as well as a lack of late biological response in epithelial ERα knockout uteri provide strong evidence that ERα is required to mediate the full biochemical and biological uterine response to estrogens (Hewitt et al. 2010a; Winuthayanon, et al. 2014; Winuthayanon et al. 2010).
Numerous studies have demonstrated some of the molecular mechanisms of E2-induced uterine epithelial cell proliferative responses in animal models. The transcription factor CCAAT Enhancer Binding Protein Beta (C/EBPβ) is involved in hormone-induced uterine proliferation (Mantena, et al. 2006). Maximum uterine expression of C/EBPβ is induced 1 h after E2 treatment in both epithelial and stromal cells (Mantena et al. 2006; Ramathal, et al. 2010). ICI 182,786 (ER antagonist) strongly inhibited E2-induced Cebpb transcript in the uterus suggesting an ER-dependent expression of C/EBPβ (Bagchi, et al. 2006). In addition, loss of epithelial ERα in the uterus did not alter E2-induced Cebpb expression, indicating that Cebpb expression is independent of epithelial ER (Winuthayanon et al. 2010), and suggesting the stimulation was through a paracrine mechanism via stromal ERα. This points to the action of estrogen through ERα as the major mediator of C/EBPβ expression in the uterus. Indeed, the deletion of C/EBPβ (C/EBPβ−/−) leads to a lack of the E-induced uterine proliferative response (Mantena et al. 2006) as reflected by the absence of mitotic activity, S-phase activity and an increase in apoptotic activity in the uterine epithelial cells (Ramathal et al. 2010). In addition to a blunted uterine growth response to hormones, the C/EBPβ−/− females also exhibit complete infertility (Bagchi et al. 2006), due to implantation and decidualization defects (Mantena et al. 2006).
Pan et al. demonstrated that the uterine expression of minichromosome maintenance proteins (MCMs), a complex required for DNA synthesis initiation, is induced after E2 treatment, specifically MCM2 and MCM3 (Pan, et al. 2006). MCM2 activity is crucial and required for DNA synthesis in uterine epithelial cells (Ray and Pollard 2012). Further study demonstrated E2-mediated induction of the transcription factor KLF4, which then targets the Mcm2 promoter (Ray and Pollard 2012).
Mice lacking ERβ
ERβ-null mice have provided insight into the importance of ERβ to female fertility and studies to date indicate ERβ plays a particularly important role in ovarian function. Four different lines of ERβ-null mice have been described. The βERKO mouse, made using homologous recombination, was first described by Krege et al. in 1998 (Krege, et al. 1998), and the ERβKO or Ex3βERKO, was described by Dupont et al. in 2000. (Dupont et al. 2000), and by Binder et al, 2013 (Binder, et al. 2013). Cre mediated recombination was employed in both lines to disrupt exon 3 (Binder et al. 2013; Dupont et al. 2000) of the murine Esr2 (ERβ) gene. As described to date, the reproductive, endocrine and ovarian phenotypes of both lines are indistinguishable, with both exhibiting female subfertility. In 2002, Shughrue et al. reported the third line of ERβKO animals, however, no uterine or ovarian phenotypes were reported (Shughrue, et al. 2002). Recently, ERβKOSTL-/L- animals, which contain LoxP sites flanking exon 3 of Esr2, were generated using the Cre/loxP recombination system (Antal, et al. 2008). Interestingly, female mice from this recently described ERβKOSTL-/L- colony were reported to be sterile due to an ovarian defect while Ex3βERKO (Binder et al. 2013) are subfertile, due to ovulatory defects.
Mice lacking ER α and β
The two reported lines of compound ER-null mice are the αβERKO, described by Couse et al. in 1999 (Couse, et al. 1999), and the ERαβKO, described by Dupont et al. in 2000 (Dupont et al. 2000). Both were generated by cross breeding animals heterozygous for the respective individual ER-null mice and as described to date, exhibit comparable reproductive, endocrine and ovarian phenotypes. The most striking phenotype is the unique trans-differentiation of the ovarian granulosa cells to sertoli-like cells in follicles of αβERKO females which is age dependent. To date, no manipulation of the individual αERKO or βERKO mouse lines can reproduce this novel phenotype. This model clearly uncovered that both ER signaling systems are required to maintain the proper differentiation state of the adult granulosa cells.
Mice lacking Cyp19
Estrogens are produced by aromatase cytochrome P450, the product of Cyp19 gene. Female mice with disruption of circulating estrogen production exhibit altered reproduction (Fisher et al. 1998; Honda, et al. 1998; Toda et al. 2001). There are 3 animal models of Cyp19-null mice (called ArKO). Fisher et al reported the first mouse line in 1998, which disrupted exon 9 of Cyp19 gene, as the region is highly conserved (Fisher et al. 1998). Later in 1998, Honda et al reported a mouse line with targeted disruption of exons 1 and 2 of the Cyp19 gene (Honda et al. 1998). Subsequently, Toda et al generated the most recent mouse line of Cyp19-null in 2001 with a targeted disruption of exon 9 of the Cyp19 gene (Toda et al. 2001). These ArKO female phenotypes are indistinguishable (Fisher et al. 1998; Honda et al. 1998; Toda et al. 2001), with similarity to the αβERKO mice with a clear metabolic syndrome (Couse et al, 1999) and infertility due to ovarian dysfunction marked by cystic follicles and a failure to respond to exogenous gonadotropins. Interestingly, the phenotype of the original ArKO mice (Fisher et al. 1998) were also shown to exhibit the same age related ovarian phenotype (Britt et al, 2002) as the αβERKO mice, indicating that hormone mediated ER action is required.
