Abstract
Background
Bisphosphonates are the most widely used treatment for osteoporosis. They accumulate in the bone for years, and therefore, their inhibitory effects on osteoclasts may persist after drug discontinuation. The ideal duration of therapy remains controversial.
Questions/Purposes
The purpose of this study is to review the literature to determine the (1) indications for drug holiday, (2) the duration of drug holiday, (3) the evaluation during drug holiday, and (4) the proper treatment and maintenance after drug holiday.
Methods
A review of two electronic databases (PubMed/MEDLINE and EMBASE) was conducted using the term “(Drug holiday),” in January 29, 2015. Inclusion criteria were as follows: (1) clinical trials and case control, (2) human studies, (3) published in a peer-review journal, and (4) written in English. Exclusion criteria were as follows: (1) case reports, (2) case series, and (3) in vitro studies.
Results
The literature supports a therapeutic pause after 3–5 years of bisphosphonate treatment in patients with minor bone deficiencies and no recent fragility fracture (low risk) and in patients with moderate bone deficiencies and/or recent fragility fracture (moderate risk). In these patients, a bone health reevaluation is recommended every 1–3 years. Patients with high fracture risk should be maintained on bisphosphonate therapy without drug holiday.
Conclusion
The duration and length of drug holiday should be individualized for each patient. Evaluation should be based on serial bone mass measurements, bone turnover rates, and fracture history evaluation. If after drug therapy, assessments show an increased risk of fracture, the patient may benefit from initiating another treatment. Raloxifene, teriparatide, or denosumab are available options.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-015-9469-1) contains supplementary material, which is available to authorized users.
Keywords: osteoporosis, bisphosphonates, drug holiday
Introduction
Osteoporosis is a chronic metabolic disease characterized by an increase in bone turnover, progressive loss of bone mass, microarchitectural deterioration, and increased fracture risk [7]. It is predominantly diagnosed using bone mineral density (BMD) techniques; however, BMD alone does not predict fracture risk. In 2008, the WHO developed the Fracture Risk Assessment Tool (FRAX) index to predict the risk of osteoporotic fracture; it reports the 10-year risk of hip fracture and the 10-year risk of major osteoporotic fracture using the patient’s own clinical risk factors. Currently, the National Osteoporosis Foundation recommends treatment for patients who have had a previous osteoporotic fracture, patients with a BMD T score of <−2.5, and patients over the age of 50 years who have low bone mass (T score −1.0 to −2.5) and a risk probability of >3% for hip fracture or >20% for major osteoporotic fracture as obtained using the FRAX algorithm [41]. Bisphosphonates are still the most widely used pharmacologic treatment for osteoporosis [64]. They bind to bone hydroxyapatite, impair the osteoclast ability to resorb bone, induce osteoclast apoptosis, and increase BMD reducing the risk of fractures by 50–70% [5, 19, 22, 44]. Bisphosphonates accumulate in the bone, and therefore, their inhibitory effects on osteoclasts may persist for years after drug discontinuation [7, 34]. This mechanism has led to controversy regarding the ideal duration of therapy and whether the drug provides protection after being discontinued [20, 54]. There has been limited data addressing the benefits of this type of “drug holiday,” and little is known regarding the initiation and duration of the holiday. In addition, controversy exists regarding if and when bisphosphonates should be resumed or whether another treatment option should be explored [39].
The purpose of this current study was to review the literature to evaluate the clinical evidence to determine the following: (1) when drug holiday is indicated, (2) the duration of drug holiday, (3) the follow-up during drug holiday, and (4) the proper treatment and maintenance after drug holiday.
Methods
Search Strategy and Criteria
A review of two electronic databases (PubMed/MEDLINE and EMBASE) was conducted using the following search term: “(Drug holiday)” in January 29, 2015. No filters were applied. The search parameters yielded to 887 records. After duplicates were removed and records were screened by title, abstract, and full text, 65 records were included. Reference lists of selected articles were also reviewed to ensure that no pertinent articles were omitted; this yielded to 30 more records for a total of 95 records.
Inclusion criteria were as follows: (1) clinical trials and case control, (2) human studies, (3) published in a peer-review journal, and (4) written in English. Exclusion criteria were as follows: (1) case reports, (2) case series, and (3) in vitro studies.
