Abstract
Light-emitting diodes (LEDs) have been used to provide illumination in industrial and commercial environments. LEDs are also used in TVs, computers, smart phones, and tablets. Although the light emitted by most LEDs appears white, LEDs have peak emission in the blue light range (400–490 nm). The accumulating experimental evidence has indicated that exposure to blue light can affect many physiologic functions, and it can be used to treat circadian and sleep dysfunctions. However, blue light can also induce photoreceptor damage. Thus, it is important to consider the spectral output of LED-based light sources to minimize the danger that may be associated with blue light exposure. In this review, we summarize the current knowledge of the effects of blue light on the regulation of physiologic functions and the possible effects of blue light exposure on ocular health.
Introduction
Lighting sources and technology have experienced a revolution in the last 15–20 years. Lighting sources and technology, especially in non-commercial or industrial illumination applications, have traditionally been slow to change [1]. In most homes, the incandescent bulb and Edison socket have been omnipresent. In the past 10 years, we have seen significant use of other technologies, such as compact fluorescent lamps (CFLs), replacing incandescent sources. However, this transition has often been driven by legislation, which has focused on energy-efficient sources instead of consumer desire for different light sources. The general user quickly noted the difference in the quality of CFL source but not necessarily in the specifics of its power spectrum. Simultaneously, the development and performance of high brightness light-emitting diodes (LEDs) have experienced tremendous advances [2]. The coupling of a blue-light LED with a phosphor has also been used to produce a white light source, the white-light LED. This solid-state fluorescent analog has become known as solid-state lighting (SSL). This approach is now considered the next generation of illumination due to the many inherent and potential advantages over current technologies.
In addition to use for general illumination, LEDs quickly became the choice for mobile devices, such as smart phones [3]. The small size of LEDs and the limited screen size make them ideal for these applications. The potential for the use of LEDs for backlighted liquid crystal displays (LCDs) in laptop computers was also quickly realized. This transition was driven by the fragility of the microfluorescent lamps used for illumination and consumer desire for thinner screens. LEDs have now become the dominant technology for backlighted tablet displays, such as iPads and e-readers, and large LCD television sets. This now means that blue light prevails in red, green, and blue (RGB) and SSL illumination systems that did not exist a decade ago. The ways in which people read have also changed. Light is now being used directly for illumination in smart phones, tablets, and readers instead of for reflection, which is typical for reading from paper.
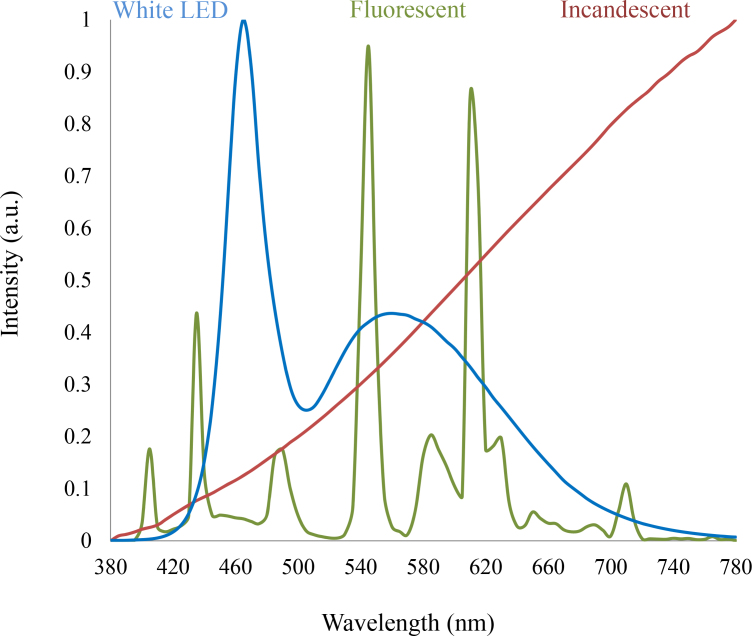
The white-light LED (i.e., the most common type of LED) is essentially a bichromatic source that couples the emission from a blue LED (peak of emission around 450–470 nm with a full width at half max of 30–40 nm) [4] with a yellow phosphor (peak of emission around 580 nm with a full width at half max of 160 nm) that appears white to the eye when viewed directly [5]. The specific pump wavelength of the phosphor in the range 450–470 nm depends critically on the absorption properties of the phosphor. Although the white-light LED can be considered the SSL analog of the fluorescent source, the power spectrum of the white-light LED is considerably different from traditional, fluorescent, or incandescent white light sources [6] (Figure 1).
Figure 1.
A comparison of the power spectrum of a standard white-light LED, a tricolor fluorescent lamp, and an incandescent source. The radically different power spectrums can look similar when viewed directly by the eye, irrespective of how much blue emission is present.
Early commercial devices lacked sophistication, adopting the currently available LED technology that was small, 350×350 mm2, and operated at low drive currents, typically 20 mA, producing 1–16 mW of power. The last decade has seen the scaling of LEDs to larger areas, 1×1 μm2, and higher drive currents of >350 mA with significantly increased power output >1,000 mW [2]. During this period, LED devices were also optimized for use in illumination applications, and reflected from a surface instead of emitted directly.
In addition, white-light LEDs degrade over time primarily through bleaching of phosphors so that they no longer efficiently absorb blue light [7]. This shifts the color temperature of the device over time, with a corresponding change in the color-rendering index but, more importantly, an increasing blue emission from the device with time.
In this review, we summarize the current knowledge of the effects of blue light on the regulation of physiologic function and the effects of blue light exposure on ocular health. Finally, we discuss the available data to determine whether long-term exposure to blue light is safe or whether additional studies are needed to fully understand the effects of blue light exposure on ocular health.
Non-image-forming photoreception
In mammals, photoreception occurs only in the retina [8] by three types of photoreceptor: cones, rods, and the intrinsically photosensitive retinal ganglion cells (ipRGCs). The classical photoreceptors (e.g., rods and cones) are mostly responsible for the image-forming vision, whereas the ipRGCs play a major role in non-image-forming photoreception, that is, the photoreceptive system that regulates circadian photic entrainment, pupillary light response, and other important biologic functions (Figure 2).
