Abstract
Multiple myeloma (MM) is a malignancy of clonal plasma cells, resulting in an increased production of ineffective immunoglobulins with suppression of non-involved immunoglobulins. Patients with MM are at increased risk of infectious complications, particularly streptococcal and staphylococcal infections. This study evaluated the impact of prophylactic antibiotics on the incidence of serious bacterial infections (SBIs) during the first 2 months of treatment in patients with newly diagnosed MM. Patients with MM receiving initial chemotherapy were randomized on a 1:1:1 basis to daily ciprofloxacin (C; 500 mg twice daily), trimethoprim-sulfamethoxazole (T; DS twice daily) or observation (O) and evaluated for SBI (Eastern Cooperative Oncology Group ⩾grade 3) for the first 2 months of treatment. From July 1998 to January 2008, 212 MM patients were randomized to C (n = 69), T (n = 76) or O (n = 67). The incidence of SBI was comparable among groups: C = 12.5%, T = 6.8% and O = 15.9%; P = 0.218. Further, any infection during the first 2 months was also comparable (20% vs 23% vs 22%, respectively, P = 0.954). We demonstrate that prophylactic antibiotics did not decrease the incidence of SBI (⩾grade 3) within the first 2 months of treatment. We conclude that routine use of prophylactic antibiotics should not be mandated for patients receiving induction chemotherapy.
Keywords: antibiotics, multiple myeloma, infections
INTRODUCTION
Multiple myeloma (MM) is a malignancy of clonal plasma cells, resulting in an increased production of ineffective immunoglobulins with suppression of non-involved immunoglobulins. Treatment of MM is virtually always associated with further immunosuppression, at least in the immediate term, until the malignant clone is eradicated and normal immunoglobulins are produced. Infections are a major cause of morbidity in patients with MM and is related to deficits in both humoral and cellular immunity, reduced mobility and performance status, which may be associated with both the disease and its treatment. One study reported that up to 10% of patients die of infective causes within the first 2 months of diagnosis.1 Pneumonias and urinary sepsis caused by common bacterial pathogens including Streptococcus pneumoniae, Haemophilus influenza and Escherichia coli are most frequent.2–5 In a retrospective study evaluating the incidence of infection throughout the MM patient’s disease course, the first 2 months of initial chemotherapy emerged as a particularly high-risk period during which nearly half of the patients experienced at least one clinically significant infection.6 Over the course of MM, infections occur at the rate of 1.46–4.68 infections per patient-year.6–8 During the first 2 months of initial chemotherapy, the incidence is 2–3 times higher. These early infections are serious with approximately one-third of them proving fatal. Even when the early infection is not fatal, it frequently leads to substantial delays and dose reduction in subsequent chemotherapy with its attendant increased risk of treatment failure.6 We conducted a preliminary randomized study to evaluate the efficacy of prophylactic trimethoprim-sulfamethoxazole (TMP-SMX) to prevent these early infections.8 In 54 evaluable patients, the incidence of severe infections was eight in control patients and one in TMP-SMX, leading to four and one infection deaths, respectively. The rate of clinical bacterial infection was 2.43 infections per patient-year for controls and 0.29 infections per patient-year for the treated group.
Owing to further compromise of an already incompetent immune system by initial chemotherapy and the previous suggestion that prophylactic antibiotics may reduce infectious complications, this phase 3 cooperative group study was designed to evaluate the impact of prophylactic antibiotics on the incidence of serious bacterial infections (SBIs) during the first 2 months of treatment.
PATIENTS AND METHODS
Patients with symptomatic and untreated MM receiving chemotherapy were eligible for participation in the study. Patients must not have had an active infection during the 7 days prior to initiation of chemotherapy and must have been off all antibiotics for the prior 7 days. Patients were required to receive myelosuppressive and/or immunosuppressive chemotherapy. The protocol was modified in May 2004 to include high-dose dexamethasone alone (40 mg per day on days 1–4, 9–12 and 17–20 on the first cycle and a minimum of 40 mg per day on days 1–4 of the second cycle). Patients must have had a serum creatinine ≤5 mg/dl. Patients with documented hypersensitivity to quinolones or sulfa-based agents were excluded from participation.
