Abstract
Background
Central retinal vein occlusion (CRVO) is a common retinal vascular abnormality associated with conditions such as hypertension, diabetes, glaucoma, and a wide variety of hematologic disorders. Macular edema (ME) represents an important vision‐threatening complication of CRVO. Intravitreal steroids (IVS), such as triamcinolone acetonide, have been utilized to treat macular edema stemming from a variety of etiologies and may be a treatment option for CRVO‐ME.
Objectives
To explore the effectiveness and safety of intravitreal steroids in the treatment of CRVO‐ME.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014 Issue 10), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2014), EMBASE (January 1980 to November 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 13 November 2014. For all included primary studies, we used The Science Citation Index (3 December 2014) and manually reviewed reference lists to identify other possible relevant trials.
Selection criteria
We included randomized controlled trials (RCTs) that compared intravitreal steroids, of any dosage and duration of treatment of at least six months, with observation for the treatment of CRVO‐ME.
Data collection and analysis
Two review authors independently screened titles and abstracts identified from the electronic searches and assessed full‐text articles from potentially eligible trials. Two review authors independently assessed trial characteristics, risk of bias, and extracted data from included trials. We contacted investigators of included trials for desired data not provided in the trial reports.
Main results
We included two RCTs that enrolled a total of 708 participants with CRVO‐ME. SCORE compared triamcinolone acetonide intravitreal injections (n = 165) with observation (n = 72); GENEVA compared dexamethasone intravitreal implants (n = 290) with sham injections (n = 147). We observed characteristics indicative of high risk of bias due to incomplete outcome data in SCORE and selective outcome reporting in GENEVA. Loss to follow‐up was high with 10% in the steroid groups and almost twice as much (17%) in the observation group. GENEVA enrolled participants with both branch and central retinal vein occlusion, but did not present subgroup data for the CRVO‐ME population. A qualitative assessment of the results from GENEVA indicated that the dexamethasone implant was not associated with improvement in visual acuity after six months among participants with CRVO‐ME. Although the SCORE investigators reported that participants treated with 1 mg (n = 82) or 4 mg (n = 83) triamcinolone intravitreal injections were five times more likely to have gained 15 letters or more in visual acuity compared with participants in the observation group (1 mg; risk ratio (RR): 5.27; 95% confidence interval (CI) 1.62 to 17.15; 4 mg RR 4.92; 95% CI 1.50 to 16.10) by the eighth‐month follow‐up examination, the average visual acuity decreased in all three groups. However, eyes treated with triamcinolone lost fewer letters than participants in the observation group at 8 months (1 mg mean difference (MD): 8.70 letters, 95% CI 1.86 to 15.54; 4 mg MD: 9.80 letters, 95% CI 3.32 to 16.28). A higher incidence of adverse events was noted with IVS therapy when compared with observation alone. As many as 20% to 35% of participants experienced an adverse event in the IVS groups compared with 8% of participants in the observation group of the SCORE study. The GENEVA investigators reported 63% in the treatment arm versus 43% in the observation arm experienced an adverse event. The most commonly encountered adverse events were elevated intraocular pressure, progression of cataracts, and retinal neovascularization. We graded the quality of evidence as low due to study limitations, imprecision of treatment estimates, and selective outcome reporting.
Authors' conclusions
The two RCTs reviewed herein provide insufficient evidence to determine the benefits of IVS for individuals with CRVO‐ME. The improvement in visual acuity noted in the SCORE trial should be interpreted with caution as outcome data were missing for a large proportion of the observation group. Adverse events were observed more often with IVS treatment compared with observation/no treatment.
Plain language summary
Steroids inserted into the eye versus observation for macular edema secondary to central retinal vein occlusion
Review question
We aimed to examine the benefits and harms of inserting steroids into the eye for treating macular edema secondary to central retinal vein occlusion (CRVO‐ME).
Background
Central retinal vein occlusion (CRVO) is a common abnormality of the blood vessels in the retina (back portion of the eye which receives visual images). CRVO usually presents as a painless loss of vision in one eye of people over the age of 40 who often have other health issues such as high blood pressure, diabetes, glaucoma, and blood diseases. Macular edema (ME) is the swelling of the macula (central area of the retina responsible for detailed vision, such as reading and seeing colors). ME is a complication of CRVO and is the primary reason for loss of vision in this condition. Steroids inserted into the eye, either by injection or an implanted device, have been used to treat ME caused by eye disorders other than CRVO. While steroids can lead to improvements in vision, the effect usually lasts only a few months and there is a risk of developing glaucoma, cataracts, and other complications.
Study characteristics
The review authors searched the medical literature up to 13 November 2014 and included two randomized controlled trials (GENEVA and SCORE) that had evaluated steroids in 708 participants with CRVO‐ME. Both trials included participants with similar baseline characteristics with respect to age, gender, and co‐morbidities. GENEVA was conducted in 24 countries across the world and SCORE was conducted in the US. Both trials compared two different doses of steroid, but the investigators of the two trials used different steroidal agents and different methods of delivery (implant versus injection). Both trials received full or partial sponsorship from the manufacturer of the drugs.
Key results
Neither trial provided sufficient evidence to determine whether steroids had improved visual acuity after six months of treatment. Due to the limited evidence, we are unable to determine reliably whether steroid implants improved vision in eyes with CRVO‐ME. Although the SCORE trial showed that more eyes in the steroid injection groups had improvement in vision compared with eyes in the observation group, participants treated with steroids and those not treated with steroids both lost vision on average at eight months. The GENEVA investigators reported no difference in vision outcomes between participants treated with steroids and those not treated with steroids after six months of treatment; however the GENEVA study was not limited to participants with only CRVO‐ME and included participants with other retinal disease. Both trials showed that patients treated with steroids were at increased risk for high eye pressure ‐ requiring additional medications to lower the eye pressure ‐ and developing cataracts.
Quality of the evidence
The overall quality of the evidence was low due to clinical differences between studies, incomplete information available to assess outcomes, and lack of masking which may lead to biased study results.
Background
Description of the condition
Central retinal vein occlusion (CRVO) is a common retinal vascular abnormality associated with conditions such as hypertension, diabetes, glaucoma, and a wide variety of hematologic disorders (Eye Disease Case‐Control Study 1996). Patients usually are aged 40 years or older and report sudden painless loss of vision in one eye upon waking. Funduscopic evaluation typically reveals intraretinal hemorrhages in all four quadrants ("blood and thunder appearance"), dilation, and tortuosity of the retinal veins. A histopathological study of 29 eyes suggested that central vein occlusion results from the formation of a thrombus at the lamina cribrosa (Green 1981).
Macular edema (ME) represents an important vision‐threatening complication of CRVO. ME formation is presumed to occur from a hypoxic environment in the retina that leads to changes in retinal capillaries, including an increase in capillary permeability and plasma leakage (Ip 2004). Decreased visual acuity results from disruption of photoreceptor function by an edematous and hemorrhagic macula and, in some cases, ischemic retinal damage (Mandelcorn 2007).
Description of the intervention
At the time this review was originally prepared and published, there was no accepted treatment for CRVO‐ME (Gewaily 2009). As of this update, the accepted treatments for CRVO are intravitreal administration of anti‐vascular endothelial growth factor (anti‐VEGF) inhibitors, such as ranibizumab, bevacizumab, and aflibercept (Braithwaite 2014); however, intravitreal steroids may still be used where anti‐VEGF agents are not readily available. The CRUISE 2007 trial results showed significant improvements in vision for patients treated with intravitreal ranibizumab as early as one month after initiation of treatment. The GALILEO 2014 study showed a mean of 60% of patients treated with aflibercept (vs 32% in the control group) gained 15 letters or more in vision improvement at 52 weeks and the COPERNICUS 2013 trial results indicated that 56% of patients treated with aflibercept gained 15 letters from baseline (vs 12% in the control group). Bevacizumab is used off‐label for CRVO therapy. Laser photocoagulation has not been shown to be effective in treating CRVO‐ME (CVOS 1995). There continues to be interest in other treatment modalities, including medical therapy with anticoagulants, fibrinolytics, acetazolamide, isovolemic hemodilution, and angiostatic agents. Surgical options include vitrectomy, chorioretinal anastomosis, direct venous cannulation with injection of fibrinolytics, and radial optic neurotomy (Mohamed 2007). However, none of the aforementioned interventions (other than anti‐VEGF therapy as noted above) has been proven effective in treating CRVO‐induced cystoid macular edema.
Intravitreal steroids, such as triamcinolone acetonide, have been utilized to treat macular edema stemming from a variety of etiologies, including retinal vein occlusion, diabetic retinopathy, uveitis, pseudophakic cystoid macular edema, and exudative macular degeneration (Antcliff 2001; Bashshur 2004; Conway 2003; Jonas 2005b). Although past attempts using topical or systemic steroids failed to improve visual outcomes, intravitreal administration, in the form of an injection or surgical implant, may serve as a method to increase local concentration of the drug while minimizing systemic side effects (Jonas 2005b). Promising results using intravitreal anti‐VEGF therapy (CRUISE 2007COPERNICUS 2013; GALILEO 2014) lend support to intravitreal injection as an alternative to systemic therapy.
How the intervention might work
Corticosteroids have been shown to reduce edema resulting from breaks in the blood‐retina barrier by reducing both intraocular inflammation and capillary permeability (Jonas 2005b). The increased capillary permeability that occurs in macular edema may be mediated partially by vascular endothelial growth factor (VEGF). In cases in which macular ischemia has occurred due to CRVO, VEGF is further upregulated (Pe'er 1998). Corticosteroids have been demonstrated to decrease the induction of VEGF by pro‐inflammatory mediators, such as platelet activating factor, in a dose‐dependent manner (Nauck 1997; Nauck 1998). In addition, studies have found that intravitreal steroid injection led to significant improvements in retinal response density as measured by multifocal electroretinography (mf‐ERG) in both foveal and parafoveal regions in patients with CRVO‐induced macular edema, although those improvements did not correlate directly with improvements in visual acuity (Moschos 2007).
Why it is important to do this review
The prognosis of CRVO can be very poor. Approximately half of non‐ischemic eyes with an initial visual acuity of 20/50 or worse have a visual acuity of 20/250 or worse three years after event onset (CVOS 1997). Macular edema remains the primary cause of decreased vision. At the time of the original publication of this review (Gewaily 2009), there was no standard of care. The use of intravitreal steroids has been proposed over the last few years and multiple studies of this intervention are underway or have been completed. This review does not compare intravitreal steroid therapy with intravitreal anti‐VEGF therapy as our goal is to evaluate whether intravitreal steroid injections are beneficial to patients with CRVO‐ME. Further, the use of intravitreal steroids for CRVO‐induced cystoid macular edema must be weighed against potential complications such as glaucoma, endophthalmitis, and cataracts. This review was designed to explore the benefits and medical risks of using intravitreal steroids in the treatment of CRVO‐ME.
Objectives
To explore the effectiveness and safety of intravitreal steroids in the treatment of CRVO‐ME.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomized controlled trials (RCTs) in our review. We included relevant observational studies retrieved from electronic search results in our discussion of the topic, but not in statistical analyses.
Types of participants
We placed no restrictions with respect to the age or gender of participants enrolled in the primary studies. We included studies of individuals with either unilateral or bilateral macular edema secondary to CRVO.
Types of interventions
This review was limited to a comparison of intravitreal steroids with observation (in this case natural history, i.e., no treatment, sham treatment, or placebo) for CRVO‐ME.
Intravitreal steroid administration could have taken the form of an injection or surgical implantation. We included trials with any dosage and duration of treatment of at least six months.
Types of outcome measures
Primary outcomes
The pre‐specified primary outcome of this review was the proportion of eyes with improved best‐corrected visual acuity at six months of follow‐up compared with baseline visual acuity (Gewaily 2008). We defined a significant improvement in visual acuity as a gain of greater than or equal to 0.1 LogMAR unit (or standard equivalent) compared with visual acuity at the time of CRVO diagnosis. For this review we also reported other measures of improvement in best‐corrected visual acuity as reported by the included studies (e.g., proportion of eyes with gain of 15 letters or more visual acuity). When available, we also reported improvements in visual acuity for longer follow‐up intervals.
Secondary outcomes
Secondary outcomes for comparison of interventions included:
Mean change in best‐corrected visual acuity at six months of follow‐up;
Mean change in macular thickness using optical coherence tomography (OCT).
-
Mean change in macular edema grade by:
Area of leakage using fluorescein angiography (FA):
Area of edema using FA and stereoscopic photography.
Mean change in electroretinogram recordings.
Complications: all named complications will be tabulated.
Number of interventions performed: frequency and time intervals will be tabulated.