Female reproductive phenotypes in mice with disrupted estrogen signaling
Females within each respective model exhibit a similar phenotypic syndrome. Female mice lacking ERα or aromatase are infertile due to dysfunction of numerous physiological systems, including the ovary and uterus, whereas ERβ–null females exhibit reduction or loss of fecundity that is largely attributable to ovarian dysfunction. A level of caution is warranted when making phenotypic comparisons between the ER-null and Cyp19-null models because sensitivity to maternally derived estrogens may provide a more normal developmental environment during gestation in Cyp19-null mice and sensitivity to dietary estrogens during adulthood is able to abate several phenotypes in Cyp19-null mice (Britt, et al. 2002).
The reported uterine phenotypes of these models are summarized in Table 2. All lines of ER-null females exhibit uteri that possess the expected tissue compartments, myometrium, endometrial stroma, and epithelium (Couse 1999; Couse and Korach 1999; Hewitt et al. 2010a). However, in females lacking functional ERα or Cyp19, uteri are overtly hypoplastic and exhibit severely reduced weights relative to wild-type littermates (Britt, et al. 2001; Couse and Korach 1999; Fisher et al. 1998; Toda et al. 2001), whereas ERβ-null uteri are grossly normal and normally responsive to ovarian-derived steroids (Couse and Korach 1999). The uterus of ERα-null females is severely hypotrophic, poorly organized, and possesses a paucity of glandular structures (Hewitt et al. 2010a; Korach et al. 1996). The luminal and glandular epithelial cells in ERα-null uteri are severely immature with fewer glands present in the adults (Nanjappa, et al. 2015) and consistently exhibit a cuboidal morphology, versus the tall columnar morphology and basal location of the nucleus of an “estrogenized” epithelium in WT uteri. Therefore, fetal, neonatal and perinatal development of the female reproductive tract in mice is largely independent of ERα – and ERβ–mediated actions, but estrogen responsiveness and sexual maturation of the adult uterus are ablated after the loss of functional ERα. The totality of the ERα-null phenotype and lack of any overt uterine abnormalities in ERβ-null females suggest that ERβ has little meaningful function in mediating estrogen actions in the uterus. Moreover, ERαβ-null also demonstrated a similar uterine phenotype as ERα-null (Walker and Korach 2004). Weihua et al. reported that ERβ-null females exhibited a slightly aberrant uterine growth response after estrogen replacement; however, the uterine bioassay was conducted in immature intact, not ovariectomized adult, animals (Weihua, et al. 2000). In addition, Wada-Hiraike et al showed that in immature females, loss of ERβ leads to increased uterine epithelial proliferation induced by E2 compared to Wild Type uteri (Wada-Hiraike, et al. 2006). Although ERβ-null females are subfertile, when pregnancies are established they are sustained to term (Krege et al. 1998), indicating uterine competence. More recent findings suggest that loss of ERβ leads to complete sterility due to a defect in ovarian function (Antal et al. 2008; Dupont et al. 2000).
Mice with uterine specific deletion of ERα
Selectively deleting ERα in the uterus postpubertally, using the Cre/LoxP recombination system, by crossing PgrCre+ with Esr1f/f animals (Esr1d/d), leads to a hypoplastic uterus that lacks a decidual response (Pawar, et al. 2015). Our laboratory has described uterine epithelial cell selective deletion of ERα, using the Cre/LoxP recombination system, by crossing Wnt7aCre+ (Huang, et al. 2012) with Esr1f/f animals (Hewitt et al. 2010a) (ERα Epi-cKO). The expression of ERα in the uterine luminal and glandular epithelium of these animals was ablated, while the ERα expression in the stromal cells and other uterine cells remains intact (Winuthayanon et al. 2010). The epithelial ERα was ablated not only in the uterus in this mouse line (Winuthayanon et al. 2010), but also in the oviduct (Winuthayanon, in press, eLife). As expected, based on findings in the global ERα knockouts, loss of uterine epithelial ERα has no effect on female reproductive tract development. Uterine histological analysis showed a similar uterine morphology as wild type control (Winuthayanon et al. 2010). The ERα Epi-cKO uteri are sensitive to 24 h treatment of E2, as the uterine epithelial proliferation is preserved. However, ERα Epi-cKO uteri lack a complete uterine response to E2, following a three-day uterine bioassay, which demonstrated a blunted growth response and increased apoptotic activity in ERα Epi-cKO compared to the control uteri. Additionally, a lack of ERα expression in the uterine epithelial cells contributes to complete infertility, due to oviduct, and uterine implantation and decidulaization defects (Pawar et al. 2015; Winuthayanon et al. 2010) (Winuthayanon, in press, eLife). This suggests that uterine epithelial ERα is dispensable for early uterine proliferative responses but crucial for a complete adult biological response induced by E2, as well as for establishing pregnancy.
Mice with mutated DNA binding domains of ERα
To date, there are two mouse lines with mutations that are designed to disrupt the DNA binding function of the ERα that have been “knocked-in” (KI) at the ERα gene locus. The first line was generated by replacing critical P-box amino acids E207 and G208 with alanines (ERαAA). This line was named “Non-genomic ER knock-in” (NERKI), as these mutations were intended to restrict ERα signaling to the non-genomic and tethered mechanisms. Female NERKI+/− animals that have one mutated allele and one WT allele (Jakacka, et al. 2002) were infertile, exhibiting a highly novel hyperplastic uterine phenotype, so NERKI+/− males were crossed with ERα null heterozygous (WT/KO) females to produce mice with one NERKI mutated allele and one deleted Esr1 allele, called ERα KIKO or ERαAA/- as described by O’Brien et al. in 2006 (O'Brien, et al. 2006). The second line of DNA-binding domain knock-in animals were created through mutation of four amino acids in the first zinc finger of the Esr1 gene, substituting Y at position 201 with E, and in the critical P box, K at position 210 with A, K at position 214 with A, and R at position 215 with E as described by Ahlbory-Dieker et al. in 2009 (called ERαEAAE/EAAE) (Ahlbory-Dieker et al. 2009).