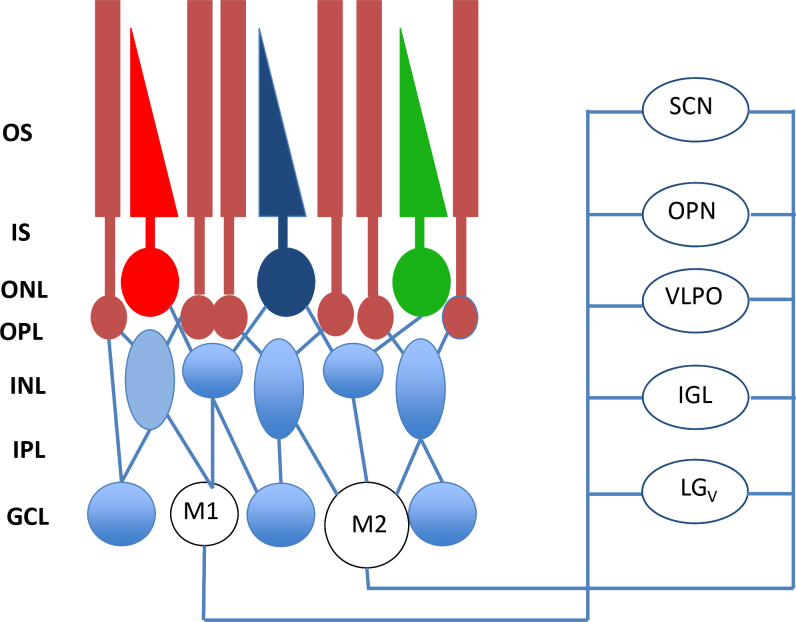
Figure 2.
In addition to the classical photoreceptors (rods and cones), ipRGCs are present in the retina. Recent studies have shown that at least two types of intrinsically photosensitive retinal ganglion cells (ipRGCs) have been identified: M1 and M2. Most of the M1 cells project to the suprachiasmatic nucleus (SCN) of the hypothalamus whereas the number of M1 and M2 projecting to the olivary pretectal nucleus (OPN) is similar (55% from M1 cells versus 45% from M2 cells). The M1 cells are considerably smaller but respond with significantly larger depolarizations and light-induced currents than do the M2 cells. Other neural targets of ipRGCs not shown in the figure include the preoptic area, sub-paraventricular zone, anterior hypothalamic nucleus, lateral hypothalamus, medial amygdaloid nucleus, lateral habenula, lateral geniculate nucleus (dorsal division), bed nucleus of the stria terminalis, periaqueductal gray, and superior colliculus. OS=outer segments; IS=inner segments; ONL=outer nuclear layer; OPL=outer plexiform layer; INL=inner nuclear layer; IPL=inner plexiform layer; GCL=ganglion cell layer; from [31] with permission.
The idea that the mammalian retina is capable of non-image-forming photoreception emerged during the 1990s when a series of studies indicated that mice lacking rod photoreceptors (rd/rd) have a normal phase response curve (PRC) to light [9], with an action spectrum that peaks around 480 nm [10]. This result suggested that a photo pigment different from rhodopsin (λmax 498 nm), short wavelength sensitive opsin (λmax 460 nm), and middle wavelength sensitive opsin (λmax 508 nm) [11] was responsible for the entrainment of circadian rhythms. Additional studies reported that mice lacking rods and cones were still capable of synchronizing their circadian rhythms to light-dark cycles [12], thus demonstrating that an undiscovered photo pigment/photoreceptor in the mammalian retina was responsible for the photoentrainment of circadian rhythms.
The most likely candidate to emerge as the circadian retinal photo pigment is a mammalian homolog of Xenopus melanopsin (aka Opn4) [13-15]. In mammals, melanopsin mRNA (and protein) is expressed only in a small population (about 3–5%) of the RGCs [14,16] that are directly photosensitive and have an absorption peak around 470–480 nm [17-19]. These RGCs express pituitary adenylate cyclase-activating polypeptide (PACAP) [20] and form the retinohypothalamic tract (RHT) [16,21]. The RHT is responsible for conveying the light information from RGCs to the part of the brain that controls circadian rhythms within the whole body [22,23]. The RGCs that express melanopsin were named intrinsically photosensitive RGCs (ipRGCs), and these cells were no longer intrinsically photosensitive in melanopsin knockout (KO) mice, although the cell number, morphology, and projections remained unchanged [24].
Additional studies have also shown that melanopsin KO mice entrained to light-dark photoperiods, albeit the response to light was attenuated in the KO animals as the magnitude of the phase-shift is about half (40%) of that of wild-type mice at each of the three non-saturating irradiance levels [25]. A saturating white light pulse also produced a diminished phase shift in the KO animals [26]. The length of the free-running period that follows the exposure to constant light is reduced (to about 55–65% of that of controls) in melanopsin KO animals [25,26].
Melanopsin has also been implicated in regulation of the pupillary light reflex (PLR). Transgenic mice lacking rod and cone photoreceptors (rdcl) retain a PLR, and this response is driven by a photo pigment with peak sensitivity of around 479 nm [27]. Melanopsin KO animals showed a PLR indistinguishable from that of the wild-type mice at low irradiances, but at high irradiances, the reflex was incomplete. This result suggests that the melanopsin-associated system and the classical rod/cone system are complementary functions [28,29]. Thus, the current view is that no single photoreceptor type is necessary for the synchronization of circadian rhythms with external light-dark cycles [30,31].
Finally, mice with the melanopsin gene ablated only in ipRGCs have normal outer retinal function but lack non-image-forming visual responses, such as circadian photoentrainment, light modulation of activity, and PLR [32]. Thus, the ipRGCs represent the site of integration of non-image-forming photo responses in mammals.
Further studies have also shown that melanopsin-based photoreception is involved in the modulation of sleep [33-36] and mood and learning [37], and recent data have also indicated that melanopsin-based photoreception may be involved in the regulation of metabolism [38]. Finally, it has been reported that loss of the melanopsin gene abolishes circadian control in some parameters of cone electroretinogram, causing significant attenuation of the diurnal variation in cone vision [39]. Melanopsin signaling may influence intraretinal signaling by acting on dopaminergic neurons [40]. Therefore, these data suggest melanopsin-dependent regulation of visual processing within the retina.
Melanopsin also plays an important role in mediating human circadian rhythms. Several studies have reported that in humans, the action spectra for melatonin suppression has a lambda max (λmax) of around 460 nm, suggesting that melanopsin is a key player in the photic regulation of melatonin levels [41-43]. Additional studies have also shown that blue light in the range of 460–480 nm is more effective compared to monochromatic light of 555 nm in phase-shifting the human circadian clock [44,45]. Finally, a recent study expanded these previous results by showing that light in the 555 nm range may significantly affect the synchronization of the circadian system to light exposure of short duration or to low irradiance, whereas light in the 460 nm range is more effective in phase-shifting the circadian system than exposure to light of longer duration and higher irradiance [46]. Additional studies have also shown that exposure to blue light can increase alertness [47-50] and stimulate cognitive functions [51-53]. A recent study reported that exposure to light-emitting e-readers at bedtime may negatively affect sleep and the circadian system [54]. Finally, blue light may also be used to treat seasonal affective disorders [55], and mutations in the melanopsin gene may increase the susceptibility to developing seasonal affective disorders [56,57]. However, another study reported that exposure to blue-enriched light was less effective compared to full-spectrum light in the treatment of seasonal affective disorder [58].