Results/Discussion
Bisphosphonates: Efficacy of Treatment
FDA-approved bisphosphonates for the prevention and treatment of postmenopausal osteoporosis include the following: Fosamax (alendronate), Actonel (risedronate), Boniva (ibandronate), and Reclast (zoledronic acid). These have passed through phase III clinical trials demonstrating a statistically significant risk reduction in the incidence of vertebral fractures as well as other associated osteoporotic fractures [4]. The fracture risk reduction ranges from 47 to 70% for vertebral fractures [4, 5], 28 to 50% for hip fractures [5, 37], and 19 to 38% for other nonvertebral fractures [29]. In addition, they have all demonstrated improved BMD in postmenopausal women [2, 5, 19, 29, 45].
Long-Term Effects of Bisphosphonates
There has been controversy regarding the use of long-term bisphosphonate therapy. The Fracture Intervention Trial Long-term Extension (FLEX) compared the effects of continuing and discontinuing alendronate after 5 years of treatment. This study demonstrated no risk reduction of nonvertebral fractures after 10 years of treatment compared with treatment of 5 years followed by 5 years of placebo. In addition, despite the fact that the placebo group showed a decrease at total hip and spine BMD, mean values remained at or above pretreatment levels from 10 years earlier [7, 8]. These results suggest that discontinuation of alendronate for up to 5 years does not appear to significantly increase fracture risk; however, women at very high risk of clinical vertebral fractures may benefit by continuing beyond 5 years [7]. Sporensen et al. conducted a similar study investigating the effects of risedronate therapy on fracture risk after 5 years of treatment, demonstrating that the effects of risedronate over 3 years on vertebral fracture risk and BMD are maintained with 2 years of further treatment [58]. In the 7-year extension of this study, the incidence of new vertebral fractures during years 6 and 7 was similar for both treatment and placebo groups with overall maintenance of BMD at the femoral neck [40].
The HORIZON-PFT study evaluated the long-term effects of zoledronic acid (ZOL) for up to 6 years. The women who received ZOL for 3 years were randomized to three additional years of ZOL or placebo. The results were similar to those found in the FLEX study. In the ZOL group, the BMD and biochemical markers remained constant and new morphometric vertebral fractures were lower than in the placebo group, but there was no difference in the risk of other fractures, including hip fractures. They suggested that those with a higher vertebral fracture risk may benefit by continued treatment. This benefit has been not demonstrated for further prevention of hip fractures [5].
There is no clear benefit for overall osteoporotic fracture risk with continuing treatment over 5 years in patients with low risk of fracture, and patients with well-recognized risks for fracture (baseline history of fracture, age older than 70 years, and those remaining in the osteoporotic T-score range) could be more likely to fracture despite continued bisphosphonate therapy. These results suggest no significant advantage of continuing drug therapy beyond 5 years in low-risk fracture patients.
Safety: Why Stop?
Prescriptions for oral bisphosphonates for osteoporosis treatment and prevention have declined by 53% in a 4-year period (2008–2012). Similarly, the number of intravenous bisphosphonate packages sold for osteoporosis treatment has decreased by 22% from 2010 to 2012. This decline may be attributed to the concerns regarding the optimal treatment duration and potential safety risks of using long-term bisphosphonates [64]. Osteonecrosis of the jaw, atypical femoral fractures (AFFs), and esophageal cancer are the most common adverse effects reported to the FDA.
Osteonecrosis of the Jaw
According to the American Association of Oral and Maxillofacial Surgeons Task Force, to be considered bisphosphonate-related osteonecrosis of the jaw (BRONJ), all of the following criteria must be met the following: (1) exposed bone in the maxillofacial region that has persisted for more than 8 weeks, (2) current or previous treatment with a bisphosphonate, and (3) no history of radiation therapy to the jaw [41]. Although studies have demonstrated an increased risk of osteonecrosis of the jaw (ONJ) in patients with malignancy that received monthly intravenous bisphosphonates [14, 63, 65], the association between ONJ and oral bisphosphonates therapy for the prevention and treatment of osteoporosis is epidemiologically different. The Predicting Risk of Osteonecrosis with Bisphosphonate Exposure (PROBE) study reported an increase in the prevalence of ONJ with increased duration of exposure (highest with ≥4 years of treatment) [35]. ONJ may be attributed to bone formation suppression; therefore, ideal treatment should improve bone remodeling. Teriparatide, hyperbaric oxygen, low-level laser therapy, and growth factor therapy have all been used as alternatives alone or in combination with the conventional palliative treatment (antibiotics and/or surgical) [25, 33, 59]. Future research is needed to establish the association between bisphosphonates and ONJ [41].