The NERKI+/− females have normal uterine development but exhibit hyperplastic uteri, and are hypersensitive to estrogen (Jakacka et al. 2002). These NERKI+/− are infertile and exhibit a uterine abnormality of enlarged hyperplastic endometrial glands despite possessing normal levels of circulating sex steroids (Jakacka et al. 2002).
ERαAA/- females have normal uterine development. Initially, O’Brien et al. reported that ERαAA/- females, with mutation of the DNA binding domain, maintained proliferative responses induced by E2 (O'Brien et al. 2006). However, in subsequent studies, no uterine proliferation was observed (Hewitt, et al. 2010b; Hewitt, et al. 2009). Ahlbory-Dieker et al. showed that, unlike the NERKI+/−, females heterozygous for the ERαEAAE mutation are fertile. The homozygous ERαEAAE/EAAE females have normal reproductive tract development but uteri are severely hypoplastic, similar to global ERα-null uteri. Additionally, ERαEAAE/EAAE uteri do not respond to E treatment, as normally estrogen-responsive uterine and liver genes are not regulated in ERαEAAE/EAAE (Ahlbory-Dieker et al. 2009; Hewitt et al. 2014). The females from these two mouse lines with point mutations in the DNA binding domain of ERα are infertile. Thus the physiological function of the DNA binding domain of ERα is crucial for female reproduction. ERα ChIP-seq analysis of the ERαAA/- uterus revealed that the DBD mutation, rather than completely disrupting DNA binding instead altered the motif specificity, so that ERαAA could bind HRE motifs normally occupied by progesterone receptor (Pgr or PR). Additionally, this HRE binding lead to E2 regulation of uterine transcripts that are normally progesterone responsive (Hewitt et al. 2014). This novel ERαAA binding activity may also explain the hyperplastic phenotype of the heterozygous ERαAA/+ females where the normally activated uterine HRE sites are occupied by the mutant ERαAA and thus blocking the dampening activity of uterine PR at those sites. Adding to this abnormal regulation is the expression of ERαAA in all uterine cells at all times, whereas, the PR is restricted to epithelial cells and is dynamically induced in the stromal cells during the estrous cycle. Additionally, the phenotype also indicates the specificity of the action at the HRE requires the proper activity of the PR to elicit the dampening action.
Mice with mutated AF-1 or AF-2 domains of ERα
As discussed in the Receptor Structure section, AF-1 and AF-2 are important for ER transcriptional activity (Fig.1). Amino acids 2-128 were deleted from exon 1 of Esr1, which removes the AF-1 domain, and knocked into a mouse line (called ERαAF-10) (Billon-Gales, et al. 2009). There are three reported mouse lines with mutation in the AF-2 domain of ERα. One with a single point mutation in ERα of G at position 525 to L in the ligand binding domain (LBD), called “Estrogen-nonresponsive ERα Knock-in or ENERKI” (ERαG525L) (Sinkevicius, et al. 2008). Amino acids 543-549 were deleted from the LBD of ERα, removing helix 12 and thus AF-2 functionality, to create a second mouse line (called ERαAF-20) (Billon-Gales, et al. 2011). Two point mutations in the AF-2 of the LBD of ERα were knocked into a mouse (L543A and L544A; called AF2ERKI/KI animals) (Arao, et al. 2011). ERαAF-10, ERαG525L, ERαAF-20 and AF2ERKI/KI females are all sterile (Arao et al. 2011; Billon-Gales et al. 2009; Billon-Gales et al. 2011; Sinkevicius et al. 2008).
ERαAF-10 females exhibited minimal uterine wet weight gain compared to ER+/+ uteri after treatment with E2 pellets for 2 consecutive weeks, while ERαAF-20 females did not respond (Abot, et al. 2013; Billon-Gales et al. 2009; Billon-Gales et al. 2011). This indicates that the ERα AF-2 functional domain contributes to minimal uterine weight increase induced by E2 in the absence of AF-1. Both lines of AF-2 mutated animals (ERαG525L and AF2ERKI/KI) display severely hypoplastic uteri, and lack uterine growth response to E2 treatment (Arao et al. 2011; Billon-Gales et al. 2011; Sinkevicius et al. 2008). Interestingly, uterine wet weight can be increased by using the synthetic ERα agonist PPT in ERαG525L or by using the ER antagonists ICI 182,780 or tamoxifen in AF2ERKI/KI females (Arao et al. 2011; Sinkevicius et al. 2008). The ability of the antagonists to mediate responses seems to be due to a unique conformation of the LBD of the AF2ER that leads to AF-1-dependent transcriptional activity (Arao et al. 2013; Arao et al. 2011). Arao et al. also demonstrated that the uterine response to ICI or tamoxifen includes increased DNA synthesis in the uterine epithelial cells of AF2ERKI/KI (Arao et al. 2011). The growth factor IGF-1 induced minimal uterine epithelial proliferation in ERαG525L, and was not seen in AF2ERKI/KI uteri (Arao et al. 2011; Sinkevicius et al. 2008). Together, these findings indicated that both AF-1 and AF-2 activation domains of ERα contribute to a normal regulation of the complete biological response of uterine growth and reproductive functions. As the AF domains mediate ER-co-regulator interaction (Table 1), this emphasizes the importance of effective ERα co-activator protein recruitment for successful uterine E2 response. Similarly, mice lacking sufficient SRC-1 co-activator (SRC1−/−), exhibit measurably diminished uterine response to E2 (Xu, et al. 1998).
