A salt-inducible Mn-catalase from Anabaena plays a vital role in combating oxidative and salinity stress.
Abstract
Catalases, enzymes that detoxify H2O2, are widely distributed in all phyla, including cyanobacteria. Unlike the heme-containing catalases, the physiological roles of Mn-catalases remain inadequately characterized. In the cyanobacterium Anabaena, pretreatment of cells with NaCl resulted in unusually enhanced tolerance to oxidative stress. On exposure to H2O2, the NaCl-treated Anabaena showed reduced formation of reactive oxygen species, peroxides, and oxidized proteins than the control cells (i.e. not treated with NaCl) exposed to H2O2. This protective effect correlated well with the substantial increase in production of KatB, a Mn-catalase. Addition of NaCl did not safeguard the katB mutant from H2O2, suggesting that KatB was indeed responsible for detoxifying the externally added H2O2. Moreover, Anabaena deficient in KatB was susceptible to oxidative effects of salinity stress. The katB gene was strongly induced in response to osmotic stress or desiccation. Promoter-gfp analysis showed katB to be expressed only in the vegetative cells but not in heterocysts. Biochemically, KatB was an efficient, robust catalase that remained active in the presence of high concentrations of NaCl. Our findings unravel the role of Mn-catalase in acclimatization to salt/oxidative stress and demonstrate that the oxidative stress resistance of an organism can be enhanced by a simple compound such as NaCl.
Cyanobacteria evolved more than 3 billion years ago and are believed to be the progenitors of plant chloroplasts (Brock, 1973; Fay, 1992). Oxygen liberated by cyanobacteria during photosynthesis was responsible for the initial oxygenation of Earth’s atmosphere, which consequently allowed the aerobic life to occur and flourish (Schopf, 1975). Due to their intimate association with oxygen, it is expected that cyanobacteria have developed several strategies to overcome the deleterious effects caused by the reactive oxygen species (ROS), which form due to the partial reduction of oxygen (Banerjee et al., 2012a, 2013). Also, during the course of their evolution, cyanobacteria have been exposed to virtually all natural (high-intensity light, salinity, desiccation, etc.) and anthropogenic stresses (heavy metals, herbicides, etc.), many of which ultimately culminate in oxidative stress (Dadheech, 2010). For these reasons, cyanobacteria have been proposed to be excellent model systems to study mechanisms of oxidative stress resistance.
In biological systems, hydrogen peroxide (H2O2) is a key ROS that threatens cell survival. H2O2 is produced by dismutation of O2· by superoxide dismutases or directly by several oxidases (Collén et al., 1995). H2O2 can directly permeate into cells; hence, H2O2 toxicity arises whenever H2O2 is present in the environment. Inside cells, H2O2 is known to directly inactivate enzymes such as dehydratases that contain iron-sulfur clusters (Jang and Imlay, 2010). In the presence of Fe2+, H2O2 produces OH· (Fenton reaction), the most destructive radical that can damage molecules in its vicinity at diffusion-limited rates (Halliwell and Gutteridge, 1986). H2O2 generated within cells is detoxified by several enzymes, of which peroxidases and catalases are the two major classes. Interestingly, glutathione peroxidases and ascorbate peroxidases, which play a major role in decomposing H2O2 in animals and plants, respectively, are not widespread in cyanobacteria (Tel-Or et al., 1985), but catalases and peroxiredoxins (Prxs) are well represented (Zamocky et al., 2008).
Catalases are subdivided into three categories: (1) typical monofunctional catalases, (2) bifunctional catalase peroxidases (KatG), and (3) binuclear manganese catalases (Mn-catalases). Of the three, the first two, which are haem-containing, have been extensively characterized from several prokaryotic and eukaryotic organisms (Zamocky et al., 2008). The distribution of Mn-catalases is restricted to prokaryotes and archae (Amo et al., 2002). Mn-catalases have been relatively poorly characterized, and the crystal structures of only two Mn-catalases (from Thermus thermophilus and Lactobacillus plantarum) have been reported so far (Antonyuk et al., 2000; Barynin et al., 2001). These structures have shown that Mn-catalases are 4-helix bundle proteins belonging to the Ferritin-like superfamily (Andrews, 2010).
Nitrogen-fixing strains of cyanobacteria such as Anabaena are economically important, as these are employed as biofertilizers in the paddy fields of Southeast Asia (Venkataraman, 1979). Various abiotic stresses (heavy metals, drought, salinity, herbicides, etc.) that affect plant growth and limit crop production worldwide also adversely affect the biofertilizer potential of Anabaena. It should be noted that all these stresses cause overproduction of ROS (Choudhury et al., 2013). Thus, studying the detailed basis of oxidative stress resistance in Anabaena is essential for development of novel biofertilizers, which may be used under adverse conditions. The role played by various proteins in overcoming oxidative stress in the filamentous, heterocystous, nitrogen-fixing, cyanobacterium Anabaena PCC 7120 has garnered remarkable interest in the recent past (Cha et al., 2007; Agrawal et al., 2014; Panda et al., 2014; Banerjee et al., 2015; Tailor and Ballal, 2015).
In the cyanobacterium Anabaena PCC 7120, genes representing the typical catalases or KatGs were absent, but genome analysis revealed the presence of two open reading frames (ORFs), alr0998 (katA) and alr3090 (katB), that encoded a Mn-catalase (Kaneko et al., 2001). Earlier, zymographic analysis had shown no detectable catalase activity in the control (i.e. unstressed) or the H2O2-treated Anabaena PCC 7120 (Banerjee et al., 2012b). Among the two Mn-catalases, overproduction of KatA (encoded by alr0998) was shown to protect Anabaena from oxidative stress mediated by H2O2 (Banerjee et al., 2012b), whereas the physiological/biochemical functions of KatB (encoded by alr3090) remained unknown.
In this study, we have elucidated the molecular basis of the unusual tolerance shown by the NaCl-treated Anabaena cells to oxidative stress imposed by H2O2. Several lines of evidence showed the KatB protein to be responsible for this protective phenomenon. Furthermore, Anabaena katB was found to be transcriptionally induced in response to salt, and, interestingly, the katB promoter was found to be active only in the vegetative cells and not in heterocysts. In Anabaena, the KatB protein was cytosolic, and once synthesized the protein was relatively stable in vivo. Compared with the wild-type Anabaena, the mutant strain lacking KatB was sensitive to salt stress. In the presence of salt, exposure to H2O2 led to severe oxidative stress in the katB mutant, eventually resulting in cell death. These data confirm the role of KatB in overcoming salinity/oxidative stress, and to our knowledge, this is the first exhaustive physiological characterization of a Mn-catalase from any photosynthetic organism.
RESULTS
Salt-Pretreated Anabaena PCC 7120 Is Protected from Oxidative Stress Mediated by H2O2
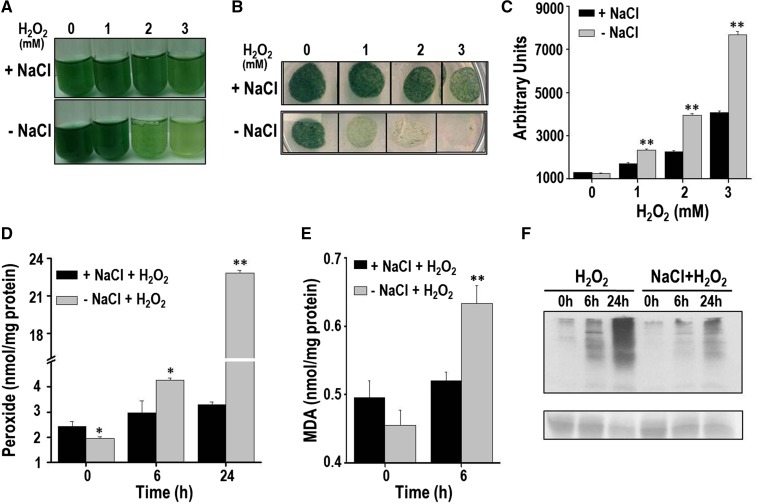
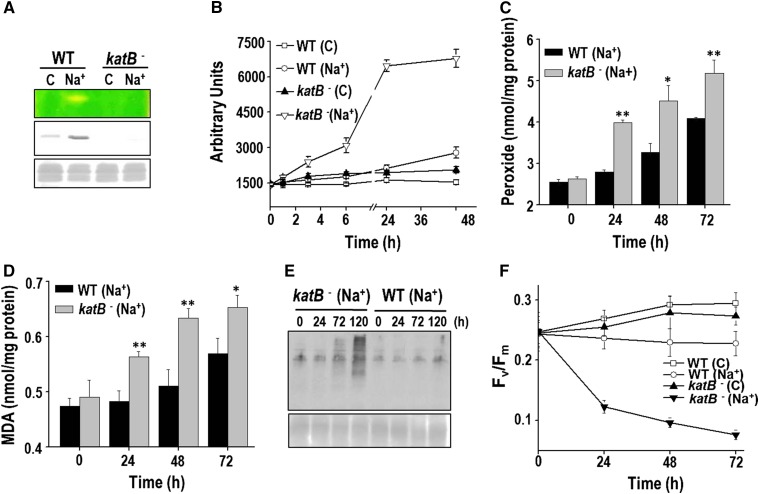
A wild-type Anabaena PCC 7120 culture was split into two parts, with one part being subjected to NaCl treatment whereas the other served as the control. Subsequently, both cultures were exposed to different concentrations of H2O2 (0, 1, 2, and 3 mm) and monitored (Fig. 1). With a higher concentration of H2O2 (2 or 3 mm), greater bleaching of pigments was observed in the control cells than the salt-stressed cells (Fig. 1A). The control cells subjected to H2O2 stress failed to grow on BG11 plates, indicting a loss in viability. On the other hand, the salt-stressed Anabaena cells survived H2O2 treatment and could grow on plates (Fig. 1B). The cellular oxidative stress in the above-mentioned cells was monitored with the probe dichloro dihydrofluorescein diacatate (DCHFDA). At all the concentrations of H2O2 tested, considerably reduced amounts of ROS were observed in the NaCl-pretreated cells compared with the control cells (Fig. 1C). In good agreement with the increased levels of ROS observed, the control cells also showed considerably higher accumulation of total peroxides than cells pretreated with NaCl (Fig. 1D). As oxidative stress is known to enhance lipid peroxidation and protein oxidation, these aspects were also monitored. After H2O2 exposure, Anabaena PCC 7120 pretreated with salt showed significantly reduced levels of malondialdehyde (MDA; an end product of lipid peroxidation) compared with the control cells (Fig. 1E). After 24 h of H2O2 treatment, the cellular proteins in the control cells were severely oxidized, whereas proteins from the NaCl-pretreated cells showed noticeably reduced oxidative damage (Fig. 1F). In conclusion, H2O2 caused severe oxidative stress in Anabaena, but Anabaena cells that were pretreated with NaCl were remarkably protected from these deleterious effects.