Atypical Femoral Fractures
In 2014, the American Society for Bone and Mineral Research (ASBMR) Task Force published the revised AFF case definition: “the fracture must be located along the femoral diaphysis from just distal to the lesser trochanter to just proximal to the supracondylar flare. In addition, at least four of five major features must be present. None of the minor features is required but have sometimes been associated with these fractures” [55]. Major features include minimal or no associated trauma, fracture line originating in the lateral cortex with transverse or short oblique orientation, presence of a medial spike in complete fractures, noncomminution or minimal comminution, and periosteal or endosteal thickening of the lateral cortex at the fracture site. Minor features include generalized cortical thickening of the femoral diaphyses, prodromal symptoms such as pain in the groin or thigh, bilaterality, and delayed healing [55, 56]. The pathogenesis of AFFs remains unclear, but the current consensus is that AFFs are stress or insufficiency fractures that develop over time. Since bisphosphonates (BPs) localize in areas of developing stress fractures, they suppress the targeted intracortical remodeling at that site, thereby impairing the process by which stress fractures normally heal [55].
Previous studies report conflicting results on the association between atypical fractures and the use of BPs [2, 60]. Part of the discrepancy may be due to the variability of the defined outcomes, with higher risk of AFF in studies using the ASMBR criteria as compared to studies using mainly diagnosis codes [27]. In addition, an increase in the incidence of BP-associated AFF in patients who received treatment for more than 10 years compared with patients who underwent 6–8 years of treatment has been reported [21].
According to the 2014 Task Force, although there is robust evidence for an association, causality between BPs and AFF has not been established. The absolute risk for AFF is low, and there is no current consensus on the extent to which cumulative use of BPs increases the risk of this rare type of fracture. Therefore, while the benefit of BPs likely outweighs the risk of AFF early on in treatment, long-term therapy may be associated with higher risk [55].
Esophageal Cancer
Although evidence has been inconclusive, there has been concern about the association between long-term BP use and esophageal cancer [1]. Using the UK’s General Practice Research Database (GPRD), a retrospective study evaluating patients treated with BPs between 1996 and 2005 found no difference in esophageal cancer risk between the patients treated with BPs when compared with patients receiving no treatment [13]. Using the same database, a case control study by Green et al. found that the incidence of esophageal cancer increased in patients with multiple prescriptions for oral BPs and was significantly higher in patients who had ten or more prescriptions [28]. These studies are preliminary in nature, and therefore, there is no concrete evidence supporting an increased risk of esophageal cancer after BP use.
Treatment Options
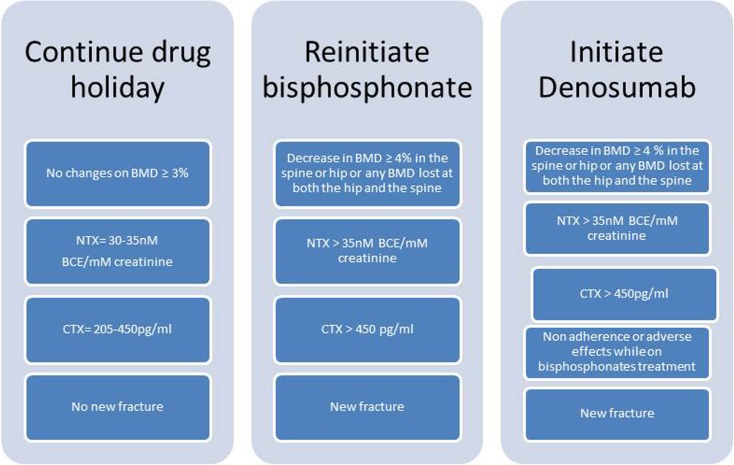
After initiation of BP therapy, it is recommended that patients should have a yearly physician follow-up. Medical and fracture history, physical examination, laboratory testing for osteoporosis and bone turnover markers (BTMs), bone densitometry (every 2 years), and vertebral imaging (if there is a decrease in height measurements or new back pain) should be performed at each follow-up visit. After thorough evaluation, the patient should be considered for one of the following treatments: (1) continuation of BP treatment, (2) initiation of a drug holiday, or (3) change in pharmacologic treatment (Fig. 1).
Fig. 1.
Osteoporosis treatment options [BMD bone mineral density, NTX N-telopeptide cross-links, CTX carboxyterminal collagen cross-link].