Mice with altered localization of ERα
A mutated mouse ERα that remains sequestered outside the nucleus (ERαH2NES), is unable to mediate transcriptional responses in a cell based assay, but maintains estrogen induced MAPK phosphorylation (Burns, et al. 2011). Targeting steroid receptors to the membrane involves palmitoylation, which is facilitated by HSP27 (Levin 2011). The palmitoylation promotes interaction with caveolin-1, which then results in localization of the receptor in membrane caveolin rafts. Two laboratories have mutated the palmitoylation site of the mouse ERα, and created knock in mouse models to study the effect of disabling this mechanism in vivo (Adlanmerini, et al. 2014; Pedram, et al. 2014). Both mouse lines have ovarian defects, but differ in several aspects (Table 2). Both involved knocking in an ERα with the same mutation of cysteine 451 to alanine. The first, C451A-ERα, exhibits normal uterine development and E2 induced growth response (Adlanmerini et al. 2014), whereas the nuclear-only ERα [NOER] has a hypoplastic ERα-null like uterus that fails to respond to E2 (Pedram et al. 2014). Both models have elevation in LH, but only the NOER has elevated E2. These mixed results remain to be reconciled to definitively illustrate the role of membrane associated ERα in these physiological systems.
Conclusions
Female reproduction is a complex staged series of physiological responses occurring in multiple organ systems activated by estrogen and estrogen receptors. Cell based studies have uncovered that cellular signaling mechanisms for ER are multifaceted regarding gene regulation. Because of the complexity with what is known about female reproduction and fertility, the mechanisms and activities cannot be clearly studied or tested in cell based systems. The development of gene targeting has allowed the evaluation of the physiological roles of estrogen action and estrogen receptor functionality under natural biological conditions. It is now apparent from the experimental and clinical reports outlined in this review that the primary mediator of female reproduction is ERα. What functional aspects of the ERα action are required will be forthcoming with the continued use of new technologies and experimental approaches, which will lead to a better understanding for the potential origins of infertility, reproductive tract disease and development of reproductive therapeutics.
Acknowledgements
Research support for studies reported in this review was provided by the Division of Intramural Research of the NIEHS/NIH 1ZIAES070065 to KSK.
Footnotes
Declaration of interest: The authors have no conflict of interest.
References
- Aagaard MM, Siersbaek R, Mandrup S. Molecular basis for gene-specific transactivation by nuclear receptors. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2011;1812:824–835. doi: 10.1016/j.bbadis.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Berges H, Laurell H, Gourdy P, et al. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154:2222–2233. doi: 10.1210/en.2012-2059. [DOI] [PubMed] [Google Scholar]
- Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessieres E, Kim SH, et al. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A. 2014;111:E283–290. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlbory-Dieker DL, Stride BD, Leder G, Schkoldow J, Trolenberg S, Seidel H, Otto C, Sommer A, Parker MG, Schutz G, et al. DNA binding by estrogen receptor-alpha is essential for the transcriptional response to estrogen in the liver and the uterus. Molecular Endocrinology. 2009;23:1544–1555. doi: 10.1210/me.2009-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade PM, Silva I, Borra RC, de LGR, Baracat EC. Estrogen regulation of uterine genes in vivo detected by complementary DNA array. Hormone and Metabolic Research. 2002;34:238–244. doi: 10.1055/s-2002-32136. [DOI] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ER beta-null mutant. Proc Natl Acad Sci U S A. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonson P, Omoto Y, Humire P, Gustafsson JA. Generation of ERalpha-floxed and knockout mice using the Cre/LoxP system. Biochem Biophys Res Commun. 2012;424:710–716. doi: 10.1016/j.bbrc.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Arao Y, Hamilton KJ, Coons LA, Korach KS. Estrogen receptor alpha L543A, L544A mutation changes antagonists to agonists which correlates with the ligand binding domain dimerization associated with DNA binding activity. J Biol Chem. 2013 doi: 10.1074/jbc.M113.463455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arao Y, Hamilton KJ, Ray MK, Scott G, Mishina Y, Korach KS. Estrogen receptor alpha AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc Natl Acad Sci USA. 2011;108:14986–14991. doi: 10.1073/pnas.1109180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi MK, Mantena SR, Kannan A, Bagchi IC. Role of C/EBP beta in steroid-induced cell proliferation and differentiaion in the uterus: Functional implications for establishment of early pregnancy. Placenta. 2006;27:A13–A13. doi: 10.4161/cc.5.9.2712. [DOI] [PubMed] [Google Scholar]
- Biddie SC, John S, Hager GL. Genome-wide mechanisms of nuclear receptor action. Trends Endocrinol Metab. 2010;21:3–9. doi: 10.1016/j.tem.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon-Gales A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, et al. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17 beta-estradiol. Proc Natl Acad Sci U S A. 2009;106:2053–2058. doi: 10.1073/pnas.0808742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon-Gales A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, et al. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci U S A. 2011;108:13311–13316. doi: 10.1073/pnas.1105632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder AK, Rodriguez KF, Hamilton KJ, Stockton PS, Reed CE, Korach KS. The absence of ER-beta results in altered gene expression in ovarian granulosa cells isolated from in vivo preovulatory follicles. Endocrinology. 2013;154:2174–2187. doi: 10.1210/en.2012-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder AK, Winuthayanon W, Hewitt SC, Couse JF, Korach KS. Knobil and Neill's Physiology of Reproduction. Elsevier; Eds TM Plant & AJ Zeleznik: 2015. Steroid Receptors in the Uterus and Ovary. pp. 1099–1193. [Google Scholar]
