Abstract
In this review we discuss the role of ATP synthase as a molecular drug target for natural and synthetic antimi-crobial/antitumor peptides. We start with an introduction of the universal nature of the ATP synthase enzyme and its role as a biological nanomotor. Significant structural features required for catalytic activity and motor functions of ATP synthase are described. Relevant details regarding the presence of ATP synthase on the surface of several animal cell types, where it is associated with multiple cellular processes making it a potential drug target with respect to antimicrobial peptides and other inhibitors such as dietary polyphenols, is also reviewed. ATP synthase is known to have about twelve discrete inhibitor binding sites including peptides and other inhibitors located at the interface of α/β subunits on the F1 sector of the enzyme. Molecular interaction of peptides at the β DEELSEED site on ATP synthase is discussed with specific examples. An inhibitory effect of other natural/synthetic inhibitors on ATP is highlighted to explore the therapeutic roles played by peptides and other inhibitors. Lastly, the effect of peptides on the inhibition of the Escherichia coli model system through their action on ATP synthase is presented.
Keywords: F1Fo ATP synthase, ATPase, E. coli ATP synthase, antimicrobial peptides, antitumor peptides, enzyme inhibitors
1. INTRODUCTION
ATP synthase is the primary means of cellular energy production in all animals, plants, and almost all microorganisms. ATP, the universal energy currency, is generated by ATP synthase by oxidative or photophosphorylation in the membranes of bacteria, mitochondria, and chloroplasts. The overall reaction sequence is: ATP synthase + ADP + Pi ↔ ATP Synthase + ATP. ATP generation requires a mechanical rotation mechanism in which ATP synthase subunits rotate at approximately 100 times per second in order to convert food into energy by oxidation. ATP synthase works like a motor and is indeed one of the smallest biological nanomotors found in all livings systems. An average human leading a normal life is expected to generate approximately 2.0 million kg of ATP from ADP and Pi (inorganic phosphate) in a 75-year lifetime. [1–3]. The structural and functional activity of ATP synthase enzymes are essentially the same in all prokaryotes and eukaryotes [4–8]. The total number of protons required to synthesize one ATP molecule among different organisms ranges from three to four, with the possibility that cells can vary this ratio to suit their physiological and environmental conditions [9–11].
2. ATP SYNTHASE ENZYMES
All the metabolic and physiological processes performed by living organisms require energy. This source of energy is adenosine triphosphate (ATP). Consequently, ATP is the universal energy currency used by all cells from bacteria to human. The third phosphate bond of ATP is extremely unstable and its hydrolysis releases a significant amount of free energy (~7kcal/mol). The continuous use of ATP in a multitude of functions means every cell must generate ATP on a constant basis.
ATP synthase is one of the oldest and most highly conserved enzymes. Consequently, ATP synthases, from the inner membrane of mitochondria and chloroplast thylakoid membranes, show identical structural and functional properties to their counterparts from the plasma membrane of bacteria. ATP synthase (EC 3.6.3.14) is a general term for an enzyme that can synthesize adenosine triphosphate (ATP) from adenosine diphosphate and inorganic phosphate. ATP synthase molecules are membrane-bound transporters that couple ion movement through a membrane with the synthesis or hydrolysis of an ATP nucleotide. A variety of membrane- bound ATP synthases evolved to fulfill the explicit needs of different cell types. Based on the particular function, these enzymes are categorized as F-, V-, A-, P-, or E-type ATP synthase [12–15]. Synthesis and hydrolysis of ATP is the sole function of all these forms of ATP synthases.