When Should Treatment Be Continued for More Than 3–5 Years?
Studies have shown an increased vertebral fracture risk in patients with ≥1 vertebral fractures at baseline independent of BMD and no vertebral fracture in patients with BMD-defined osteoporosis [3, 52]. Treatment should be continued in high-risk patients with the following: (1) low BMD (−2.5 or less), (2) history of a previous vertebral fracture, (3) ongoing high-dose glucocorticoid therapy, or (4) high bone turnover markers (BTMs) [19, 53, 61]. In these high-risk patients the benefits of long-term therapy outweigh the risks, and long-term treatment must not be denied because of possible late side effects. It is also recommended to continue treatment if the patient continues to show improvement in the BMD values and bone markers are not severely suppressed, type I collagen cross-linked N-telopeptide (NTX) <15.
Patients with a femoral neck T-score above −2.0 have a low risk of vertebral fracture and are unlikely to benefit from continued treatment. For those who have discontinued treatment with BPs, there appears to be no difference in fracture rates compared to those who continued active therapy. Physicians should critically reassess BMD and risk profile after 3–5 years of therapy to avoid treatment in patients at low risk [41].
When Should Drug Holiday Be Instituted?
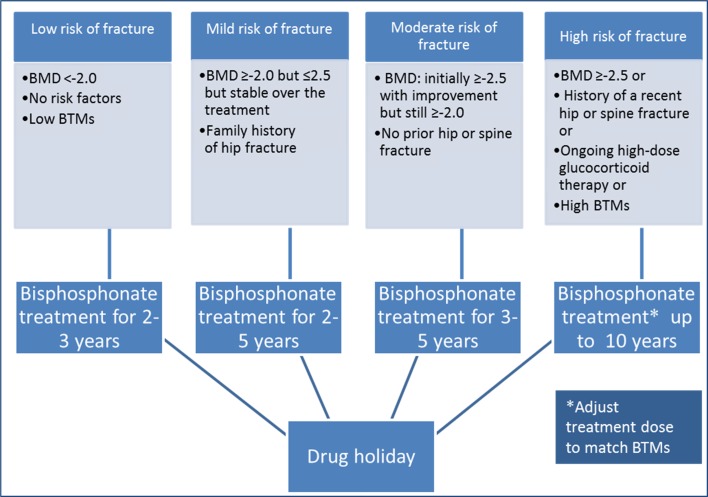
The goal of the drug holiday is to maintain the fracture risk at low level while off-therapy. Previous data has shown that the risk of morphometric vertebral fractures did not increase after discontinuing alendronate for up to 5 years, but the risk of nonvertebral fractures increased with lower baseline BMD or prevalent fracture [7, 52]. Thus, drug holidays should be recommended for patients taking alendronate with a lower risk of fractures, such as those with a BMD in the osteopenic range (T-score −1 to −2.5) or the normal range (T-score >−1.0) after 2–5 years of BP treatment and no history of fracture (Fig. 2) [51].
Fig. 2.
Duration of bisphosphonate treatment and indication of drug holiday [BMD bone mineral density, BTMs bone turnover markers].
Studies supporting drug holiday show that there is no increased risk of fractures, other than morphometric vertebral fractures, after stopping 3 years of treatment with either zoledronate or risedronate [6, 58]. There is no enough information about fracture risk after discontinuing ibandronate therapy.
An observational study to evaluate the risk for classical hip fractures (femoral neck and intertrochanteric fractures) after BP discontinuation using BMD and hip fracture end points showed an increased risk of classical hip fracture in women compliant with BP therapy (medication possession ratio 66–100%) for 2 years who subsequently discontinued, but not in women with higher compliance (MPR ≥80%) or those receiving treatment for at least 3 years. The authors suggest that a drug holiday after 2–3 years of therapy could be reasonably safe in terms of hip fracture risk for at least 1 year. For women who are compliant for a longer duration, extended periods of discontinuation could be safe [20]. The protection after therapy withdrawal decreases after 3 to 5 years of discontinuation [50]. For patients who have discontinued treatment for longer than 5 years, there is no data reporting an optimal time for reinitiation of treatment. The role of repeated assessment of BMD, BTMs, and other clinical indicators is currently being studied [3].
