Abstract
Mouse pancreatic stem cells have been isolated from mouse pancreata. This study evaluated the efficacy of isolating mouse pancreatic stem cells using mice of different ages. The pancreata of newborn mice, 8-week-old mice, and 24-week-old mice were harvested and digested by using collagenase. The “duct-like” cells in the digested pancreatic tissue were then inoculated into 96-well plates, cloned by limiting dilution, and cultured in DMEM with 20% FBS. Pancreatic stem cells were isolated from the pancreata of all newborn mice, while cells could only be isolated from 10% of the pancreata of 8-week-old mice and could not be isolated from the pancreata of any 24-week-old mice. These data suggest that young mice may have some pancreatic stem cells and that older mice may only have a few pancreatic stem cells. These data also indicate that it is extremely difficult to isolate pancreatic stem cells from older mice, suggesting that future research focus its efforts on finding methods of isolating pancreatic stem cells from adult mice.
Keywords: Mouse pancreatic stem cells, Age dependent, Diabetes, ES medium, Feeder cells, Pancreatic islet transplantation
INTRODUCTION
Diabetes is one of the most increasingly prevalent and serious metabolic diseases. The reduction of insulin biosynthesis by pancreatic β-cells is closely associated with the onset and progression of diabetes. It is therefore important to search for ways to produce sufficient numbers of insulin-producing cells for transplantation in diabetes. While there is renewed interest in islet transplantation due to the recent success of this procedure (8,12,16,17,19,20,22), efforts are hindered by the limited supply of donor pancreata. Pancreatic stem/progenitor cells could become a useful tool for β-cell replacement therapy in diabetic patients since the cells are abundantly available in the pancreas of these patients and in donor organs. It was thought that pancreatic stem/progenitor cells were predominantly derived from the precursor cells residing among pancreatic epithelial duct cells or duct-associated cells both during embryonic development and later in life (1). Islet cell neogenesis from ducts has been observed in experimental injury models in rats (1,26) and in transgenic mice overexpressing certain growth factors or cytokines (5,23,27). Mouse pancreatic stem cells were recently established from the pancreata of newborn mice without genetic manipulation (17). These pancreatic stem cells have the potential to differentiate into not only insulin-producing cells but also hepatocytes (17,27). The isolation technique used might be useful for identification and isolation of human pancreatic stem/progenitor cells. This study attempted to isolate pancreatic stem cells from the pancreata of newborn mice, 8-week-old mice, and 24-week-old mice, in order to evaluate the isolation efficiency of mouse pancreatic stem cells.
MATERIALS AND METHODS
Isolation and Culture of Mouse Pancreatic Stem Cells and Islets
The review committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences approved these studies. Islets were removed from newborn (0-week-old), 8-week-old, and 24-week-old C57BL/6 mice (CLEA Japan, Inc., Meguro, Tokyo, Japan) using a modified method reported previously (17). Briefly, 2 ml of cold M199 medium (Life Technologies Japan, Tokyo, Japan) containing 2 mg/ml collagenase (Roche Boehringer Mannheim, Indianapolis, IN, USA) was injected into the cannulated common bile duct (14). The pancreas was removed, and an Optiprep® density gradient (Sigma-Aldrich, St. Louis, MO, USA) was used to isolate the islets.
The tissue collagenase was digested (2 mg/ml) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies Japan) with 20% lot-limited fetal bovine serum (FBS; BIO-WEST, Inc., Logan, UT, USA; S1560 Lot. #SO5094S1560). Once the cells had attached and spread, cells with a fibroblast morphology (nonductal cells) were removed using a rubberscrapper (Life Technologies Japan). The “duct-like” cells were cultured in DMEM with 20% FBS in 96-well plates (Life Technologies Japan) and cloned by limiting dilution.