Before discussing the detailed structure of F1Fo ATP synthase (see Fig. 1A), it would be appropriate to briefly describe other types of ATPases. The F-type ATP synthase (for ‘phosphorylation Factor’, and also known as H+-transporting ATPases or F1Fo-ATPases) are extraordinarily conserved among organisms and are the principal enzymes performing ATP synthesis in living systems. They are located in the plasma membranes of bacteria, in the thylakoid membranes of chloroplasts, and in the inner membranes of mitochondria. In certain bacteria, Na+-transporting F-ATP synthase is also present. The V-type ATP synthase (for ‘Vacuole’) is found in the eukaryotic endomembrane systems, e.g. in vacuoles, the Golgi apparatus, endosomes, lysosomes, and in the plasma membrane of prokaryotes and certain specialized eukaryotic cells. V-ATPases hydrolyze ATP to drive a proton pump, but cannot work in reverse to synthesize ATP [16, 17]. The A-type ATP synthases (A-ATPases, for ‘Archaea’) are found solely in Archaea and have a similar function to F-ATPases (reversible ATPases). A-type ATPases may have arisen as an adaptation to different cellular needs and the more extreme environmental conditions faced by Archaeal species. The P-type ATP synthases (P-ATPases, also known as E1-E2 ATPases) are found in bacteria and in a number of eukaryotic plasma membranes and organelles. P-ATPases function to transport a variety of different compounds, including ions and phospholipids, across a membrane using ATP hydrolysis for energy. There are many different classes of P-ATPases, each of which transport a specific type of ion: H+, Na+, K+, Mg2+, Ca2+, Ag+ and Ag2+, Zn2+, Co2+, Pb2+, Ni2+, Cd2+, Cu+ and Cu2+. The E-type ATP synthases (E-ATPases, for ‘Extracellular’) are membrane-bound cell surface enzymes that have broad substrate specificity, hydrolyzing other NTPs besides ATP, as well as NDPs – although their most likely substrates are ATP, ADP, and UTP, as well as extracellular ATP [18–22].
Fig. (1). Structure of Escherichia coli ATP synthase and peptide binding siteβDELSEED.
(A) Water soluble F1 form of E. coli enzyme in backbone form showing catalytic Pi binding subdomain triangle, polyphenol, and peptide binding sites in space fill form. Membrane bound Fo sector is also show in backbone form. Polyphenol and peptide binding sites are identified with circles at the interface of α/β subunits. (B) Enlarged peptide binding pocket in wireframe form identifies the involved residues. Figure was generated by PDB file 1H8E [44] using Rasmol [179].
The F1Fo ATP synthase has a long research history and is the prime focus of this review. The F1 particle was first isolated by Ephraim Racker in 1961 (for factor 1). The name Fo comes from the oligomycin inhibition of the membrane-embedded portion of ATP synthase. Fundamentally, F1Fo-ATP synthase is structurally and functionally similar whatever the source. In its simplest form, as shown in Fig. (1A), Escherichia coli ATP synthase contains eight different subunits, namely α3β3γεab2c10. The total molecular mass is ~530 kDa. F1 corresponds to α3β3γδε and Fo to ab2c. The yeast ATP synthase is one of the most complex known enzymes with ~20 different subunit types [7, 8, 23]. In plants ATP synthase is also present in chloroplasts (CFoF1-ATP synthase). The enzyme is integrated into the thylakoid membrane where; the CF1-part inserts into the stroma, and is integral to the dark reactions of photosynthesis (Calvin cycle) and ATP synthesis. In chloroplasts, the structure is the same except that there are two isoforms. In mitochondria, there are 7–9 additional subunits depending on the source, but in toto they contribute only a small fraction of additional mass and may have regulatory functions [24–26]. ATP hydrolysis and synthesis occur on three catalytic sites in the F1 sector, whereas proton transport occurs through the membrane embedded Fo sector. The γ-subunit forms a coiled coil of α-helices that extends into the central space of the α3β3 hexagon. Proton gradient-driven clockwise rotation of γ (as viewed from the outer membrane) leads to ATP synthesis and anticlockwise rotation of γ results in ATP hydrolysis. In recent nomenclature, the rotor consists of γεcn, and the stator consists of α3β3δab2 [27–29]. The function of the stator is to prevent co-rotation of catalytic sites with the rotor. Current understanding of the F1Fo structure and mechanism has been thoroughly reviewed by Senior’s group and others [1–3, 29–42].