Drug holidays have also shown to improve the safety of long-term BP treatment. BPs localize preferably at sites of high bone turnover, as in the microcrack areas caused by overloading. If intracortical remodeling to repair the microcracks is suppressed as in BP treatment, they can evolve into a full fracture. Drug holidays instead may increase bone remodeling in those areas. This is suggested by a 70% decline in the risk of AFF one year after stopping BP treatment, and a decrease in the rate of contralateral femoral fractures if the BP treatment is stopped soon after an AFF has occurred [50, 55].
Monitoring Drug Holiday
There is no data reporting information on how to accurately monitor the drug holiday. In the absence of randomized clinical trials, empiric approaches are necessary [36]. Follow-up evaluating BMD, biochemical BTMs, and an updated assessment of fracture risk should be done ideally every year after risedronate treatment (lowest skeletal affinity and most rapid offset of effect on BMD and BTMs), every 1 to 2 years after alendronate treatment, and every 2 to 3 years after ZOL treatment (highest affinity and slowest offset of effect) [17, 57]. It has been shown that the response rates to antiresorptive therapy based on changes in BMD and BTMs, even among highly compliant patients, range approximately from 70 to 85% in randomized control trials [10].
Dual-energy X-ray absorptiometry (DXA) has been recommended as the preferred method to monitor changes in BMD during therapy, with the posteroanterior (PA) spine as the preferred site. A measured decline of ≤3% suggests stability or no real change in bone mass [11]. Changes of >3% at the PA spine and total hip are indicative of a clinically meaningful change [9, 12]. During antiresorptive treatment, there is a statistically significant relationship between increasing BMD and reduction of fracture risk [30]. Although this is true, other studies have shown that reductions in risk of fractures could be anticipated even if BMD did not increase or stays stable [61]. Failure of BP treatment occurs when there is ≥4% loss at the hip or spine in BMD or when there is any BMD loss at both the hip and the spine simultaneously [15]. Chiha et al. evaluated patients on drug holidays and observed no significant difference in mean lumbar spine BMD over 4 years of drug holiday but reported a statistically significant decline in the femoral neck BMD after 2 years [16].
Unfortunately, there have been a limited number of studies evaluating the association between BMD and fracture risk during drugs holidays. BTMs may provide additional data superior to those obtained from BMD alone, especially when BMD is hard to interpret like in elderly patients with spondylosis and recurrent fractures. The primary BTMs include the following: bone alkaline phosphatase, osteocalcin, C-terminal propeptides of type I collagen (CTX) and NTX. Urine bone resorption markers include breakdown products of type I collagen such as pyridonium cross-links (PYR and D-PYR), CTX and NTX. The level of these markers reflects rates of bone remodeling with an intraassay and interassay variability of less than 10% [24]. The level of the BTMs is effectively decreased by BP therapy in a pattern depending on the marker, the BP, the dose regimen, and the disease.
Although there are few studies that show the change in the level of BTMs after a drug holiday [62], the trends of these markers during drug holidays have not yet been established and it is not clear if BTMs predict the risk of future fracture. However, significant increases in BTMs suggest that the benefit of bisphosphonate therapy may be diminishing and may be an indication to return to active therapy. It is not clear when to restart treatment based on BTMs; an NTX value >35 nM bone collagen equivalent (BCE)/mM creatinine and a CTX value >450 pg/mL are levels to consider retreating the patient. In summary, reinitiation of BPs treatment should be considered in patients with high levels in BTMs, a significant decrease in BMD (>4%), or a new fracture event [41].
When Should We Consider a New Treatment?