Figure 1.
H2O2 stress tolerance of the salt-pretreated Anabaena PCC 7120. A, Three-day-old Anabaena culture was reinoculated in fresh growth medium and divided into two parts. One part was treated with 100 mm NaCl (+NaCl) for 20 h, whereas the other served as the control (-NaCl). Subsequently, these cultures were stressed with different concentrations of H2O2 as indicated in the figure and photographed after 2 d. B, The above-mentioned Anabaena cultures after 2 d of treatment with H2O2 were spotted (20 μL each) on BG11 agar plate, incubated under continuous illumination, and photographed after 14 d of incubation. C, ROS production in response to H2O2. The control (-NaCl) or NaCl-treated (+NaCl) Anabaena cells were exposed to H2O2 for 16 h. Subsequently, cells were incubated with DCHFDA (10 µm final concentration) for 20 min, and fluorescence emission (λex = 490 nm, λem = 520 nm) from cells was measured immediately on a spectrofluorimeter. The relative fluorescence in arbitrary units (AUs) of both types of cultures is shown in the figure. Error bars show SE (n = 5). ** indicates significant differences at P < 0.01 compared with the corresponding control (-NaCl) cells (Student’s t test). D, Production of total peroxides in cells pretreated with NaCl (+NaCl) or control (-NaCl) cells on exposure to 1 mm H2O2. Error bars represent SE (n = 3). Asterisks indicate significant differences (*P < 0.05 and **P < 0.01) compared with the corresponding control (-NaCl) cells. E, TBARS assay to determine lipid peroxidation. The MDA produced in the control or the NaCl-pretreated cells (on exposure to H2O2) was measured at the time point indicated. Error bars represent SE (n = 3). ** indicates significant differences at P < 0.01 compared with the corresponding control (-NaCl) cells (Student’s t test). F, Detection of oxidized proteins. At the time points indicated, protein extracts were prepared from the control or +NaCl Anabaena cultures, and derivatized with dinitrophenol (DNP). Subsequently, these proteins were resolved on SDS-PAGE, transferred to nitrocellulose membrane, and probed with the monoclonal DNP antiserum. Chemiluminescent detection was performed as described in “Materials and Methods.” A Ponceau S-stained part of the blot is shown as loading control below the western blot. The experiment was repeated thrice and consistent results were obtained.
Anabaena Pretreated with NaCl Shows High Catalase (KatB) Activity, and Anabaena Overproducing KatB Is Protected from Oxidative Stress Caused by H2O2
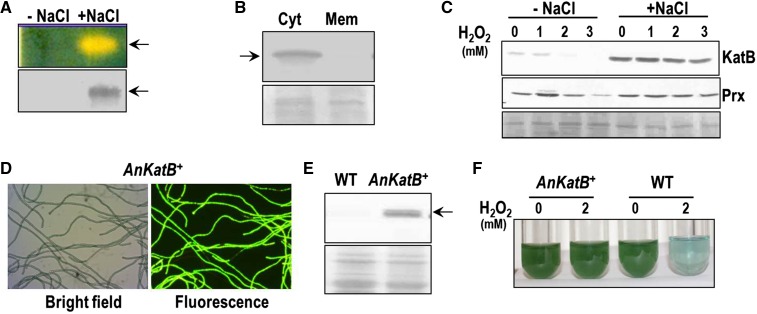
As the salt-pretreated Anabaena PCC 7120 was protected from oxidative stress mediated by H2O2, catalase activity of these cultures was monitored (Fig. 2). Zymographic analysis of the salt-stressed Anabaena showed profuse catalase activity (indicated by a zone of clearance), whereas the control cells showed none (Fig. 2A). Western-blotting analysis of native gels showed the zone of clearance matched the signal obtained from the KatB, but not the KatA, antiserum (Fig. 2A). The KatB protein was clearly detected in the soluble fraction (and not in the membrane preparation) of Anabaena PCC 7120 whole-cell extracts, indicating that the protein was cytosolic in nature (Fig. 2B). KatB could barely be detected in the above-mentioned control Anabaena cells treated with H2O2, whereas in the NaCl-treated cells, KatB production was elevated and remained virtually unaltered in the presence of H2O2 (Fig. 2C). Expression of another antioxidant enzyme, 2-Cys-Prx (which is induced by H2O2), was monitored in the salt-pretreated Anabaena PCC 7120 subjected to H2O2 stress. Although a distinct induction of 2-Cys-Prx was observed on treatment of control Anabaena cells with 1 mm H2O2, no such increase was observed in the salt-pretreated cells (Fig. 2C).
Figure 2.
Expression of KatB in Anabaena PCC 7120. A, The cell free extracts of control (-NaCl) and NaCl-treated (+NaCl) Anabaena PCC 7120 were assayed for in gel catalase activity (zymogram, top) or probed with the KatB antiserum at 1:10,000 dilution on a western blot (bottom). The experiment was repeated thrice with similar results and a representative image is shown. B, Localization of KatB. The salt-stressed Anabaena cells were lysed with glass beads, and the membrane proteins in the lysate were separated from the cytosolic proteins. Cytosolic and membrane proteins were resolved on SDS-PAGE, transferred onto a nitrocellulose membrane, and probed with the KatB antiserum. The Ponceau S-stained part of the nitrocellulose membrane is shown as a loading control at the bottom. C, The cell-free extracts of control (-NaCl) or NaCl-pretreated (+NaCl) Anabaena exposed to H2O2 were resolved on SDS-PAGE and electroblotted on to nitrocellulose membrane. These were probed with the KatB antiserum (top, KatB) or the Alr4641 (2-Cys-Prx) antiserum (bottom, Prx). The Ponceau S-stained part of the nitrocellulose membrane is shown as a loading control at the bottom. D, Bright field and fluorescence micrograph of KatB overexpressing Anabaena strain (AnKatB+). E, Overexpression of KatB protein in Anabaena. The cell-free extracts of the wild-type Anabaena PCC 7120 (WT) and AnKatB+ were resolved by SDS-PAGE, immunoblotted, and probed with anti-KatB antiserum. Ponceau S-stained loading control is shown at the bottom. F, The wild-type Anabaena PCC 7120 (WT) or Anabaena overexpressing KatB (AnKatB+) cells were treated with H2O2 (2 mm) for 2 d and photographed.
Further, to validate the contribution of KatB in overcoming oxidative stress mediated by H2O2, KatB was overexpressed in Anabaena PCC 7120 employing an Anabaena-Escherichia coli shuttle vector, pAMKatB. In AnKatB+ (Anabaena PCC 7120 strain transformed with pAMKatB) both katB and gfp are cotranscribed but independently translated, resulting in coexpression of both proteins. The AnKatB+ cells that appeared on the selection medium were verified by monitoring expression of GFP (Fig. 2D). When probed with the KatB antiserum (at 1:15,000 dilution), the recombinant AnKatB+ showed considerable production of the KatB protein (Fig. 2E). Treatment with H2O2 resulted in marked bleaching of the wild-type Anabaena PCC 7120, but the AnKatB+ cells were protected from this damage (Fig. 2F).