With age, the lens becomes more yellowish, and thus, the spectrum of blue light transmission dramatically decreases through the years. It is suspected that one reason older individuals experience sleep problems is the lack of blue light during the daytime. Ayaki et al. [59] reported that after cataract extraction, sleep quality improved dramatically because more blue light could pass through the intraocular lens. In addition, there has been a discussion on whether a clear or yellow lens is preferable [60]. Of course, the yellow lens may protect the retina, but the clear lens provides more blue light during the day, providing better quality of sleep [61]. Consistent with this result, Sletten et al. [62] reported that in older people, acute exposure to blue light, but not to green light, significantly decreased their alertness and suppressed their sleep and melatonin production compared to young people. However, another study reported that in older patients with decreased lens transmittance, melatonin was not significantly suppressed following blue light exposure [43]. Thus, whether the yellowing of the lens associated with aging really affects the non-image-forming photoreception is still a matter of debate.
Light-induced damage to the retina
Several investigations have shown that exposure to light of specific wavelengths or intensity may induce severe damage to the retina [63,64]. This type of damage is called light-induced damage. Light can induce damage via three mechanisms: photomechanical, photothermal, and photochemical. Photomechanical damage is due to a rapid increase in the amount of energy captured by the RPE, which may cause irreversible damage to the RPE and lead to photoreceptor damage. This type of retinal damage depends on the amount of energy absorbed and not on the spectral composition of the light. Photothermal damage occurs when the retina and the RPE are exposed to brief (100 ms to 10 s) but intense light that induce a significant increase in the temperature of these tissues [63,64].
A more common type of retinal/RPE damage is photochemical damage, which occurs when the eyes are exposed to light of high intensity in the visible range (390–600 nm). The current view suggests that there are two distinct types of photochemical damage. The first type is associated with short but intense exposure to light affecting the RPE, and the second type is associated with longer but less intense light exposure, affecting the outer segment of the photoreceptors. Short (up to 12 h) exposure to blue light may induce damage in the RPE of the rhesus monkey [65], and a clear relationship has been found between the extent of the damage and the oxygen concentration [66,67]. The fact that many different antioxidants can reduce the damage suggests that this type of damage is associated with oxidative processes [68,69]. Experimental data suggest that lipofuscin is the chromophore involved in the mediation of light-induced retinal damage following the exposure to blue light [70-73].
The second type of light-induced photochemical damage occurs with longer (12–48 h) but less intense light exposure. This type of damage was initially observed in albino rats [74] but has also been observed in other species. The cones seem to be more vulnerable compared to the rods [75]. Several lines of evidence suggest that the visual photo pigments (e.g., rhodopsin and cone opsins) are involved in this type of damage. Early studies [76-78] also provided evidence that the action spectrum for light-induced photoreceptor damage is similar to the absorption spectrum of rhodopsin, but later studies indicated that blue light (400–440 nm) might be more damaging [79-81]. Grimm et al. [82] provided an explanation for this phenomenon, demonstrating that in vivo bleached rhodopsin may be regenerated not only via a metabolic pathway (e.g., via the visual cycle) but also via a photochemical reaction called photoreversal of bleaching [83] that is most effective with blue light. Photoreversal of bleaching augments the capability of rhodopsin molecules to absorb photons by several orders of magnitude, thus allowing the molecules to reach the critical number of photons required to induce damage in the retinal cells [84].
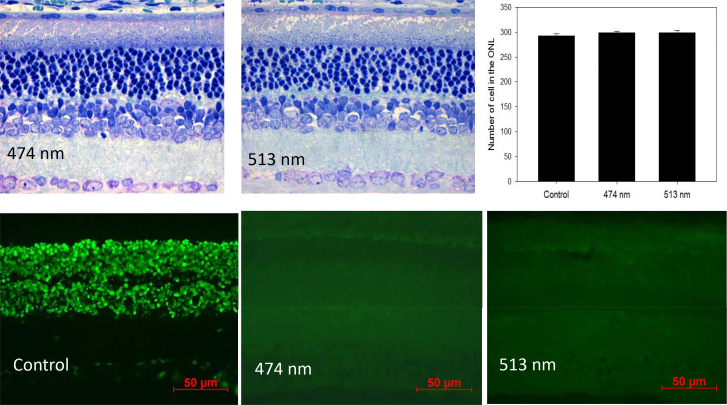
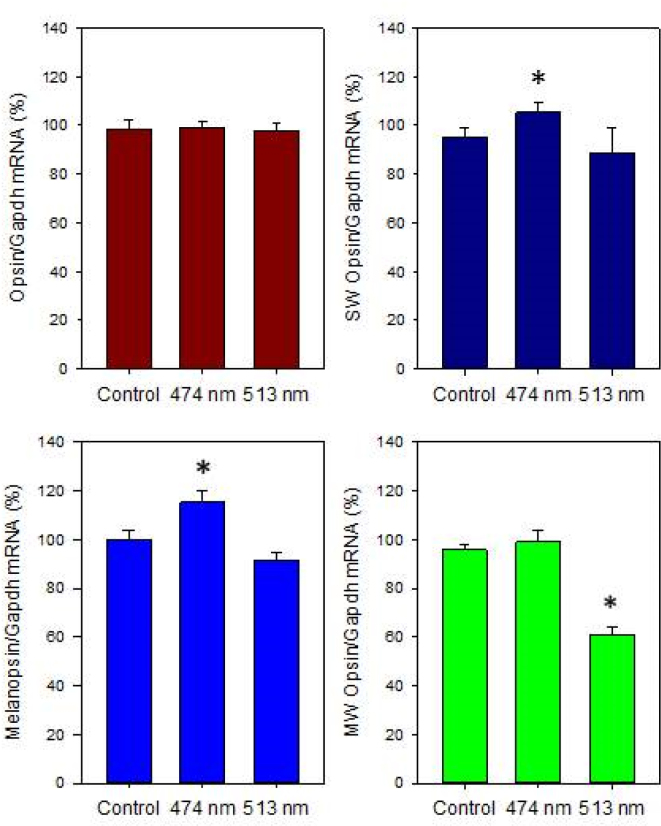
This process can further increase the potential production of reactive oxygen species (ROS); thus, the oxidative damage can lead to the accumulation and build-up of lipofuscin in the RPE. The build-up of lipofuscin in the RPE can affect the ability of the RPE to provide nutrients to the photoreceptors, affecting photoreceptor viability [85]. Moreover, when lipofuscin absorbs blue light, the material becomes phototoxic, which can lead to further damage in the RPE and in the photoreceptors [70]. The data from our laboratory indicate that in albino rats, exposure to blue light (λmax 474 nm, 1×10−1 μW/cm2) acutely suppressed melatonin levels [6] while exposure to blue light for 4 h/day for 30 days did not produce significant effects on photoreceptor viability (Figure 3). However, this treatment produced a small (10–20%) but significant reduction in the levels of melanopsin and short wavelength opsin mRNAs in rats exposed to white or green (λmax513 nm) light (Figure 4).