If a patient has stopped BP therapy, but assessments show an increased risk of fractures, the patient may benefit from initiating another treatment. Options available are selective estrogen receptor modulators, hormone therapy, teriparatide, or denosumab [46]. Raloxifene is a selective estrogen receptor modulator that agonizes estrogen receptor activity in the bone and antagonizes estrogen receptor activity in the breast tissue. It is indicated to reduce the risk of invasive breast cancer in postmenopausal woman with osteoporosis and decrease the risk of vertebral fractures by 30% in patients with a prior vertebral fracture and by 55% in patients without a vertebral fracture over a 3-year study period. Reduction in the risk of nonvertebral fracture, however, has not been documented [18, 23, 32]. Hormone therapy is an alternative treatment considered primarily for women within the first few years of menopause. It has shown to reduce the risk of vertebral and hip fractures by 23% but should be used in the lowest effective doses for the shortest duration due to the reported increased risks of myocardial infarction, stroke, invasive breast cancer, pulmonary emboli, and deep vein thrombosis [47]. Consequently, the FDA does not include osteoporosis treatment in the label for estrogen. Parathyroid hormone (PTH 1-34), also known as Teriparatide, is the only anabolic agent approved for the treatment of osteoporosis in postmenopausal women, men at high risk of fracture, and for glucocorticoid therapy-associated osteoporosis [49]. Teriparatide reduces the risk of vertebral fractures by 65% and nonvertebral fractures by 53% after an average of 18 months of treatment. It is administered by daily subcutaneous injection and is contraindicated in patients with osteosarcoma, Paget’s disease, prior radiation therapy, bone metastases or skeletal malignancy, and hypercalcemia [42]. Randomized trials have demonstrated enhanced fracture healing and spine fusion [31, 43] It is recommended by the Task Force on AFFs to replace antiresorptive agents for those who appear not to heal on conservative therapy [55]. An alternative treatment that has gained attention is Denosumab. Denosumab is a monoclonal antibody that binds to receptor activator of nuclear factor kappa B ligand (RANKL) with high affinity and specificity blocking the interaction of RANKL with the receptor (RANK). RANKL is a protein expressed by osteoblastic stromal cells that mediates osteoclast differentiation, activation, and survival and is therefore responsible for osteoclast-mediated bone resorption [38]. Denosumab has been found to increase BMD in both trabecular bone and cortical bone as well as decrease bone turnover in treatment-naïve or previously alendronate-treated postmenopausal women [48]. This drug is administered every 6 months and therefore may be recommended for patients who were suboptimally adherent to BP therapy [26].
In conclusion, bisphosphonate therapy has significantly lowered the risk of vertebral and appendicular fragility fractures in osteoporotic patients. Current research now supports the development of a therapeutic pause after 3–5 years of treatment. In patients with minor bone deficiencies (T-scores between −1.5 and −2.0) and no recent fragility fracture, antiresorptive drug therapy can be discontinued. In patients with moderate bone deficiencies (T-scores between −2.0 and −2.5) and/or recent fragility fracture, drug therapy can be temporarily halted but bone health should be reevaluated every 1–3 years. Lastly, in patients with true osteoporosis (T-score <−2.5) who have improved bone density, continued treatment is recommended. The dosage should be adjusted according to BTM levels. An NTX <20 nM BCE/mM creatinine suggests suppression of bone resorption; therefore, lowering the dose of BPs should be considered. Serial DXAs and bone markers should be monitored annually for all patients. Reinstitution of drug therapy should be considered when there is a decline of DXA by 4–5% or a rise of bone marker levels above normal (urine NTX >35 nM BCE/mM creatinine, serum CTX >450 pg/mL). Patient-centered care based on bone mass, bone turnover rates, and fracture history should all be considered when determining the most effective treatment strategy. Actual recommendations could change depending on longer follow-up studies on BP treatment.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)
Compliance with Ethical Standards
Conflict of Interest
Jordan Villa, MD and Arianna Gianakos, BS have declared that they have no conflict of interest. Joseph M. Lane, MD, reports personal fees from Bone Therapeutics, Graftys, Agnovos, CollPlant, Harvest, ISTO, and Kuros, outside the work.