Patients were randomized on a 1:1:1 basis to receive either a daily quinolone (C; ciprofloxacin; Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA) 500 mg every 12 h or ofloxacin (Janssen Pharmaceuticals, Titusville, NJ, USA) 400 mg every 12 h, T; trimethoprim-sulfamethoxazole (Hoffman-La Roche, Philadelphia, PA, USA) (DS = 160 mg trimethoprim and 800 mg sulfamethoxazole every 12 h) or C; observation (O) for the first 2 months of treatment (Figure 1). Patients were evaluated for SBI (grade 3 or 4) during the first 2 months of myelotoxic/suppressive therapy. The patients were then observed without anti-bacterial prophylaxis for one additional month on study continuing regular myeloma chemotherapy. Severe bacterial infections were defined based upon the Eastern Cooperative Oncology Group toxicity scale. A grade 3 infection was defined as a severe, systemic infection or an infection requiring hospitalization. A grade 4 infection was defined as life-threatening, for example, sepsis. Prophylactic antibiotics were modified for renal insufficiency. Patients who developed hypersensitivity reactions were followed for the study without antibiotics. Patients were then followed for response at 3 months, 6 months, 1 year and 2 years.
Figure 1.
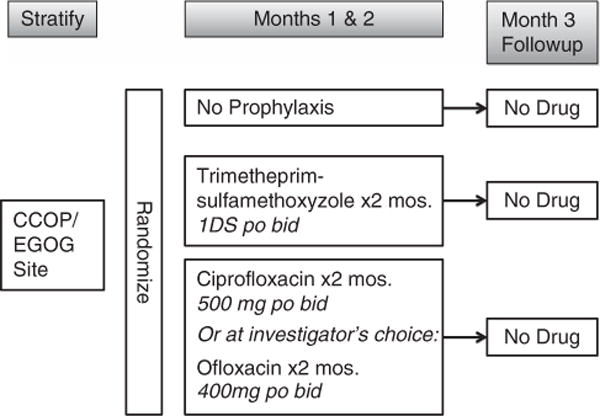
Study schema.
STATISTICAL ANALYSIS
All statistical tests were conducted at the 0.05 significance level. Differences in infection rates were compared across all three arms with Fisher’s exact test. If this test was statistically significant, then comparisons of the infection rates for T vs C and O vs C were planned. The target accrual was 70 patients per arm. This provided 92% power to detect a difference of 0.31 vs 0.08 in the proportion of patients with serious infection in any pair of treatment arms.
Secondary end points included: (a) the incidence of non-bacterial infections, (b) the incidence of serious infection, the third month OFF of antibiotic prophylaxis, (c) whether protection against infection is associated with an improved response rate and (d) overall survival. Fisher’s exact test with follow-up pairwise comparisons as described above were used to evaluate (a) and (b). For (c), response rates at months 3 and 6 and years 1 and 2 were analyzed as an ordinal multinomial repeated measures model with arm, time and arm*time interaction as the fixed effects, using generalized estimating equation methodology. For (d), product-limit survival curves (censored at 40 months) for each arm were compared using the log rank procedure (although the sample size is small for a survival analysis and there will be low power). SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for all calculations.
RESULTS
From July 1998 to January 2008, 212 untreated, symptomatic MM patients being treated with myelotoxic/suppressive chemotherapy were randomized to C (n = 69), T (n = 76) or O (n = 67) for the first 2 months of treatment. Patient demographics are shown in Table 1: there were no statistical difference in gender, race, type of induction chemotherapy, performance status or age between the three groups. Over 90% of patients in each arm enrolled in the study were evaluable at the primary end point of SBI at 2 months. The few patients who were not evaluable at the 2-month primary end point are indicated in the patient flow shown in Figure 2. The incidence of serious infection (⩾grade 3 Eastern Cooperative Oncology Group toxicity criteria and/or hospitalization) was comparable among groups: C = 10 (12.5%), T = 5 (6.8%) and O = 9 (15.9%); (P = 0.218), which was the primary end point (Table 2). The incidence of any infection during the first 2 months was also comparable (20.3% vs 23.0% vs 22.2%, respectively, P = 0.954). The risk of SBI was highest in the second month of prophylaxis (Figure 3). There were 24 grade 3 infections and 3 grade 4 infections involving respiratory (n = 11), sepsis/bacteremia (n = 6), urinary (n = 3), joint/bone (n = 2), unknown (n = 2) or various GI tract (n = 2) and skin (n = 1) (Table 3). Eight patients expired within the first 2 months: two from MM, three cardiac, two respiratory, one renal (unrelated to MM or treatment) occurring in the C (4), T (1) and O (2). Secondary end points failed to show a difference in the incidence of non-bacterial infections (P = 1.00), incidence of severe bacterial infection during the third month in the absence of prophylaxis (C = 3.1%, T = 2.7% and O = 4.82%; P = 0.799), initial response to therapy (P = 0.858) or overall survival (P = 0.863).