- Brelivet Y, Rochel N, Moras D. Structural analysis of nuclear receptors: From isolated domains to integral proteins. Mol Cell Endocrinol. 2012;348:466–473. doi: 10.1016/j.mce.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Dyson M, Wreford NG, Jones MEE, Simpson ER, Findlay JK. The ovarian phenotype of the aromatase knockout (ArKO) mouse. Journal of Steroid Biochemistry and Molecular Biology. 2001;79:181–185. doi: 10.1016/s0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Britt KL, Kerr J, O'Donnell L, Jones ME, Drummond AE, Davis SR, Simpson ER, Findlay JK. Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1389–1397. doi: 10.1096/fj.01-0992com. [DOI] [PubMed] [Google Scholar]
- Bulynko YA, O'Malley BW. Nuclear Receptor Coactivators: Structural and Functional Biochemistry. Biochemistry. 2011;50:313–328. doi: 10.1021/bi101762x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Li Y, Arao Y, Petrovich RM, Korach KS. Selective mutations in estrogen receptor alpha D-domain alters nuclear translocation and non-estrogen response element gene regulatory mechanisms. J Biol Chem. 2011;286:12640–12649. doi: 10.1074/jbc.M110.187773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20:1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Cheung E, Kraus WL. Genomic Analyses of Hormone Signaling and Gene Regulation. Annu Rev Physiol. 2010;72:191–218. doi: 10.1146/annurev-physiol-021909-135840. [DOI] [PubMed] [Google Scholar]
- Couse J. Reproductive phenotypes in estrogen receptor knockout mice: Contrasting roles for estrogen receptor-alpha and estrogen receptor-beta. Biology of Reproduction. 1999;60:88–88. [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Molecular Endocrinology. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Curtis SH, Korach KS, and Signaling. In Hormones. Academic Press; Ed BW O'Malley. San Diego: 1999. Steroid receptor knockout models: Phenotypes and responses illustrate interactions between receptor signaling pathways in vivo. pp. 357–380. [DOI] [PubMed] [Google Scholar]
- Deblois G, Giguere V. Nuclear receptor location analyses in mammalian genomes: from gene regulation to regulatory networks. Mol Endocrinol. 2008;22:1999–2011. doi: 10.1210/me.2007-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Farnham PJ. Insights from genomic profiling of transcription factors. Nat Rev Genet. 2009;10:605–616. doi: 10.1038/nrg2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck KC, Eckel JE, Gennings C, Zacharewski TR. Identification of temporal patterns of gene expression in the uteri of immature, ovariectomized mice following exposure to ethynylestradiol. Physiological genomics. 2003;15:127–141. doi: 10.1152/physiolgenomics.00058.2003. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EM, Andrade J, Shupnik MA. Novel actions of estrogen to promote proliferation: Integration of cytoplasmic and nuclear pathways. Steroids. 2009;74:622–627. doi: 10.1016/j.steroids.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Huang C, Schiff R. More on FOX News: FOXA1 on the horizon of estrogen receptor function and endocrine response. Breast Cancer Res. 2011;13:307. doi: 10.1186/bcr2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Ma X, Ostmann AB, Das SK. GPR30 activation opposes estrogen-dependent uterine growth via inhibition of stromal ERK1/2 and estrogen receptor alpha (ERalpha) phosphorylation signals. Endocrinology. 2011;152:1434–1447. doi: 10.1210/en.2010-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Dahlman-Wright K. The gene regulatory networks controlled by estrogens. Mol Cell Endocrinol. 2011;334:83–90. doi: 10.1016/j.mce.2010.09.002. [DOI] [PubMed] [Google Scholar]
- George CL, Lightman SL, Biddie SC. Transcription factor interactions in genomic nuclear receptor function. Epigenomics. 2011;3:471–485. doi: 10.2217/epi.11.66. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Saunders PT. Estrogen dependent signaling in reproductive tissues - a role for estrogen receptors and estrogen related receptors. Mol Cell Endocrinol. 2012;348:361–372. doi: 10.1016/j.mce.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Gilfillan S, Fiorito E, Hurtado A. Functional genomic methods to study estrogen receptor activity. J Mammary Gland Biol Neoplasia. 2012;17:147–153. doi: 10.1007/s10911-012-9254-4. [DOI] [PubMed] [Google Scholar]
- Green CD, Han JDJ. Epigenetic regulation by nuclear receptors. Epigenomics. 2011;3:59–72. doi: 10.2217/epi.10.75. [DOI] [PubMed] [Google Scholar]
- Grontved L, Hager GL. Impact of chromatin structure on PR signaling: transition from local to global analysis. Mol Cell Endocrinol. 2012;357:30–36. doi: 10.1016/j.mce.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Helsen C, Kerkhofs S, Clinckemalie L, Spans L, Laurent M, Boonen S, Vanderschueren D, Claessens F. Structural basis for nuclear hormone receptor DNA binding. Mol Cell Endocrinol. 2012;348:411–417. doi: 10.1016/j.mce.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Collins J, Grissom S, Deroo B, Korach KS. Global uterine genomics in vivo: microarray evaluation of the estrogen receptor alpha-growth factor cross-talk mechanism. Molecular Endocrinology. 2005;19:657–668. doi: 10.1210/me.2004-0142. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Kissling GE, Fieselman KE, Jayes FL, Gerrish KE, Korach KS. Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010a;24:4660–4667. doi: 10.1096/fj.10-163428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Li L, Grimm SA, Chen Y, Liu L, Li Y, Bushel PR, Fargo D, Korach KS. Research Resource: Whole-Genome Estrogen Receptor alpha Binding in Mouse Uterine Tissue Revealed by ChIP-Seq. Mol Endocrinol. 2012;26:887–898. doi: 10.1210/me.2011-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Li L, Grimm SA, Winuthayanon W, Hamilton KJ, Pockette B, Rubel CA, Pedersen LC, Fargo D, Lanz RB, et al. Novel DNA Motif Binding Activity Observed In Vivo With an Estrogen Receptor alpha Mutant Mouse. Molecular Endocrinology. 