Human/Animal Rights
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
N/A
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Abrahamsen B, Eiken P, Eastell R. More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360(17):1789. doi: 10.1056/NEJMc096026. [DOI] [PubMed] [Google Scholar]
- 2.Anastasilakis AD, Polyzos SA, Gkiomisi A, Bisbinas I, Gerou S, Makras P. Comparative effect of zoledronic acid versus denosumab on serum sclerostin and dickkopf-1 levels of naive postmenopausal women with low bone mass: a randomized, head-to-head clinical trial. J Clin Endocrinol Metab. 2013;98(8):3206–3212. doi: 10.1210/jc.2013-1402. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long? N Engl J Med. 2012;366(22):2051–2053. doi: 10.1056/NEJMp1202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/S0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 5.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-pivotal fracture trial (PFT) J Bone Miner Res. 2012;27(2):243–254. doi: 10.1002/jbmr.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the fracture intervention trial long-term extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 8.Bone HG, Hosking D, Devogelaer JP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 9.Bonnick SL. HSA: beyond BMD with DXA. Bone. 2007;41(1 Suppl 1):S9–12. doi: 10.1016/j.bone.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Bonnick SL. Osteoporosis in men and women. Clin Cornerstone. 2006;8(1):28–39. doi: 10.1016/S1098-3597(06)80063-3. [DOI] [PubMed] [Google Scholar]
- 11.Bonnick SL, Johnston CC, Jr, Kleerekoper M, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4(2):105–110. doi: 10.1385/JCD:4:2:105. [DOI] [PubMed] [Google Scholar]
- 12.Bonnick SL, Shulman L. Monitoring osteoporosis therapy: bone mineral density, bone turnover markers, or both? Am J Med. 2006;119(4 Suppl 1):S25–31. doi: 10.1016/j.amjmed.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA. 2010;304(6):657–663. doi: 10.1001/jama.2010.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartsos VM, Zhu S, Zavras AI. Bisphosphonate use and the risk of adverse jaw outcomes: a medical claims study of 714,217 people. J Am Dent Assoc. 2008;139(1):23–30. doi: 10.14219/jada.archive.2008.0016. [DOI] [PubMed] [Google Scholar]
- 15.Chapurlat RD, Palermo L, Ramsay P, Cummings SR. Risk of fracture among women who lose bone density during treatment with alendronate. The Fracture Intervention Trial. Osteoporos Int. 2005;16(7):842–848. doi: 10.1007/s00198-004-1770-7. [DOI] [PubMed] [Google Scholar]
- 16.Chiha M, Myers LE, Ball CA, Sinacore JM, Camacho PM. Long-term follow-up of patients on drug holiday from bisphosphonates: real-world setting. Endocr Pract. 2013;19(6):989–994. doi: 10.4158/EP12425.OR. [DOI] [PubMed] [Google Scholar]
- 17.Compston JE, Bilezikian JP. Bisphosphonate therapy for osteoporosis: the long and short of it. J Bone Miner Res. 2012;27(2):240–242. doi: 10.1002/jbmr.1542. [DOI] [PubMed] [Google Scholar]
- 18.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the fracture intervention trial. JAMA. 1998;280(24):2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int. 2008;19(11):1613–1620. doi: 10.1007/s00198-008-0604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27(12):2544–2550. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- 22.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple outcomes of raloxifene evaluation (MORE) investigators. JAMA. 1999;282(7):637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 24.Farooki A. NCCN bone health task force: key recommendations. J Natl Compr Cancer Netw. 2014;12(5 Suppl):813–816. doi: 10.6004/jnccn.2014.0196. [DOI] [PubMed] [Google Scholar]
- 25.Fliefel R, Troltzsch M, Kuhnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int J Oral Maxillofac Surg. 2015. [DOI] [PubMed]
- 26.Freemantle N, Satram-Hoang S, Tang ET, et al. Final results of the DAPS (denosumab adherence preference satisfaction) study: A 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23(1):317–326. doi: 10.1007/s00198-011-1780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res. 2013;28(8):1729–1737. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris ST, Watts NB, Genant HK, et al. Effects of Risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral efficacy with Risedronate therapy (VERT) study group. JAMA. 1999;282(14):1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 30.Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002;87(4):1586–1592. doi: 10.1210/jcem.87.4.8415. [DOI] [PubMed] [Google Scholar]
- 31.Inoue G, Ueno M, Nakazawa T, et al. Teriparatide increases the insertional torque of pedicle screws during fusion surgery in patients with postmenopausal osteoporosis. J Neurosurg Spine. 2014;21(3):425–431. doi: 10.3171/2014.5.SPINE13656. [DOI] [PubMed] [Google Scholar]
- 32.Komm BS, Chines AA. An update on selective estrogen receptor modulators for the prevention and treatment of osteoporosis. Maturitas. 2012;71(3):221–226. doi: 10.1016/j.maturitas.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Kwon YD, Lee DW, Choi BJ, Lee JW, Kim DY. Short-term teriparatide therapy as an adjunctive modality for bisphosphonate-related osteonecrosis of the jaws. Osteoporos Int. 2012;23(11):2721–2725. doi: 10.1007/s00198-011-1882-9. [DOI] [PubMed] [Google Scholar]
- 34.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18(2):75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 35.Lo JC, O’Ryan FS, Gordon NP, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68(2):243–253. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126(1):13–20. doi: 10.1016/j.amjmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 37.McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. hip intervention program study group. N Engl J Med. 2001;344(5):333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 38.McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 39.McNabb B, Vittinghoff E, Eastell R, et al. A model of BMD changes after alendronate discontinuation to guide postalendronate BMD monitoring. J Clin Endocrinol Metab. 2014;99(11):4094–4100. doi: 10.1210/jc.2014-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellstrom DD, Sorensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75(6):462–468. doi: 10.1007/s00223-004-0286-7. [DOI] [PubMed] [Google Scholar]
- 41.Moeney D. Briefing information for the September 9, 2011 joint meeting of the advisory. Committee for Reproductive Health Drugs and the Drug Safety and Risk. Management Advisory Committee. 2011;Appendix 8.