KatB Is Induced in Response to Osmotic Up-Shock and Desiccation
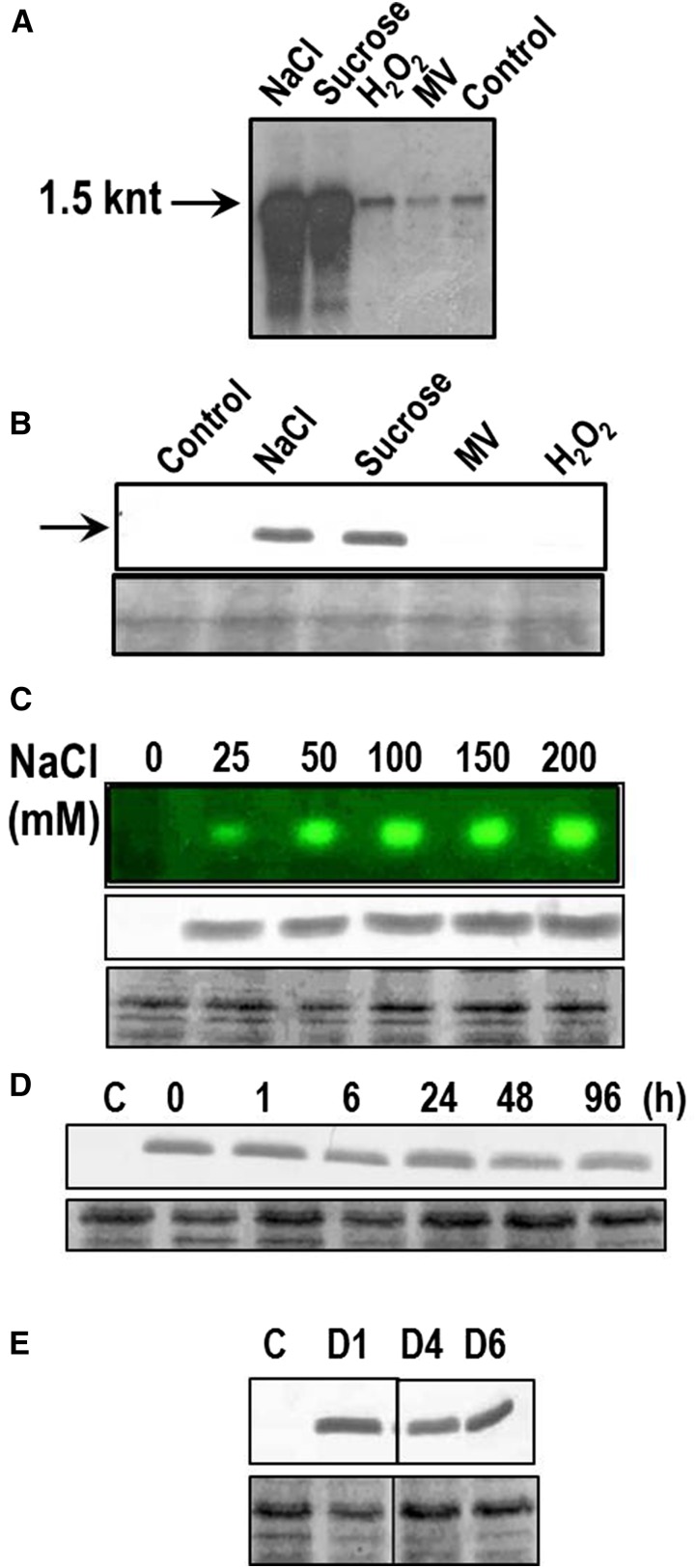
Expression of the katB gene was monitored at the level of RNA and protein under various stresses (Fig. 3). The wild-type Anabaena PCC 7120 cells were exposed to NaCl, Suc, or direct oxidative stress-causing agents like H2O2 or methyl viologen (MV) as indicated in Figure 3A. Compared with the control cells, a many-fold induction of the katB transcript (approximately 1.5 knt) was observed in response to salt and Suc stresses. In contrast, no induction of katB was observed with H2O2 and MV (Fig. 3A). In agreement with the transcriptional analysis, the 26-kD KatB protein was found to be synthesized in cells stressed with salt or Suc but not in the H2O2/MV-treated cells (Fig. 3B).
Figure 3.
Expression analysis of KatB under different abiotic stresses. A, Northern-blotting hybridization analysis. Total RNA was isolated from untreated Anabaena PCC 7120 (control) or from cells treated with 150 mm NaCl, 300 mm Suc, 1 mm H2O2 (H2O2), or 2 μm MV. Subsequently, RNA (5 μg/lane) was resolved on formaldehyde-agarose gels, transferred onto a nylon membrane, and probed with the DIG-labeled katB ORF. The approximately 1.5-knt transcript is shown by an arrow. The northern blotting-hybridization experiment was repeated four times with consistent results. B, Induction of the KatB protein in Anabaena. Total proteins (20 μg/lane) were isolated from Anabaena cells treated with 150 mm (NaCl) or 300 mm (Suc) or 2 μm (MV) or 1 mm H2O2 (H2O2) and probed with the KatB antiserum (1:10,000 dilution). The 20-kD KatB protein is shown by an arrow. C, Anabaena cells were treated with different concentrations of NaCl as indicated in the figure for 20 h. Subsequently, cell-free extracts were prepared and employed for zymographic analysis (top) or probed with the KatB antiserum on western blots (middle). Representative zymogram is depicted and the Ponceau S-stained part of the nitrocellulose membrane is shown as loading control at the bottom. D, Stability of the KatB protein. Anabaena cells were exposed to 150 mm NaCl for 16 h, thoroughly washed, reinoculated in medium lacking NaCl, and the KatB protein content was monitored on western blots with the KatB antiserum at different intervals of time as indicated. The Ponceau S-stained loading control is shown at the bottom. E, KatB expression in response to desiccation. Cell-free extracts of the control Anabaena cells (C) or the desiccated Anabaena cells after 1 d (D1), 4 d (D4), or 6 d (D6) were probed with the KatB antiserum on western blots. The Ponceau S-stained loading control is shown at the bottom.
With increasing concentrations of NaCl, a concomitant rise in synthesis of the KatB protein as well as the catalase activity was observed on western blots and zymograms, respectively (Fig. 3C). Anabaena cells were exposed to NaCl for 16 h, thoroughly washed, reinoculated in a medium lacking NaCl, and the content of the KatB protein was monitored. Once synthesized in vivo, the KatB protein was relatively stable, and even 4 d after the withdrawal of NaCl, KatB could be distinctly visualized (Fig. 3D). It should be noted that KatB expression was also observed in the wild-type control Anabaena PCC 7120 when a lower dilution of antiserum (1:5,000) was used for western blots (Fig. 2C). However, this basal level of expression in the untreated cells (i.e. control) was inadequate to form a zone of clearance on zymograms (Fig. 3C; Banerjee et al., 2012b). In their natural habitat, cyanobacteria are periodically exposed to desiccation. Hence, induction of KatB in response to desiccation stress was monitored. No KatB could be detected in the control cells, whereas after day 1 of desiccation and beyond, expression of KatB was clearly observed (Fig. 3E).
Identification of Transcriptional Start Site by Rapid Amplification of cDNA Ends
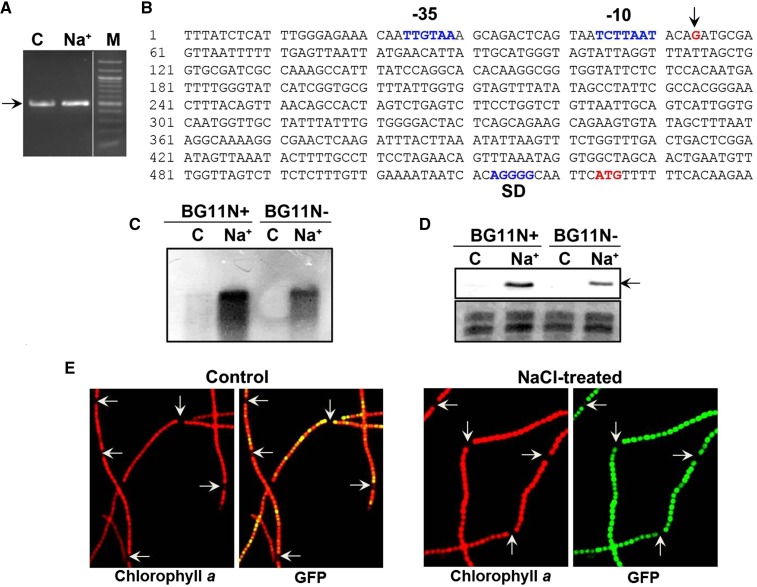
As distinct induction of katB was observed in response to various abiotic stresses, it was desired to identify its promoter (Fig. 4). Rapid amplification of cDNA ends (RACE) with the total RNA isolated from the wild-type Anabaena PCC 7120 control or the NaCl-treated cells showed an approximately 450-bp cDNA product (Fig. 4A). Sequence analysis of the product (in both the cases) identified the start of the transcript to be 410 nt upstream of the translational start of the katB ORF (Fig. 4B). The -10 and -35-like promoter sequences identified upstream of the transcriptional start site are indicated in Figure 4B.
Figure 4.