3. FORMATION OF ATP THE ENERGY CURRENCY
A total of six nucleotide binding sites exist on the F1 sector of ATP synthase. The three that are catalytic are mainly contributed by the β-subunit, and the three that are non-catalytic are mainly contributed by α-subunits. The three catalytic sites are designated as βTP, PDP, and βE by x-ray crystallographers based on the binding of ATP, ADP, and Pi respectively [43, 44]. Pi initially binds to the βE (the empty site) for ATP synthesis. The synthesis reaction in the three catalytic sites is interdependent and occurs successively. The three catalytic sites are known to have different affinities for nucleotides at any given moment. Each catalytic site undergoes a conformational change that results in the following sequence: substrate (ADP+Pi) binding → ATP synthesis → ATP release. Boyer [4, 45, 46] predicted that catalysis requires the sequential involvement of three catalytic sites, each of which changes its binding affinity for substrate and product as it proceeds through the cyclical mechanism. Boyer named this sequence the “binding change mechanism.” In Fo, a proton motive force is converted to a mechanical rotation of the rotor shaft, which drives conformational changes of the catalytic domains in F1 to synthesize ATP. The reverse reaction hydrolysis of ATP induces reverse conformational changes of the Fo sector and consequently reverses rotation of the rotor shaft. These conformational changes in the catalytic sites are linked to rotation of the γ-subunit. Yoshida and Kinosita, with colleagues in Japan, and subsequently by several other labs [10, 47–51], have observed the γ-subunit rotation in an isolated α3β3γ subcomplex. The catalytic function of ATP synthase with respect to ATP hydrolysis or synthesis in F1Fo and its relationship to the mechanical rotation of γ-subunit is not the focus of this review. However, a better understanding of the structure and function of F1Fo would illuminate the possible pathways to developing ATP synthase as a molecular drug target and its use in nanotechnology and nanomedicine [52–55]. Hence, understanding the structure and catalytic function of ATP synthase, particularly Pi binding leading to ATP formation, is of paramount importance to embarking on the details of its inhibition by peptides [2, 3, 56–59].
4. SIGNIFICANCE OF INORGANIC PHOSPHATE (Pi) BINDING
Understanding Pi binding can reveal a wealth of information on the reaction mechanism of ATP synthesis, hydrolysis, and the γ-subunit rotation induced conformational changes of αβ-subunits. These relationships are appreciated from the following two central questions. (I) What causes the ATP synthase to bind ADP and Pi rather than ATP at catalytic sites? The interesting fact is that in active cells, the cytoplasmic concentrations of ATP and Pi are approximately in the 2–5 mM range, whereas the ADP concentration is at least 10–50-fold lower. However, it has been established from the equilibrium binding assays that both ADP and ATP bind to catalytic sites of purified F1 and detergent solubilized F1Fo with nearly comparable binding affinities [60–63]. Apparently, a specific mechanism favors the selective binding of ADP into catalytic sites while simultaneously obstructing access to ATP during proton driven rotation and ATP synthesis. One hypothesis is that during ATP synthesis, proton gradient driven rotation of subunits drives an empty catalytic site to bind Pi tightly, thus stereochemically preventing ATP binding and resulting in ADP binding [30]. (II) How does subunit rotation affect the Pi binding [45, 64, 65]? Theoretical and experimental evidence suggests that Pi binding appears to be “energy linked”, implying that it is linked directly to subunit rotation [24, 66, 67]. Thus, the details of Pi binding are not only necessary for understanding the mechanism of ATP synthesis, but as suggested earlier, molecular features of Pi binding derived from mutational and biochemical studies may in the near future, assist in the development of potent and novel molecular drug inhibitors of ATP synthase [2, 59, 68, 69].
Selective Pi binding, carried out by the catalytic site Pi binding subdomain residues, is the key for ATP formation. Residues αPhe-291, αSer-347, αGly-351, αArg-376, βLys-155, βArg-182, βAsn-243, βArg-246, and other highly conserved αVISIT-DG sequence residues are found in close proximity to bound phosphate analogs AlF3 or SO42− in the X-ray crystallographic structure of ATP synthase catalytic sites, which suggests their involvement in preferential Pi binding [44, 70]. [E. coli residue numbers are used throughout]. Moreover, Orris et al. [71] showed by X-ray crystallography that the covalent adduct formed by NBD-Cl (7-chloro-4-nitrobenzo-2-oxa-1, 3,-diazole) is specifically in the βE catalytic site, thus the protection afforded by Pi against the NBD-Cl inhibition of ATP synthase indicates that Pi binding occurs at the βE catalytic site.
For mitochondrial ATP synthase Perez et al. [72] showed that Pi protects against the NBD-Cl inhibition of ATPase activity, providing a means to measure Pi binding. Alteration of the Pi protection against the NBD-Cl assay for E. coli purified F1 or membrane bound F1Fo resulted in defining the relationship between Pi binding and catalysis for eight residues, namely aPhe-291, aSer-347, aGly-351, aArg-376, βLys-155, βArg-182, βAsn-243, and βArg-246. The following five residues; αSer-347, αArg-376, βLys-155, βArg-182, and βArg-246 grouped in a triangular fashion, were found to be involved in Pi binding. Three other residues; αPhe-291, αGly-351, and βAsn-243, though important for function and overall structural maintenance, are not directly involved in Pi binding [35, 53, 73–78]. The presence of Pi binding residues in the catalytic site causes the preferential binding of ADP over ATP. As mentioned elsewhere [3] there are other residues, such as rest of the αVISIT-DG sequence residues, in close proximity to Pi binding subdomain in the catalytic sites that appear to be potential candidates for direct or indirect Pi binding and require further characterization.