Table 1.
Demographic and clinical characteristics at randomization
| All (%)a | Ciprofloxacin | Trimethoprim-sulfamethoxyzole | Observation | ||
|---|---|---|---|---|---|
| n = 212 | n = 69 | n = 76 | n = 67 | ||
| Male | 133 (62.7%) | 46 | 44 | 43 | |
| White | 160 (77.7%) | 54 | 51 | 55 | |
| Infection in last 6 months | 28 (13.5%) | 13 | 7 | 8 | |
| Regimen | MP | 34 (16.5%) | 11 | 13 | 10 |
| VBMCP | 22 (10.7%) | 6 | 8 | 8 | |
| VAD | 63 (30.6%) | 24 | 17 | 22 | |
| Other | 87 (42.2%) | 28 | 36 | 23 | |
| ECOG performance status | Mean | 1.0 | 1.0 | 1.1 | 0.8 |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 4 | 3 | 4 | 4 | |
| Age | Mean | 63.8 | 63.7 | 62.6 | 65.0 |
| Minimum | 32.4 | 32.4 | 36.7 | 36.2 | |
| Maximum | 89.3 | 86.0 | 85.7 | 89.3 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group; MP, melphalan and prednisone; VBMCP, vincristine, carmustine, melphalan, cyclophosphamide and prednisone; VAD, vincristine, adriamycin and dexamethasone.
Not statistically significant difference between the groups.
Figure 2.
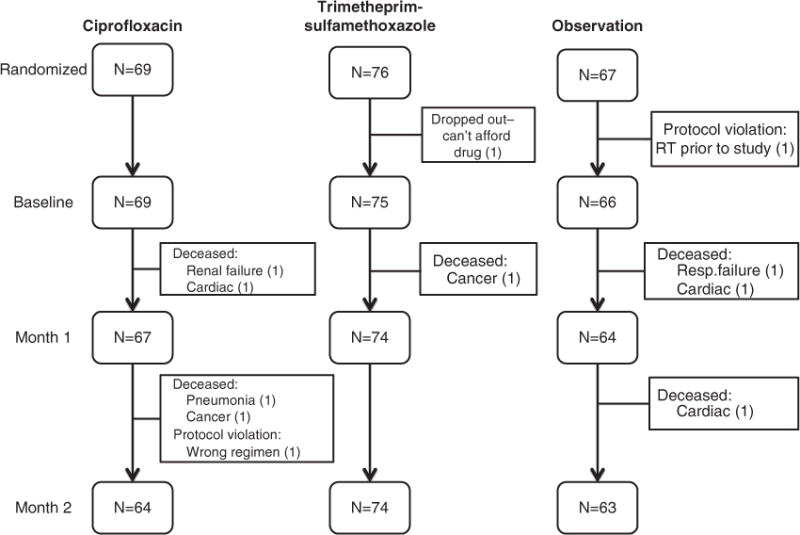
Patient flow.
Table 2.
Infection incidence during study period
| Outcome (P-value) | Treatment arm | n at risk | n | % | 95% confidence interval | ||
|---|---|---|---|---|---|---|---|
| Severe bacterial infections during first 2 months (P = 0.218) | C | 64 | 8 | 12.5 | 5.6 | – | 23.2 |
| T | 74 | 5 | 6.8 | 2.2 | – | 15.1 | |
| O | 63 | 10 | 15.9 | 7.9 | – | 27.3 | |
| Any infection during first 2 months (P = 0.954) | C | 64 | 13 | 20.3 | 11.3 | – | 32.2 |
| T | 74 | 17 | 23.0 | 14.0 | – | 34.2 | |
| O | 63 | 14 | 22.2 | 12.7 | – | 34.5 | |
| Severe infections during the first month after prophylaxis (P = 0.799) | C | 64 | 2 | 3.1 | 0.0 | – | 7.4 |
| T | 74 | 2 | 2.7 | 0.0 | – | 6.4 | |
| O | 63 | 3 | 4.8 | 0.0 | – | 10.0 | |
Abbreviation: C, ciprofloxacin; O, observation; T, trimethoprim-sulfamethoxyzole.
Figure 3.