RACE analysis and expression of the katB promoter-gfp gene fusion (pAM3090prom). A, RACE was performed with RNA isolated from control Anabaena cells (C) or cells treated with 150 mm NaCl (Na+) for 16 h. The approximately 450-bp DNA fragment is shown by an arrow. Then 20 µL and 5 µL of the PCR reaction was loaded in the C and Na+ lane, respectively. M, 100-bp DNA marker. B, Sequence analysis of the RACE product. The transcriptional start site (G, in red color) is indicated by an arrow. The nucleotide sequence corresponding to the −10 and −35 region of the katB promoter, the ribosome binding site (SD), and the translational start codon (ATG) are denoted. C, The wild-type Anabaena PCC 7120 cells grown in BG11 medium with (BG11N+) or without combined nitrogen (BG11N-) were treated with 150 mm NaCl (Na+). The untreated cells (C) served as control in both types of media. RNA was isolated from both control (C) and treated (Na+) cells and resolved on formaldehyde-agarose gel, transferred onto anylon membrane, and probed with the DIG-labeled katB DNA. D, Protein extracts (60 μg/lane) from the control (C) and treated (Na+) Anabaena PCC 7120 cells grown in BG11N+ or BG11N- were resolved by SDS-PAGE (14% gel) and probed with the KatB antiserum on western blots. The 26-kD KatB protein is depicted by an arrow. E, Fluorescence micrographs (1500×). An3090prom cells were grown in medium without combined nitrogen (BG11N-) and treated with NaCl. Anabaena filaments were visualized under a fluorescence microscope using Hg-Arc lamp; chlorophyll a fluorescence (excitation BP, 546–612 nm and emission LP, 515 nm), and GFP fluorescence (excitation BP, 450–490 nm and emission LP, 515 nm). Heterocysts are depicted by arrows.
Nitrogen Status Influences Production of the KatB Protein and the katB Promoter Is Not Active in Heterocysts
The wild-type Anabaena PCC 7120 cells grown under nitrogen-fixing conditions (BG11N-) or in the presence of combined nitrogen (BG11N+) were treated with NaCl and monitored for production of the katB transcript or the KatB protein. Without NaCl, no katB transcript or KatB protein was observed in cells cultured in BG11N- medium, while minor synthesis of the same was observed in cells from BG11N+ medium (Fig. 4, C and D). On treatment with NaCl, the katB transcript as well as the KatB protein was detected in Anabaena cells grown in BG11N- medium, but their level was several-fold lower than that observed in the corresponding cells grown in BG11N+ medium (Fig. 4, C and D).
To monitor its promoter activity, the katB promoter (identified by RACE analysis) and its adjacent DNA were cloned upstream of the gfp (GFP) reporter gene in the reporter vector, pAM1956 (pAM3090prom), and transferred into Anabaena PCC 7120 (strain named An3090prom). For in vivo validation, An3090prom was treated with NaCl and subjected to microscopic analysis. In the absence of NaCl, relatively weak fluorescence was observed when filaments were grown in BG11N- (Fig. 4E, left), whereas bright green fluorescence was observed in filaments treated with NaCl (Fig. 4E, right), indicating that the cloned katB promoter was indeed functional. Interestingly, microscopic analysis showed strong GFP fluorescence in the vegetative cells, whereas hardly any fluorescence was observed in heterocysts, demonstrating that the katB promoter was active in vegetative cells but not in heterocysts (Fig. 4E).
GFP fluorescence was monitored and quantified under control conditions or in response to salt stress. In the absence of NaCl (i.e. control conditions), an approximately 6-fold lower florescence was observed in cells grown in BG11N- than those cultured in BG11N+. Although a 6- to 7-fold increase in GFP fluorescence was observed on treatment with NaCl, florescence of cells grown in BG11N- was still 3- to 4-fold lower than the corresponding BG11N+-grown cells exposed to NaCl (Supplemental Fig. S1).
Inactivation of the katB Gene Results in Enhanced Sensitivity to Salt Stress
To evaluate the in vivo contribution of katB, insertional inactivation of the katB gene in Anabaena PCC 7120 was performed (Fig. 5) employing a strategy described by Neunuebel and Golden (2008). On zymographic/western-blotting analysis, KatB activity/protein could be observed only in the wild-type Anabaena PCC 7120 cells but not in the mutant (AnKatB-; Fig. 5A). Both the wild type and the katB mutant showed similar sensitivity to exogenously added H2O2 (data not shown).
Figure 5.
Sensitivity of the KatB mutant to salt stress. A, Catalase activity in the wild-type Anabaena (WT) cells or the katB mutant (katB-) was monitored on zymogram (top). Production of the KatB protein was monitored on western blots with the KatB antiserum (middle). The Ponceau S-stained part of the nitrocellulose membrane is shown as loading control at the bottom. C, Control cells; Na+, cells treated with NaCl. B, ROS production in response to NaCl. The wild type (WT) or the katB mutant (katB-) was treated with 100 mm NaCl, and the total ROS were measured with the fluorescent probe DCHFDA. The relative fluorescence in arbitrary units (AUs) of both types of cultures without NaCl (control, C) or in the presence of NaCl (Na+) is depicted. Error bars represent SE (n = 4). C, Production of total peroxides in cells on treatment with 100 mm NaCl. Error bars represent SE (n = 3). Asterisks indicate significant differences (*P < 0.05 and **P < 0.01) compared with the corresponding wild-type cells. D, Lipid peroxidation. The MDA produced in the wild type (WT) or the katB mutant (katB-) in response to 100 mm NaCl. Error bars show SE (n = 3). Asterisks indicate significant differences (*P < 0.05 and **P < 0.01) compared with the corresponding wild-type cells. E, Detection of oxidized proteins. Protein extracts were prepared from the two different cell types at the indicated time points and derivatized with DNP. Subsequently, these proteins were resolved on SDS-PAGE, transferred to nitrocellulose membrane, and probed with the monoclonal DNP antiserum. Chemiluminescent detection was performed as described in “Materials and Methods.” F, Changes in the Fv/Fm of the wild type or the katB mutant (katB-) in response to NaCl (100 mm). Error bars represent SE (n = 4).
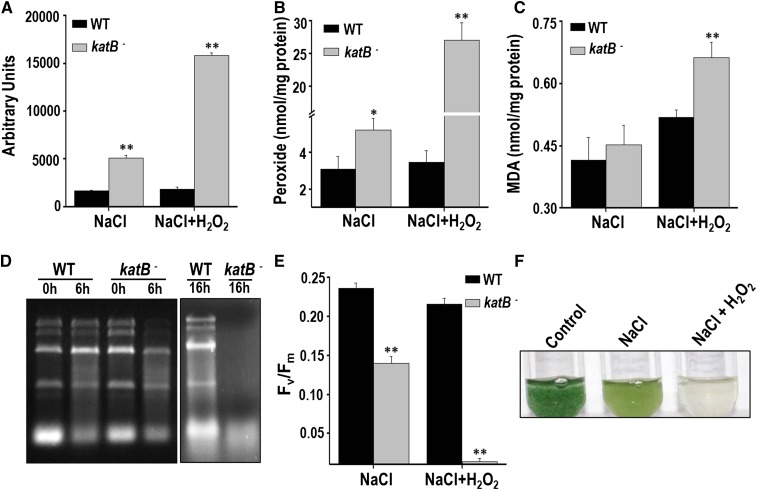
The overall cellular oxidative stress in response to NaCl was monitored with DCHFDA. After as early as 3 h, a distinct increase in the ROS levels could be observed in the katB mutant (Fig. 5B). Although increased ROS levels were observed in the wild-type cells by 48 h, these levels were several-fold lower than that observed in the katB mutant. Treatment with NaCl led to increased accumulation of peroxides in the mutant compared with the wild type (Fig. 5C). In addition, the mutant showed a higher level of lipid peroxidation (Fig. 5D) and protein oxidation (Fig. 5E) than the wild type. The maximum photochemical efficiency of PSII (Fv/Fm), was monitored in the two above-mentioned cell types. A severe reduction was observed in Fv/Fm of the KatB mutant with time, whereas, on the contrary, only a minor reduction of the same was detected in the wild-type cells (Fig. 5F). In the presence of NaCl, the wild-type Anabaena PCC 7120 showed reduction in growth compared with the control cells, but interestingly, no growth was observed in AnKatB- cells (Supplemental Fig. S2A). On longer exposure to NaCl, AnKatB- (but not the wild-type Anabaena PCC 7120) appeared to be severely bleached (Supplemental Fig. S2B). These results demonstrate that KatB is essential to reduce the oxidative stress burden brought about by salt stress in Anabaena PCC 7120.