After single cell cloning, the mouse pancreatic stem cells were maintained in specific culture condition with lot-limited FBS (17) during the early studies (first study using a 0-week-old pancreas and first to fifth studies using 8-week-old pancreata) or in culture condition of mouse ES cells (18) during the later studies (studies except first study using a 0-week-old pancreas and first to fifth studies using 8-week-old pancreata).
The current study maintained mouse pancreatic stem cells in DMEM with 20% FBS (BIO-WEST, Inc., S1560 Lot. #SO5094S1560) during the early studies (first study using a 0-week-old pancreas and first to fifth studies using 8-week-old pancreata) or complete ES cell media with 15% FBS (Millipore, Billerica, MA, USA) on feeder layers of mitomycin C (Sigma-Aldrich)-treated STO cells [Sandos inbred mice fibroblast cell line with 6-thioguanine and ouabain resistance; American Type Culture Collection (ATCC), Manassas, VA, USA] during the later studies (studies except first study using a 0-week-old pancreas and first to fifth studies using 8-week-old pancreata).
ES Cell Culture and Differentiation
Mouse ES cells (ATCC) were maintained in and differentiated using a modification of a method that was reported previously (3,7,17). ES cells differentiated into definitive endoderm in stage 1, into gut tube endoderm in stage 2, and into pancreatic progenitors in stage 3.
Semiquantitative RT-PCR
Total RNA was extracted from cells using a method that was reported previously (13). Semiquantitative RT-PCR was performed using a modification of a method that was reported previously (17). In brief, the RNA was reverse-transcribed into cDNA using SuperScriptII Reverse Transcriptase (Life Technologies Japan). Polymerization reactions were performed in a Perkin-Elmer 9700 Thermocycler with 3 μl cDNA (20 ng RNA equivalents), 160 μmol/L cold dNTPs, 10 pmol appropriate oligonucleotide primers, 1.5 mmol/L MgCl2, and 5 U AmpliTaq Gold DNA polymerase (Perkin-Elmer, Norwalk, CT, USA). The oligonucleotide primers and cycle number used for semiquantitative PCR are shown in Table 1. The steps taken to vali-date these measurements were previously reported (13).
Table 1.
List of Gene-Specific Primers
| Gene | Forward/Reverse Primer |
|---|---|
| Sox17 | ctgccctgccgggatggcacggaatc/ttctggccctcaggtcgggtcggcaac |
| Foxa2 | tggtcactggggacaagggaa/gcaacaacagcaatagagaac |
| Hnf1β | cacagccctcaccagcagcc/gactgcctgggctctgctgc |
| Hnf4α | acacgtccccatctgaaggtg/cttccttcttcatgccagccc |
| Pdx1 | cctgcgtgcctgtacatggg/tttccacgcgtgagctttgg |
| Hnf6 | gggtgagccatgagccggtg/catagccgcgccgggatgag |
| Insulin-1 | ccagctataatcagagacca/gtgtagaagaagccacgct |
| Insulin-2 | tccgctacaatcaaaaaccat/gctgggtagtggtgggtcta |
| Glucagon | actcacagggcacattcacc/ccagttgatgaagtccctgg |
| NeuroD | gcgctcaggcaaaagccc/gccattgatgctgagcggcg |
| Pax6 | cagtcacagcggagtgaatc/cgcttcagctgaagtcgcat |
| Isl-1 | agatatgggagacatgggcgat/acacagcggaaacactcgatg |
| GAPDH | accacagtccatgccatcac/tccaccaccctgttgctgta |
Sox 17, sex-determining region Y-box17; Foxa2, forkhead box protein a2; Hnf, hepatocyte nuclear factor; Pdx1; pancreatic and duodenal homeobox factor-1; NeuroD, neuronal differentiation 1; Isl-1, Islet or Insulin gene enhancer protein; Pax6, paired box gene 6; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Cell Induction and Differentiation Into Insulin-Producing Cells
Cell induction was performed using a modification of a method that was reported previously (17). In brief, the cells were cultured in DMEM with 10% FBS, 10 nM exendin-4, 10 mM nicotinamide, 10 ng/ml keratinocyte growth factor (KGF; all from Sigma-Aldrich), 100 nM pancreatic and duodenal homeobox factor 1 (Pdx1) protein, and 100 nM β-cell E-box transactivator/neuronal differentiation 1 (BETA2/NeuroD) proteins for 2 weeks. Pdx1 and BETA2/NeuroD proteins were generated by cDNA as previously described (18).