Figure 3.
Top panels. The exposure to blue light (λmax 474), green light (λmax 513), or fluorescent light at the intensity of 1×10−1 μW/cm2 for 4 h/day for 30 days did not produce a significant change in the number of cells in the photoreceptor layers of the Sprague-Dawley rats (n=6; see [121] for details about the methods used to quantify cells in the photoreceptor layer). Lower panels. The exposure to blue or green light-emitting diodes (LEDs) for 4 h in the middle of the day did not induce apoptosis. Terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) assay: 4- to 6-week-old Sprague-Dawley rats (n=6) were anesthetized (75 mg/kg ketamine and 23 mg/kg xylazine), kept on heating pads (37 °C), and exposed to blue or green light for 4 h. The pupils were dilated with 1% atropine and 2.5% phenylephrine eye drops 45 min before the light exposure. Rats were then killed 16 h after the exposure to blue light or green light. The eyes were explanted and fixed using freshly prepared 4% polyformaldehyde in PBS, pH 7.4 for 20 min at room temperature. They were washed 3X with PBS, permeabilized with freshly prepared 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice (2–8 °C), and then the TUNEL reaction was performed according to the instructions included in the manual (In Situ cell Death Detection kit). The slides were incubated in a humidified container for 60 min at 37 °C in the dark. Slides were rinsed 3X with PBS, and samples were analyzed under a fluorescence microscope (Zeiss Axioskop).
Figure 4.
Different light treatments did not affect rhodopsin mRNA levels (one-way ANOVA, p>0.1). Exposure to blue light (λmax 474) at the intensity of 1×10−1 μW/cm2 for 4 h/day for 30 days produced significant changes in the mRNA levels of short wavelength sensitive (SW) opsin, melanopsin, and medium wavelength sensitive opsin (* one-way ANOVA followed by Holm-Sidak tests, p<0.05). Rats were exposed to blue, green, or white light-emitting diodes (LEDs) every day (4 h) for 30 days in the middle of the day (11:00 to 15:00) and then returned to a 12 h:12 h light-dark cycle. The intensity of the light during the light phase of the 12 h:12 h light-dark cycle was about 400–450 lux. Every day, the pupils were dilated with 1% atropine and 2.5% phenylephrine eye drops 45 min before exposure to blue, green, or white light-emitting diodes (LEDs). After 30 days, the rats were killed, and the retinas were explanted, immediately frozen, and stored at −80 °C. mRNA was then extracted, and mRNA levels were measured using real-time quantitative PCR (qPCR; see [122] for details about primers and qPCR conditions).
In this context, two recent studies on the effect of blue light exposure on the RPE and cone-like cells (661W, murine photoreceptor-derived cells [86]) should be mentioned. In the first study, Arnault et al. [87] reported that in the primary porcine RPE, exposure to light with irradiance similar to that of natural sunlight, that is, light in the range of 415–455 nm, was the most effective in reducing cell viability.
In the second study, Kuse et al. [88] reported that 661W cells are more sensitive to light-induced damage when exposed to light emitted by blue (464 nm) LEDs than when exposed to green (522 nm) or white LEDs (wavelength peak at 456 and 553 nm) of the same intensity (0.38 mW/cm2). The exposure to blue light, unlike the exposure to white and green LEDs, also produced a significant increase in ROS and induced cell damage. Similar results were also observed in primary retinal cells [88]. These data support the idea that exposure to blue light in the range of 400–470 nm (even at low levels) may damage photoreceptors and retinal pigment epithelium cells.
Although most studies have focused on the acute effect of light exposure, several have also investigated the cumulative effect of light. For example, Noell [89] reported that a single 5 min exposure to light did not induce significant damage in photoreceptor cells, whereas a series of 5 min exposures led to significant photoreceptor damage. Furthermore, the time between exposures affects the cumulative effect of light [90-92]. In some cases, intermittent light exposure may produce even more pronounced damage than an equivalent amount of light in a single exposure [93]. In addition, the type of illumination to which the animals had been exposed before the experimental treatment influenced the extent of the retinal damage following light exposure. For example, rats raised in complete darkness showed greater susceptibility to light-induced retinal damage [89], and rats raised in an 800 lux light-dark cycle were more resistant to light-induced retinal damage compared to animals raised in a 5 lux light-dark cycle [94]. Finally, light-induced damage to photoreceptors increases with age. The exposure to light that might affect adult animals might not induce retinal damage in young animals [95]. In this context, with age, superoxide dismutase 1 (SOD1) and protective enzymes do not function as well due to zinc deficiency. SOD1 does not function well because the enzyme activity is controlled by zinc. Imamura et al. have shown that even with normal light that contains some blue light, fluorescent light damaged the retina tremendously in the SOD1 knockout mouse, which is similar to an aging mouse [96]. However, nothing happened in the normal mouse. The protective mechanism of the retina is important. From that point of view, the protective function of lutein, or blue-blocking pigment, on the retina is also considered. Ozawa et al. published research showing that when lutein was given, retina photodamage was alleviated [97].
Finally, the severity of light-induced retinal damage changes with the time of the day [98-102]. For example, rats are three to four times more susceptible to light damage at night (01:00) than during the day (09:00 and 17:00). The circadian dependency of light-induced photoreceptor damage appears to involve mechanisms that control cAMP and c-fos levels (see [63] for a review), both of which are under the control of the retinal circadian clock [103,104]. Exposure to blue light during the night might have more negative effects compared to the same exposure during the daytime. However, in this case, this assumption is based on the experimental data obtained from nocturnal rodents. Thus, it is difficult to determine whether light-induced retinal damage has a daily rhythm in humans, and further studies on diurnal animal models (e.g., non-human primates) are required to address this important point.