Adverse outcomes
We compared all reported systemic and ocular complications and adverse events related to the use of intravitreal corticosteroids compared with the control group that were mentioned in the primary studies. Specific adverse outcomes of interest included the development of sterile/nonsterile endophthalmitis, an increase in mean intraocular pressure (IOP) or need for anti‐glaucomatous therapy, and cataract formation and/or progression.
Economic outcomes
We planned to report cost‐benefit analyses and other relevant economic data, but there were no relevant economic data reported in the included studies.
Quality‐of‐life outcomes
We planned to report quality‐of‐life outcomes, but did not find any relevant quality‐of‐life data in the included primary studies.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014 Issue 10), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2014), EMBASE (January 1980 to November 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 13 November 2014.
See Appendices for details of the search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), mRCT (Appendix 4), ClinicalTrials.gov (Appendix 5) and the ICTRP (Appendix 6).
Searching other resources
For the previous version of this review, we conducted manual searches by reviewing the reference lists of all reviews and non‐randomized studies that compared intravitreal steroids to observation for CRVO‐ME for additional relevant papers. For this update, we also reviewed the reference lists from reports of included studies. We used the Science Citation Index to search for studies that had cited included primary trials (3 December 2014). We contacted researchers in the field currently working on RCTs on the topic to seek information on additional current, past, or unpublished trials.
Data collection and analysis
Selection of studies
Two authors independently reviewed all titles and abstracts retrieved from the electronic and manual searches and judged potential relevance based on the inclusion criteria for this review. The authors classified each abstract as: (a) definitely relevant, (b) possibly relevant, or (c) definitely not relevant. The authors retrieved the full reports from records assessed as (a) or (b). The authors reviewed the full reports and classified the studies as: (1) include, (2) awaiting assessment, or (3) exclude. A third review author resolved any discrepancies at each stage. We contacted the authors of studies classified as (2) for further information. Whenever the contacted authors did not respond within two weeks, we used the available data. We documented titles of excluded studies, along with primary reason(s) for exclusion. Reports identified in languages other than English were translated by colleagues for screening and classification.
Data extraction and management
Two review authors independently extracted data from each included primary study using data extraction forms developed by the Cochrane Eyes and Vision Group. We extracted data pertaining to study characteristics, participants (including the stated inclusion and exclusion criteria), intervention and control group descriptions, primary and secondary outcome data, and relevant corollary notes. We made efforts to contact primary investigators to obtain missing or unclearly reported data. The authors discussed discrepancies in data extracted independently to reach a consensus. One author entered data into Review Manager 5.3 (RevMan 2014) and the second author verified the entered data.
Assessment of risk of bias in included studies
Two review authors independently evaluated the included trials for risks of bias following the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the potential risk of bias in the included trials by examining selection bias (sequence generation and allocation concealment before randomization), performance bias (masking of participants and personnel), detection bias (masking of outcome assessors), attrition bias (incomplete outcome data), as well as reporting bias (selective outcome reporting). We also assessed whether other aspects of trial design or conduct could have biased the individual study results. We judged studies on each risk of bias domain to be at 'high risk,' 'low risk,' or 'unclear risk' of bias. We discussed discrepancies between authors regarding risk of bias assessments to come to a consensus and reported the rationale for each judgment in Characteristics of included studies tables. We contacted authors of trial reports for additional information when descriptions of study methods needed to judge risk of bias were unclear or not reported.
Measures of treatment effect
Dichotomous data
We analyzed the primary outcome of interest ‐ the proportion of patients with a gain of visual acuity greater than or equal to 0.1 logMAR (or standard equivalent) at six months of follow‐up ‐ and the occurrence of adverse events as dichotomous variables. We presented dichotomous data as risk ratios (RR) with 95% confidence intervals (CIs).
Continuous data
We measured the mean changes in visual acuity and macular thickness as continuous variables, and calculated the mean differences with 95% CIs.
Ordinal data
We planned to analyze and report the number of complications and the economic and quality of life data as ordinal data.
Unit of analysis issues
The unit of analysis was the individual (one eye per person).
Dealing with missing data
The GENEVA study reports pooled data for CRVO and branch retinal vein occlusion (BRVO). Since the population of interest for our review was participants with CRVO, we needed subgroup analysis data for the CRVO cases from GENEVA. We contacted the GENEVA investigators and trial sponsor (Allergan, Inc.) for outcome data for the CRVO participants, a pre‐specified subgroup analysis of the trial. Unfortunately, our request for the CRVO data from the GENEVA study was denied by the trial sponsor (email correspondence). We did not impute any data for either GENEVA or SCORE and used the data as available for all analyses.
Assessment of heterogeneity
We assessed both clinical and methodological heterogeneity among the included trials. Based on clinical and methodological heterogeneity, we did not conduct meta‐analyses. In future updates, if additional RCTs are found that meet the inclusion criteria and can be included in meta‐analyses, we will assess statistical heterogeneity through inspection of forest plots and the results of Chi2 tests and the I2 statistic. We will consider I2 values ≥ 60% to indicate substantial statistical heterogeneity.
Assessment of reporting biases
We could not assess publication bias through inspection of funnel plots because we had too few included studies. We will examine funnel plots to identify any evidence of publication bias if future trials are found and the same outcome is reported from 10 or more trials. We assessed selective outcome reporting by comparing trial protocols with published results of included studies.
Data synthesis
Due to heterogeneity in the types of intravitreal steroid evaluated, data reporting, and follow‐up periods we were not able to perform meta‐analyses. We will perform meta‐analysis when RCTs are found that meet the inclusion criteria and substantial heterogeneity across studies is absent. We will use a random‐effects model for meta‐analysis, or a fixed‐effect model whenever fewer than three trials report the same outcome.
Subgroup analysis and investigation of heterogeneity
We analyzed dosages of steroid used and delivery method of the corticosteroid (i.e., injection versus surgical implant) separately in this review. In future updates, we will investigate heterogeneity through quantitative subgroup analyses based on the type of CRVO (i.e., ischemic versus non‐ischemic) and baseline measurements (i.e., visual acuity and macular thickness) when sufficient data are available.
Sensitivity analysis
We did not perform sensitivity analyses in this review. In future updates, we plan to assess the impact of excluding studies that have high risk of attrition bias using sensitivity analysis. We also plan to examine the impact of both unpublished studies and industry‐funded trials on overall results.
Summary of findings
We did not prepare a summary of findings table as no meta‐analysis was performed due to differences in intervention groups between the included studies. Two authors independently graded the overall quality of the evidence for each outcome using the GRADE classification (www.gradeworkinggroup.org/).
Results
Description of studies
Results of the search
Electronic searches, inherently designed to include published and unpublished trials, conference proceedings, and papers written in languages other than English, originally yielded a total of 177 records as of November 2008. We screened the full‐text reports corresponding to seven potentially relevant records. A preliminary review of the articles yielded no eligible RCTs.
In November 2014 the electronic searches were updated; we screened 286 additional titles and abstracts and 37 records from clinical trials registries (Figure 1). After screening titles and abstracts, we classified 19 records as 'definitely relevant' or 'possibly relevant.' After review of the full‐text, 15 reports from two RCTs met our inclusion criteria and were included in the review (GENEVA; SCORE); three studies were not RCTs and were excluded (Chuang 2010; Jain 2012; Muni 2010); and one study is ongoing (NCT01660802). We entered reports from the two included trials into the Science Citation Index which resulted in 355 unique citations. We identified and excluded one additional potentially relevant trial for this review (ROVO).
1.
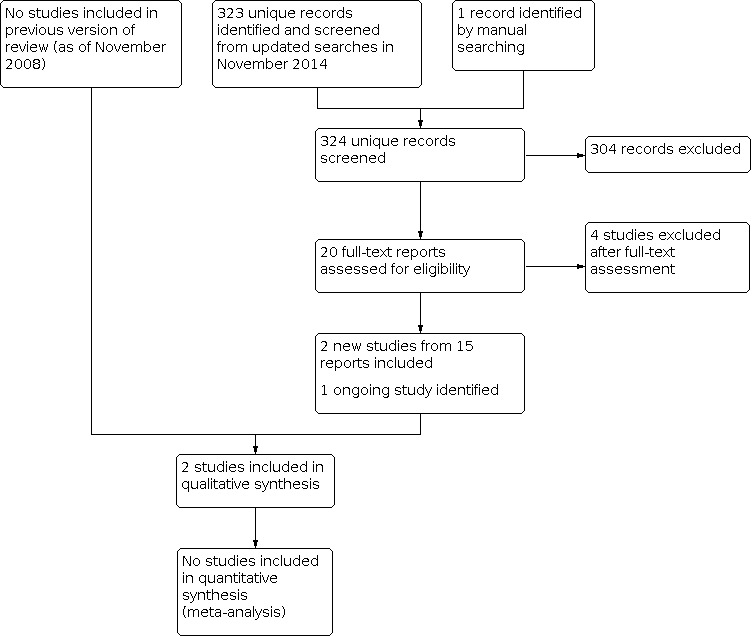
Study flow diagram.
Included studies
We identified two RCTs that directly compared intravitreal steroids with observation for CRVO‐ME (GENEVA; SCORE). Summary information regarding the two RCTs are included below with detailed information provided in the Characteristics of included studies table.
Types of participants
A total of 1538 participants, 708 with CRVO‐ME, were enrolled in the two trials (GENEVA; SCORE). The baseline characteristics of participants in both trials were similar with respect to age, gender, and co‐morbidities (e.g., diabetes mellitus, hypertension, and coronary artery disease). SCORE investigators enrolled 271 participants from the US with CRVO, best‐corrected visual acuity ranging from 20/40 to 20/400 and retinal thickness ≥ 250 microns. GENEVA investigators at multiple study sites around the world enrolled participants with CRVO (n = 437) or BRVO (n = 930) and visual acuity between 20/50 and 20/200 with retinal thickness ≥ 300 microns. Both trials enrolled one study eye per participant; when both eyes of a participants were eligible, the eye with the shorter duration of macular edema was selected in the GENEVA study (the SCORE study did not report how the study eye was selected if both eyes were eligible).
Types of interventions
Both trials compared two different doses of intravitreal steroid with control, but different steroids and methods of intravitreal delivery were used between the two trials. 1 mg and 4 mg intravitreal injections of triamcinolone acetonide (with retreatment at four‐month intervals) were compared to observation in the SCORE study while 0.35 mg and 0.70 mg dexamethasone intravitreal implants were compared to sham injection in the GENEVA study. After the first six months, participants in GENEVA were eligible for retreatment with either dose of the dexamethasone implant and followed for an additional 12 months in an open‐label extension.
Types of outcomes
Both trials used the Early Treatment of Diabetic Retinopathy Scale (ETDRS) to measure visual acuity; investigators reported the proportion of participants who gained 15 letters or more of best‐corrected visual acuity (BCVA) and the mean change in BCVA from baseline to a predetermined time point. The mean change in retinal thickness from baseline to a time point, based on optical coherence tomography of central subfield retinal thickness, was reported in both trials. Neither trial reported mean change in macular edema grade (assessed by area of leakage or area of edema), mean change in electroretinogram recordings, number of interventions performed, economic outcomes, or quality‐of‐life outcomes.
The greatest source of variation in terms of outcomes was the follow‐up interval. There was no overlap in the time points at which any of the above outcome measures were reported in the two trials. SCORE investigators followed up participants at four‐month intervals for 12 months, and then annually for 36 months. The GENEVA trial follow‐up schedule was 1, 7, 30, 60, 90 and 180 days post‐treatment.
Excluded studies
See the 'Characteristics of excluded studies' table for details of the 13 studies that were screened and excluded. Ten studies were not RCTs, one study was not eligible as intravitreal steroids were compared with laser treatment, one study was of participants with CRVO but not all had macular edema, and one study was excluded because follow‐up was shorter than six months.
Risk of bias in included studies
Figure 2 provides a summary of our judgements for each risk of bias domain for the included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged both GENEVA and SCORE to be at low risk of selection bias because the investigators used appropriate methods to generate the randomization sequence and to conceal each allocation before assigning it.
Masking of study participants and personnel (performance bias)
Although participants in SCORE were masked to the doses of steroid injections (1 mg vs 4 mg), they were not masked to treatment assignment of observation versus steroid injection, suggesting a high risk of bias. A needle‐less applicator was used to apply a sham injection in the control group in GENEVA. Thus, participants allocated to implants were masked to the dose (0.35 mg and 0.70 mg), but not to the treatment assignment (steroid implant versus no implant), resulting in high risk of bias. GENEVA and SCORE investigators were also unmasked to the treatment assignment (intravitreal steroid versus no steroid), although they were masked to the dosage assignment within the groups receiving steroids.
Masking of outcome assessors (detection bias)
Both SCORE and GENEVA used masked observers and technicians to assess OCT and visual acuity measurements. Thus, they both were at low risk of detection bias.