RESULTS
Isolation of Pancreatic Stem Cells
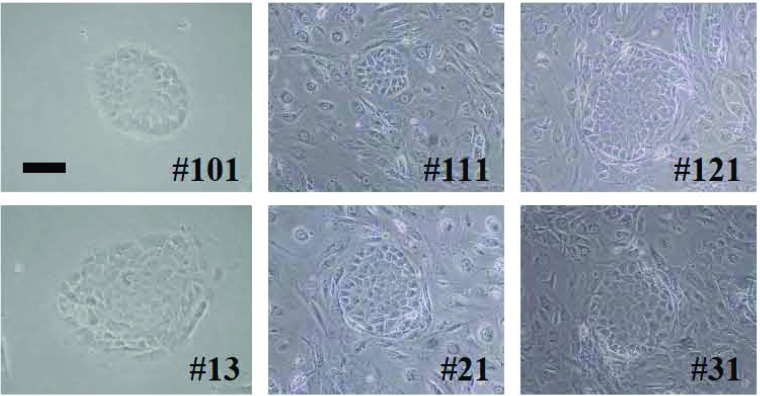
Pancreatic stem cells were isolated from all three pancreata from newborn mice (efficacy: 100%). On the other hand, pancreatic stem cells were isolated from only 3 of 30 pancreata of 8-week-old mice (10%) and from none of the 30 pancreata of 24-week-old mice (0%, Table 2). Each pancreatic stem cell formed “cobblestone” morphology (Fig. 1).
Table 2.
Isolation Efficacy of Mouse Pancreatic Stem Cells
| Old | Suc#/Iso# | Clone# | Name | |
|---|---|---|---|---|
| Mouse | 0 w | 3/3 (100%) | 6 | HN#101–106 |
| 4 | HN#111–114 | |||
| 8 | HN#121–128 | |||
| Mouse | 8 w | 3/30 (10%) | 15 | HN#1–15 |
| 7 | HN#21–27 | |||
| 3 | HN#31–33 | |||
| Mouse | 24 w | 0/30 (0%) |
Suc#/Iso#, successful isolation number of pancreatic stem cells/total isolation number; w, weeks.
Figure 1.
Isolation and culture of pancreatic stem cells. Morphology of pancreatic stem cells from newborn (HN#101, HN#111, HN#121) and 8-week-old mice (HN#13, HN#21, HN#31). The “duct-like” cells from digested pancreatic tissue were inoculated onto 96-well plates, cloned by limiting dilution, and cultured in DMEM with 20% FBS or complete ES cell media on feeder layers of mitomycin C-treated STO cells (Sandos inbred mice fibroblast cell line with 6-thioguanine and ouabain resistance). Scale bar: 100 μm.