The Salt-Pretreated katB Mutant Is Extremely Sensitive to H2O2
To conclusively prove the involvement of KatB in protection of the NaCl-treated Anabaena PCC 7120 from externally added H2O2, the katB mutant was subjected to the regimen of salt stress followed by exposure to H2O2 (Fig. 6). Compared with the salt-treated wild-type Anabaena cells, a more than 8-fold excess of ROS and total peroxides was detected in the NaCl-treated katB mutant on the addition of H2O2 (Fig. 6, A and B). Similarly, higher levels of lipid peroxides were observed in the katB mutant (Fig. 6C). In the absence of sufficient catalase activity, H2O2 is known to degrade cellular RNA in Anabaena (Banerjee et al., 2012b). The salt-treated KatB mutant showed complete degradation of RNA after 24 h of H2O2 treatment. Meanwhile, RNA from the wild-type Anabaena PCC 7120 was remarkably protected from this damage, and the rRNA bands could be clearly observed (Fig. 6D). Exposure to H2O2 not only led to a drastic reduction in Fv/Fm but also caused severe bleaching of the NaCl-treated katB mutant (Fig. 6, E and F). All these results confirm the crucial role played by KatB in overcoming the deleterious effects of H2O2 in Anabaena pretreated with salt.
Figure 6.
Exposure of the NaCl-treated katB- Anabaena to H2O2. The wild type (WT) or the KatB mutant (katB-) was pretreated with 100 mm NaCl for 16 h and subsequently exposed to 1 mm H2O2. A, Total ROS production at the end of 6 h was measured with DCHFDA. Error bars show SE (n = 3). ** indicates significant differences at P < 0.01 compared with the corresponding wild type. B, Production of total peroxides after 16 h of treatment with H2O2. Error bars represent SE (n = 3). Asterisks indicate significant differences (*P < 0.05 and **P < 0.01) compared with the corresponding wild type. C, Lipid peroxidation. The MDA produced in the wild type (WT) or the katB mutant (katB-) is shown. Error bars show SE (n = 3). ** indicates significant differences at P < 0.01 compared with the corresponding wild type. D, Total RNA was isolated from the NaCl-treated cells that were exposed to H2O2 for 6 or 14 h. The total RNA (5 µg) was resolved on formaldehyde-agarose gels and photographed. The experiment was repeated at least thrice and the representative image is shown. E, Fv/Fm of the NaCl-treated wild type or the katB mutant (katB-) in response to 16 h of exposure to H2O2 (1 mm). Error bars show SE (n = 5). ** indicates significant differences at P < 0.01 compared with the corresponding wild type. F, The NaCl-treated katB- was exposed to 1 mm H2O2 and photographed after 2 d.
KatB Is a Robust Catalase
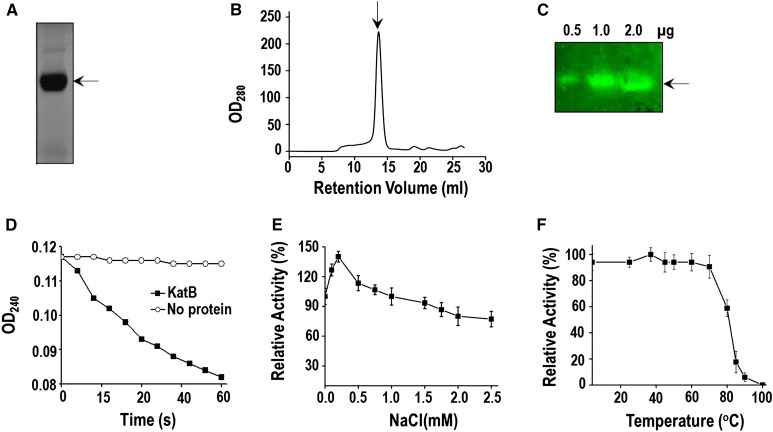
Bioinformatic analysis showed katB (alr3090) from Anabaena PCC 7120 to encode a protein (230 amino acids) that contained a domain specific for Mn-catalase. For biochemical characterization, the alr3090 ORF (with 6 additional in-frame His codons) was overexpressed in E. coli and purified (Bihani et al., 2013). The biochemical/biophysical characteristics of the purified KatB protein are shown in Figure 7. When resolved on native polyacrylamide gel, the purified KatB protein appeared as a single band (Fig. 7A). Gel filtration analysis of KatB revealed the presence of a single peak (approximately 109 kD), indicating the presence of single oligomeric species (Fig. 7B). Dynamic light scattering also showed the KatB protein solution to be monodispersive (Supplemental Fig. S3A). On zymographic analysis, the purified KatB protein showed a distinct zone of clearance in native gels, indicating that it was indeed active (Fig. 7C). These observations were corroborated by spectroscopic analysis (Fig. 7D). KatB could tolerate high NaCl concentration and even in the presence of 2.5 m NaCl, 77% activity (compared with the untreated enzyme) was retained (Fig. 7E). CD spectropolarimetric analysis showed KatB to be a largely alpha helical protein (Supplemental Fig. S3B). Interestingly, the KatB protein displayed a Tm of 83°C, indicating that the protein was fairly thermostable (Supplemental Fig. S3C). To verify its ability to withstand high temperatures, KatB was exposed to elevated temperatures for 10 min and assayed at room temperature. No loss in activity was observed even when KatB was exposed to temperature as high as 80°C. Subsequently, on increasing the temperature further, a rapid decline was observed, and only 10% of the original activity remained when the protein was exposed to 90°C (Fig. 7F).
Figure 7.
Alr3090 (KatB) is a robust Mn-catalase. A, Purified KatB protein was resolved on 10% nondenaturing native PAGE and visualized by staining with Coomassie Brilliant Blue. Similar gel profiles were obtained in four different experiments and a representative image is shown. B, Size exclusion chromatography profile of the purified KatB protein using Superdex 200 10/300 GL column pre-equilibrated with buffer (20 mm Tris, 50 mm NaCl, pH 7.5). C, The recombinant KatB protein was assayed for catalase activity on zymogram. The amount of the KatB protein is indicated. D, The catalase activity of KatB protein was measured (at room temperature) by monitoring the decrease in A240 (OD240). E, Catalase activity of the purified KatB protein was monitored at different concentrations of NaCl as indicated. The rate of KatB activity in the absence of NaCl was considered as 100%, and other rates were calculated accordingly. Error bars represent SE (n = 5). F, The purified KatB protein (2 µg) was incubated in the presence of different temperatures for 10 min. Subsequently, employing H2O2 as substrate, the catalase activity was measured (at room temperature) by monitoring the decrease in A240. The activity of KatB incubated at room temperature was considered to be 100%, and the other rates were calculated accordingly. Error bars represent SE (n = 4).
DISCUSSION
Catalases, being highly active enzymes, play an important role in detoxifying H2O2 in all three phylogenetic domains, i.e. Eukaryota, Bacteria, and Archaea. Mn-catalases form a minor gene family in cyanobacteria, but remarkably, all nitrogen-fixing cyanobacteria have at least one ORF that encodes a Mn-catalase (Banerjee et al., 2013). In this study, a comprehensive physiological characterization of a Mn-catalase (KatB) from the nitrogen-fixing cyanobacterium Anabaena PCC 7120 was performed.
Large-scale genome sequencing analysis has shown the presence of Mn-catalase-like genes in several prokaryotes. However, assignment of some of these as Mn-catalase appears to be ambiguous. The Mn-catalase (Z1921P) from E. coli 0157:H7 could be purified as a soluble hexameric protein, but surprisingly, it did not show any catalase activity (Whittaker, 2012). The catalase activity of the KatB protein was detected in the extracts of the salt-stressed Anabaena PCC 7120 and the purified KatB too showed H2O2-detoxification ability, indicating that, unlike Z1921P, KatB was indeed a functional catalase. In contrast to other Mn-catalases that are mostly hexameric (Antonyuk et al., 2000; Barynin et al., 2001), gel filtration analysis indicated the purified KatB to be tetrameric (Fig. 7B). However, x-ray crystallography-based structural analysis has shown KatB to exist as a very compact hexamer (S.C. Bihani, D. Chakravarty, and A. Ballal, unpublished observations), which in all likelihood is the reason for the increased mobility observed during gel filtration. Earlier, KatA from Anabaena was shown to be a cytosolic, thermostable enzyme, whereas KatG from Synechococcus PCC 7942 was found to be inactivated at temperatures above 50°C (Banerjee et al., 2012b). In this study, the KatB protein was observed to be cytosolic in Anabaena PCC 7120 (Fig. 2B), and the purified KatB protein showed intact secondary structure along with unaltered catalase activity even on exposure to 80°C (Supplemental Fig. S3B; Fig. 7F), indicating that KatB, like KatA, is also a thermostable protein.
Although Anabaena PCC 7120 shows the presence of two genes encoding Mn-catalases, the intrinsic catalase activity in the unstressed Anabaena (i.e. under control conditions) is very low. In comparison, unstressed Synechocystis PCC 6803 (Banerjee et al., 2012b) and Synechococcus PCC 7942 (Gupta and Ballal, 2015) both show inherently higher levels of catalase, which can be easily detected on zymograms. This correlates well with the fact that both Synechocystis PCC 6803 and Synechococcus PCC 7942 tolerate more H2O2 than Anabaena PCC 7120 (Pascual et al., 2010; Gupta and Ballal, 2015). Deletion of katG in Synechococcus and Synechocystsis results in severe sensitivity to exogenously added H2O2, but intriguingly, these mutants show a normal phenotype in the absence of any stresses (Tichy and Vermaas, 1999; Perelman et al., 2003). The Anabaena PCC 7120 katB mutant too shows a normal growth phenotype in the absence of any stress, but in contrast to the two above-mentioned cyanobacteria, the mutant showed no difference in sensitivity to H2O2 when compared with the unstressed wild-type Anabaena PCC 7120. Clearly, the lack of sufficient catalase activity makes both the wild-type and the mutant extremely sensitive to H2O2.