- 42.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 43.Ohtori S, Inoue G, Orita S, et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine (Phila Pa 1976) 2013;38(8):E487–92. doi: 10.1097/BRS.0b013e31828826dd. [DOI] [PubMed] [Google Scholar]
- 44.Pazianas M, van der Geest S, Miller P. Bisphosphonates and bone quality. Bonekey Rep. 2014;3:529. doi: 10.1038/bonekey.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. vertebral efficacy with risedronate therapy (VERT) study group. Osteoporos Int. 2000;11(1):83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 46.Rizzoli R, Reginster JY, Boonen S, et al. Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int. 2011;89(2):91–104. doi: 10.1007/s00223-011-9499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 48.Roux C, Hofbauer LC, Ho PR, et al. Denosumab compared with Risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone. 2014;58:48–54. doi: 10.1016/j.bone.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028–2039. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 50.Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011;364(18):1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994–1001. doi: 10.2146/ajhp090506. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz AV, Bauer DC, Cummings SR, et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res. 2010;25(5):976–982. doi: 10.1002/jbmr.11. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz AV, Garnero P, Hillier TA, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(7):2380–2386. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sebba A. Osteoporosis: how long should we treat? Curr Opin Endocrinol Diabetes Obes. 2008;15(6):502–507. doi: 10.1097/MED.0b013e328317ca83. [DOI] [PubMed] [Google Scholar]
- 55.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 56.Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 57.Silverman SL, Cummings SR, Watts NB. Consensus panel of the ASBMR, ISCD, and NOF. Recommendations for the clinical evaluation of agents for treatment of osteoporosis: consensus of an expert panel representing the American Society for Bone and Mineral Research (ASBMR), the International Society for Clinical Densitometry (ISCD), and the National Osteoporosis Foundation (NOF) J Bone Miner Res. 2008;23(1):159–165. doi: 10.1359/jbmr.070905. [DOI] [PubMed] [Google Scholar]
- 58.Sorensen OH, Crawford GM, Mulder H, et al. Long-term efficacy of Risedronate: a 5-year placebo-controlled clinical experience. Bone. 2003;32(2):120–126. doi: 10.1016/S8756-3282(02)00946-8. [DOI] [PubMed] [Google Scholar]
- 59.Thumbigere-Math V, Sabino MC, Gopalakrishnan R, et al. Bisphosphonate-related osteonecrosis of the jaw: clinical features, risk factors, management, and treatment outcomes of 26 patients. J Oral Maxillofac Surg. 2009;67(9):1904–1913. doi: 10.1016/j.joms.2009.04.051. [DOI] [PubMed] [Google Scholar]
- 60.Vestergaard P, Schwartz F, Rejnmark L, Mosekilde L. Risk of femoral shaft and subtrochanteric fractures among users of bisphosphonates and raloxifene. Osteoporos Int. 2011;22(3):993–1001. doi: 10.1007/s00198-010-1512-y. [DOI] [PubMed] [Google Scholar]
- 61.Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab. 2000;85(1):231–236. doi: 10.1210/jcem.85.1.6267. [DOI] [PubMed] [Google Scholar]
- 62.Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008;19(3):365–372. doi: 10.1007/s00198-007-0460-7. [DOI] [PubMed] [Google Scholar]
- 63.Wilkinson GS, Kuo YF, Freeman JL, Goodwin JS. Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: a population-based analysis. J Natl Cancer Inst. 2007;99(13):1016–1024. doi: 10.1093/jnci/djm025. [DOI] [PubMed] [Google Scholar]
- 64.Wysowski DK, Greene P. Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the united states, 2002-2012. Bone. 2013;57(2):423–428. doi: 10.1016/j.bone.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 65.Zavras AI, Zhu S. Bisphosphonates are associated with increased risk for jaw surgery in medical claims data: is it osteonecrosis? J Oral Maxillofac Surg. 2006;64(6):917–923. doi: 10.1016/j.joms.2006.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)