2014;28:899–911. doi: 10.1210/me.2014-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Li Y, Li L, Korach KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem. 2010b;285:2676–2685. doi: 10.1074/jbc.M109.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, O'Brien JE, Jameson JL, Kissling GE, Korach KS. Selective Disruption of ER alpha DNA-Binding Activity Alters Uterine Responsiveness to Estradiol. Molecular Endocrinology. 2009;23:2111–2116. doi: 10.1210/me.2009-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilser VJ, Thompson EB. Structural Dynamics, Intrinsic Disorder, and Allostery in Nuclear Receptors as Transcription Factors. Journal of Biological Chemistry. 2011;286:39675–39682. doi: 10.1074/jbc.R111.278929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Hong S, Young Nah H, Yoon Lee J, Chan Gye M, Hoon Kim C, Kyoo Kim M. Analysis of estrogen-regulated genes in mouse uterus using cDNA microarray and laser capture microdissection. J Endocrinol. 2004;181:157–167. doi: 10.1677/joe.0.1810157. [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Park SH, Choi KC, Leung PC, Jeung EB. Identification of estrogen-regulated genes by microarray analysis of the uterus of immature rats exposed to endocrine disrupting chemicals. Reproductive biology and endocrinology : RB&E. 2006;4:49. doi: 10.1186/1477-7827-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia EY, Goodson ML, Zou JX, Privalsky ML, Chen HW. Nuclear receptor coregulators as a new paradigm for therapeutic targeting. Adv Drug Deliv Rev. 2010;62:1227–1237. doi: 10.1016/j.addr.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7:e44285. doi: 10.1371/journal.pone.0044285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PX, Chandra V, Rastinejad F. Structural Overview of the Nuclear Receptor Superfamily: Insights into Physiology and Therapeutics. Annu Rev Physiol. 2010;72:247–272. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- Johnson AB, O'Malley BW. Steroid receptor coactivators 1, 2, and 3: Critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol. 2012;348:430–439. doi: 10.1016/j.mce.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Choi IH, Delage-Mourroux R, Ediger TR, Martini PGV, Montano M, Sun J, Weis K, Katzenellenbogen JA. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. Journal of Steroid Biochemistry and Molecular Biology. 2000;74:279–285. doi: 10.1016/s0960-0760(00)00104-7. [DOI] [PubMed] [Google Scholar]
- Kim H, Ku SY, Sung JJ, Kim SH, Choi YM, Kim JG, Moon SY. Association between hormone therapy and nerve conduction study parameters in postmenopausal women. Climacteric. 2011;14:488–491. doi: 10.3109/13697137.2011.553972. [DOI] [PubMed] [Google Scholar]
- Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20:1479–1493. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide A, Zhao C, Naganuma M, Abrams J, Deighton-Collins S, Skafar DF, Koide S. Identification of regions within the F domain of the human estrogen receptor alpha that are important for modulating transactivation and protein-protein interactions. Mol Endocrinol. 2007;21:829–842. doi: 10.1210/me.2006-0203. [DOI] [PubMed] [Google Scholar]
- Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science. 1994;266:1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, et al. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent progress in hormone research. 1996;51:159–186. discussion 186-158. [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, McEwan IJ. Allosteric Modulators of Steroid Hormone Receptors: Structural Dynamics and Gene Regulation. Endocr Rev. 2012;33:271–299. doi: 10.1210/er.2011-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Zakharov MN, Khan SH, Miki R, Jang H, Toraldo G, Singh R, Bhasin S, Jasuja R. The dynamic structure of the estrogen receptor. J Amino Acids. 2011;2011:812540. doi: 10.4061/2011/812540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. Journal of Steroid Biochemistry and Molecular Biology. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. 2010:603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Laudet V, Gronemeyer H. The Nuclear Receptor FactsBook. Academic Press; 2001. [Google Scholar]
- Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir JM, Corbo L. Cracking the estrogen receptor's posttranslational code in breast tumors. Endocr Rev. 2011;32:597–622. doi: 10.1210/er.2010-0016. [DOI] [PubMed] [Google Scholar]
- Levin ER. Minireview: Extranuclear Steroid Receptors: Roles in Modulation of Cell Functions. Molecular Endocrinology. 2011;25:377–384. doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, O'Malley BW. Expanding functional diversity of the coactivators. Trends Biochem Sci. 2005;30:126–132. doi: 10.1016/j.tibs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Krum SA, Rhodes DR, Liu XS, Brown M. Coactivator Function Defines the Active Estrogen Receptor Alpha Cistrome. Molecular and Cellular Biology. 2009;29:3413–3423. doi: 10.1128/MCB.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. Genomic Collaboration of Estrogen Receptor alpha and Extracellular Signal-Regulated Kinase 2 in Regulating Gene and Proliferation Programs. Molecular and Cellular Biology. 2011;31:226–236. doi: 10.1128/MCB.00821-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantena SR, Kannan A, Cheon YP, Li Q, Johnson PF, Bagchi IC, Bagchi MK. C/EBPbeta is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci U S A. 2006;103:1870–1875. doi: 10.1073/pnas.0507261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JH, Rao NA, Stunnenberg HG. Genome-wide interplay of nuclear receptors with the epigenome. Biochim Biophys Acta. 2011;1812:818–823. doi: 10.1016/j.bbadis.2010.10.005. [DOI] [PubMed] [Google Scholar]
- McEwan IJ. Molecular mechanisms of androgen receptor-mediated gene regulation: structure-function analysis of the AF-1 domain. Endocr Relat Cancer. 2004;11:281–293. doi: 10.1677/erc.0.0110281. [DOI] [PubMed] [Google Scholar]