Incomplete outcome data
We observed a much higher proportion of missing data in the observation group (17%) compared to both steroid injection groups (10%) at the 12 month visit in the SCORE trial. Analysis was performed based on participants with available 12‐month data assuming all missing data were missing at random, leading us to judge SCORE as having a high risk of bias for incomplete outcome data.
GENEVA investigators reported the combined number of BRVO and CRVO participants with missing data and reasons for the missing data at the end of six months which were consistent across treatment groups. We could not determine the amount of, or reasons for, missing data among the CRVO subgroup and therefore judged GENEVA to have an unclear risk of bias for incomplete outcome data.
Selective reporting
We judged SCORE to have a low risk of bias for selective outcome reporting as all primary and secondary outcomes were reported according to prespecified methods. Based on information provided in the GENEVA trial registry, a number of secondary outcomes (e.g., contrast sensitivity, vessel leakage and quality of life assessments) were to be measured from one to 12 months, the results of which were not provided in the clinical trial registry results section, six month report or 12 month report (for either BRVO or CRVO). Macular thickness was a secondary outcome for only one of the trials, but the six month report used pooled data from both trials to analyze this outcome. Although GENEVA’s six month report described key efficacy variables for a prespecified CRVO subgroup analysis, there was no information about central retinal thickness for just the CRVO subgroup. Thus we judged GENEVA to have a high risk of bias.
Other potential sources of bias
For the SCORE trial, study drugs were provided by the pharmaceutical company Allergan which also provided funding for study site visits and conducted secondary analyses. It is unclear whether this support could have introduced bias. Furthermore, the intravitreal triamcinolone group (both 1 mg and 4 mg) had additional safety visits at 4 days and 4 weeks to make visual acuity and IOP measurements and to perform a dilated fundus examination. Thus, participants in the steroid groups may have perceived that they were getting superior care which may have introduced additional performance bias. GENEVA had a similar source of bias: personnel from Allergan ‐ the study sponsor ‐ participated in the design of the study and in data analysis and interpretation, and also supervised the preparation of the final manuscript. This involvement of the sponsor was judged to result in a high risk of bias. Furthermore, GENEVA conducted two separate trials for regulatory purposes with slightly different primary end points. The final 6 month results from the two GENEVA trials were pooled, possibly introducing external sources of bias.
Effects of interventions
Due to differences in comparisons and outcome measurements between the two included trials, we did not combine trial results in meta‐analysis. Rather, we discuss the relevant trial results for each study. Neither trial reported mean change in macular edema grade (assessed by area of leakage or area of edema), mean change in electroretinogram recordings, number of interventions performed, economic outcomes, or quality‐of‐life outcomes. We assessed the overall quality of evidence as low due to study limitations, imprecision, and selective outcome reporting.
Dexamethasone implant versus sham injection
One trial compared dexamethasone implants (two doses: 0.35 mg and 0.70 mg) with sham injections in 437 participants with CRVO‐ME (GENEVA). Outcome data for both primary and secondary outcomes at six months were described for participants with CRVO‐ME in a difficult to read figure without sufficient information to estimate steroid effects (i.e., risk ratios or mean differences) or corresponding variances (i.e., confidence intervals, standard deviations, or standard errors). The insufficient reporting of outcomes among the participants with CRVO‐ME prevented us from analyzing results regarding the effectiveness of individual or combined doses of the dexamethasone implant compared with sham injection. Based on a qualitative assessment of the published figures and narrative description of the effects of the two doses of the dexamethasone implant in the subgroup of participants with CRVO, there did not appear to be a difference between either dose group and the sham group at six months with respect to gaining 15 letters or more BCVA or mean change in BCVA from baseline. Change in macular thickness was not reported in the figures or in the text for the CRVO subgroup. We assessed the quality of evidence comparing dexamethasone implants with sham injections as low due to limitations of the trial and lack of quantitative results for eyes with CRVO‐ME for any outcome.
Triamcinolone acetonide versus observation
One trial compared triamcinolone acetonide (two doses: 1 mg and 4 mg) with observation in 271 participants with CRVO‐ME (SCORE). SCORE investigators did not designate a six‐month evaluation for primary or secondary outcomes specified for this review. We therefore evaluated each of the primary and secondary outcomes at time points beyond six months as reported. At the 12‐month primary follow‐up time for the trial, visual acuity data were available for 238 (88%) of 271 total participants. At 24 months, the longest follow‐up in the study, visual acuity data were available for 151 (56%) of 271 total participants.
Improvement in visual acuity
Both doses of triamcinolone acetonide (1 mg and 4 mg) were associated with a greater proportion of participants who experienced a gain of 15 letters or more of BCVA when compared with observation at 8 months (1 mg RR: 5.27, 95% CI 1.62 to 17.15; 4 mg RR: 4.92, 95% CI 1.50 to 16.10), 12 months (1 mg RR: 3.87, 95% CI 1.54 to 9.70; 4 mg RR: 3.74, 95% CI 1.49 to 9.41), 16 months (1 mg RR: 2.63, 95% CI 1.11 to 6.28; 4 mg RR: 2.87, 95% CI 1.21 to 6.78), 20 months (1 mg RR: 2.52, 95% CI 0.98 to 6.46; 4 mg RR: 2.08, 95% CI 0.78 to 5.51), and 24 months (1 mg RR: 3.55, 95% CI 1.29 to 9.82; 4 mg RR: 2.99, 95% CI 1.05 to 8.52) follow‐up (Analysis 1.1; Analysis 2.1). Results were similar when results from the two triamcinolone acetonide groups were combined and compared with the observation group (RR: 5.09, 95% CI 1.62 to 16.02 at 8 months; RR: 3.80, 95% CI 1.57 to 9.21 at 12 months; RR: 2.75, 95% CI 1.22 to 6.21 at 16 months; RR: 2.30, 95% CI 0.94 to 5.65 at 20 months; and RR: 3.29, 95% CI 1.23 to 8.79 at 24 months) (Analysis 3.1).
1.1. Analysis.

Comparison 1: 1 mg triamcinolone versus observation, Outcome 1: Gain of 15 letters or more (ETDRS)
2.1. Analysis.

Comparison 2: 4 mg triamcinolone versus observation, Outcome 1: Gain of 15 letters or more (ETDRS)
3.1. Analysis.

Comparison 3: Combined doses of triamcinolone versus observation, Outcome 1: Gain of 15 letters or more (ETDRS)
The large relative effects seen in both doses compared with observation across each time point suggest that eyes treated with triamcinolone acetonide may be up to five times more likely to experience improvement in BCVA than those not treated. However, none of the differences between groups (each dose separately or the two dose groups combined compared to control) were statistically significant at 20 months follow‐up, and the amount of missing data throughout the 24 months of follow‐up adds uncertainty to the size and precision of these estimates. For these reasons, we graded the quality of evidence for this outcome as low.
Change in visual acuity
Overall there was a decrease in the average BCVA from baseline through 24 months of follow‐up in all three groups. Participants treated with either dose of triamcinolone acetonide lost approximately six to 11 fewer letters than participants in the observation group at 8 months (1 mg MD: 8.70 letters, 95% CI 1.86 to 15.54; 4 mg MD: 9.80 letters, 95% CI 3.32 to 16.28), 12 months (1 mg MD: 10.90 letters, 95% CI 3.80 to 18.00; 4 mg MD: 10.90 letters, 95% CI 3.80 to 18.00), 16 months (1 mg MD: 9.90 letters, 95% CI 2.47 to 17.33; 4 mg MD: 7.80 letters, 95% CI 0.02 to 15.58), 20 months (1 mg MD: 7.30 letters, 95% CI ‐1.23 to 15.83; 4 mg MD: 4.30 letters, 95% CI ‐4.23 to 12.83), and 24 months (1 mg MD: 6.30 letters, 95% CI ‐3.22 to 15.82; 4 mg MD: ‐0.93 letters, 95% CI ‐0.93 to 17.53) (Analysis 1.2; Analysis 2.2). Results were similar when doses were combined and compared with the observation group (MD: 9.25 letters, 95% CI 3.61 to 14.90 at 8 months; MD: 10.90 letters, 95% CI 4.80 to 17.00 at 12 months; MD: 8.87 letters, 95% CI 2.28 to 15.45 at 16 months; MD: 5.82 letters, 95% CI ‐1.54 to 13.19 at 20 months; and MD: 7.30 letters, 95% CI ‐0.72 to 15.32 at 24 months) (Analysis 3.2).
1.2. Analysis.

Comparison 1: 1 mg triamcinolone versus observation, Outcome 2: Change in visual acuity (ETDRS) from baseline
2.2. Analysis.

Comparison 2: 4 mg triamcinolone versus observation, Outcome 2: Change in visual acuity (ETDRS) from baseline
3.2. Analysis.

Comparison 3: Combined doses of triamcinolone versus observation, Outcome 2: Change in visual acuity (ETDRS) from baseline
The smaller reductions in loss of BCVA in the 1 mg and 4 mg triamcinolone groups, or the two groups combined, compared with the observation group were not statistically significant at 20 and 24 months follow‐up. Again, the imprecision of these estimates and the amount of missing data adds further uncertainty to these estimates across all follow‐up periods. Therefore, we graded the quality of evidence for this outcome as low.
Change in macula thickness
Participants in all three groups had a decrease in the median center point thickness throughout the 24 months of follow‐up (Table 1). Given the way these data were reported (median and interquartile range) we were not able to estimate a treatment effect for this outcome. Qualitatively, no clinically meaningful difference in the decrease in center point thickness between groups throughout 24 months follow‐up was observed. We assessed the quality of evidence for this outcome as low due to lack of quantitative data and missing data at 24 months follow‐up.
1. Change in macular thickness* from baseline in SCORE study.
| 1 mg triamcinolone | 4 mg triamcinolone | Observation | ||||
| Follow‐up time | No. of participants | Median change from baseline (IQR) | No. of participants | Median change from baseline (IQR) | No. of participants | Median change from baseline (IQR) |
| 8 months | 73 | ‐173 (‐306 to ‐17) | 80 | ‐219 (‐351 to ‐63) | 70 | ‐198 (‐326 to ‐102) |
| 12 months | 72 | ‐196 (‐390 to ‐62) | 78 | ‐261 (‐407 to ‐79) | 68 | ‐277 (‐418 to ‐40) |
| 16 months | 61 | ‐242 (‐426 to ‐108) | 59 | ‐259 (‐396 to ‐91) | 61 | ‐315 (‐427 to ‐145) |
| 20 months | 55 | ‐296 (‐450 to ‐60) | 47 | ‐249 (‐362 to ‐102) | 44 | ‐343 (‐458 to ‐102) |
| 24 months | 48 | ‐286 (‐458 to ‐119) | 45 | ‐236 (‐421 to ‐63) | 43 | ‐304 (‐465 to ‐108) |
*change reported in micrometers IQR: interquartile range mg: milligram
Adverse events
Overall, more adverse events occurred in both the 4 mg triamcinolone acetonide group (35%) and 1 mg group (20%) compared with the observation group (8%) in the SCORE study through 12 months of follow‐up. Almost twice as many participants were reported to have experienced an ocular adverse event in the GENEVA trial steroid groups compared to the control group, with 63% of participants in the 0.7 mg and 62% of participants in the 0.35 mg dexamethasone implant groups experiencing an ocular adverse event compared with 43% of participants in the sham injection group. The most common adverse events were related to elevated IOP and cataract in both trials (Table 2; Table 3). Although less common, vitreous hemorrhage and retina hemorrhage and detachment also were reported from both trials. Ocular adverse events that required additional medical treatment were reported from SCORE.