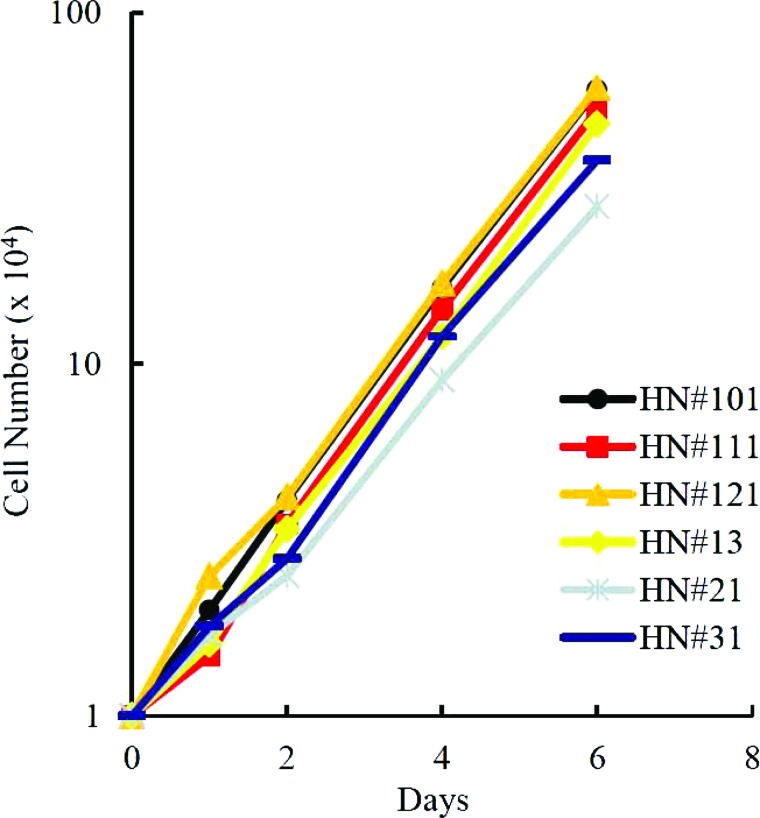
Growth Activity of the Pancreatic Stem Cells
One of the pancreatic stem cells from each isolate, named HN#101, HN#111, HN#121, HN#13, HN#21, or HN#23, was evaluated for growth activity. Each cell divided actively beyond population doubling level (PDL) 100 (passage 25) without growth inhibition (Fig. 2). These data suggest that pancreatic stem cells from different aged mice have a similar growth activity.
Figure 2.
Growth activity of the pancreatic stem cells. HN#101, HN#111, HN#121, HN#13, HN#21, and HN#31 cells were cultured in complete ES cell media on feeder layers of mitomycin C-treated STO cells and evaluated for their growth activity.
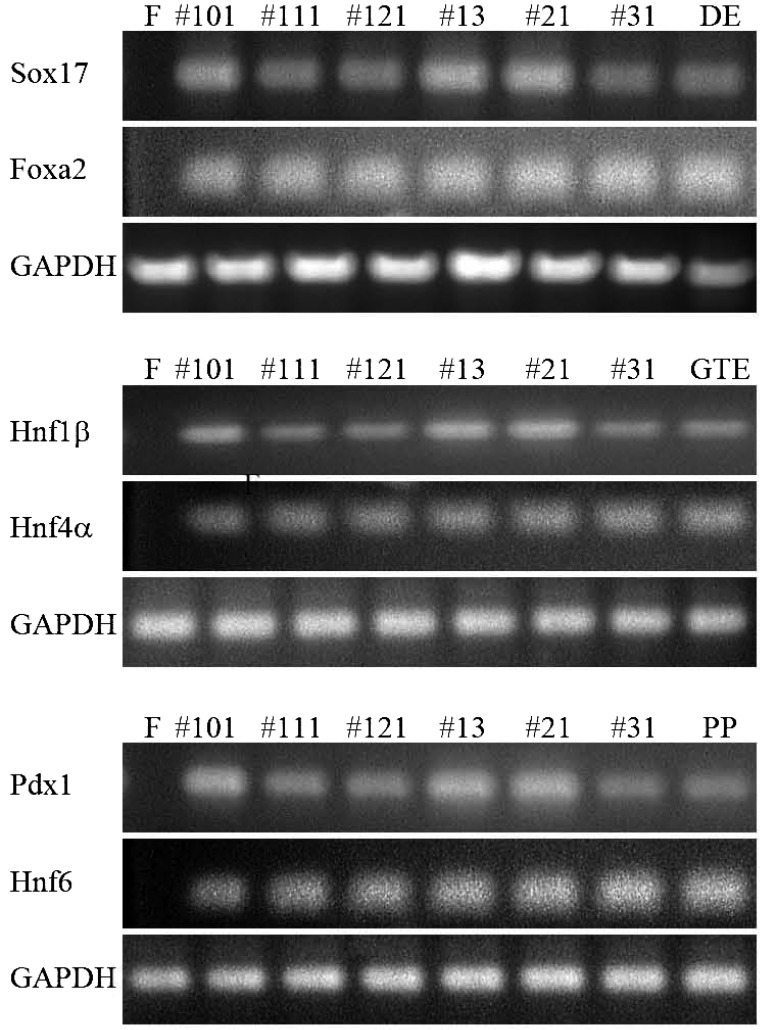
Gene Expression of the Pancreatic Stem Cells
An RT-PCR analysis of endodermal/pancreatic progenitor cell markers was performed to investigate gene expression in each clone. Differentiated cells from ES cells, generated by a stepwise differentiation protocol that relies on intermediates thought to be similar to cell populations present in the developing embryo (3,7), were used as a positive control. The marker gene expression patterns of the definitive endoderm [sex-determining region Y-box17 (Sox17), forkhead box protein a2 (Foxa2)], gut tube endoderm [hepatocyte nuclear factor 1β (HNF1β), HNF4α], and pancreatic progenitors (Hnf6, Pdx1) were detected in each cell (Fig. 3). These data suggest similar gene expression in each cell culture.
Figure 3.
Gene expression of the pancreatic stem cells in culture condition 3. RT-PCR analysis of endodermal/pancreatic progenitor cell markers was used to investigate gene expression in HN#101, HN#102, HN#103, HN#13, HN#21, and HN#31 cells. F, feeder cells (STO cells, negative control); #101, HN#101 cells at PDL 100; #111, HN#111 cells at PDL 100; #121, HN#121 cells at PDL 100; #13, HN#13 cells at PDL 