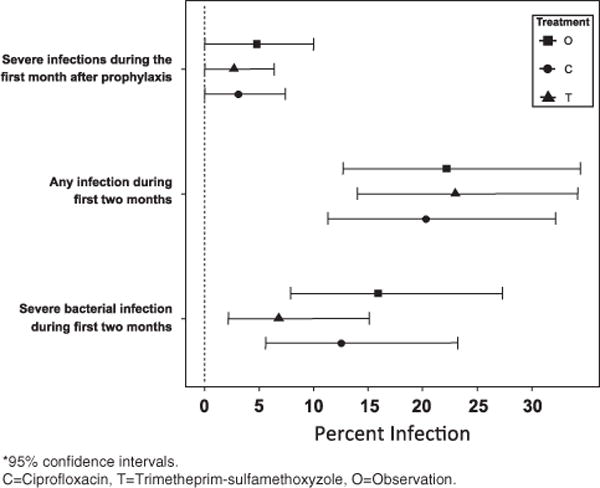
Severity and infection incidence at 1 and 2 months during prophylaxis.
Table 3.
Summary of reported infections
| n | |
|---|---|
| Severity | |
| Grade 3 | 24 |
| Grade 4 | 3 |
| Sites | n |
| Respiratory | 11 |
| GI | 2 |
| Sepsis/bacteremia | 6 |
| Urinary tract | 3 |
| Joint/bone | 2 |
| Skin | 1 |
| Unknown | 2 |
Note: multiple infections for some patients were counted in these totals.
DISCUSSION
The use of prophylactic antibiotics did not decrease the incidence of serious infection (⩾grade 3 and/or hospitalization) nor of any infection within the first 2 months of treatment. Infection prophylaxis did not affect the incidence of infection upon completion of 2 months of therapy (nor, ultimately, at any time during the subsequent 2 years), the response to therapy (data not shown) or to overall survival (data not shown).
Our results are different than older studies, showing an increased risk of infection during initial therapy with regimens prior to the initiation of novel therapies. Interestingly, the incidence of infections with modern novel therapies, immumomodulatory-based or proteasome inhibitor-based, shows similar patterns. Rajkumar et al.9 reported the incidence of severe infections (Eastern Cooperative Oncology Group ⩾grade 3) in newly diagnosed MM patients treated with lenalidomide and dexamethasone (either high or low dose, 480 and 160 mg monthly, respectively). All the patients were recommended to receive either a quinolone or other broad-spectrum antibiotic. The incidence of severe infections in the first 2 months of treatment was 9.4% in the high-dose dexamethasone arm and 6.4% in the low-dose dexamethasone arm, which did not achieve statistical significance. This is a similar incidence of severe infection rates observed in the current study. Gay et al.10 reported the incidence of grade >3 infections in their study of lenalidomide, dexamethasone and daily clarithromycin (BiRD regimen). They observed a 16.7% incidence throughout the course of the study even in the presence of continuous exposure to a macrolide antibiotic (the incidence limited to the first 2 months is not available).
For those patients considered high risk for bacterial infections, those patients with a history of repeated infections, possibly severely low immunoglobulin levels, those receiving more intense induction regimens (for example, VDT-PACE (bortezomib, dexamethasone, thalidomide, cisplatin, adriamycin, cyclophosphamide and etoposide), may benefit from prophylactic antibiotics. However, the choice of prophylactic antibiotics remains unanswered because a significant difference in the incidence of severe bacterial infections was not observed between a quinolone and TMP-SMX. Prior to the incorporation of immunomodulatory agents into our treatment armamentarium, TMP-SMX-based antibiotic prophylaxis was routinely employed to decrease the risk of Pneumocystis (carinii) jiroveci infections. Of note, we did not observe any Pneumocystis jiroveci infections in any patient in this trial. Further, most of our patients received substantially higher levels of corticosteroids than is routinely utilized in our current treatment algorithms with low-dose dexamethasone. Thus, it does not appear necessary to include Pneumocystis jiroveci prophylaxis in the management of MM patients. Finally, one can consider passive immunization through the use of intravenous immunoglobulin (IVIG) to reduce the risk of infectious complications. Prophylactic IVIG has been studied in plateau phase following initial treatment with some benefit in reducing the risk of infection: IVIG has been shown to have some benefit in reducing infection rates in patients in plateau phase,11 but no effect has been demonstrated in newly diagnosed patients.12