2. Adverse events in GENEVA.
| Total number of adverse events inGENEVA | Comparison of dexamethasone with sham | ||||
| 0.35 mg dexamethasone | 0.70 mg dexamethasone | sham injection | 0.35 mg vs sham group (RR, CI) | 0.70 mg vs sham group (RR, CI) | |
| Elevated IOP: | |||||
| ≥ 10 mm Hg | NR | NR | NR | ‐ | ‐ |
| ≥ 35 mm Hg | NR | NR | NR | ‐ | ‐ |
| Ocular hypertension: | 16/414 (3.9%) | 17/427 (4.0%) | 3/426 (0.7%) | 5.49 (1.61, 18.69) | 5.65 (1.67, 19.15) |
| Eye pain: | 17/414 (4.1%) | 31/427 (7.3%) | 16/426 (3.8%) | 5.49 (1.61, 18.69) | 5.65 (1.67, 19.15) |
| Conjunctiva: | |||||
| Hemmorhage | 72/414 (17.4%) | 85/427 (19.9%) | 63/426 (14.8%) | 1.18 (0.86, 1.60) | 1.35 (1.00, 1.81) |
| Hyperemia | 27/414 (6.5%) | 28/427 (6.6%) | 20/426 (4.7%) | 1.39 (0.79, 2.44) | 1.40 (0.80, 2.44) |
| Cornea/lens: | |||||
| Infection/endophthalmitis | NR | NR | NR | ‐ | ‐ |
| Cataract/lens opacity | 17/414 (4.1%) | 31/427 (7.3%) | 19/426 (4.5%) | 0.92 (0.49, 1.75) | 1.63 (0.93, 2.84) |
| Iris neovascularization: | NR | NR | NR | ‐ | ‐ |
| Vitrious: | |||||
| Hemorrhage | NR | NR | NR | ‐ | ‐ |
| Detachment | 12/414 (2.9%) | 12/427 (2.8%) | 8/426 (1.9%) | 1.54 (0.64, 3.74) | 1.50 (0.62, 3.62) |
| Retina: | |||||
| Neovascularization | 4/414 (1.0%) | 3/427 (0.7%) | 11/426 (2.6%) | 0.37 (0.12, 1.17) | 0.27 (0.08, 0.97) |
| Detachment | NR | NR | NR | ‐ | ‐ |
| Hemorrhage | 8/414 (1.9%) | 12/427 (2.8%) | 10/426 (2.3%) | 0.82 (0.33, 2.07) | 1.20 (0.52, 2.74) |
| Maculopathy | 22/414 (5.3%) | 19/427 (4.4%) | 23/426 (5.4%) | 0.98 (0.56, 1.74) | 0.82 (0.46, 1.49) |
| Required additional therapy: | |||||
| IOP lowering medications | NR | NR | NR | ‐ | ‐ |
| Cataract surgery | NR | NR | NR | ‐ | ‐ |
| Photocoagulation | NR | NR | NR | ‐ | ‐ |
| Pars plana vitrectomy | NR | NR | NR | ‐ | ‐ |
CI: 95% confidence interval IOP: intraocular pressure mg: milligram mm Hg: millimeter of mercury NR: not reported RR: risk ratio
3. Adverse events in SCORE.
| Total number of adverse events inSCORE | Comparison of triamcinolone with observation | ||||
| 1 mg triamcinolone | 4 mg triamcinolone | observation | 1 mg vs observation group (RR, CI) | 4 mg vs observation group (RR, CI) | |
| Elevated IOP: | |||||
| ≥ 10 mm Hg | 15/92 (16.3%) | 24/91 (26.4%) | 2/88 (2.3%) | 7.17 (1.69, 30.47) | 11.60 (2.83, 47.65) |
| ≥ 35 mm Hg | 5/92 (5.4%) | 8/91 (8.8%) | 1/88 (1.1%) | 4.78 (0.57, 40.13) | 7.74 (0.99, 60.59) |
| Ocular hypertension: | NR | NR | NR | ‐ | ‐ |
| Eye pain: | NR | NR | NR | ‐ | ‐ |
| Conjunctiva: | |||||
| Hemmorhage | NR | NR | NR | ‐ | ‐ |
| Hyperemia | NR | NR | NR | ‐ | ‐ |
| Cornea/lens: | |||||
| Infection/endophthalmitis | 0/92 (0%) | 0/91 (0%) | 0/88 (0%) | N/A | N/A |
| Cataract/lens opacity | 20/92 (21.7%) | 25/91 (27.5%) | 12/88 (13.6%) | 1.59 (0.83, 3.06) | 2.01 (1.08, 3.76) |
| Iris neovascularization: | 9/92 (9.8%) | 4/91 (4.4%) | 2/88 (2.3%) | 4.30 (0.96, 19.37) | 1.93 (0.36, 10.29) |
| Vitrious: | |||||
| Hemorrhage | 4/92 (4.3%) | 0/91 (0%) | 4/88 (4.5%) | 4.30 (0.96, 19.37) | 1.93 (0.36, 10.29) |
| Detachment | NR | NR | NR | ‐ | ‐ |
| Retina: | |||||
| Neovascularization | 2/92 (2.2%) | 2/91 (2.2%) | 4/88 (4.5%) | 0.48 (0.09, 2.55) | 0.48 (0.09, 2.57) |
| Detachment | 0/92 (0%) | 0/91 (0%) | 0/88 (0%) | N/A | N/A |
| Hemorrhage | NR | NR | NR | ‐ | ‐ |
| Maculopathy | NR | NR | NR | ‐ | ‐ |
| Required additional therapy: | |||||
| IOP lowering medications | 18/92 (19.6%) | 32/91 (35.2%) | 7/88 (8.0%) | 2.46 (1.08, 5.60) | 4.42 (2.06, 9.49) |
| Cataract surgery | 0/92 (0%) | 4/91 (4.4%) | 0/88 (0%) | N/A | N/A |
| Photocoagulation | 9/92 (9.8%) | 3/91 (3.3%) | 5/88 (5.7%) | 1.72 (0.60, 4.94) | 0.58 (0.14, 2.36) |
| Pars plana vitrectomy | 2/92 (2.2%) | 0/91 (0%) | 1/88 (1.1%) | 1.91 (0.18, 20.72) | N/A |
CI: 95% confidence interval IOP: intraocular pressure mg: milligram mm Hg: millimeter of mercury NR: not reported RR: risk ratio
Discussion
Summary of main results
When the original version of this review was published in 2009, no RCTs had yet been completed (Gewaily 2009). With the recent completion and publication of results from two RCTs ‐ SCORE and GENEVA ‐ we have more information regarding the effects of two different methods of administration of intravitreal steroids for CRVO‐ME. Based on the available published evidence from GENEVA, neither dose of the dexamethasone implant appeared to provide clinically meaningful improvements in BCVA at six months compared with the sham injection group. Throughout longer follow‐up periods, results from SCORE suggested that intravitreal injections of triamcinolone acetonide may provide benefits in terms of a greater proportion of participants experiencing an improvement in BCVA as well as an overall smaller decrease in BCVA compared with observation alone. However, the imprecision of the effect estimates and considerable amount of missing data through follow‐up in SCORE warrants caution in interpreting these results. Furthermore, although the differences between treatment and observation groups with respect to the mean changes in BCVA reported by SCORE are striking, the modest improvements in BCVA are overshadowed by an overall loss of BCVA from baseline. As much as 25% of study eyes in the treatment group continued to experience moderate losses of BCVA.
Adverse events were noted in at least a quarter of the participants in SCORE given intravitreal steroids. GENEVA reported a higher incidence of adverse events: over 60% in dexamethasone implant groups and over 40% in the control group. Although the control groups in both trials experienced adverse events, the treatment groups had noticeably more. The most common adverse events were elevations of IOP and progression of cataracts. Additional side effects, such as retinal neovascularization, were also reported but were rare. In both SCORE and GENEVA, retinal neovascularization was seen more often in observation groups than in steroid groups, which may suggest that intravitreal steroids help to reduce neovascularization, a known complication of untreated CRVO‐ME.
Overall completeness and applicability of evidence
Athough both RCTs included in the update of this review addressed the primary objective of the review, neither trial provided complete data for the primary or secondary outcomes of interest at six months as pre‐specified for this review. We also observed significant heterogeneity in terms of the actual steroids used, retreatment schedules, and follow‐up intervals which limited our ability to draw conclusions. In the SCORE study, retreatment was common, making it difficult to assess a specific treatment schedule. Adding to the insufficient reporting of outcome data, one trial included participants with both CRVO‐ and BRVO‐ME, and did not clearly report outcomes for the CRVO‐ME participants separately (GENEVA). At the time this review was originally conceived, observation was considered standard practice for treating CRVO‐ME based on results from CVOS 1995. In the time since the initial publication of this review, standard of care has evolved with evidence for intravitreal steroids and the introduction of newer treatment options including anti‐vascular growth factor (anti‐VEGF) agents. Thus, observation may no longer be the most appropriate method of managing CRVO against which to compare the efficacy of steroids.
Quality of the evidence
Neither RCT was free of potential biases which may call into question the validity and reliability of study results. Of note, in neither SCORE nor GENEVA were participants masked to the assigned group (steroid vs observation) which could have resulted in performance bias affecting the observed outcomes. In SCORE, there was a differential proportion of missing data between the two steroid groups and the observation group. Incomplete documentation of the reasons for loss of follow‐up data makes it difficult to determine whether the reasons for missing data were treatment‐related. When comparing the published reports and trial registrations for GENEVA, we noticed potential selective outcome reporting as not all pre‐specified outcomes and subgroup analyses (CRVO participants) had been published. Furthermore, GENEVA pooled results from two independent trials, which limits our ability to comment on any potential variability in the participants, methods, or results from each trial independently. Due to these study limitations, imprecision in effect estimates, and inconsistency in results, we graded the overall quality of the evidence as low.
Potential biases in the review process
A potential bias in the review process is that the level of visual acuity improvement specified for this review was not reported completely by the included studies; we relied on the way that the authors of the GENEVA and SCORE trials reported this outcome (15 letters or more improvement). We also included the GENEVA trial which enrolled participants who did not meet the eligibility criteria specified for this review (participants with branch retinal vein occlusion were included in addition to participants with CRVO). However, given that randomization was stratified by branch and central retinal vein occlusion and the trial investigators pre‐specified subgroup analyses by type of retinal vein occlusion, it was appropriate to include this trial. We also judged the control group in the GENEVA trial, which received a sham injection, to be comparable to observation. We were not able to evaluate the presence of publication bias through inspection of funnel plots.
Agreements and disagreements with other studies or reviews
Based on unpublished data related to participants with CRVO in GENEVA, a report from the Aberdeen Health Technology Assessment Group sponsored by the National Institute for Health Research Health Technology Assessment Programme (UK) showed no difference between the 0.70 mg dexamethasone implant and sham group in the proportion of participants who gained 15 letters or more BCVA at six months or in the mean change in BCVA from baseline to six‐month follow‐up (Shyangdan 2010). The conclusions based on the unpublished data for the CRVO participants in GENEVA are consistent with our qualitative assessment of the limited information in this subgroup of participants provided in the published reports. In terms of adverse events, the 0.70 mg dexamethasone implant was also shown to be associated with an increase in the frequency of adverse events (i.e., increased IOP and ocular hypertension, retinal neovascularization and retinal detachment).
Results from the observational studies cited in the previous publication of this review are consistent with the findings from SCORE with regards to improvements in visual acuity (Gewaily 2009). Bashshur 2004 found improvements in visual acuity in individuals treated with 4 mg of triamcinolone acetonide after 10 to 12 months of follow‐up, although this study excluded ischemic CRVOs. The Cheng 2009 study, which included both ischemic and non‐ischemic CRVO cases, also found improvement in visual acuity amongst treated patients. In general, visual acuity improvements were more noticeable in non‐ischemic CRVO as reported by subgroup analyses performed in the Gelston 2006 and Jonas 2005a studies. The results from the Jonas 2005a study seem to agree with those from GENEVAin that visual acuity returned to baseline approximately five months after intravitreal triamcinolone acetonide treatment in all CRVO participants after an initial significant improvement of visual acuity from baseline. This change was more pronounced in non‐ischemic CRVO.
Macular edema resolution was not emphasized in the observational studies. The only quantitative results related to macular edema came from the Bashshur 2004 and Cheng 2009 studies. The Bashshur 2004 study found noticeable improvements in macular edema with treatment but 2 of 20 treated eyes re‐developed macular edema during the study. OCT results in the Cheng 2009 study revealed a significant decrease in macular edema among treated eyes, although edema reoccurred in 6 of 22 study eyes.
Adverse events were consistent across observational studies and agree with the included trials. The most common complication was an increase in IOP. In all four observational studies, immediate paracentesis of the anterior chamber was performed alongside the intravitreal steroid injection, either conditionally or routinely. The Gelston 2006 and Jonas 2005a studies showed transient increases in IOP which eventually returned to baseline at later follow‐up visits. Retinal toxicity reported in rabbit models (Lang 2007; Ruiz‐Morena 2007) and some human cases (Kivilcim 2000) was not observed in SCORE or GENEVA.
Two other interesting (but rare) potential complications to note include the unmasking of ocular syphilis and cytomegalovirus retinitis after intravitreal steroid treatment. These were reported in small case reports by Mushtaq 2009 and Sekiryu 2008 respectively. It is worth noting that in both case series' some of the affected individuals had complicating histories including preexisting immunosuppression or infection (Appendix 7).
Similar to GENEVA and SCORE, a number of case series' reported sustained improvements in BCVA and some reported relapses or only transient improvements in BCVA (Batioglu 2007; Goff 2006; Gregori 2006; Ip 2003; Ip 2004; Krepler 2005; Moschos 2007; Ozdek 2005; Park 2003; Williamson 2005). They also agree that improvements in macular edema are transient. On the other hand, individual case reports indicated that patients were more likely to have improved BCVA (Al‐Dhibi 2007; Ip 2002; Jonas 2002; Karacorlu 2004; Paques 2005).
Authors' conclusions
Implications for practice.