5. ATP SYNTHASE AND DISEASE STATES
It is well known that failure of the ATP synthase complex can result in a wide variety of diseases and that this enzyme may also be used as a therapeutic drug target in the treatment of many diseases such as cancer, tuberculosis, obesity, neuropathies, Alzheimer’s, microbial infections, mitochondrial diseases, immune deficiency, cystic fibrosis, diabetes, ulcers, and Parkinson’s [2, 56, 79, 80]. For example, one of the forms of Leigh syndrome, a neurodegenerative disease, is the result of mutation in the a-subunit of ATP synthase [81]. The c-subunit of ATP synthase is involved in both lysosomal storage diseases and Batten disease. Alzheimer’s disease patients show accumulation of α-subunit and low expression of β-subunit in the cytosol. The presence of circulating subunit F6 has been associated with hypertension [82, 83]. Furthermore, ATP synthase is a possible molecular target for antiobesity drugs. The inhibition of non-mitochondrial ATP synthase resulted in the inhibition of cytosolic lipid droplet accumulation [84]. The presence of ATP synthase on the surface of multiple animal cell types is also correlated with several other cellular processes including angiogenesis, intracellular pH regulation, and programmed cell death [85–90]. Angiostatin, a known inhibitor of angiogenesis, was shown to bind to ATP synthase on the surface of human endothelial cells. The transport of H+ across the plasma membrane by mitochondrial ATP synthase was associated with cytolysis of tumor cells and is the basis for angiostatin’s antiproliferative effect on endothelial cells because of its interaction with the α-subunit of ATP synthase [91].
The potential use of ATP synthase protection against microbial infections is straight forward because it is an appropriate target enzyme for antimicrobial agents. Protection against dental cavities caused by the microbe Streptococcus mutans presents a nice example for this potential. S. mutans is an important microbial agent in the pathogenesis of dental cavities through acid production and biofilm formation. Inhibition of S. mutans ATP synthase provides a prophylactic effect against S. mutans metabolism by arresting biofilm formation and acid production [92, 93]. Another instructive example is the case of Mycobacterium tuberculosis ATP synthase, where two mutations in its c-subunit (D32V and A63P) confer resistance to the tuberculosis drug diarylquinoline [94, 95], providing insight into the causes of drug resistance against tuberculosis. Hence, a better understanding of ATP synthase inhibition and its interaction with known inhibitors may be of value in the treatment of these and other diseases.
The importance of ATP synthase as a promising target for drug development is also evident from the fact that many antibiotics such as efrapeptins, aurovertins, and oligomycins inhibit its function. Efrapeptins and aurovertins inhibit both synthesis and hydrolysis of ATP by ATP synthase [96, 97]. Oligomycin on the other hand is a potent inhibitor of ATP synthase by binding in the Fo sector and blocking proton conduction. One study showed that oligomycin induces an apoptotic suicide response in cultured human lymphoblastoid and other mammalian cells within 12–18 hrs, but not in ρo cells that are depleted of a functional mitochondrial respiratory chain [98]. Another similar study suggested that oligomycin interaction with components of mitochondrial pathways may lead to apoptosis of select cells via CD 14 [99]. Thus, it is quite possible that some degree of similar inhibition, or interactions between ATP synthase and other inhibitors, may occur and play a significant role in apoptosis via mitochondrial pathways [68, 69, 79].
6. NON PEPTIDE ATP SYNTHASE INHIBITORS
A wide variety of natural and synthetic products are known to bind and inhibit ATP synthase [2, 3, 56, 59, 68, 69, 100–104]. 7-chloro-4-nitrobenzo-2-oxa-1, 3-diazole (NBD-Cl), sodium azide (NaN3), aluminum fluoride (AlFx), scandium fluoride (ScFx), beryllium fluoride (BeFx) are known inhibitors. (In a biochemical reaction Al, Sc, and Be can be coordinated by different numbers of fluoride ions. The presence of F- species is indicated with x). Several naturally occurring antibiotics such as oligomycin, efrapeptins, aurovertins, leucinostatins, a number of polyphenols like resveratrol, piceatannol, quercetin, morin, epicatechin, and peptides to be discussed later are additional inhibitors of ATP synthase [2, 35, 53, 59, 68, 69, 97, 101, 105–112]. Fig. (3) shows inhibitory effects of some inhibitors.