100; #21, HN#21 cells at PDL 100; #31, HN#31 cells at PDL 100; DE, differentiated definitive endoderm cells from ES cells (positive control); GTE, differentiated gut tube endoderm cells from ES cells (positive control); PP, differentiated pancreatic progenitors cells from ES cells (positive control); Sox17, sex-determining region Y box 17; Foxa2, forkhead box A2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Hnf, hepatocyte growth factor; Pdx1, pancreatic and duodenal homeobox factor 1.
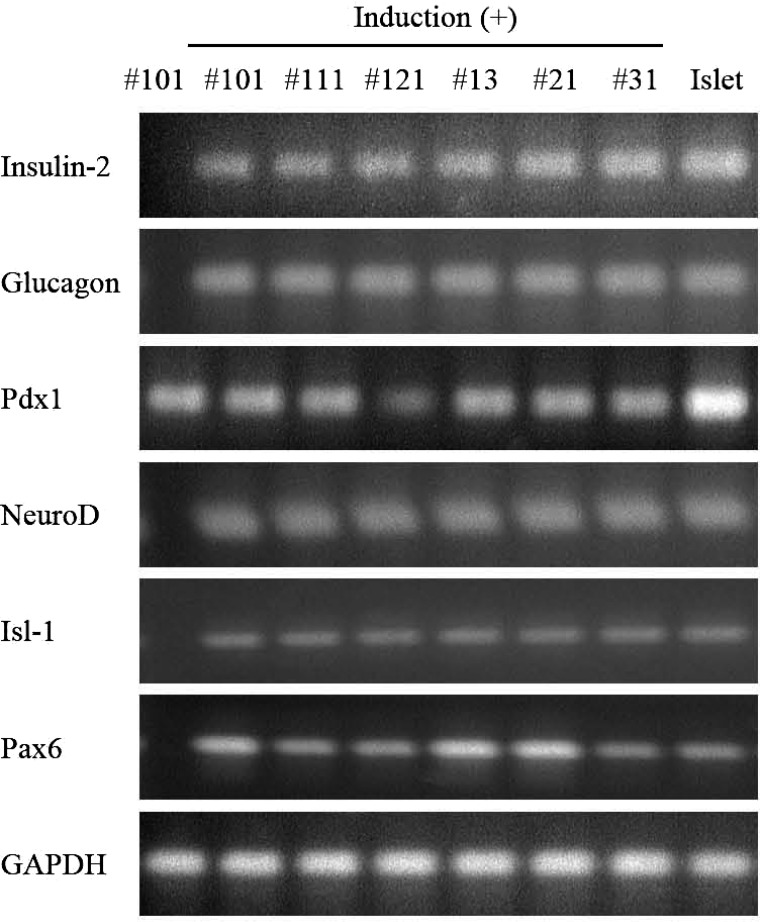
Differentiation Ability of the Pancreatic Stem Cells
HN#101, HN#111, HN#121 (all 0 week) and HN#13, HN#21, or HN#23 (all 8 weeks) cells were induced to differentiate into insulin-producing cells by Pdx1 and BETA2/NeuroD protein transduction (11,13) with exendin-4 for 2 weeks. Treatment induced the expression of insulin-2, BETA2/NeuroD, islet 1 (Isl-1), and paired box 6 (Pax6) transcription factors and glucagon in the differentiated cells (Fig. 4). These data suggest that each clone could differentiate into insulin-producing cells.
Figure 4.
Differentiation ability of the pancreatic stem cells after culture for 2 months. HN#101, HN#102, HN#103, HN#13, HN#21, and HN#31 cells were induced to differentiate into insulin-producing cells using 10 nM exendin-4, 10 mM nicotinamide, 10 ng/ml KGF, 100 nM Pdx1 protein, and 100nM BETA2/NeuroD protein for 2 weeks. PCR was performed in a Perkin-Elmer 9700 Thermocycler with 2 μl cDNA (20 ng RNA equivalent) from the cells. The oligonucleotide primers and cycle number used for semiquantitative PCR are shown in Table 1. #101, HN#101 cells at PDL 100; #111, HN#111 cells at PDL 100; #121, HN#121 cells at PDL 100; #13, HN#13 cells at PDL 100; #21, HN#21 cells at PDL 100; #31, HN#31 cells at PDL 100; Islet, mouse islets (positive control); Isl-1, islet 1; Pax6, paired homeobox 6.
DISCUSSION