In the original version of this review, it was not possible to draw reliable conclusions about the benefits and harms from intravitreal steroids in people with CRVO‐ME. Despite the completion of two RCTs (GENEVA; SCORE) uncertainty remains regarding the overall benefits of intravitreal steroids in terms of reducing vision loss associated with CRVO or improving vision compared with no treatment. Furthermore, there is a clear increase in adverse events when using intravitreal steroids including increased IOP, cataract progression, and retinal detachment.
Implications for research.
In this review we evaluated two different agents and methods of administering intravitreal steroids (triamcinolone acetonide injection and dexamethasone implant). We did not plan to make a head‐to‐head comparison of these two methods for corticosteroid delivery, but it is possible that the type of delivery (injection versus implant) and the length of time over which the steroid is delivered, as well as the type of steroid itself, could affect outcomes. Results from both GENEVA and SCORE suggest that retreatments may be necessary in order to maintain the benefits. The need for dexamethasone retreatment is further supported by a recent case study (Joshi 2013) and the unpublished data from GENEVA (Shyangdan 2010) which suggest that the effects of dexamethasone implants may not last for six months and may begin to wane after 60 days. In SCORE, participants in the 1 mg and 4 mg triamcinolone treatment groups were eligible for retreatment every four months, with participants in both groups having an average of two injections in the first 12 months. Therefore, the number and frequency of treatments and retreatments remains unclear.
Results from the SCORE study indicate sustained visual improvements when compared with observation up to 24 months after steroid injection, however about 25% of treated participants had significant visual loss. Thus, there is room for improvement in therapy. Results from CVOS 1995 indicated that visual acuity improvements may not be linked to ME changes, as also observed in SCORE. The failure of anatomical improvements to correlate with functional outcomes associated with the use of intravitreal steroids underscores the need to identify a possible alternative mechanism for the salvage of visual acuity. Research into alternate agents with other mechanisms of action, such as neuroprotective factors, may prove fruitful. As multiple randomized controlled trials have shown success with anti‐VEGF treatments for CRVO‐ME (Braithwaite 2014), further research will need to incorporate these agents as a standard treatment for CRVO‐ME.
What's new
| Date | Event | Description |
|---|---|---|
| 9 September 2021 | Review declared as stable | This review is no longer being updated as the use of anti‐VEGF therapy has largely made this intervention obsolete. |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 1 July 2015 | New search has been performed | Issue 8, 2015: Searches updated 13 November 2014 |
| 1 July 2015 | New citation required but conclusions have not changed | Issue 8, 2015: Two studies included (GENEVA; SCORE) |
| 16 June 2010 | Amended | External source of support added. |
| 10 February 2009 | Amended | A reference has been added for the SCORE study. |
Acknowledgements
We thank the Cochrane Eyes and Vision Group (CEVG) editorial team for their assistance in devising and running the electronic searches as well as providing general advice towards the completion of this systematic review. We also thank Jennifer Christian, PharmD, MPH, PhD for assisting with assessment of the risk of bias of included primary studies. We thank Marilita Moschos, Maurizio Battaglia Parodi, and Roberta Scherer for their comments on earlier versions of the review and Barbara Hawkins and Nitin Verma for their peer review comments on this updated version.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Macular Edema, Cystoid #2 MeSH descriptor Edema #3 MeSH descriptor Macula Lutea #4 macula* near/3 oedema #5 macula* near/3 edema #6 CME or CMO #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 MeSH descriptor Retinal Vein Occlusion #9 MeSH descriptor Retinal Vein #10 retina* near/3 (vein* or occlu* or obstruct* or clos* or stricture* or steno* or block* or embolism*) #11 CRVO or CVO or RVO #12 (#8 OR #9 OR #10 OR #11) #13 MeSH descriptor Steroids #14 MeSH descriptor Triamcinolone #15 MeSH descriptor Triamcinolone Acetonide #16 triamcin* or acetonide or kenalog* #17 steroid* or glucorticoid* #18 (#13 OR #14 OR #15 OR #16 OR #17) #19 (#6 AND #12 AND #18)
Appendix 2. MEDLINE (OvidSP) search strategy
1 randomized controlled trial.pt. 2 (randomized or randomised).ab,ti. 3 placebo.ab,ti. 4 dt.fs. 5 randomly.ab,ti. 6 trial.ab,ti. 7 groups.ab,ti. 8 or/1‐7 9 exp animals/ 10 exp humans/ 11 9 not (9 and 10) 12 8 not 11 13 exp macular edema cystoid/ 14 exp edema/ 15 exp macula lutea/ 16 (macula$ adj3 oedema).tw. 17 (macula$ adj3 edema).tw. 18 (CME or CMO).tw. 19 or/13‐18 20 exp retinal vein occlusion/ 21 exp retinal vein/ 22 ((vein$ or occlu$ or obstruct$ or clos$ or stricture$ or steno$ or block$ or embolism$) adj3 retina$).tw. 23 (CRVO or CVO or RVO).tw. 24 or/20‐23 25 exp steroids/ 26 exp triamcinolone/ 27 exp triamcinolone acetonide/ 28 (triamcin$ or acetonide$ or kenalog$).tw. 29 (steroid$ or glucocorticoid$).tw. 30 or/25‐29 31 19 and 24 and 30 32 12 and 31
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1 exp randomized controlled trial/ 2 exp randomization/ 3 exp double blind procedure/ 4 exp single blind procedure/ 5 random$.tw. 6 or/1‐5 7 (animal or animal experiment).sh. 8 human.sh. 9 7 and 8 10 7 not 9 11 6 not 10 12 exp clinical trial/ 13 (clin$ adj3 trial$).tw. 14 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15 exp placebo/ 16 placebo$.tw. 17 random$.tw. 18 exp experimental design/ 19 exp crossover procedure/ 20 exp control group/ 21 exp latin square design/ 22 or/12‐21 23 22 not 10 24 23 not 11 25 exp comparative study/ 26 exp evaluation/ 27 exp prospective study/ 28 (control$ or prospectiv$ or volunteer$).tw. 29 or/25‐28 30 29 not 10 31 30 not (11 or 23) 32 11 or 24 or 31 33 exp retina macula cystoid edema/ 34 exp eye edema/ 35 exp retina macula lutea/ 36 (macula$ adj3 oedema).tw. 37 (macula$ adj3 edema).tw. 38 (CME or CMO).tw. 39 or/33‐38 40 exp retinal vein occlusion/ 41 exp retina vein/ 42 ((vein$ or occlu$ or obstruct$ or clos$ or stricture$ or steno$ or block$ or embolism$) adj3 retina$).tw. 43 (CRVO or CVO or RVO).tw. 44 or/40‐43 45 exp steroids/ 46 exp triamcinolone/ 47 exp triamcinolone acetonide/ 48 (triamcin$ or acetonide$ or kenalog$).tw. 49 (steroid$ or glucocorticoid$).tw. 50 or/45‐49 51 39 and 44 and 50 52 51 and 32
Appendix 4. metaRegister of Controlled Trials search strategy
(Central Retinal Vein Occlusion) AND (Macular Edema)
Appendix 5. ClinicalTrials.gov search strategy
Interventional Studies | macular edema AND retinal vein occlusion | (steroid OR glucocorticoid OR triamcinolone)
Appendix 6. ICTRP search strategy
Macular Edema = Title AND Central Retinal Vein Occlusion = Condition
Appendix 7. Other complications for intravitreal steroids: select studies
| Study (in chronological order) | Complication | Corollary notes |
| Clemens 2013 | Onset and formation of macular hole | ― |
| Turaka 2013 | Anterior migration of implant | Patient had history of pars plana vitrectomy and scleral buckle retinal detachment surgery; prior treatment for CRVO‐ME with multiple intravitreal bevacizumab and dexamethasone injections |
| Bakri 2012 | Worsening of vitreomacular traction | Patient had diabetes, hypertension, anemia and rheumatoid arthritis |
| Pardo‐Lopez 2012 | Diffuse corneal edema and anterior migration of implant | Patient treated for BRVO‐ME; patient had prior cataract surgery with intraoperative violation of the lens posterior capsula; implant removed and treated with corneal transplantation |
| Hirasawa 2009 | Secondary macular hole | Patient had mild ROP that resolved spontaneously |
| Mushtaq 2009 | Syphilitic infection of the eye | May have had previous syphilis infection that re‐activated locally |
| Sekiryu 2008 | CMV retinitis | Patient was immunocompetent |
| Roth 2008 | Corneal epithelial defect | Thought to be related to pretreatment with povidone‐iodine solution |
| Lattanzio 2007 | Macular hole progression | Occurred despite improvement in CME and VA |
| Konstantopoulos 2007 | Phacoanaphylactic glaucoma | Required vitrectomy with cataract surgery |
| Retinal detachment | In 2 patients with previous retinal tears | |
| Nkeme 2006 | Pseudo‐endophthalmitis | ― |
| Transient hypertony | Occurred in 53% eyes | |
| Full‐thickness macular hole | ― | |
| Jain 2006 | Pseudocataract | Result of adherence of triamcinolone particles to posterior lens |
| Aggermann 2006 | Endophthalmitis with retinal necrosis | Clinically resembles herpetic retinopathies |
| Yang 2005 | Refractory ocular hypertension | ― |
| Srinivasan 2005 | Conjunctival necrosis overlaying sclera entry site | Patient treated for BRVO‐ME |
| Kaushik 2004 | Intractable glaucoma | ― |
| Benz 2003 | Mycobacterium chelonae abscess | Patient treated for diabetic macular edema Eventual enucleation of eye |
Data and analyses
Comparison 1. 1 mg triamcinolone versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Gain of 15 letters or more (ETDRS) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 8 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 12 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.3 16 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.4 20 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.5 24 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Change in visual acuity (ETDRS) from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 8 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.3 16 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.4 20 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.5 24 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. 4 mg triamcinolone versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Gain of 15 letters or more (ETDRS) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 8 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.2 12 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.3 16 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.4 20 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1.5 24 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Change in visual acuity (ETDRS) from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 8 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.3 16 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.4 20 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.5 24 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Combined doses of triamcinolone versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Gain of 15 letters or more (ETDRS) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 8 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.2 12 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.3 16 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.4 20 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.5 24 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Change in visual acuity (ETDRS) from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 8 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.3 16 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.4 20 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.5 24 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
GENEVA.