We conclude that prophylactic anti-bacterial antibiotics should not be mandated for all patients. This is in accordance with the ‘Haemato-Oncology Task Force of the British Committee for Standards in Haematology and UK Myeloma Forum’ which also does not recommend routine anti-bacterial prophylaxis.13 Although this study did not demonstrate a requirement for anti-bacterial prophylaxis, MM patients receiving proteasome inhibitor-based therapy should receive anti-viral prophylaxis. A number of studies have demonstrated an increased risk of Herpes zoster reactivation in the setting of proteasome inhibitor treatment.13–15
Acknowledgments
We thank the members and staff of URCC and ECOG and the patients and their families. This work was supported in part by ECOG Grant numbers CA13650, CA21115 and CA23318.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002–Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–9226. doi: 10.1200/JCO.2005.03.2086. [DOI] [PubMed] [Google Scholar]
- 2.Myers BR, Hirschman SZ, Axelrod JA. Current patterns of infection in multiple myeloma. Am J Med. 1972;52:87–92. doi: 10.1016/0002-9343(72)90010-1. [DOI] [PubMed] [Google Scholar]
- 3.Savage DG, Lindenbaum J, Garret TJ. Biphasic pattern of bacterial infection in multiple myeloma. Ann Intern Med. 1982;96:47–50. doi: 10.7326/0003-4819-96-1-47. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson DR, Zolla-Pazner PS. Immunosuppression and infection in multiple myeloma. Semin Oncol. 1986;13:282–290. [PubMed] [Google Scholar]
- 5.Doughney KB, Williams DM, Penn RL. Multiple myeloma: Infectious complications. South Med J. 1988;81:855–888. doi: 10.1097/00007611-198807000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Perri RT, Hebbel R, Oken MM. Influence of treatment and response status on infection risk in multiple myeloma. Am J Med. 1981;71:935–940. doi: 10.1016/0002-9343(81)90303-x. [DOI] [PubMed] [Google Scholar]
- 7.Hargreaves RM, Lea JR, Griffiths H, Faux JA, Holt JM, Reid C, et al. Immunological factors and risk of infection in plateau phase myeloma. J Clin Pathol. 1995;48:260–266. doi: 10.1136/jcp.48.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oken MM, Pomeroy C, Weisdorf D, Bennett JM. Prophylactic antibiotics for the prevention of early infection in multiple myeloma. Am J Med. 1996;100:624–628. doi: 10.1016/s0002-9343(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gay F, Rajkumar SV, Coleman M, Kumar S, Mark T, Dispenzieri A, et al. Clarithromycin (Biaxin)-lenalidomide-low-dose dexamethasone (BiRd) versus lenalidomide-low-dose dexamethasone (Rd) for newly diagnosed myeloma. Am J Hematol. 2010;85:664–669. doi: 10.1002/ajh.21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapel HM, Lee M, Hargreaves R, Pamphilon DH, Prentice AG. Randomised trial of intravenous immunoglobulin as prophylaxis against infection in plateau-phase multiple myeloma. The UK Group for Immunoglobulin Replacement Therapy in Multiple Myeloma. Lancet. 1994;343:1059–1063. doi: 10.1016/s0140-6736(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 12.Salmon SE, Samal BA, Hayes DM, Hosley H, Miller SP, Schilling A. Role of gamma globulin for immunoprophylaxis in multiple myeloma. N Engl J Med. 1967;277:1336–1340. doi: 10.1056/NEJM196712212772503. [DOI] [PubMed] [Google Scholar]
- 13.Snowden JA, Ahmedzai SH, Ashcroft J, D’Sa S, Littlewood T, Low E, et al. Guidelines for supportive care in multiple myeloma 2011. Br J Haematol. 2011;154:76–103. doi: 10.1111/j.1365-2141.2011.08574.x. [DOI] [PubMed] [Google Scholar]
- 14.Chanan-Khan A, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. 2008;26:4784–4790. doi: 10.1200/JCO.2007.14.9641. [DOI] [PubMed] [Google Scholar]
- 15.Swaika A, Paulus A, Miller KC, Sher T, Almyroudis NG, Ball D, et al. Acyclovir prophylaxis against Varicella zoster virus reactivation in multiple myeloma patients treated with bortezomib-based therapies: A retrospective analysis of 100 patients. J Support Oncol. doi: 10.1016/j.suponc.2011.10.006. e-pub ahead of print 4 January 2012. [DOI] [PubMed] [Google Scholar]