Incidentally, Anabaena PCC 7120 shows the presence of several genes encoding Prxs, which can detoxify H2O2. Prxs such as All1541 and Alr4641 are induced by oxidizing agents, including H2O2 (Banerjee et al., 2012a, 2015), indicating that these Prxs may be the principal proteins that detoxify H2O2 under normal conditions. However, the role of KatB becomes apparent when Anabaena PCC 7120 is stressed with salt, a stressor that activates KatB synthesis (Fig. 2). Accumulation of KatB leads to substantial increase in the catalase activity, which subsequently manifests in enhanced tolerance to H2O2 (Figs. 1 and 2). These aforementioned effects are absent in the salt-treated Anabaena katB mutant, which is in fact very susceptible to H2O2 (Fig. 6). Clearly, KatB is responsible for the cross-protection observed when the salt-treated Anabaena PCC 7120 cells are exposed to H2O2. Moreover, ectopic overexpression of KatB in Anabaena PCC 7120 also protects cells from exogenously added H2O2 (Fig. 2).
Earlier, when compared with the wild-type Anabaena PCC 7120, KatA overexpressing strain showed reduced ROS levels and no increase in the 2-Cys-Prx expression on exposure to H2O2 (Banerjee et al., 2012b). Similarly, under H2O2 stress, Anabaena PCC 7120 pretreated with salt also showed reduced ROS levels and no increase in the content of the H2O2-inducible 2-Cys-Prx (Fig. 2C). Thus, irrespective of the mode of Mn-catalase overproduction, i.e. by constitutive overexpression using a plasmid-based system or by inducing its synthesis with salt, once adequately present, these proteins can competently detoxify exogenously added H2O2 and consequently reduce the ROS burden in cells.
By itself, H2O2 is a weak oxidant, but it can form the most deleterious hydroxyl radical in the presence of transition metals like iron (Halliwell and Gutteridge, 1986). The hydroxyl radical formed can directly or indirectly (via generation of lipid peroxides or protein radicals, etc.) damage all the cellular components, leading to cell death. In Anabaena, the photosynthetic pigments appear to be particularly prone to bleaching on treatment with H2O2 (Banerjee et al., 2012a, 2015). In another cyanobacterium, Microcystis aeruginosa, treatment with H2O2 led to enhanced ROS generation that destroyed pigment synthesis and membrane integrity (Qian et al., 2010). Other studies have shown protein synthesis in cyanobacteria to be a specific target of H2O2 (Nishiyama et al., 2011). Inhibition of protein synthesis prevents the repair of photosystems from photodamage, eventually leading to pigment loss and disruption of photosynthetic activity (Nishiyama et al., 2001; Nishiyama et al., 2011). Due to elevated levels of KatB, the salt-pretreated Anabaena PCC 7120 cells efficiently decompose the externally added H2O2, which in turn reduces the burden of total ROS, oxidized proteins, and lipid peroxides, consequently leading to improved survival as evidenced by protection of pigments, RNA, etc. It should be noted that the protective effect of NaCl is abolished in the KatB mutant. Thus, the presence of KatB is essential to protect the NaCl-treated cells from the toxic effects of H2O2. The type of damage caused by H2O2 and the role played by NaCl in protecting Anabaena (i.e. by inducing the KatB protein) is schematically depicted in Supplemental Figure S4.
Heterocysts are specialized cells that fix nitrogen in Anabaena. As the nitrogenase enzyme is extremely sensitive to O2, heterocysts have evolved multiple strategies to exclude oxygen (Adams and Duggan, 1999). Results with PkatB-gfp fusion construct showed lack of katB promoter activity in heterocysts, whereas abundant promoter activity was observed in the neighboring vegetative cells (Fig. 4). In the context of reduced O2 environment required in heterocysts, the absence of KatB may confer a distinct advantage, as oxygen is one of the products of catalase activity. Prxs like Alr4641 and Alr2375 are expressed in heterocysts (Banerjee et al., 2013, 2015). Possibly, the above-mentioned proteins that detoxify H2O2 without generating O2 are better suited to function in heterocysts than catalases like KatB.
It should be noted that oxidative stress is one of the most damaging consequences of salinity (Abogadallah, 2010) as well as desiccation (Dadheech, 2010) in several organisms. In this study, treatment with NaCl severely exacerbated the formation of total ROS, cellular peroxides, oxidized proteins, and lipid peroxides in the katB mutant. However, the wild-type cells that produced KatB were remarkably shielded from the above-mentioned oxidative damage (Fig. 5). In M. aeroginosa, salinity stress caused significant production of H2O2 compared with the unstressed cells (Ross et al., 2006). In Nostoc flagelliforme, a nitrogen-fixing cyanobacterium like Anabaena PCC 7120, desiccation caused an increase in the content of the intracellular H2O2 (Liang et al., 2014). In its natural habitat, Anabaena is more liable to encounter stresses like salinity or desiccation, which will eventually increase the levels H2O2 within cells. Naturally, effective decomposition of this endogenously generated H2O2 will decrease the severity of oxidative trauma caused by these stresses. In line with this, both salt and desiccation were shown to induce KatB in Anabaena (Fig. 3). Also, the katB mutant is not only susceptible to salt stress (Fig. 5) but is also sensitive to desiccation (Katoh, 2012).
In a proteomic screen, the KatB protein from Anabaena was found to be induced by arsenic, iron starvation, or on exposure to blutachlor, a herbicide used in rice fields (Narayan et al., 2011; Pandey et al., 2012; Agrawal et al., 2014), whereas expression of KatA, the other Mn catalase, was not detected. Interestingly, induction of KatB seems to be independent of H2O2, as addition of exogenous H2O2 (a membrane permeable molecule) does not induce katB in Anabaena PCC 7120 (Fig. 3A). Induction of katB by salinity or desiccation but not by its actual substrate (i.e. H2O2) is perplexing. Results described in this study (Fig. 3) clearly show that expression of katB is carefully regulated, and transcriptional activation occurs only under certain stimuli (e.g. desiccation or salinity). Although regulators that govern katB expression are unknown as yet, it may be argued that these regulators perceive stresses such as salinity or desiccation (but not H2O2 itself) and enhance KatB synthesis for eliminating H2O2 that would eventually be produced during these stresses. Identification of regulators that control katB expression will help us answer some of these questions.
CONCLUSION
The Mn-catalase, KatB, is substantially induced in response to salt stress in Anabaena. The KatB protein efficiently detoxifies H2O2; therefore, addition of NaCl protects Anabaena from ROS-mediated damage caused by H2O2. In contrast, the NaCl-treated katB mutant is very sensitive to the deleterious effects of H2O2. Analysis with the katB mutant shows that NaCl by itself can impose oxidative stress in Anabaena, but production of KatB mitigates these harmful effects to a large extent in the wild type. These results not only underscore the vital role played by KatB in overcoming different stresses in Anabaena but also demonstrate the ability of a simple compound (NaCl) to modulate the overall oxidative stress resistance of an organism by orchestrating induction of a ROS scavenging enzyme.
MATERIALS AND METHODS
Organism and Growth Conditions
Anabaena PCC 7120 cultures were grown in BG11 liquid medium, pH 7.0 with combined nitrogen (17 mm NaNO3, BG11N+) or diazotropically (BG11N-) under continuous illumination (30 μE m−2s−1), with shaking (100 rpm) or without shaking (as still culture) at 27°C ± 2°C. Assessment of growth was done by measuring the chlorophyll a content from 1 mL of the culture suspension (Mackinney, 1941). Escherichia coli cells were grown in Luria-Bertani medium at 37°C with shaking at 150 rpm in the presence of appropriate antibiotics. The antibiotics used were neomycin (12.5 μg mL−1, Neo12.5) in BG11 liquid media and neomycin (25 μg mL−1, Neo25) in BG11 agar plates for recombinant Anabaena PCC 7120; and chloramphenicol (34 μg mL−1, Cm34), kanamycin (50 μg mL−1, Kan50), or carbenicillin (100 μg mL−1, Cb100) for E. coli. The E. coli and Anabaena strains and plasmids used in the study are indicated in Supplemental Table S1.
Cloning of alr3090 (katB) into pET16b, Overproduction, and Purification of the Recombinant KatB Protein
Cloning of the katB ORF into pET16b expression vector, overproduction of the His-tagged KatB protein in E. coli BL21pLysS, and its subsequent purification by affinity chromatography were described earlier (Bihani et al., 2013). The purified KatB protein was used to immunize rabbits for generating specific antiserum. The primary and booster immunizations and subsequent collection of the antiserum were performed at a commercial facility (Merck, India).
Size Exclusion Chromatography
Size exclusion chromatography (AKTApurifier, GE Healthcare) was performed using GE Superdex 200 10/300 GL column equilibrated with buffer A (20 mm Tris, 50 mm NaCl, pH 7.2) at 25°C.