| Study characteristics | ||
| Methods |
Study design: 2 parallel‐group, randomized controlled trials Number randomized: Total: 1267 (CRVO: 437): trial 1 – 599; trial 2 – 668 Per group: 427 DEX 0.70 mg; 414 DEX 0.35 mg; 426 sham (CRVO: 136 DEX 0.70 mg; 154 DEX 0.35 mg; 147 sham) Unit of randomization: one eye per participant (when both eyes eligible the eye with the shorter duration of macular edema was selected) Exclusions after randomization: Total: 71 (BRVO and CRVO) Per group: 24 DEX 0.70 mg; 19 DEX 0.35 mg; 28 sham Losses to follow‐up: Total: 5 (BRVO and CRVO) prior to day 180 Per group: 2 DEX 0.70 mg; 0 DEX 0.35 mg; 3 sham Number analyzed: Total: 1267 (CRVO: 437): trial 1 – 599; trial 2 – 668 Per group: 427 DEX 0.70 mg; 414 DEX 0.35 mg; 426 sham (CRVO: 136 DEX 0.70 mg; 154 DEX 0.35 mg; 147 sham) Unit of analysis: one eye per participant Analysis of outcomes: intention‐to‐treat analysis including all randomized participants for the primary and secondary efficacy variables; missing data were replaced by last‐observation‐carried‐forward method for all BCVA analyses, except Kaplan‐Meier analysis Sample size calculation: 495 eyes (165 per group) was estimated to provide 81% power for detecting an 11% difference between the DEX implant groups and sham in the proportion of eyes that achieved at least a 15 letter improvement in BCVA at day 180 |
|
| Participants |
Country: 167 clinical sites in 24 countries across the world Study period: recruitment November 2004 and March 2008 Age (mean): BRVO and CRVO combined: 65 years (range 33 to 90) DEX 0.70 mg; 65 years (range 31 to 96) DEX 0.35 mg; 64 years (range 31 to 91) sham Gender (% male): BRVO and CRVO combined: 50.8% DEX 0.70 mg; 53.1% DEX 0.35 mg; 56.3% sham Underlying conditions: diabetes mellitus, hypertension, coronary artery disease Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics? yes |
|
| Interventions |
Intervention 1: dexamethasone intravitreal implant (DEX) 0.35 mg Intervention 2: dexamethasone intravitreal implant (DEX) 0.70 mg Control: sham needleless intravitreal injection Length of follow up: Planned: 6 months Actual: 6 months, with additional 12 month open‐label extension Notes: approximately half of the participants (BRVO and CRVO) had their 6‐month follow‐up evaluation after 6‐months of treatment |
|
| Outcomes |
Primary outcome, as defined in the study: time to reach a 15 letter improvement in BCVA Secondary outcomes, as defined in the study:
Measurement of outcomes in the study: BCVA was measured using the ETDRS chart at 4 meters under standardized lighting conditions; retinal thickness was measured by collecting two OCT images at the screening visit and six images during the 90 and 180 day follow‐up visits; central retinal thickness was determined from the central 1‐mm macular subfield Other outcomes: safety parameters including IOP, slit‐lamp biomicroscopy, ophthalmoscopy, and adverse events. Presence of nuclear, cortical, and posterior subcapsular lens opacities was measured during the slit‐lamp examination. Intervals at which outcomes were assessed: Participants were evaluated at baseline and days 1, 7, 30, 60, 90, and 180 post‐treatment. BCVA, IOP, biomicroscopy, ophthalmoscopy, and adverse events were evaluated at each study visit. Fluorescein angiography and vital signs were assessed at baseline and day 180. Central retinal thickness was assessed at baseline, day 90, and day 180. Outcomes related to cost of interventions: not reported Outcomes related to quality of life: not reported |
|
| Notes |
Full study name: Global Evaluation of implaNtable dExamethasone in retinal Vein occlusion with macular edemA Publication types: all types (journal articles, conference abstracts, regulatory documents, and trial registry information) Trial registration number: NCT00168298; NCT00168324 Source of funding: sponsored by Allergan, Inc., which participated in the design of the study, data analysis, and interpretation, and supervised the preparation of the manuscript and approved the final version Declarations of interest: trial authors made several disclosures including serving as a consultant, equity owner, lecturer, or patent holder in pharmaceutical companies, including Allergan (manufacturer of the DEX implant) Subgroup analyses: prospectively planned subgroup analyses based on type of retinal vein occlusion (BRVO vs CRVO) and a post hoc analysis based on duration of macular edema at baseline (but not reported or available) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized to either a sham procedure or treatment with 0.35 mg or 0.70 mg DEX implant using a 1:1:1 allocation ratio. Randomization was performed centrally (using an interactive voice response system) and stratified by the underlying cause of RVO (BRVO or CRVO). |
| Allocation concealment (selection bias) | Low risk | Randomization was performed centrally (using an interactive voice response system) and stratified by the underlying cause of RVO (BRVO or CRVO). |
| Masking of participants and personnel of the allocated intervention during the study (performance bias) | High risk | Personnel administering treatments were not masked – sham had a needleless applicator. Participants were masked to dose of implant, but not to treatment (steroid implant versus no implant). |
| Masking of outcome assessors during follow‐up examination (detection bias) | Low risk | “…key efficacy variables were collected and evaluated by follow‐up investigators who were also masked with regard to study treatment.” A central reading center with masked graders was used to evaluate OCT measurements of central retinal thickness. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Combined incomplete outcome data from both trials including BRVO and CRVO participants were reported for the 6‐month analyses. The number of participants with missing data and reasons for exclusion were consistent across treatment arms. Incomplete outcome data for CRVO participants only were not reported and the risk of bias in this subgroup could not be determined for the 6‐month analyses. |
| Selective reporting (reporting bias) | High risk | Based on the information provided in the trial registry for both trials, contrast sensitivity, vessel leakage and quality of life assessment were described as secondary outcomes to be measured from 1 to 12 months. Results were not provided for these outcomes in the clinical trial registry results section, 6‐month report, or 12‐month report for either BRVO or CRVO participants. Macular thickness was described as a secondary outcome in the trial registry for one trial only (NCT00168298), but the 6‐month reported results used the pooled data from both trials to analyze this outcome at 6 months. The 6‐month report only described “key efficacy variables” for the prespecified CRVO subgroup analysis (time to achieve ≥ 15 letters improvement, proportion with ≥ 15 letters improvement, and mean change in BCVA from baseline to day 180). The secondary outcome for central retinal thickness was not reported for the CRVO subgroup. |
| Other bias | High risk | Two separate trials were conducted for regulatory purposes with different primary endpoints determined by the regulatory agency. For the first trial the proportion of participants with at least 15 letters improvement at 180 days was the primary outcome while the time to reach a 15‐letter improvement from baseline was the primary outcome for the second trial. Results from both trials were pooled in the 6‐month report to analyze both primary outcomes. Allergan, Inc. participated in the design, data analysis, and interpretation, and supervised the preparation of the manuscript. |
SCORE.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, randomized controlled trial Number randomized: Total: 271 Per group: 92 IVTA 1 mg; 91 IVTA 4 mg; 88 observation Unit of randomization: one eye per participant Exclusions after randomization: Total: none reported Per group: none reported Losses to follow‐up: Total: 33 Per group: 9 IVTA 1 mg; 9 IVTA 4 mg; 15 observation Number analyzed: Total: 238 at 12 months Per group: 83 IVTA 1 mg; 82 IVTA 4 mg; 73 observation Unit of analysis: one eye per participant Analysis of outcomes: "The primary analysis of the SCORE‐CRVO trial is based on an observed case analysis that analyzed participants based on the arm to which they were randomized (consistent with the intention‐to‐treat principle) and treated missing 12‐month observations as missing completely at random. To be included in the primary analysis, a study participant must have had 12‐month visual acuity within a window ranging from 2 months before the target date to 3 months after the target date, with the target date defined as 12 months after the date of randomization." Sample size calculation: "target sample size of 630 participants to be divided equally across three treatment arms. Due to slow recruitment, adjust to 486 participants. When it was determined about only half of the sample size was enrolled, a series of conditional power calculations were presented to the DSMB which determined the trial should continue." |
|
| Participants |
Country: 66 clinical sites in the USA Study period: recruitment November 2004 to February 2008 with follow‐up and study close‐out through February 2009 Age (mean): 68 years (range 27 to 93 years) Gender (% male): 55% Underlying conditions: 62 (23%) participants had diabetes mellitus; 197 (73%) had hypertension; 56 (21%) had coronary artery disease; and 58 (21%) had a history of cancer Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics? yes |
|
| Interventions |
Intervention 1: intravitreal triamcinolone acetonide 1 mg; preservative‐free, single‐use, 0.05 mL dose injected into the vitreous cavity via the pars plana 3 to 4 mm posterior to the limbus Intervention 2: intravitreal triamcinolone acetonide 4 mg; preservative‐free, single‐use, 0.05 mL dose injected into the vitreous cavity via the pars plana 3 to 4 mm posterior to the limbus Control: observation Eyes assigned to observation could receive intravitreal triamcinolone when there was a loss from baseline in BCVA letter score of 15 or more that was present at 2 consecutive 4‐month–interval visits. The decrease in visual acuity had to be a result of persistent or recurrent macular edema (not because of cataract or other abnormality) that was documented on OCT. If the above criteria were met, eyes assigned to observation could receive (but were not required to receive) intravitreal triamcinolone (4 mg dose, study formulation). Length of follow up: Planned: the prespecified primary efficacy evaluation was performed at month 12 Actual: 4‐month intervals through 12 months, then annually through 36 months |
|
| Outcomes |
Primary outcome, as defined in the study: the proportion of participants who experienced a gain in visual acuity of 15 letters or more from baseline to month 12, as assessed by electronic‐ETDRS Secondary outcomes, as defined in the study: mean change in visual acuity from baseline to 12 months. Additional secondary analyses were conducted to determine the consistency of the primary results which included a last‐observation‐carried‐forward analysis and a per protocol analysis that included only study eyes with 12‐month visual acuity data and excluded participants who, before 12 months, received an alternative treatment (i.e., treatment crossovers) or a non‐protocol treatment, who did not meet the eligibility criteria, or who did not receive the treatment assigned at randomization. Measurement of outcomes in the study: BCVA was measured using the electronic‐ETDRS method at 3 meters by a masked certified tester Other outcomes: other visual acuity measures included loss in visual acuity of 15 letters or more; imaging outcomes included OCT‐measured center point thickness (study eye only) and disc areas of fluorescein leakages Intervals at which outcomes were assessed: Participants were evaluated every 4 months through first 12 months and annually through 36 months. Additional safety visits were scheduled 4 days and 4 weeks after intravitreal triamcinolone injections. Stereoscopic color fundus photographs (7 fields) were taken of the study eye at baseline and at the annual visits. Three‐field photographs were taken of the study eye at the month 4, 8, 16, 20, 28, and 32 visits, and of the fellow eye at the baseline and annual visits. Fluorescein angiograms were performed at baseline and at the month 4, 12, and 24 visits. All images were sent to the reading center and graded by a masked observer. Outcomes related to cost of interventions: not reported Outcomes related to quality of life: not reported |
|
| Notes |
Full study name: Standard Care vs COrticosteroid for REtinal Vein Occlusion Publication types: all types (journal articles, conference abstracts, regulatory documents, and trial registry information) Trial registration number: NCT00106132 Source of funding: National Eye Institute (USA) grants 5U10EY014351, 5U10EY014352, and 5U10EY014404. Allergan, Inc. donated investigational drug, partially funded site monitoring visits, and performed secondary data analyses. Declarations of interest: "The authors have no proprietary or commercial interest in any of the materials discussed in this article" Subgroup analyses: according to eyes with the following baseline characteristics: pseudophakic, duration of macular edema, visual acuity letter score, and OCT‐measured center point thickness |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "participants were randomly assigned centrally through a Web‐based data entry system maintained at the data coordinating center (EMMES Corporation, Rockville, Maryland), with equal probability to receive standard care (observation group), 1 mg of intravitreal triamcinolone, or 4 mg of intravitreal triamcinolone using a permuted blocks design with random block sizes" |
| Allocation concealment (selection bias) | Low risk | "participants were randomly assigned centrally through a Web‐based data entry system" |
| Masking of participants and personnel of the allocated intervention during the study (performance bias) | High risk | "Participants and physicians were masked to the intravitreal triamcinolone dose (1 mg vs 4 mg) but not to the treatment assignment of observation vs intravitreal triamcinolone." |
| Masking of outcome assessors during follow‐up examination (detection bias) | Low risk | Fluorescein angiograms and OCT results were assessed by a masked observer at the reading center. Visual acuity was measured at each visit by a masked technician. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with missing data at the 12‐month visit (which was the basis of the primary outcome analysis) was identical in both 1 mg and 4 mg triamcinolone intravitreal injection arms (10%). In the observation arm, 17% of participants had missing data compared with the 6.8% observed risk for the primary outcome. Reasons for missing data were not reported. Data were analyzed assuming all missing data were missing at random. |
| Selective reporting (reporting bias) | Low risk | Primary analysis reported as described in methods paper. All secondary analyses were reported according to prespecified methods as well. |
| Other bias | Unclear risk | Study drugs provided by pharmaceutical company (Allergan, Inc.) which also provided funding for study site visits, and conducted the secondary analyses. |
BCVA: best‐corrected visual acuity BRVO: branch retinal vein occlusion CRVO: central retinal vein occlusion DEX: dexamethasone ETDRS: Early Treatment Diabetic Retinopathy Study IOP: intraocular pressure IVTA: intravitreal triamcinolone acetonide OCT: optical coherence tomography
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bashshur 2004 | Not an RCT: prospective case series of 20 patients treated with an intravitreal injection of 4 mg of triamcinolone acetonide compared with a retrospective matched observation group |
| Batioglu 2007 | Not an RCT: case series of 20 patients treated with intravitreal injections of triamcinolone acetonide |
| Cheng 2009 | Not an RCT: prospective comparative non‐randomized interventional study of 22 patients who accepted an intravitreal injection of 4 mg of triamcinolone acetonide compared with 21 patients who refused intravitreal injection |
| Chuang 2010 | Not an RCT: retrospective cohort of 68 patients (39 with CRVO and 29 with BRVO); 36 patients who received an intravitreal injection of 2 mg of triamcinolone acetonide compared with 32 patients who received an intravitreal injection of 4 mg of triamcinolone acetonide |
| Gelston 2006 | Not an RCT: retrospective comparative case series of 9 patients treated with an intravitreal injection of 4 mg of triamcinolone acetonide compared with 10 patients managed by observation |
| Georgopoulos 2006 | Not an RCT: review of dexamethasone implants for macular edema, not limited to CRVO |
| Jain 2012 | Not an RCT: prospective case series of 23 patients treated with fluocinolone acetonide intravitreal implant |
| Jiang 2006 | Not an RCT: case series of 39 patients with CRVO or BRVO treated with intravitreal injections of triamcinolone acetonide |
| Jonas 2005a | Not an RCT: prospective comparative non‐randomized clinical interventional study of 12 patients who received an intravitreal injection of 20 to 25 mg of triamcinolone acetonide compared with 20 patients managed by observation |
| Manaviat 2008 | Did not meet inclusion criteria: "randomized case‐control trial" of 21 patients with macular edema and retinal vein occlusion (16 with CRVO and 5 with BRVO); 9 patients treated with an intravitreal injection of 4 mg of triamcinolone acetonide compared with 12 patients treated with aspirin and laser treatment; length of follow‐up was not consistent for all patients, range was 1 to 16 months and only three patients were followed up after 4 months |
| Muni 2010 | Not an RCT: commentary on SCORE study |
| Ramezani 2006 | Did not meet inclusion criteria: RCT of 13 participants treated with an intravitreal injection of 4 mg of triamcinolone acetonide compared with 14 patients receiving sham injection as control group; follow‐up was less than six months (4‐month follow‐up) |
| ROVO | Did not meet inclusion criteria: RCT of 90 participants with CRVO treated with radial optic neurotomy, intravitreal injection of 4 mg of triamcinolone acetonide, or sham injection; not all participants had macular edema (about 75% in the sham group) and no results are presented separately for the participants who had macular edema at baseline |
BRVO: branch retinal vein occlusion CRVO: central retinal vein occlusion RCT: randomized controlled trial SCORE: Standard Care vs COrticosteroid for REtinal Vein Occlusion study
Characteristics of ongoing studies [ordered by study ID]
NCT01660802.