This study evaluated the isolation efficacy of mouse pancreatic stem cells. Pancreatic stem cells were isolated from the pancreata of all newborn mice. On the other hand, the isolation efficacy of pancreatic stem cells from 8-week-old mice was only 10% and 0% from 24-week-old mice. These data suggest that young mice may have many pancreatic stem cells, while older mice may have only a few pancreatic stem cells. It is also extremely difficult to get clones of pancreatic stem cells from older mice. Indeed, human pancreatic stem cells could not be isolated from 20- to 60-year-old donors (15). Although it is not possible to conclude that there are few human pancreatic stem cells in older donors because the culture conditions for maintenance of human pancreatic stem cells have not yet been established, the evidence from mouse pancreatic stem cells may explain why human pancreatic stem cells have not been isolated from older donors.
As the number of diabetic patients continues to increase worldwide, pancreatic stem/progenitor cells could become a useful tool for β-cell replacement therapy since the cells are abundantly available in the pancreas of these patients and in donor organs (2,4,6,9,10,11,13,15,21,24,25). However, the technique for the isolation of pancreatic stem cells remains difficult, and it is necessary to find a more efficient process. The current study suggests that it is extremely difficult to isolate pancreatic stem cells from the pancreata of older mice, in particular. Furthermore, since the optimal culture conditions for the maintenance of human pancreatic stem cells have yet to be established, the current data may suggest that it will be extremely difficult to isolate human pancreatic stem cells. Further optimization of methods is needed to isolate human pancreatic stem cells and to maintain the undifferentiated fate of human pancreatic stem cells.