Fig. (3). Inhibition of ATP synthase.
Inhibition profiles induced by NaN3 taken from [74], resveratrol taken from [68], modified resveratrol 2-[[(4-hydroxy-2-nitrophenyl)imino]methyl] phenol taken from [100], melittin/melittin-NH2, MRP/MRP-NH2 taken from [59]. For experimental details see the associated references.
Recently there has also been a focus on polyphenol induced inhibition of ATP synthase. This is due to their natural occurrence, compatibility with the human system, and ubiquitous availability. Polyphenols are mainly naturally occurring, but are also synthetic or semisynthetic organic chemicals characterized by the presence of single or multiple phenolic structural units. The number and characteristics of the phenolic groups stimulates the unique metabolic, toxic, or therapeutic properties associated with them [113–115].
A variety of dietary flavonoids or polyphenolic compounds exert a broad range of pharmacological effects, such as protection of cells or tissues through multiple responses, including cell death, through their actions on a multitude of targets. A large body of experimental data is available on the effects of dietary polyphenolic compounds and their derivates on human health. Some polyphenols are known to block the action of enzymes and other substances that promote the growth of cancer cells [102, 116–120]. Physiological relevance of dietary polyphenols can be ascribed to their interaction with the mitochondria of eukaryotic cells, while degenerative diseases such as cancer, aging, and neurological disorders are attributed to mitochondrial dysfunction [121, 122]. Interestingly enough, experimental results suggest that the mechanism of inhibitory actions of both polyphenols and peptides on ATP synthase may be somewhat similar [59, 68, 69, 119]. X-ray crystallographic structures, in addition to biochemical assays of polyphenol and peptide inhibited ATP synthase, may help in revealing the exact inhibitory mechanism.
7. ANTIMICROBIAL AND ANTITUMOR PEPTIDES - THE POTENTIAL THERAPEUTIC DRUGS INHIBITORS OF ATP SYNTHASE
Insulin, thyroid hormone, and factor VII were among the first peptide drugs [123]. Recent interest in peptides for therapeutic applications has developed in part from the need for new antibiotics because of evolved bacterial resistance to traditional molecules [124]. The potential utility of AMPs for the development of new antibiotics is also related to the observation that there may be no general mechanism for the evolution of bacterial resistance to the activity of different peptides [2, 59, 125, 126]. Additionally, peptides are characterized by a wide spectrum of activity against multiple bacteria types and by low levels of evolved resistance [127]. Bio-active peptides can be grouped into a number of functional classes such as 1) peptide hormones and neurohormones, 2) peptide toxins, 3) antimicrobial peptides (AMPs), and 4) cell penetrating peptides, with these having a number of different effects, such as being neurotoxic, cytolytic, necrotic, hemor-rhagic, anti-inflammatory and analgesic, among others [128].
A number of peptides with antimicrobial activity known as Antimicrobial Peptides (AMPs) or Host Defense Peptides (HDPs) are in various stages of development. Possible clinical applications include anticancer activity, immunomodulation, wound healing, drug carriers, vaccine adjuvants, innate defense regulators, and both pro and anti-inflammatory agents [125]. Potential general applications under development include topical antibiotics and antiseptics, anti-inflammatory activity, nosocomial infections, and respiratory [129]. Present efforts to develop AMPs for specific therapeutic applications include therapies for oral diseases [130], biofilm infections [131], bacterial sepsis [132], antimalarial host-directed adjunctive therapy [133], and methicillin-resistant Staphylococcus aureus [134].
Since 2000 about 20 new antibiotics have been developed and 40 compounds are in clinical development. At least 15 peptides or peptide mimics for therapeutic applications are in active development [129]. A predominance of natural product compounds now present in late stage trials suggests the possibility that natural products such as AMPs may have an increased likelihood of success [135]. Potential weaknesses of AMPs as candidates for new antibiotics include weak activity, nonspecific cytotoxicity, susceptibility to proteolysis, high production costs, loss of activity, potential interference with host innate immunity, and interference with normal flora [126, 127, 136, 137].