| Study name | Safety and Efficacy Study of Dexamethasone in the Treatment of Patients With Macular Edema Following Retinal Vein Occlusion (RVO) |
| Methods | Randomized controlled trial |
| Participants |
Location: China Inclusion criteria: 18 years of age or older Presence of macular edema due to branch or central retinal vein occlusion Exclusion criteria: History of glaucoma, ocular hypertension or optic nerve head change Any active bacterial, viral, parasitic, or fungal infections in either eye History of use of intravitreal steroids or any intravitreal injectable drug in the study eye within three months prior to study start Use of oral, intravenous, intramuscular, antimetabolites, and/or alkylating agents within three months prior to study start Use of topical ophthalmic corticosteroids within two weeks of study start |
| Interventions |
Intervention 1: 700 μg Dexamethasone Posterior Segment Drug Delivery System (DEX PS DDS) intravitreal injection in the study eye on day one and month six Intervention 2: sham PS DDS intravitreal injection in the study eye on day one and 700 μg DEX PS DDS intravitreal injection month six |
| Outcomes |
Primary outcome: best‐corrected visual acuity at six months Secondary outcomes: central retinal thickness measured with optical computed tomography and fluorescein leakage on fluorescein angiography at six months |
| Starting date | August 2012 |
| Contact information | Allergan, Inc. (clinicaltrials@allergan.com) |
| Notes | Estimated enrollment: 260 Estimated study completion date: August 2014 Estimated primary completion date: June 2014 (final data collection date for primary outcome measure) Responsible party: Allergan ClinicalTrials.gov identifier: NCT01660802 Other study ID numbers: 206207‐020 Last updated: March 20, 2013 Health Authority: China: Food and Drug Administration |
Differences between protocol and review
Our review protocol stated that we would include trials with any dosage and duration of treatment (Gewaily 2008); however, after further reflection, we felt that duration of treatment less than six months may not be appropriate to compare steroid and observation groups. Intravitreal steroid treatment regimens generally consist of multiple injections administered every few months, in which case trials with less than six months duration may only measure the effects of a single injection, such as with SCORE in which injections were administered every four months. Thus, we modified the eligibility criteria to include trials with any dosage and duration of treatment of at least six months.
Due to the lack of comparability between treatment interventions between included studies, we did not conduct methods specified in the protocol for this review in regards to meta‐synthesis, subgroup analysis, and sensitivity analysis. We performed GRADE assessments of the quality of the evidence, which was not specified in the protocol for this review, but has become standard procedure for Cochrane reviews of treatment interventions.
Contributions of authors
Conceiving the review: CEVG, PBG Designing the review: PBG Coordinating the review: PBG Data collection for the review ‐ Designing search strategies: DG, PBG, CEVG ‐ Undertaking searches: CEVG, DG, PBG ‐ Screening search results: DG, PBG, KM ‐ Organizing retrieval of papers: DG ‐ Screening retrieved papers against inclusion criteria: DG, PBG, KM ‐ Appraising quality of papers: DG, PBG, KM ‐ Extracting data from papers: DG, PBG, KM ‐ Writing to authors of papers for additional information: DG, PBG ‐ Providing additional data about papers: DG, PBG ‐ Obtaining and screening data on unpublished studies: CEVG, DG, PBG Data management for the review ‐ Entering data into RevMan: DG, KM ‐ Analysis of data: DG, PBG, KM Interpretation of data ‐ Providing a methodological perspective: DG, PBG, KM ‐ Providing a clinical perspective: PBG ‐ Providing a policy perspective: PBG ‐ Providing a consumer perspective: DG, PBG Writing the review: DG, PBG, KM Providing general advice on the review: PBG Securing funding for the review: PBG Performing previous work that was the foundation of the current study: PBG Updating the review: DG, PBG, KM
Sources of support
Internal sources
Section of Ophthalmology, VA Medical Center, Providence, RI, USA
Dept. of Ophthalmology at Rhode Island Hospital, USA
External sources
Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA
-
National Institute of Health Research (NIHR), UK
Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
DG, PBG, KM: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
GENEVA {published data only}
- Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology 2011;118(12):2453-60. [DOI] [PubMed] [Google Scholar]
- Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117(6):1134-46. [DOI] [PubMed] [Google Scholar]
- Haller JA, Blumenkranz MS, Williams GA, Kuppermann BD. Treatment of persistent macular edema associated with central and branch retinal vein occlusion with extended delivery of intravitreal dexamethasone. Investigative Ophthalmology and Visual Science 2003;44(2):ARVO E-abstract 4311.
- Kuppermann BD, Haller JA, Bandello F, Loewenstein A, Jiao J, Li XY, et al. Onset and duration of visual acuity improvement after dexamethasone intravitreal implant in eyes with macular edema due to retinal vein occlusion. Retina 2014;34(9):1743-9. [DOI] [PubMed] [Google Scholar]
- Yeh WS, Haller JA, Lanzetta P, Kuppermann BD, Wong TY, Mitchell P, et al. Effect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implant. Ophthalmology 2012;119(6):1190-8. [DOI] [PubMed] [Google Scholar]
SCORE {published data only}
- Blodi BA, Domalpally A, Scott IU, Ip MS, Oden NL, Elledge J, et al. Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study system for evaluation of stereoscopic color fundus photographs and fluorescein angiograms: SCORE study report 9. Archives of Ophthalmology 2010;128(9):1140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domalpally A, Blodi BA, Scott IU, Ip MS, Oden NL, Lauer AK, et al. The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study system for evaluation of optical coherence tomograms: SCORE study report 4. Archives of Ophthalmology 2009;127(11):1461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip MS, Oden NL, Scott IU, VanVeldhuisen PC, Blodi BA, Figueroa M, et al. SCORE study report 3: study design and baseline characteristics. Ophthalmology 2009;116(9):1770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Archives of Ophthalmology 2009;127(9):1101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Erratum. Archives of Ophthalmology 2009;127(12):1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D, Blodi B, Ip M, Scott I, Warren K. Reading center evaluation of OCT images from patients enrolled in the Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) Study. Investigative Ophthalmology and Visual Science 2006;47:ARVO E-abstract 5194.
- Scott IU, Oden NL, VanVeldhuisen PC, Ip MS, Blodi BA, Antoszyk AN, et al. SCORE study report 7: incidence of intravitreal silicone oil droplets associated with staked-on vs luer cone syringe design. American Journal of Ophthalmology 2009;148(5):725-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IU, VanVeldhuisen PC, Oden NL, Ip MS, Blodi BA, Hartnett ME, et al. Baseline predictors of visual acuity and retinal thickness outcomes in patients with retinal vein occlusion: Standard Care Versus COrticosteroid for REtinal Vein Occlusion Study report 10. Ophthalmology 2011;118(2):345-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IU, VanVeldhuisen PC, Oden NL, Ip MS, Blodi BA, SCORE Study Investigator Group. SCORE study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology 2009;116(3):504-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg DV, Wahle AE, Ip MS, Scott IU, VanVeldhuisen PC, Blodi BA, et al. SCORE study report 12: development of venous collaterals in the SCORE study. Retina 2013;33(2):287-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bashshur 2004 {published data only}
- Bashshur ZF, Ma'luf RN, Allam S, Jurdi FA, Haddad RS, Noureddin BN. Intravitreal triamcinolone for the management of macular edema due to nonischemic central retinal vein occlusion. Archives of Ophthalmology 2004;122(8):1137-40. [DOI] [PubMed] [Google Scholar]
Batioglu 2007 {published data only}
- Batioglu F, Ozmert E, Akmese E. Two-year results of intravitreal triamcinolone acetonide injection for the treatment of macular edema due to central retinal vein occlusion. Annals of Ophthalmology 2007;39(4):307-12. [DOI] [PubMed] [Google Scholar]
Cheng 2009 {published data only}
- Cheng KC, Wu WC, Lin CJ. Intravitreal triamcinolone acetonide for patients with macular oedema due to central retinal vein occlusion in Taiwan. Eye 2009;23(4):849-57. [DOI] [PubMed] [Google Scholar]
Chuang 2010 {published data only}
- Chuang LH, Yeung L, Wang NK, Chen HS, Ku WC, Lai CC. Secondary ocular hypertension after intravitreal injection with 2 mg or 4 mg of triamcinolone in retinal vein occlusion. Journal of Ocular Pharmacology and Therapeutics 2010;26(4):325-8. [DOI] [PubMed] [Google Scholar]
Gelston 2006 {published data only}
- Gelston CD, Olson JL, Mandava N. Macular oedema in central retinal vein occlusion treated with intravitreal triamcinolone. Acta Ophthalmologica Scandinavica 2006;84(3):314-8. [DOI] [PubMed] [Google Scholar]
Georgopoulos 2006 {published data only}
- Georgopoulos M, Sacu S, Vecsei PV, Michels S, Kiss C, Scholda C, et al. Therapy of macular edema with an intravitreal dexamethasone implant. Spektrum Der Augenheilkunde 2006;20(5):231-3. [Google Scholar]
Jain 2012 {published data only}
- Jain N, Stinnet S, Jaffe GJ. Prospective study of a fluocinolone acetonide implant for chronic macular edema from central retinal vein occlusion: thirty-six-month results. Ophthalmology 2012;119(1):132-7. [DOI] [PubMed] [Google Scholar]
Jiang 2006 {published data only}
- Jiang Y, Wang K, Li X. Clinical observation of intravitreal injection of triamcinolone acetonide for macular edema secondary to retinal vein occlusion. Chinese Ophthalmic Research 2006;24(6):639-42. [Google Scholar]
Jonas 2005a {published data only}
- Jonas JB, Akkoyun I, Kamppeter B, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide for treatment of central retinal vein occlusion. European Journal of Ophthalmology 2005;15(6):751-8. [DOI] [PubMed] [Google Scholar]
Manaviat 2008 {published data only}
- Manaviat MR, Rashidi M. Intravitreal triamcinolone acetonide for macular edema in retinal vein occlusion. International Journal of Ophthalmology 2008;8(2):230-3. [Google Scholar]
Muni 2010 {published data only}
- Muni RH, Kertes PJ, Kohly RP. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care versus Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5: comment. Evidence-Based Ophthalmology 2010;11(1):13-5. [Google Scholar]
Ramezani 2006 {published data only}
- Ramezani A, Entezari M, Moradian S, Tabatabaei H, Kadkhodaei S. Intravitreal triamcinolone for acute central retinal vein occlusion; a randomized clinical trial. Graefes Archive for Clinical and Experimental Ophthalmology 2006;244(12):1601-6. [DOI] [PubMed] [Google Scholar]
ROVO {published data only}
- Aggermann T, Brunner S, Krebs I, Haas P, Womastek I, Brannath W, et al. A prospective, randomised, multicenter trial for surgical treatment of central retinal vein occlusion: results of the Radial Optic Neurotomy for Central Vein Occlusion (ROVO) study group. Graefe's Archive for Clinical and Experimental Ophthalmology 2013;251(4):1165-72. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01660802 {unpublished data only}
- NCT01660802. Safety and efficacy study of dexamethasone in the treatment of patients with macular edema following retinal vein occlusion (RVO). clinicaltrials.gov/ct2/show/NCT01660802 (accessed 3 February 2014).