Protein Electrophoresis, Western Blotting, and Immunodetection
Purified KatB protein was electrophoretically resolved on SDS-PAGE (14% acrylamide) or on native PAGE (10% acrylamide) and stained with Coomassie Brilliant Blue G-50. Total cellular proteins from Anabaena cultures were extracted with Laemmli’s buffer (Laemmli, 1970), electrophoretically separated on polyacrylamide gels, electroblotted on to nitrocellulose membrane (Sigma), and probed with the KatB antiserum. Western blots were repeated at least thrice with consistent results.
Catalase Activity Assay
H2O2 decomposition activity of the purified KatB protein was monitored spectrophotometrically as described earlier (Beers and Sizer, 1952). In short, various concentrations of H2O2 were incubated with the purified KatB protein in 1 mL of buffer B (20 mm Tris, pH 8.5), and decomposition of H2O2 was monitored by following the decrease in A240 in a spectrophotometer (Chemito, SPETRASCAN UV2600).
Northern Blotting-Hybridization and Dot-Blot Analysis
Isolation of total RNA from Anabaena PCC 7120 cultures and subsequent northern blotting hybridization analysis with the digoxigenin (DIG)-labeled katB DNA probe was performed as described earlier (Ballal and Apte, 2005).
RACE
The total RNA, isolated from the control wild-type Anabaena PCC 7120 cells or cells stressed with NaCl for 16 h, was treated with DNase-I and repurified using spin columns (Nucleospin RNA clean-up XS, Macherey Nagel). The reverse primer KatB_RACE_Ext (Supplemental Table S2) was employed for cDNA synthesis. After tailing of cDNA (with dATP and terminal transferase), PCR was performed with the oligodT-anchor primer and an internal gene-specific primer (KatB_RACE_Int) exactly as described (5′/3′ RACE kit, 2nd Generation, Roche).
Construction of katB Promoter-gfp Fusion
A 300-bp DNA fragment (upstream of the katB ORF) that contained the katB promoter was amplified with suitable primers (KatB_prom_Fwd and KatB_prom_Rev) and cloned just upstream of gfp (reporter gene) in pAM1956 employing the restriction enzymes KpnI and SacI (construct named as pAM3090prom). This construct was conjugally transferred into Anabaena PCC 7120; exconjugants (An3090prom) were selected on BG11N+ plates containing Neo25 and subjected to microscopic analysis.
Construction of pAMkatB and Overexpression of KatB Protein in Anabaena PCC 7120
The katB DNA fragment (693 bp) was PCR amplified from Anabaena PCC 7120 genomic DNA and cloned, downstream of strong light-inducible PpsbA1 promoter, in pFPN (Chaurasia et al., 2008) using NdeI and BamHI restriction endonucleases (plasmid called pFPNkatB). Subsequently, the katB gene along with the PpsbA1 promoter was excised out as a SalI-XmaI fragment from pFPNkatB and cloned into E. coli/Anabaena shuttle vector pAM1956 (Yoon and Golden, 1998) to generate pAMkatB plasmid. Using a conjugal E. coli donor [HB101 (pRL623 + pRL443)] (Elhai and Wolk, 1988; Elhai et al., 1997), pAMkatB was conjugally transferred into Anabaena PCC 7120 as described earlier (Elhai et al., 1997). Exconjugants were selected on BG11N+ plates containing neomycin (25 μg mL−1) and repeatedly subcultured. The transformed Anabaena strain thus obtained (designated AnKatB+) was maintained on BG11N+ plates under neomycin selection pressure.
Microscopic Techniques
Light microscopic pictures of the control wild-type Anabaena PCC 7120 or the An3090prom strain or recombinant AnKatB+ were visualized at 400× magnification on a Carl Zeiss Axioscop microscope. The images were captured with a CCD AxiocamMRc (Zeiss) camera. Fluorescence microscopy of the AnKatB+ or the An3090prom was performed at 400× magnification and green fluorescence of GFP was visualized using the Hg-Arc lamp (excitation BP, 450–490 nm and emission LP, 515 nm).
In-Gel Catalase Activity (Zymogram)
Cyanobacterial cells were resuspended in a buffer (20 mm Tris-HCl, pH 8.0), subjected to repeated cycles of freeze-thaw and vortexing (in the presence of glass beads, 600 μm diameter), and centrifuged at 14,000 g for 8 min to obtain cell-free extracts. Proteins were electrophoretically separated on native 10% polyacrylamide gels and analyzed for in-gel catalase activity. After electrophoresis, the gel was treated with 0.003% H2O2 for10 min, washed with distilled water, and stained with 1% ferric chloride and 1% potassium ferricyanide. The catalase activity was observed as a zone of clearance on a greenish-yellow background of the gel (Weydert and Cullen, 2010).
Construction of the katB Mutant (AnKatB-)
To inactivate katB gene, a suicide plasmid-based single recombination strategy was employed (Neunuebel and Golden, 2008). A 300-bp DNA fragment from katB ORF was PCR amplified from genomic DNA using 3090_SacI_Fwd (containing SacI site) and Kan_3090_Olap_Rev primers. Kanamycin expression cassette was PCR amplified from pAM1956 vector by primers 3090_Kan _Olap_Fwd and pAM1956Kan_Rev (containing XhoI site). These two fragments were combined by overlap extension PCR using 3090_SacI_Fwd and pAM1956Kan_Rev primers. The resultant DNA (300-bp katB fragment + Kanamycin expression cassette) was cloned into suicide vector pRL271 by employing XhoI and SacI restriction endonucleases (construct named as pRLkatBkan). pRLkatBkan construct was conjugally transferred to Anabaena PCC 7120, and the mutants (AnKatB-) that came up were selected by repeated subculturing on BG11 plates containing neomycin.
Assays to Determine the Content of ROS
The ROS content in Anabaena strains treated with H2O2 and/or NaCl was measured with DCHFDA (He and Häder, 2002). Briefly, DCHFDA (10 μm final concentration) was added to cells suspended in BG11 medium (3 μg chlorophyll mL−1). Cells were incubated for 20 min in dark at 25°C. Fluorescence emission (λex = 490 nm, λem = 520 nm) of the control or treated cells was measured immediately afterward. Experiments were repeated thrice and the average values are reported. For MDA estimation, 300 µL cellular extract (500 μg total protein) in 10 mm potassium phosphate buffer (pH 7.4) was reacted with 900 μL of TBA reagent (0.375% 2-thiobarbituric acid, 0.25 m HCl, 15% trichloroacetic acid, and 6 mm Na2EDTA). The reaction mixture was incubated at 95°C for 20 min, cooled to ambient temperature, and centrifuged at 10,000 rpm for 5 min at 25°C. MDA equivalents in the supernatant were estimated by measuring fluorescence (λex = 530 nm; λem = 590 nm). The lipid peroxidation values are expressed as nmoles of MDA equivalents per mg protein using 1, 1, 3, 3-tetra methoxy propane as standard. Protein oxidation (using 40 µg protein) was performed as described in the OxyBlot protein oxidation detection kit (Millipore, S7150), while the total peroxides were estimated by the Peroxoquant kit (Thermo Scientific, 23280). Known concentrations of H2O2 were employed to plot a standard curve, which was used for calculating the content of peroxides in Anabaena.
Determination of Oxidative Stress Tolerance of Anabaena Strains
Three-day-old cultures of the wild-type Anabaena PCC 7120 or AnKatB+ or AnKatB- (in triplicates) were inoculated in fresh growth medium at a chlorophyll a density of 3 μg mL−1 and treated with H2O2 (1 mm) or NaCl (100 mm) in tubes (without shaking) under illumination for 2 d. Growth was monitored in liquid cultures by determining the content of chlorophyll a (Mackinney, 1941).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The katB promoter activity in An3090prom.
Supplemental Figure S2. Growth of the wild-type Anabaena PCC 7120 or the katB mutant in the presence of NaCl.
Supplemental Figure S3. KatB size distribution, CD spectra, and melting curve analysis.
Supplemental Figure S4. A schematic model depicting the role of KatB in Anabaena.
Supplemental Table S1. E. coli, Anabaena strains, and plasmids used in this study.
Supplemental Table S2. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Dr. S.K. Apte and Dr. S. Chattopadhyay for their constant encouragement during the course of this study.