ACKNOWLEDGMENTS
The authors wish to thank Mr. Koji Oda and Ms. Noriko Imagawa (Okayama University) for their valuable technical support. This work was supported in part by the Japan Society for the Promotion of Science. The authors declare no conflict of interest.
REFERENCES
- 1. Bonner-Weir S.; Baxter L. A.; Schuppin G. T.; Smith F.E. A second pathway for regeneration of adult exocrine and endocrine pancreas: A possible recapitulation of embryonic development. Diabetes 42:1715–1720; 1993. [DOI] [PubMed] [Google Scholar]
- 2. Bonner-Weir S.; Taneja M.; Weir G. C.; Tatarkiewicz K.; Song K. H.; Sharma A.; O’Neil J. J. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA 97:7999–8004; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D’Amour K. A.; Banq A. G.; Eliazer S.; Kelly O. G.; Aqulnick A. D.; Smart N. G.; Moorman M. A.; Kroon E.; Carpenter M. K.; Baetqe E. E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24:1392–1401; 2006. [DOI] [PubMed] [Google Scholar]
- 4. Edlund H. Pancreas: How to get there from the gut? Curr. Opin. Cell Biol. 11:663–668; 1999. [DOI] [PubMed] [Google Scholar]
- 5. Gu D.; Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-gamma transgenic mice. Development 118:33–46; 1993. [DOI] [PubMed] [Google Scholar]
- 6. Heremans Y.; Van De Casteele M.; in’t Veld P.; Gradwohl G.; Serup P.; Madsen O.; Pipeleers D.; Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J. Cell Biol. 159:303–312; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kroon E.; Martinson L. A.; Kadoya K.; Banq A. G.; Kelly O. G.; Eliazer S.; Young H.; Richardson M.; Smart N. G.; Cunninqham J.; Aqulnick A. D.; D’Amour K. A.; Carpenter M. K.; Baetqe E. E. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26:443–452; 2008. [DOI] [PubMed] [Google Scholar]
- 8. Kuise T.; Noguchi H. Recent progress in pancreatic islet transplantation. World J. Transplant. 1:13–18; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noguchi H. Pancreatic stem/progenitor cells for the treatment of diabetes. Rev. Diabet. Stud. 7:105–111; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noguchi H. Production of pancreatic beta-cells from stem cells. Curr. Diabetes Rev. 6:184–190; 2010. [DOI] [PubMed] [Google Scholar]
- 11. Noguchi H.; Bonner-Weir S.; Wei F. Y.; Matsushita M.; Matsumoto S. BETA2/NeuroD protein can be transduced into cells due to an arginine- and lysine-rich sequence. Diabetes 54:2859–2866; 2005. [DOI] [PubMed] [Google Scholar]
- 12. Noguchi H.; Iwanaga Y.; Okitsu T.; Nagata H.; Yonekawa Y.; Matsumoto S. Evaluation of islet transplantation from non-heart beating donors. Am. J. Transplant. 6:2476–2482; 2006. [DOI] [PubMed] [Google Scholar]
- 13. Noguchi H.; Kaneto H.; Weir G. C.; Bonner-Weir S. PDX-1 protein containing its own antennapedia-like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes 52:1732–1737; 2003. [DOI] [PubMed] [Google Scholar]
- 14. Noguchi H.; Matsushita M.; Okitsu T.; Moriwaki A.; Tomizawa K.; Kang S.; Li S. T.; Kobayashi N.; Matsumoto S.; Tanaka K.; Tanaka N.; Matsui H. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat. Med. 10:305–309; 2004. [DOI] [PubMed] [Google Scholar]