Additional references
Aggermann 2006
- Aggermann T, Stolba U, Brunner S, Binder S. Endophthalmitis with retinal necrosis following intravitreal triamcinolone acetonide injection. Ophthalmologica 2006;220(2):131-3. [DOI] [PubMed] [Google Scholar]
Al‐Dhibi 2007
- Al-Dhibi H, Chaudhry IA, Al-Saati A, Shamsi FA. Efficacy of intravitreal triamcinolone for macular oedema due to CRVO after anti-androgen therapy for hirsutism in a young monocular female. British Journal of Ophthalmology 2007;91(11):1564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Antcliff 2001
- Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, ffytche TJ, Marshall J. Intravitreal triamcinolone for uveitic cystoid macular oedema: an optical coherence tomography study. Ophthalmology 2001;108(4):765-72. [DOI] [PubMed] [Google Scholar]
Bakri 2012
- Bakri SJ, Omar AF. Evolution of vitreomacular traction following the use of the dexamethasone intravitreal implant (Ozurdex) in the treatment of macular edema secondary to central retinal vein occlusion. Journal of Ocular Pharmacology and Therapeutics 2012;28(5):547-9. [DOI] [PubMed] [Google Scholar]
Benz 2003
- Benz MS, Murray TG, Dubovy SR, Katz RS, Eifrig CW. Endophthalmitis caused by mycobacterium chelonae abscessus after intravitreal injection of triamcinolone. Archives of Ophthalmology 2003;121(2):271-3. [DOI] [PubMed] [Google Scholar]
Braithwaite 2014
- Braithwaite T, Nanji AA, Lindsley K, Greenberg PB. Anti-vascular endothelial growth factor for macular oedema secondary to central retinal vein occlusion. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD007325. [DOI: 10.1002/14651858.CD007325.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clemens 2013
- Clemens CR, Bertelmann T, Meyer CH. Vitreous traction after Ozurdex injection. Journal of Ocular Pharmacology and Therapeutics 2013;29(1):3-4. [DOI] [PubMed] [Google Scholar]
Conway 2003
- Conway MD, Canakis C, Livir-Rallatos C, Peyman GA. Intravitreal triamcinolone acetonide for refractory chronic pseudophakic cystoid macular oedema. Journal of Cataract and Refractive Surgery 2003;29(1):27-33. [DOI] [PubMed] [Google Scholar]
COPERNICUS 2013
- Brown DM, Heier JS, Clark WL, Boyer DS, Vitti R, Berliner AJ, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. American Journal of Ophthalmology 2013;155(3):429-37. [DOI] [PubMed] [Google Scholar]
CRUISE 2007
- Varma R, Bressler NM, Suñer I, Lee P, Dolan CM, Ward J, et al. Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: results from the BRAVO and CRUISE trials. Ophthalmology 2012;119(10):2108-18. [DOI] [PubMed] [Google Scholar]
CVOS 1995
- The Central Vein Occlusion Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology 1995;102(10):1425-33. [DOI] [PubMed] [Google Scholar]
CVOS 1997
- The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Archives of Ophthalmology 1997;115(4):486-91. [DOI] [PubMed] [Google Scholar]
Eye Disease Case‐Control Study 1996
- The Eye Disease Case-Control Study Group. Risk factors for central retinal vein occlusion. Archives of Ophthalmology 1996;114(5):545-54. [PubMed] [Google Scholar]
GALILEO 2014
- Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, Schmidt-Erfurth U, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology 2014;121(1):202-8. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Goff 2006
- Goff MJ, Jumper JM, Yang SS, Fu AD, Johnson RN, McDonald HR, et al. Intravitreal triamcinolone acetonide treatment of macular edema associated with central retinal vein occlusion. Retina 2006;26(8):896-901. [DOI] [PubMed] [Google Scholar]
Green 1981
- Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Transactions of the American Ophthalmological Society 1981;79:371-422. [PMC free article] [PubMed] [Google Scholar]
Gregori 2006
- Gregori NZ, Rosenfeld PJ, Puliafito CA, Flynn HW Jr, Lee JE, Mavrofrides EC, et al. One-year safety and efficacy of intravitreal triamcinolone acetonide for the management of macular edema secondary to central retinal vein occlusion. Retina 2006;26(8):889-95. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Stern JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hirasawa 2009
Ip 2002
- Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusion. Archives of Ophthalmology 2002;120(9):1217-9. [PubMed] [Google Scholar]
Ip 2003
- Ip M, Kahana A, Altaweel M. Treatment of central retinal vein occlusion with triamcinolone acetonide: an optical coherence tomography study. Seminars in Ophthalmology 2003;18(2):67-73. [DOI] [PubMed] [Google Scholar]
Ip 2004
- Ip MS, Gottlieb JL, Kahana A, Scott IU, Altaweel MM, Blodi BA, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Archives of Ophthalmology 2004;122(8):1131-6. [DOI] [PubMed] [Google Scholar]
Jain 2006
- Jain A, Vishwanath MR, Charles SJ. Triamcinolone pseudo-cataract. Annals of Ophthalmology 2006;38(1):67-8. [DOI] [PubMed] [Google Scholar]
Jonas 2002
- Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular oedema in central retinal vein occlusion. Graefes Archive for Clinical and Experimental Ophthalmology 2002;240(9):782-3. [DOI] [PubMed] [Google Scholar]
Jonas 2005b
- Jonas JB. Intravitreal triamcinolone acetonide for treatment of intraocular oedematous and neovascular diseases. Acta Ophthalmologica Scandinavica 2005;83(6):645-63. [DOI] [PubMed] [Google Scholar]
Joshi 2013
- Joshi L, Yaganti S, Gemenetzi M, Lightman S, Lindfield D, Liolios V, et al. Dexamethasone implants in retinal vein occlusion:12-month clinical effectiveness using repeat injections as-needed. British Journal of Ophthalmology Online 2013;97(8):1040-4. [DOI] [PubMed] [Google Scholar]
Karacorlu 2004
- Karacorlu M, Ozdemir H, Karacorlu S. Intravitreal triamcinolone acetonide for the treatment of central retinal vein occlusion in young patients. Retina 2004;24(2):324-7. [DOI] [PubMed] [Google Scholar]
Kaushik 2004
- Kaushik S, Gupta V, Gupta A, Dogra MR, Singh R. Intractable glaucoma following intravitreal triamcinolone in central retinal vein occlusion. American Journal of Ophthalmology 2004;137(4):758-60. [DOI] [PubMed] [Google Scholar]
Kivilcim 2000
- Kivilcim M, Peyman GA, El-Dessouky ES, Kazi AA, Cheema R, Hegazy H. Retinal toxicity of triamcinolone acetonide in silicone-filled eyes. Ophthalmic Surgery and Lasers 2000;31(6):474-8. [PubMed] [Google Scholar]
Konstantopoulos 2007
- Konstantopoulos A, Williams CP, Newsom RS, Luff AJ. Ocular morbidity associated with intravitreal triamcinolone acetonide. Eye 2007;21(3):317-20. [DOI] [PubMed] [Google Scholar]
Krepler 2005
- Krepler K. Intravitreal triamcinolone acetonide in patients with macular oedema due to central retinal vein occlusion. Acta Ophthalmologica Scandinavica 2005;83(1):71-5. [DOI] [PubMed] [Google Scholar]
Lang 2007
- Lang Y, Zemel E, Miller B, Perlman I. Retinal toxicity of intravitreal Kenalog in albino rabbits. Retina 2007;27(6):778-88. [DOI] [PubMed] [Google Scholar]
Lattanzio 2007
- Lattanzio R, Ramoni A, Scotti F, Introini U. Macular hole and intravitreal injection of triamcinolone acetonide for macular edema due to central retinal vein occlusion. European Journal of Ophthalmology 2007;17(3):451-3. [DOI] [PubMed] [Google Scholar]
Mandelcorn 2007
- Mandelcorn MS, Mandelcorn E, Guan K, Adatia FA. Surgical macular decompression for macular edema in retinal vein occlusion. Canadian Journal of Ophthalmology 2007;42(1):116-22. [PubMed] [Google Scholar]
Mohamed 2007
- Mohamed Q, McIntosh RL, Saw SM, Wong TY. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology 2007;114(3):507-19. [DOI] [PubMed] [Google Scholar]
Moschos 2007
- Moschos MM, Brouzas D, Loukianou E, Apostolopoulos M, Moschos M. Intraocular triamcinolone acetonide for macular edema due to CRVO. A multifocal-ERG and OCT study. Documenta Ophthalmologica 2007;114(1):1-7. [DOI] [PubMed] [Google Scholar]
Mushtaq 2009
- Mushtaq B, Gupta R, Elsherbiny S, Murray PI. Ocular syphilis unmasked following intravitreal triamcinolone injection. Ocular Immunology and Inflammation 2009;17(3):213-5. [DOI] [PubMed] [Google Scholar]
Nauck 1997
- Nauck M, Roth M, Tamm M, Eickelberg O, Wieland H, Stulz P, et al. Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. American Journal of Respiratory Cell and Molecular Biology 1997;16(4):398-406. [DOI] [PubMed] [Google Scholar]
Nauck 1998
- Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. European Journal of Pharmacology 1998;341(2-3):309-15. [DOI] [PubMed] [Google Scholar]
Nkeme 2006
- Nkeme J, Glacet-Bernard A, Gnikpingo K, Zourdani A, Mimoun G, Mahiddine H, et al. Surgical treatment of persistent macular edema in retinal vein occlusion. Journal Francais d Opthalmologie 2006;29(7):808-14. [DOI] [PubMed] [Google Scholar]
Ozdek 2005
- Ozdek SC, Aydin B, Gürelik G, Bahçeci U, Hasanreisoglu B. Effects of intravitreal triamcinolone injection on macular edema and visual prognosis in central retinal vein occlusion. International Ophthalmology 2005;26(1-2):27-34. [DOI] [PubMed] [Google Scholar]
Paques 2005
- Paques M, Krivosic V, Girmens JF, Giraud C, Sahel J, Gaudric A. Decreased venous tortuosity associated with resolution of macular edema after intravitreal injection of triamcinolone. Retina 2005;25(8):1099-101. [DOI] [PubMed] [Google Scholar]
Pardo‐Lopez 2012
Park 2003
- Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. American Journal of Ophthalmology 2003;136(3):419-25. [DOI] [PubMed] [Google Scholar]
Pe'er 1998
- Pe'er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology 1998;105(3):412-6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Roth 2008
- Roth DB, Realini T, Feuer WJ, Radhakrishnan R, Gloth J, Heimmel MR, et al. Short-term complications of intravitreal injection of triamcinolone acetonide. Retina 2008;28(1):66-70. [DOI] [PubMed] [Google Scholar]
Ruiz‐Morena 2007
- Ruiz-Moreno JM, Montero JA, Bayon A, Rueda J, Vidal M. Retinal toxicity of intravitreal triamcinolone acetonide at high doses in the rabbit. Experimental Eye Research 2007;84(2):342-8. [DOI] [PubMed] [Google Scholar]
Sekiryu 2008
- Sekiryu T, Iida T, Kaneko H, Saito M. Cytomegalovirus retinitis after intravitreal triamcinolone acetonide in an immunocompetent patient. Japanese Journal of Ophthalmology 2008;52:414-6. [DOI] [PubMed] [Google Scholar]
Shyangdan 2010
- Shyangdan D, Cummins E, Lois N, Royle P, Waugh N. Dexamethasone implants in the treatment of macular oedema due to retinal vein occlusion: a single technology appraisal. www.nice.org.uk/guidance/ta229/resources/guidance-dexamethasone-intravitreal-implant-for-the-treatment-of-macular-oedema-secondary-to-retinal-vein-occlusion-pdf (accessed 5 May 2014).
Srinivasan 2005
- Srinivasan S, Prasad S. Conjunctival necrosis following intravitreal injection of triamcinolone acetonide. Cornea 2005;24(8):1027-8. [DOI] [PubMed] [Google Scholar]
Turaka 2013
- Turaka K, Kwong HM Jr, De Souza S. Intravitreal implant migration into anterior chamber in a post-vitrectomy eye with central retinal vein occlusion and persistent macular edema. Ophthalmic Surgery, Lasers and Imaging Retina 2013;44(2):196-7. [DOI] [PubMed] [Google Scholar]
Williamson 2005
- Williamson TH, O'Donnell A. Intravitreal triamcinolone acetonide for cystoid macular edema in nonischemic central retinal vein occlusion. American Journal of Ophthalmology 2005;139(5):860-6. [DOI] [PubMed] [Google Scholar]
Yang 2005
- Yang CS, Chen MJ, Chou CK, Hsu WM. Refractory severe ocular hypertension after intravitreal triamcinolone acetonide injection. Ophthalmologica 2005;219(6):413-5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gewaily 2008
- Gewaily D, Greenberg PB. Intravitreal steroids versus observation for macular edema secondary to central retinal vein occlusion. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD007324. [DOI: 10.1002/14651858.CD007324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gewaily 2009
- Gewaily D, Greenberg PB. Intravitreal steroids versus observation for macular edema secondary to central retinal vein occlusion. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007324. [DOI: 10.1002/14651858.CD007324.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