Experimental evidence indicates that wavelengths in the blue part of the spectrum (400–490 nm) can induce damage in the retina, and although the initial damage following exposure to blue light may be confined to the RPE, a damaged RPE eventually leads to photoreceptor death. Although most studies on the effects of blue light have focused on the mechanisms responsible for the damage to the photoreceptors following an acute exposure to high intensity light, some studies have reported that sub-threshold exposure to blue light can also induce damage in photoreceptors [105-107]. In addition, several authors have proposed that the amount of blue light received during an individual’s entire lifespan can be an important factor in the development of age-related macular degeneration (AMD). The use of lenses (intra- and extraocular) that block blue light (“blue-blockers”) may provide some protection against the development of AMD [60,108].
The mechanism through which long-term exposure to blue light may induce photoreceptor damage is mostly unknown. Several studies have indicated lipofuscin (absorption peak around 450 nm) is a possible mediator of the risk associated with long-term exposure to blue light–induced retinal damage [109,110]. Lipofuscin accumulates in the RPE in the form of granules located in the lysosomes of the RPE. The formation of lipofuscin begins in photoreceptors’ outer segments as a byproduct of the degradation of rod photoreceptor discs [105]. When lipofuscin absorbs blue light, ROS are produced, and these free radicals are responsible for the oxidative damage that occurs in the retina. The number of reactive oxygen species produced by lipofuscin is directly related to the spectral composition of the light, and it steadily decreases from 400 to 490 nm [73]. The accumulation of lipofuscin in the RPE, particularly in the macula, has been linked to photoreceptor death and to AMD [109]. Furthermore, the amount of lipofuscin present in the RPE increases with age (i.e., the amount of lipofuscin is low in young and high in old animals); thus, the potential for blue light to damage the retina may increase with age [111]. Finally, it has been reported that chronic exposure to blue light may accelerate photoreceptor degeneration in an animal model in the study of retinal degeneration [112].
Thus, experimental evidence obtained from different experimental models indicates that exposure to blue light in the 470–490 nm range may be less damaging to the eye compared to blue light in the 400–460 nm range. We believe that the development of LEDs with a peak emission of around 470–490 nm may represent an important advancement in the safety of LEDs for ocular health [6] (Figure 3).
Light exposure and age-related macular degeneration in humans
A series of studies in many animal models have shown that exposure to blue light may represent a risk for the development of AMD or other retinal pathologies [113,114]. However, the real risk from artificial light (white or blue) exposure in humans is difficult to assess, since light therapy has been in use for only a few years and in a small number of individuals. In addition, individual susceptibility to blue light damage varies significantly among individuals, making the assessment of the risk associated with repeated exposure to blue light in the etiology of AMD difficult.
Previous epidemiological studies have indicated that chronic exposure to visible and blue light may be a cofactor in the development of AMD [115-117]. However, Darzin et al. [118] found no significant relationships between blue light and the development of AMD. Okuno et al. [119] evaluated the blue-light hazards from many different light sources and reported that the exposure (even for less than a minute) to blue light from the sun, arc-welding lamps, and the arc of discharge lamps is hazardous to the retina, whereas the exposure to blue light from fluorescent lamps or LEDs does not pose a significant hazard.
Thus, it is clear that many different factors are involved in the pathogenesis of AMD. This observation, together with the limited data in terms of number of subjects or length of treatment, makes it difficult to predict the association between blue light exposure and the development of AMD.
Finally, ultraviolet (UV) light is a risk factor for age-related macular degeneration. UV is mostly blocked by the cornea or lens; therefore, only visible light can penetrate the eye and reach the retina. A recent study by Narimatsu et al. [120] conducted with an animal model reported that blocking UV light and blue light with yellow-tinted intraocular lenses materials (400–450 nm) could protect the retina [120]. Thus, reducing the amount of blue light reaching the retina in the range 400–450 nm may also be important for the protection of the retina.
Conclusions
The use of blue light is becoming increasingly prominent in our society, and a large segment of the world population is now subjected to daily exposure (from a few minutes to several hours) of artificial light at an unusual time of the day (night). Because light has a cumulative effect and many different characteristics (e.g., wavelength, intensity, duration of the exposure, time of day), it is important to consider the spectral output of the light source to minimize the danger that may be associated with blue light exposure. Thus, LEDs with an emission peak of around 470–480 nm should be preferred to LEDs that have an emission peak below 450 nm. Although we are convinced that exposure to blue light from LEDs in the range 470–480 nm for a short to medium period (days to a few weeks) should not significantly increase the risk of development of ocular pathologies, this conclusion cannot be generalized to a long-term exposure (months to years). Finally, we believe that additional studies on the safety of long-term exposure to low levels of blue light are needed to determine the effects of blue light on the eye.
Acknowledgments
This work was supported by grants from the National Institutes of Health Grants R01EY022216 by the NASA Cooperative Agreement NCC 9–58 with the National Space Biomedical Research Institute to G.T.
References
- 1.Ferguson I, Melton A, Xu T, Jamil M, Fenwick W. What would Edison do with solid state lighting? Proc. SPIE 7784, Tenth International Conference on Solid State Lighting 2010; 77840A. [Google Scholar]
- 2.Pimputkar S, Speck J, DenBaars S, Nakamura S. Prospects for LED lighting. Nat Photonics. 2009;3:180–2. [Google Scholar]
- 3.Schubert F. Light-Emitting Diodes. Cambridge University Press; 2006; pp. 434. [Google Scholar]
- 4.Nakamura S, Chichibu S. Introduction to Nitride Semiconductor Blue Lasers and Light Emitting Diodes. 2000; CRC Press; 1st 386 pages. [Google Scholar]
- 5.Nakamura S. Present performance of InGaN-based blue/green/yellow LEDs. Light-Emitting Diodes: Research, Manufacturing, and Applications. Proc SPIE. 1997;xxx:26. [Google Scholar]
- 6.Ferguson I, Melton A, Li N, Nicol D. Park, Tosini G. Imitating Broadband Diurnal Light Variations Using Solid State Light Sources. Journal of Light & Visual Environment. 2008;32:63–8. [Google Scholar]
- 7.Brinkley S, Pfaff N, Denault K, Zhang Z, Hintzen H, Seshadri R, Nakamura S, DenBaars S. Robust thermal performance of Sr2SiN8:Eu2+: An efficient red emitting phosphor for light emitting diode based white lighting. Appl Phys Lett. 2011;99:241106. [Google Scholar]
- 8.Yamazaki S, Goto M, Menaker M. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J Biol Rhythms. 1999;14:197–201. doi: 10.1177/074873099129000605. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10452331&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 9.Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1991;169:39–50. doi: 10.1007/BF00198171. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1941717&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd). and normal CBA/N (+/+). mice. J Comp Physiol. 1996;178:797–802. doi: 10.1007/BF00225828. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8667293&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 11.Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–74. doi: 10.1085/jgp.200609490. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16567464&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman MS, Lucas RJ, Soni B, von Schantz M, Muñoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–4. doi: 10.1126/science.284.5413.502. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10205061&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Provencio I, Jiang G, DeGrip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–5. doi: 10.1073/pnas.95.1.340. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9419377&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–5. doi: 10.1523/JNEUROSCI.20-02-00600.2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10632589&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellingham J, Chaurasia SS, Melyan Z, Liu C, Cameron MA, Tarttelin EE, Iuvone PM, Hankins MW, Tosini G, Lucas RJ. Evolution of melanopsin photoreceptors: Discovery and characterization of a new melanopsin gene in non-mammalian vertebrates. PLoS Biol. 2006;4:e254. doi: 10.1371/journal.pbio.0040254. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16856781&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11834834&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berson DM, Dunn FA, Takao M. Phototransduction by ganglion cells innervating the circadian pacemaker. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11834835&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 18.Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–9. doi: 10.1038/nature03345. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15674243&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 19.Bailes HJ, Lucas RJ. (2013. Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm. supporting activation of G(q/11. and G(i/o. signalling cascades. Proc Biol Sci. 2013;280:20122987. doi: 10.1098/rspb.2012.2987. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23554393&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11756521&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11713469&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 22.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=4116104&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 23.Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2465060&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 24.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12808468&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4. requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–6. doi: 10.1126/science.1076848. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12481141&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 26.Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–3. doi: 10.1126/science.1076701. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12481140&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 27.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–6. doi: 10.1038/88443. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11369943&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 28.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–7. doi: 10.1126/science.1077293. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12522249&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 29.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–7. doi: 10.1126/science.1086179. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12829787&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 30.Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, Price LL, Provencio I, Skene DJ, Brainard GC. (2014. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24287308&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul KN, Saafir TN, Tosini G. The role of retinal photoreceptors in the regulation of circadian rhythms. Rev Endocr Metab Disord. 2009;10:271–8. doi: 10.1007/s11154-009-9120-x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19777353&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18545654&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–73. doi: 10.1038/nn.2179. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19160505&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 34.Altimus CM, Güler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci USA. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19060203&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4 (−/−. mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19513122&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muindi F, Zeitzer JM, Colas D, Heller HC. The acute effects of light on murine sleep during the dark phase: importance of melanopsin for maintenance of light-induced sleep. Eur J Neurosci. 2013;37:1727–36. doi: 10.1111/ejn.12189. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23510299&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–8. doi: 10.1038/nature11673. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23151476&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aytürk DG, Castrucci AM, Carr DE, Keller SR, Provencio I. Lack of Melanopsin Is Associated with Extreme Weight Loss in Mice upon Dietary Challenge. PLoS One. 2015;10:e0127031. doi: 10.1371/journal.pone.0127031. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26011287&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–95. doi: 10.1016/j.cub.2005.12.045. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16488873&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 40.Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci USA. 2008;105:14181–6. doi: 10.1073/pnas.0803893105. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18779590&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11487664&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11507175&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Najjar RP, Chiquet C, Teikari P, Cornut PL, Claustrat B, Denis P, Cooper HM, Gronfier C. Aging of non-visual spectral sensitivity to light in humans: compensatory mechanisms? PLoS One. 2014;9:e85837. doi: 10.1371/journal.pone.0085837. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24465738&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12970330&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 45.Rüger M, St Hilaire MA, Brainard GC, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a single 6.5 h pulse of short-wavelength light. J Physiol. 2013;591:353–63. doi: 10.1113/jphysiol.2012.239046. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23090946&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20463367&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lockley SW, Gooley JJ. Circadian photoreception: spotlight on the brain. Curr Biol. 2006;16:R795–7. doi: 10.1016/j.cub.2006.08.039. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16979545&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 48.Viola AU, James LM, Schlangen LJ, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34:297–306. doi: 10.5271/sjweh.1268. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18815716&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 49.Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37:271–81. doi: 10.5665/sleep.3396. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24501435&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Najjar RP, Wolf L, Taillard J, Schlangen LJ, Salam A, Cajochen C, Gronfier C. Chronic artificial blue-enriched white light is an effective countermeasure to delayed circadian phase and neurobehavioral decrements. PLoS One. 2014;9:e102827. doi: 10.1371/journal.pone.0102827. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25072880&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vandewalle G, Gais S, Schabus M, Balteau E, Carrier J, Darsaud A, Sterpenich V, Albouy G, Dijk DJ, Maquet P. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex. 2007;17:2788–95. doi: 10.1093/cercor/bhm007. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17404390&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 52.Vandewalle G, Schmidt C, Albouy G, Sterpenich V, Darsaud A, Rauchs G, Berken PY, Balteau E, Degueldre C, Luxen A, Maquet P, Dijk DJ. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18043754&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daneault V, Hébert M, Albouy G, Doyon J, Dumont M, Carrier J, Vandewalle G. Aging reduces the stimulating effect of blue light on cognitive brain functions. Sleep. 2014;37:85–96. doi: 10.5665/sleep.3314. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24381372&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 2015;112:1232–7. doi: 10.1073/pnas.1418490112. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25535358&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light therapy for Seasonal Affective Disorder with blue narrow-band light-emitting diodes (LED. Biol Psychiatry. 2006;59:502–7. doi: 10.1016/j.biopsych.2005.07.006. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16165105&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 56.Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, Provencio I. A missense variant (P10L. of the melanopsin (OPN4. gene in seasonal affective disorder. J Affect Disord. 2009;114:279–85. doi: 10.1016/j.jad.2008.08.005. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18804284&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roecklein KA, Wong PM, Miller MA, Donofry SD, Kamarck ML, Brainard GC. Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neurosci Biobehav Rev. 2013;37:229–39. doi: 10.1016/j.neubiorev.2012.12.009. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23286902&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordijn MC, ’t Mannetje D, Meesters Y. The effects of blue-enriched light treatment compared to standard light treatment in Seasonal Affective Disorder. J Affect Disord. 2012;136:72–80. doi: 10.1016/j.jad.2011.08.016. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21911257&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 59.Ayaki M, Muramatsu M, Negishi K, Tsubota K. Improvements in sleep quality and gait speed after cataract surgery. Rejuvenation Res. 2013;16:35–42. doi: 10.1089/rej.2012.1369. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23145881&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mainster MA. Violet and blue light blocking intraocular lenses: photoreception versus photoreception. Br J Ophthalmol. 2006;90:784–92. doi: 10.1136/bjo.2005.086553. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16714268&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayaki M, Negishi K, Suzukamo Y, Tsubota K. Color of intra-ocular lens and cataract type are prognostic determinants of health indices after visual and photoreceptive restoration by surgery. Rejuvenation Res. 2015;18:145–52. doi: 10.1089/rej.2014.1613. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25526429&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 2009;24:73–84. doi: 10.1177/0748730408328973. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19227580&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 63.Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15610977&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 64.Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010;29:113–34. doi: 10.1016/j.preteyeres.2009.11.004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19951742&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ham WT, Ruffolo JJ, Mueller HA, Clarke AM, Moon ME. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest Ophthalmol Vis Sci. 1978;17:1029–35. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=100464&dopt=Abstract [PubMed] [Google Scholar]
- 66.Ruffolo JJ, Ham WT, Mueller HA, Millen JE. Photochemical lesions in the primate retina under conditions of elevated blood-oxygen. Invest Ophthalmol Vis Sci. 1984;25:893–8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6746232&dopt=Abstract [PubMed] [Google Scholar]
- 67.Jaffe GJ, Irvine AR, Wood IS, Wood IS, Severinghaus JW, Pino GR, Haugen C. Retinal phototoxicity from the operating microscope: the role of inspired oxygen. Ophthalmology. 1988;95:1130–41. doi: 10.1016/s0161-6420(88)33065-4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3068607&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 68.Dillon J. The photophysics and photobiology of the eye. J Photochem Photobiol B. 1991;10:23–40. doi: 10.1016/1011-1344(91)80209-z. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1955945&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 69.Organisciak DT, Winkler BS. Retinal light damage: practical and theoretical considerations. Prog Retin Eye Res. 1994;13:1–29. [Google Scholar]
- 70.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1981–9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10845625&dopt=Abstract [PubMed] [Google Scholar]
- 71.Rózanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995;270:18825–30. doi: 10.1074/jbc.270.32.18825. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7642534&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 72.Rózanowska M, Wessels J, Boulton M, Burke JM, Rodgers MA, Truscott TG, Sarna T. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic Biol Med. 1998;24:1107–12. doi: 10.1016/s0891-5849(97)00395-x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9626564&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 73.Pawlak A, Rozanowska M, Zareba M, Lamb LE, Simon JD, Sarna T. Action spectra for the photoconsumption of oxygen by ocular lipofuscin and lipofuscin extracts. Arch Biochem Biophys. 2002;403:59–62. doi: 10.1016/S0003-9861(02)00260-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12061802&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 74.Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol Vis Sci. 1966;5:450–73. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=5929286&dopt=Abstract [PubMed] [Google Scholar]
- 75.Sykes SM, Robison WG, Waxler M. Kuwabara T. Damage to the monkey retina by broad-spectrum fluorescent light. Invest Ophthalmol Vis Sci. 1981;20:425–34. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7216664&dopt=Abstract [PubMed] [Google Scholar]
- 76.Noell WK, Albrecht R. Irreversible effects on visible light on the retina: role of vitamin A. Science. 1971;172:76–9. doi: 10.1126/science.172.3978.76. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=5546288&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 77.Organisciak DT, Noell WK. The rod outer segment phospholipid/opsin ratio of rats maintained in darkness or cyclic light. Invest Ophthalmol Vis Sci. 1997;16:188–90. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=832982&dopt=Abstract [PubMed] [Google Scholar]
- 78.Williams TP, Howell WL. Action spectrum of retinal light-damage in albino rats. Invest Ophthalmol Vis Sci. 1983;24:285–7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6832904&dopt=Abstract [PubMed] [Google Scholar]
- 79.Ham WT, Mueller HA, Ruffolo JJ, Clarke AM. Sensitivity of the retina to radiation damage as a function of wavelength. Photochem Photobiol. 1979;29:735–43. doi: 10.1111/j.1751-1097.1979.tb07759.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=109869&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 80.Van Norren D, Schellekens P. Blue light hazard in rat. Vision Res. 1990;30:1517–20. doi: 10.1016/0042-6989(90)90032-g. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2247961&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 81.Rapp LM, Smith SC. Evidence against melanin as the mediator of retinal phototoxicity by short-wavelength light. Exp Eye Res. 1992;54:55–62. doi: 10.1016/0014-4835(92)90069-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1541341&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 82.Grimm C, Wenzel A, Williams TP, Rol P, Hafezi F, Remé C. Rhopopsin-mediated blue-light damage to the rat retina: Effect of photoreversal of bleaching. Invest Ophthalmol Vis Sci. 2001;42:497–505. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11157889&dopt=Abstract [PubMed] [Google Scholar]
- 83.Williams TP. Photoreversal of rhopospin bleaching. J Gen Physiol. 1964;47:679–89. doi: 10.1085/jgp.47.4.679. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14127606&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keller C, Grimm C, Wenzel A, Hafezi F, Remé C. Protective effect of halothane anesthesia on retinal light damage: inhibition of metabolic rhodopsin regeneration. Invest Ophthalmol Vis Sci. 2001;42:476–80. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11157886&dopt=Abstract [PubMed] [Google Scholar]
- 85.Steinberg RH. Survival factors in retinal degenerations. Curr Opin Neurobiol. 1994;4:515–24. doi: 10.1016/0959-4388(94)90052-3. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7812140&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 86.Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:764–8. doi: 10.1167/iovs.03-1114. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14985288&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arnault E, Barrau C, Nanteau C, Gondouin P, Bigot K, Viénot F, Gutman E, Fontaine V, Villette T, Cohen-Tannoudji D, Sahel JA, Picaud S. Phototoxic action spectrum on a retinal pigment epithelium model of age-related macular degeneration exposed to sunlight normalized conditions. PLoS One. 2013;8:e71398. doi: 10.1371/journal.pone.0071398. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24058402&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuse Y, Ogawa K, Tsuruma K, Shimazawa M, Hara H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci Rep. 2014;4:5223. doi: 10.1038/srep05223. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24909301&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Noell WK. Effects of environmental lighting and dietary vitamin A on the vulnerability of the retina to light damage. Photochem Photobiol. 1979;29:717–23. doi: 10.1111/j.1751-1097.1979.tb07756.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=451011&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 90.Lawwill T. Effects of prolonged exposure of rabbit retina to low-intensity light. Invest Ophthalmol. 1973;12:45–51. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=4691946&dopt=Abstract [PubMed] [Google Scholar]