Glossary
- DCHFDA
dichloro dihydrofluorescein diacatate
- Fv/Fm
maximum photochemical efficiency of PSII in the dark-adapted state
- MDA
malondialdehyde
- MV
methyl viologen
- ORF
open reading frame
- Prx
peroxiredoxin
- ROS
reactive oxygen species
- RACE
Rapid amplification of cDNA ends
- DIG
digoxigenin
References
- Abogadallah GM. (2010) Antioxidative defense under salt stress. Plant Signal Behav 5: 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DG, Duggan PS (1999) Heterocyst and akinete differentiation in cyanobacteria. New Phytol 144: 3–33 [Google Scholar]
- Agrawal C, Sen S, Singh S, Rai S, Singh PK, Singh VK, Rai LC (2014) Comparative proteomics reveals association of early accumulated proteins in conferring butachlor tolerance in three N(2)-fixing Anabaena spp. J Proteomics 96: 271–290 [DOI] [PubMed] [Google Scholar]
- Amo T, Atomi H, Imanaka T (2002) Unique presence of a manganese catalase in a hyperthermophilic archaeon, Pyrobaculum calidifontis VA1. J Bacteriol 184: 3305–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SC. (2010) The Ferritin-like superfamily: evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim Biophys Acta 1800: 691–705 [DOI] [PubMed] [Google Scholar]
- Antonyuk SV, Melik-Adamyan VR, Popov AN, Lamzin VS, Hempstead PD, Harrison PM, Artymyuk PJ, Barynin VV (2000) Three-dimensional structure of the enzyme dimanganese catalase from Thermus thermophilus at 1 angstrom resolution. Crystallogr Rep 45: 105–116 [Google Scholar]
- Ballal A, Apte SK (2005) Differential expression of the two kdp operons in the nitrogen-fixing cyanobacterium Anabaena sp. strain L-31. Appl Environ Microbiol 71: 5297–5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Ballal A, Apte SK (2012a) A novel glutaredoxin domain-containing peroxiredoxin ‘All1541’ protects the N2-fixing cyanobacterium Anabaena PCC 7120 from oxidative stress. Biochem J 442: 671–680 [DOI] [PubMed] [Google Scholar]
- Banerjee M, Ballal A, Apte SK (2012b) Mn-catalase (Alr0998) protects the photosynthetic, nitrogen-fixing cyanobacterium Anabaena PCC7120 from oxidative stress. Environ Microbiol 14: 2891–2900 [DOI] [PubMed] [Google Scholar]
- Banerjee M, Chakravarty D, Ballal A (2015) Redox-dependent chaperone/peroxidase function of 2-Cys-Prx from the cyanobacterium Anabaena PCC7120: role in oxidative stress tolerance. BMC Plant Biol 15: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Raghavan PS, Ballal A, Rajaram H, Apte SK (2013) Oxidative stress management in the filamentous, heterocystous, diazotrophic cyanobacterium, Anabaena PCC7120. Photosynth Res 118: 59–70 [DOI] [PubMed] [Google Scholar]
- Barynin VV, Whittaker MM, Antonyuk SV, Lamzin VS, Harrison PM, Artymiuk PJ, Whittaker JW (2001) Crystal structure of manganese catalase from Lactobacillus plantarum. Structure 9: 725–738 [DOI] [PubMed] [Google Scholar]
- Beers RF Jr, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195: 133–140 [PubMed] [Google Scholar]
- Bihani SC, Chakravarty D, Ballal A (2013) Purification, crystallization and preliminary crystallographic analysis of KatB, a manganese catalase from Anabaena PCC 7120. Acta Crystallogr Sect F Struct Biol Cryst Commun 69: 1299–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock TD. (1973) Evolutionary and ecological aspects of cyanophytes. In Carr NG, Whitton BA, eds, The Biology of Blue-Green Algae. Blackwell, Oxford, UK, pp 487–500 [Google Scholar]
- Cha MK, Hong SK, Kim IH (2007) Four thiol peroxidases contain a conserved GCT catalytic motif and act as a versatile array of lipid peroxidases in Anabaena sp. PCC7120. Free Radic Biol Med 42: 1736–1748 [DOI] [PubMed] [Google Scholar]
- Chaurasia AK, Parasnis A, Apte SK (2008) An integrative expression vector for strain improvement and environmental applications of the nitrogen fixing cyanobacterium, Anabaena sp. strain PCC7120. J Microbiol Methods 73: 133–141 [DOI] [PubMed] [Google Scholar]
- Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8: e23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collén J, Del Rio MJ, García-Reina G, Pedersén M (1995) Photosynthetic production of hydrogen peroxide by Ulvarigida C. Ag (Chlorophyta). Planta 196: 225–230 [Google Scholar]
- Dadheech N. (2010) Desiccation tolerance in cyanobacteria. Afr J Microbiol Res 4: 1584–1593 [Google Scholar]
- Elhai J, Wolk CP (1988) Conjugal transfer of DNA to cyanobacteria. Methods Enzymol 167: 747–754 [DOI] [PubMed] [Google Scholar]
- Elhai J, Vepritskiy A, Muro-Pastor AM, Flores E, Wolk CP (1997) Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J Bacteriol 179: 1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. (1992) Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev 56: 340–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Ballal A (2015) Unraveling the mechanism responsible for the contrasting tolerance of Synechocystis and Synechococcus to Cr(VI): Enzymatic and non-enzymatic antioxidants. Aquat Toxicol 164: 118–125 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (1986) Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys 246: 501–514 [DOI] [PubMed] [Google Scholar]
- He YY, Häder DP (2002) UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: protective effects of ascorbic acid and N-acetyl-L-cysteine. J Photochem Photobiol B 66: 115–124 [DOI] [PubMed] [Google Scholar]
- Jang S, Imlay JA (2010) Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol Microbiol 78: 1448–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Nakamura Y, Wolk CP, Kuritz T, Sasamoto S, Watanabe A, Iriguchi M, Ishikawa A, Kawashima K, Kimura T, Kishida Y, Kohara M, et al. (2001) Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res 8: 205–213, 227–253 [DOI] [PubMed] [Google Scholar]
- Katoh H. (2012) Desiccation-inducible genes are related to N(2)-fixing system under desiccation in a terrestrial cyanobacterium. Biochim Biophys Acta 1817: 1263–1269 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Liang W, Wang L, Shi J, Lei X, Yang J, Wu S, Chen W (2014) Differential expression of antioxidant proteins in the drought-tolerant cyanobacterium Nostoc flagelliforme under desiccation. POJ 7: 205–212 [Google Scholar]
- Mackinney G. (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140: 315–322 [Google Scholar]
- Narayan OP, Kumari N, Rai LC (2011) Iron starvation-induced proteomic changes in Anabaena (Nostoc) sp. PCC 7120: exploring survival strategy. J Microbiol Biotechnol 21: 136–146 [DOI] [PubMed] [Google Scholar]
- Neunuebel MR, Golden JW (2008) The Anabaena sp. strain PCC 7120 gene all2874 encodes a diguanylate cyclase and is required for normal heterocyst development under high-light growth conditions. J Bacteriol 190: 6829–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N (2011) Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant 142: 35–46 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20: 5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda B, Basu B, Rajaram H, Kumar Apte S (2014) Methyl viologen responsive proteome dynamics of Anabaena sp. strain PCC7120. Proteomics 14: 1895–1904 [DOI] [PubMed] [Google Scholar]
- Pandey S, Rai R, Rai LC (2012) Proteomics combines morphological, physiological and biochemical attributes to unravel the survival strategy of Anabaena sp. PCC7120 under arsenic stress. J Proteomics 75: 921–937 [DOI] [PubMed] [Google Scholar]
- Pascual MB, Mata-Cabana A, Florencio FJ, Lindahl M, Cejudo FJ (2010) Overoxidation of 2-Cys peroxiredoxin in prokaryotes: cyanobacterial 2-Cys peroxiredoxins sensitive to oxidative stress. J Biol Chem 285: 34485–34492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelman A, Uzan A, Hacohen D, Schwarz R (2003) Oxidative stress in Synechococcus sp. strain PCC 7942: various mechanisms for H2O2 detoxification with different physiological roles. J Bacteriol 185: 3654–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Yu S, Sun Z, Xie X, Liu W, Fu Z (2010) Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat Toxicol 99: 405–412 [DOI] [PubMed] [Google Scholar]
- Ross C, Santiago-Vázquez L, Paul V (2006) Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa. Aquat Toxicol 78: 66–73 [DOI] [PubMed] [Google Scholar]
- Schopf JW. (1975) Precambrian paleobiology: problems and perspectives. Annu Rev Earth Planet Sci 3: 213–249 [Google Scholar]
- Tailor V, Ballal A (2015) Over-expression of Alr4642, a novel Prx-like peroxiredoxin, defends the cyanobacterium Anabaena PCC7120 from oxidative stress. J Appl Phycol 27: 2261–2270 [Google Scholar]
- Tel-Or E, Huflejt M, Packer L (1985) The role of glutathione and ascorbate in hydroperoxide removal in cyanobacteria. Biochem Biophys Res Commun 132: 533–539 [DOI] [PubMed] [Google Scholar]
- Tichy M, Vermaas W (1999) In vivo role of catalase-peroxidase in synechocystis sp. strain PCC 6803. J Bacteriol 181: 1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman GS. (1979) Algal inoculation in rice field. In NC Brady, ed, Nitrogen and Rice. International Rice Research Institute, Los Banos, Philippines [Google Scholar]
- Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5: 51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker JW. (2012) Non-heme manganese catalase--the ‘other’ catalase. Arch Biochem Biophys 525: 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Golden JW (1998) Heterocyst pattern formation controlled by a diffusible peptide. Science 282: 935–938 [DOI] [PubMed] [Google Scholar]
- Zamocky M, Furtmüller PG, Obinger C (2008) Evolution of catalases from bacteria to humans. Antioxid Redox Signal 10: 1527–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.