- 15. Noguchi H.; Naziruddin B.; Jackson A.; Shimoda M.; Ikemoto T.; Fujita Y.; Chujo D.; Takita M.; Kobayashi N.; Onaca N.; Hayashi S.; Levy M. F.; Matsumoto S. Characterization of human pancreatic progenitor cells. Cell Transplant. 19:879–886; 2010. [DOI] [PubMed] [Google Scholar]
- 16. Noguchi H.; Naziruddin B.; Shimoda M.; Chujo D.; Takita M.; Sugimoto K.; Itoh T.; Onaca N.; Levy M. F.; Matsumoto S. A combined continuous density/osmolality gradient for supplemental purification of human islets. Cell Med. 3:33–41; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noguchi H.; Oishi K.; Ueda M.; Yukawa H.; Hayashi S.; Kobayashi N.; Levy M. F.; Matusmoto S. Establishment of mouse pancreatic stem cell line. Cell Transplant. 18:563–571; 2009. [DOI] [PubMed] [Google Scholar]
- 18. Noguchi H.; Saitoh I.; Kataoka H. U.; Watanabe M.; Noguchi N.; Fujiwara T. Culture conditions of mouse pancreatic stem cells. Cell Med. 5:63-68; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noguchi H.; Ueda M.; Hayashi S.; Kobayashi N.; Nagata H.; Iwanaga Y.; Okitsu T.; Matsumoto S. Comparison of M-Kyoto solution and histidine-tryptophan-ketoglutarate solution with a trypsin inhibitor for pancreas preservation in islet transplantation. Transplantation 84:655–658; 2007. [DOI] [PubMed] [Google Scholar]
- 20. Noguchi H.; Ueda M.; Nakai Y.; Iwanaga Y.; Okitsu T.; Nagata H.; Yonekawa Y.; Kobayashi N.; Nakamura T.; Wada H.; Matsumoto S. Modified two-layer preservation method (M-Kyoto/PFC) improves islet yields in islet isolation. Am. J. Transplant. 6:496–504; 2006. [DOI] [PubMed] [Google Scholar]
- 21. Noguchi H.; Xu G.; Matsumoto S.; Kaneto H.; Kobayashi N.; Bonner-Weir S.; Hayashi S. Induction of pancreatic stem/progenitor cells into insulin-producing cells by adenoviral-mediated gene transfer technology. Cell Transplant. 15:929–938; 2006. [DOI] [PubMed] [Google Scholar]
- 22. Shapiro A. M.; Lakey J. R.; Ryan E. A.; Korbutt G. S.; Toth E.; Warnock G. L.; Kneteman N. M.; Rajotte R. V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343:230–238; 2000. [DOI] [PubMed] [Google Scholar]
- 23. Song S. Y.; Gannon M.; Washington M. K.; Scoqqins C. R.; Meszoely I. M.; Goldenrinq J. R.; Marino C. R.; Sandqren E. P.; Coffey R. J. Jr.; Wright C. V.; Leach S. D. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor α. Gastroenterology 117:1416–1426; 1999. [DOI] [PubMed] [Google Scholar]
- 24. Street C. N.; Lakey J. R.; Shapiro A. M.; Imes S.; Rajotte R. V.; Ryan E. A.; Lyon J. G.; Kin T.; Avila J.; Tsujimura T.; Korbutt G. S. Islet graft assessment in the Edmonton Protocol: Implications for predicting long-term clinical outcome. Diabetes 53:3107–3114; 2004. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki A.; Nakauchi H.; Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes 53:2143–2152; 2004. [DOI] [PubMed] [Google Scholar]
- 26. Wang R. N.; Kloppel G.; Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 38:1405–1411; 1995. [DOI] [PubMed] [Google Scholar]
- 27. Yamamoto T.; Yamato E.; Taniguchi H.; Shimoda M.; Tashiro F.; Hosoi M.; Sato T.; Fujii S.; Miyazaki J. I. Stimulation of cAMP signaling allows isolation of clonal pancreatic precursor cells from adult mouse pancreas. Diabetologia 49:2359–2367; 2006. [DOI] [PubMed] [Google Scholar]