- 91.Lawwill T, Crockett S, Currier G. Retinal damage secondary to chronic light exposure, thresholds and mechanisms. Doc Ophthalmol. 1977;44:379–402. doi: 10.1007/BF00230089. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=413705&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 92.Griess GA, Blankenstein MF. Additivity and repair of actinic retinal lesions. Invest Ophthalmol Vis Sci. 1981;20:803–7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7195384&dopt=Abstract [PubMed] [Google Scholar]
- 93.Organisciak DT, Jiang YL, Wang HM, Pickford M, Blanks JC. Retinal light damage in rats exposed to intermittent light. Comparison with continuous light exposure. Invest Ophthalmol Vis Sci. 1989;30:795–805. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2722438&dopt=Abstract [PubMed] [Google Scholar]
- 94.Penn JS, Naash ML, Anderson RE. Effect of light history on retinal antioxidants and light damage susceptibility in the rat. Exp Eye Res. 1987;44:779–88. doi: 10.1016/s0014-4835(87)80041-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3653273&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 95.O’Steen WK, Anderson KV, Shear CR. Photoreceptor degeneration in albino rats: dependency on age. Invest Ophthalmol Vis Sci. 1974;13:334–9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=4823176&dopt=Abstract [PubMed] [Google Scholar]
- 96.Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci USA. 2006;103:11282–7. doi: 10.1073/pnas.0602131103. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16844785&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ozawa Y, Sasaki M, Takahashi N, Kamoshita M, Miyake S, Tsubota K. Neuroprotective effects of lutein in the retina. Curr Pharm Des. 2012;18:51–6. doi: 10.2174/138161212798919101. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22211688&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duncan TE, O’Steen WK. The diurnal susceptibility of rat retinal photoreceptors to light induced damage. Exp Eye Res. 1985;41:497–507. doi: 10.1016/s0014-4835(85)80007-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=4085578&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 99.White MP, Fisher LJ. Degree of light damage to the retina varies with time of day of bright light exposure. Physiol Behav. 1987;39:607–13. doi: 10.1016/0031-9384(87)90160-0. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3588706&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 100.Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Circadian-dependent retinal light damage in rats. Invest Ophthalmol Vis Sci. 2000;41:3694–701. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11053264&dopt=Abstract [PubMed] [Google Scholar]
- 101.Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM, Organisciak DT. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem Photobiol. 2002;75:547–53. doi: 10.1562/0031-8655(2002)075<0547:efacro>2.0.co;2. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12017483&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 102.Wong P, Organisciak DT, Ziesel AC, Chrenek MA, Patterson ML. (Circadian effects on retinal light damage. In: The Retina and Circadian Rhythms 2014; (Eds G. Tosini, P.M. Iuvone, D.G. McMahon and S.P Collin. pp131–170. Springer. [Google Scholar]
- 103.Fukuhara C, Liu C, Ivanova TN, Chan GC, Storm DR, Iuvone PM, Tosini G. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neurosci. 2004;24:1803–11. doi: 10.1523/JNEUROSCI.4988-03.2004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14985420&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Humphries A, Carter DA. Circadian dependency of nocturnal immediate-early protein induction in rat retina. Biochem Biophys Res Commun. 2004;320:551–6. doi: 10.1016/j.bbrc.2004.06.006. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15219864&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 105.Andley UP, Chylack LT. Recent Studies on photodamage to the eye with special reference to clinical and therapeutic procedures. Photodermatol Photoimmunol Photomed. 1990;7:98–105. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2081122&dopt=Abstract [PubMed] [Google Scholar]
- 106.Boulton M, Rozanowska M, Rozanowski B. Retinal Photodamage. J Photochem Photobiol B. 2001;64:144–61. doi: 10.1016/s1011-1344(01)00227-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11744401&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 107.Chu R. Blue light irradiation inhibits the production of HGF by human retinal pigment epithelium cells in vitro. Photochem Photobiol. 2006;82:1247–50. doi: 10.1562/2006-04-19-RA-880. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16740060&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 108.Margrain TH, Boulton M, Marshall J, Sliney DH. Do blue light filters confer protection against age-related macular degeneration? Prog Retin Eye Res. 2004;23:523–31. doi: 10.1016/j.preteyeres.2004.05.001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15302349&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 109.Wolf G. Lipofuscin and macular degeneration. Nutr Rev. 2003;61:342–6. doi: 10.1301/nr.2003.oct.342-346. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14604266&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 110.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal photobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15862166&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 111.Delori FC, Goger DC, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–66. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11431454&dopt=Abstract [PubMed] [Google Scholar]
- 112.Thomas BB, Seiler MJ, Aramant RB, Samant D, Qiu G, Vyas N, Arai S, Chen Z, Sadda SR. Visual functional effects of constant blue light in a retinal degenerate rat model. Photochem Photobiol. 2007;83:759–65. doi: 10.1562/2006-09-19-RA-1044. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17115798&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 113.Cruickshanks KJ, Klein R, Klein BEK. Sunlight and age-related macular degeneration—the Beaver Dam Eye Study. Arch Ophthalmol. 1993;111:514–8. doi: 10.1001/archopht.1993.01090040106042. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8470986&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 114.Klein R, Klein BEK, Jensen SC, Cruickshanks KJ. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol. 1998;116:506–13. doi: 10.1001/archopht.116.4.506. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9565051&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 115.Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006;84:4–15. doi: 10.1111/j.1600-0420.2005.00627.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16445433&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 116.Taylor HR, Muñoz B, West S, Bressler NM, Bressler SB, Rosenthal FS. Visible light and risk of age-related macular degeneration. Trans Am Ophthalmol Soc. 1990;88:163–73. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2095019&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 117.Taylor HR, West S, Munoz B, Rosenthal FS, Bressler SB, Bressler NM. The long-term effects of visible-light on the eye. Arch Ophthalmol. 1992;110:99–104. doi: 10.1001/archopht.1992.01080130101035. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1731731&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 118.Darzins P, Mitchell P, Heller RF. Sun exposure and age-related macular degeneration—an Australian case-control study. Ophthalmology. 1997;104:770–6. doi: 10.1016/s0161-6420(97)30235-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9160021&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 119.Okuno T, Saito H, Ojima J. Evaluation of blue-light hazards from various light sources. Dev Ophthalmol. 2002;35:104–12. doi: 10.1159/000060814. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12061267&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 120.Narimatsu T, Ozawa Y, Miyake S, Kubota S, Yuki K, Nagai N, Tsubota K. Biological effects of blocking blue and other visible light on the mouse retina. Clin Experiment Ophthalmol. 2014;42:555–63. doi: 10.1111/ceo.12253. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24304494&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 121.Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Iuvone PM, Tosini G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci USA. 2009;106:15043–8. doi: 10.1073/pnas.0904400106. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19706469&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sakamoto K, Liu C, Kasamatsu M, Iuvone PM, Tosini G. Intraocular injection of kainic acid does not abolish the circadian rhythm of arylalkylamine N-acetyltransferase mRNA in rat photoreceptors. Mol Vis. 2006;12:117–24. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16518309&dopt=Abstract [PubMed] [Google Scholar]