- Meyer CA, Tang Q, Liu XS. Minireview: applications of next-generation sequencing on studies of nuclear receptor regulation and function. Mol Endocrinol. 2012;26:1651–1659. doi: 10.1210/me.2012-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: Structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Moggs JG, Tinwell H, Spurway T, Chang HS, Pate I, Lim FL, Moore DJ, Soames A, Stuckey R, Currie R, et al. Phenotypic anchoring of gene expression changes during estrogen-induced uterine growth. Environmental Health Perspectives. 2004;112:1589–1606. doi: 10.1289/txg.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa MK, Medrano TI, March AG, Cooke PS. Neonatal uterine and vaginal cell proliferation and adenogenesis are independent of estrogen receptor 1 (ESR1) in the mouse. Biology of Reproduction. 2015;92:78. doi: 10.1095/biolreprod.114.125724. [DOI] [PubMed] [Google Scholar]
- O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- O'Malley BW, Malovannaya A, Qin J. Minireview: nuclear receptor and coregulator proteomics--2012 and beyond. Mol Endocrinol. 2012;26:1646–1650. doi: 10.1210/me.2012-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Deng Y, Pollard JW. Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci U S A. 2006;103:14021–14026. doi: 10.1073/pnas.0601271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nature reviews. Genetics. 2009;10:669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar S, Laws MJ, Bagchi IC, Bagchi MK. Uterine Epithelial Estrogen Receptor-alpha Controls Decidualization via a Paracrine Mechanism. Mol Endocrinol. 2015;29:1362–1374. doi: 10.1210/me.2015-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-Localized Estrogen Receptor alpha Is Required for Normal Organ Development and Function. Developmental cell. 2014;29:482–490. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nature Reviews Endocrinology. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaynor SD, Stradtman EW, Jr., Kim HG, Shen Y, Chorich LP, Schreihofer DA, Layman LC. Delayed puberty and estrogen resistance in a woman with estrogen receptor alpha variant. The New England journal of medicine. 2013;369:164–171. doi: 10.1056/NEJMoa1303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramathal C, Bagchi IC, Bagchi MK. Lack of CCAAT enhancer binding protein beta (C/EBPbeta) in uterine epithelial cells impairs estrogen-induced DNA replication, induces DNA damage response pathways, and promotes apoptosis. Molecular and Cellular Biology. 2010;30:1607–1619. doi: 10.1128/MCB.00872-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Pollard JW. KLF15 negatively regulates estrogen-induced epithelial cell proliferation by inhibition of DNA replication licensing. Proc Natl Acad Sci U S A. 2012;109:E1334–1343. doi: 10.1073/pnas.1118515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi MA, Dhakal P, Kubota K, Chakraborty D, Lei T, Larson MA, Wolfe MW, Roby KF, Vivian JL, Soares MJ. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 2014;155:1991–1999. doi: 10.1210/en.2013-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Kim K. Nuclear receptor-mediated transactivation through interaction with Sp proteins. Prog Nucleic Acid Res Mol Biol. 2004;77:1–36. doi: 10.1016/S0079-6603(04)77001-4. [DOI] [PubMed] [Google Scholar]
- Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 2008;41:263–275. doi: 10.1677/JME-08-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Askew GR, Dellovade TL, Merchenthaler I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, et al. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149:2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N-Engl-J-Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Takeo T, Sakuma Y. Diametrically opposite effects of estrogen on the excitability of female rat medial and lateral preoptic neurons with axons to the midbrain locomotor region. Neurosci Res. 1995;22:73–80. doi: 10.1016/0168-0102(95)00885-w. [DOI] [PubMed] [Google Scholar]
- Tang Q, Chen Y, Meyer C, Geistlinger T, Lupien M, Wang Q, Liu T, Zhang Y, Brown M, Liu XS. A comprehensive view of nuclear receptor cancer cistromes. Cancer Res. 2011;71:6940–6947. doi: 10.1158/0008-5472.CAN-11-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda K, Takeda K, Okada T, Akira S, Saibara T, Kaname T, Yamamura K, Onishi S, Shizuta Y. Targeted disruption of the aromatase P450 gene (Cyp19) in mice and their ovarian and uterine responses to 17beta-oestradiol. J Endocrinol. 2001;170:99–111. doi: 10.1677/joe.0.1700099. [DOI] [PubMed] [Google Scholar]
- Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta−/− mice. Proc Natl Acad Sci U S A. 2006;103:18350–18355. doi: 10.1073/pnas.0608861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker VR, Korach KS. ILAR journal / National Research Council. Vol. 45. Institute of Laboratory Animal Resources; 2004. Estrogen receptor knockout mice as a model for endocrine research. pp. 455–461. [DOI] [PubMed] [Google Scholar]
- Wall EH, Hewitt SC, Case LK, Lin CY, Korach KS, Teuscher C. The role of genetics in estrogen responses: a critical piece of an intricate puzzle. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:5042–5054. doi: 10.1096/fj.14-260307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Suzuki A, Kobayashi M, Takahashi E, Itamoto M, Lubahn DB, Handa H, Iguchi T. Analysis of temporal changes in the expression of estrogen-regulated genes in the uterus. J Mol Endocrinol. 2003;30:347–358. doi: 10.1677/jme.0.0300347. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Saji S, Makinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A. 2000;97:5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Korach KS. Uterine epithelial cell estrogen receptor alpha-dependent and -independent genomic profiles that underlie estrogen responses in mice. Biology of Reproduction. 2014;91:110. doi: 10.1095/biolreprod.114.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- Yang J, Singleton DW, Shaughnessy EA, Khan SA. The F-domain of estrogen receptor-alpha inhibits ligand induced receptor dimerization. Mol Cell Endocrinol. 2008;295:94–100. doi: 10.1016/j.mce.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Yi P, Wang Z, Feng Q, Pintilie GD, Foulds CE, Lanz RB, Ludtke SJ, Schmid MF, Chiu W, O'Malley BW. Structure of a biologically active estrogen receptor-coactivator complex on DNA. Mol Cell. 2015;57:1047–1058. doi: 10.1016/j.molcel.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;25:552–563. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- Binder AK, Winuthayanon W, Hewitt SC, Couse JF, Korach KS. Steroid Receptors in the Uterus and Ovary. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction. Elsevier; 2015. pp. 1099–1193. [Google Scholar]
- Conaway RC, Conaway JW. Function and regulation of the Mediator complex. Current opinion in genetics & development. 2011;21:225–230. doi: 10.1016/j.gde.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia EY, Goodson ML, Zou JX, Privalsky ML, Chen HW. Nuclear receptor coregulators as a new paradigm for therapeutic targeting. Adv Drug Deliv Rev. 2010;62:1227–1237. doi: 10.1016/j.addr.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AB, O'Malley BW. Steroid receptor coactivators 1, 2, and 3: Critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol. 2012;348:430–439. doi: 10.1016/j.mce.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler BR, Archer TK, Kinyamu HK. Emerging roles of the 26S proteasome in nuclear hormone receptor-regulated transcription. Biochimica Et Biophysica Acta-Gene Regulatory Mechanisms. 2011;1809:109–118. doi: 10.1016/j.bbagrm.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Yu Y, Cheng Y. Structure characterization of the 26S proteasome. Biochim Biophys Acta. 2011;1809:67–79. doi: 10.1016/j.bbagrm.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Roeder RG. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nature Reviews Genetics. 2010;11:761–772. doi: 10.1038/nrg2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Orkin SH. The SWI/SNF complex--chromatin and cancer. Nature reviews. Cancer. 2004;4:133–142. doi: 10.1038/nrc1273. [DOI] [PubMed] [Google Scholar]
- Wu SC, Zhang Y. Minireview: role of protein methylation and demethylation in nuclear hormone signaling. Molecular Endocrinology. 2009;23:1323–1334. doi: 10.1210/me.2009-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Berges H, Laurell H, Gourdy P, et al. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154:2222–2233. doi: 10.1210/en.2012-2059. [DOI] [PubMed] [Google Scholar]
- Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessieres E, Kim SH, et al. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A. 2014;111:E283–290. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlbory-Dieker DL, Stride BD, Leder G, Schkoldow J, Trolenberg S, Seidel H, Otto C, Sommer A, Parker MG, Schutz G, et al. DNA binding by estrogen receptor-alpha is essential for the transcriptional response to estrogen in the liver and the uterus. Molecular Endocrinology. 2009;23:1544–1555. doi: 10.1210/me.2009-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ER beta-null mutant. Proc Natl Acad Sci U S A. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonson P, Omoto Y, Humire P, Gustafsson JA. Generation of ERalpha-floxed and knockout mice using the Cre/LoxP system. Biochem Biophys Res Commun. 2012;424:710–716. doi: 10.1016/j.bbrc.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Arao Y, Hamilton KJ, Ray MK, Scott G, Mishina Y, Korach KS. Estrogen receptor alpha AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc Natl Acad Sci USA. 2011;108:14986–14991. doi: 10.1073/pnas.1109180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon-Gales A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, et al. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17 beta-estradiol. Proc Natl Acad Sci U S A. 2009;106:2053–2058. doi: 10.1073/pnas.0808742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon-Gales A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, et al. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci U S A. 2011;108:13311–13316. doi: 10.1073/pnas.1105632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder AK, Winuthayanon W, Hewitt SC, Couse JF, Korach KS. Steroid Receptors in the Uterus and Ovary. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction. Elsevier; 2015. pp. 1099–1193. [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Curtis Hewitt S, Goulding EH, Eddy EM, Korach KS. Studies using the estrogen receptor alpha knockout uterus demonstrate that implantation but not decidualization-associated signaling is estrogen dependent. Biology of Reproduction. 2002;67:1268–1277. doi: 10.1095/biolreprod67.4.1268. [DOI] [PubMed] [Google Scholar]
- Curtis SW, Clark J, Myers P, Korach KS. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor alpha knockout mouse uterus. Proc Natl Acad Sci U S A. 1999;96:3646–3651. doi: 10.1073/pnas.96.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Kissling GE, Fieselman KE, Jayes FL, Gerrish KE, Korach KS. Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010a;24:4660–4667. doi: 10.1096/fj.10-163428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Li Y, Li L, Korach KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem. 2010b;285:2676–2685. doi: 10.1074/jbc.M109.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- Pawar S, Laws MJ, Bagchi IC, Bagchi MK. Uterine Epithelial Estrogen Receptor-alpha Controls Decidualization via a Paracrine Mechanism. Mol Endocrinol. 2015;29:1362–1374. doi: 10.1210/me.2015-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-Localized Estrogen Receptor alpha Is Required for Normal Organ Development and Function. Developmental cell. 2014;29:482–490. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, et al. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149:2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda K, Takeda K, Okada T, Akira S, Saibara T, Kaname T, Yamamura K, Onishi S, Shizuta Y. Targeted disruption of the aromatase P450 gene (Cyp19) in mice and their ovarian and uterine responses to 17beta-oestradiol. J Endocrinol. 2001;170:99–111. doi: 10.1677/joe.0.1700099. [DOI] [PubMed] [Google Scholar]
- Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta−/− mice. Proc Natl Acad Sci U S A. 2006;103:18350–18355. doi: 10.1073/pnas.0608861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Korach KS. Uterine epithelial cell estrogen receptor alpha-dependent and -independent genomic profiles that underlie estrogen responses in mice. Biology of Reproduction. 2014;91:110. doi: 10.1095/biolreprod.114.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]