Natural defenses against pathogens include a wide variety of systems in both plants and animals and include various types of oligopeptides and peptides [138]. AMPs are a component of vertebrate innate immunity that have been present in most living organisms for over 2.6 billion years [139] and were first described in insects as an inducible system of protection against bacterial infection [140–142]. AMPs are generally cationic and amphipathic molecules of less than 50 amino acids residues. They have been isolated from all investigated phyla, including microbes, plants, invertebrates, and vertebrates. AMPs have been shown to exhibit inhibitory activity against Gram-positive and Gram-negative bacteria, fungi, parasites, and viruses [139]. Plant AMPs also exhibit activity against human pathogens [136]. A large number of AMPs are known to have selective anticancer activity as well [143]. AMPs have a neutralizing effect on bacterial endotoxins that are a primary cause of lethality in sepsis [144–146] and may have multiple additional inhibitory properties with unclear modes of actions [137].
There are 2065 entries in the Antimicrobial Peptide Database (APD) [147], (http://aps.unmc.edu/AP/main.php), of which 1664 (80.6%) are identified as having antibacterial activity, 732 (35.4%) have antifungal activity, 141 (6.8%) have anticancer activity, and 125 (6.0%) have antiviral activity. The mean length of all peptides in the APD is 30.63 residues and the mean net charge is +3.11. Identification of secondary structure among database AMPs shows 14.67% (N=303) are α-helical, 4.35% (N=90) are in β-conformation, and 2.76% (N=57) are α+β. 17.96% (N=371) were found to have disulfide bonds and 5.52% (N=114) were rich in unusual amino acids [147]. While the updated APD is sufficient to serve our purpose in this article, interested readers may refer to other databases dedicated to AMPs listed in the APD links. Additional information on helical AMPs from both synthetic and natural sources can be found in YADAMP, which was built based on the information from the APD as well as other literature sources [148].
Of all APD listed animal derived AMPs, 1490 (56.5%) are from amphibians. Following the discovery of AMPs in insects, biochemically active substances in frog skin were identified as bioactive peptides [149–151]. AMPs are gene-encoded and produced by phagocytes and epithelial cells [128]. Frogs and toads secrete AMPs from granular glands of the skin, typically in response to infection or environmental stress [152] and at concentrations as high as mg/g of wet skin [150]. The single largest source of AMPs found in the APD is from amphibian skin with 842 (40.7%) of all listed AMPs from this source. The first amphibian AMPs identified were the magainins from skin secretions of the frog Xenopus laevis [149]. Currently known amphibian AMPs were derived from the European toad in the family Bufonidae, South American tree frog species of the family Hylidae, and species of frogs in the family Ranidae in Europe, North America and South America [146, 153]. Frog skin produces a variety of AMPs with up to 100 unique amino acid sequences per species [154]. Based on structural similarity and species of origin, there are four identified classes of amphibian AMPs: 1) magainins from Xenopus, 2) dermaseptins from species in the genus Phyllomedusa, 3) bombinins and bombinin H from European toads, and 4) temporins, brevinins, esculentins, ranalexins, and ranauerins from species in the genus Rana [146].
Most AMPs are cationic, between 10 and 50 residues in length, and frequently include a C-terminal amide group. Mode of action studies indicate that AMPs appear to interact with negatively charged phospholipids and then insert into the bacterial cell membrane, or they may also move across the cell membrane by passive transport and there disrupt a number of cellular processes. AMPs are associated with a number of other antimicrobial processes as well, including cell proliferation and angiogenesis [139]. Several mechanisms have been hypothesized regarding the activity of AMPs, such as membrane permeabilization and cell death by either a “barrel-stave” model [155] or a “torodial pore” model proposed for magainins from Xenopus skin [156–158]. Dermaseptins appear to cause a non-pore-dependent cytolytic activity that causes membrane bilayer micellization and disintegration [159]. Laughlin and Ahmad [59] showed that several cationic α-helical amphibian AMPs such as Ascaphin-8, XT-7, Citropin 1.1, Aurein 2.2, Aurein 2.3, Maculatin 1.1, Melittin-related peptide, Carein 1.8, Carein 1.9, Maganin II, Maganin II -amide, and dermaseptin have reversible inhibitory effects on the ATPase activity of E. coli ATP synthase. ATP synthase is present of the plasma membrane of bacteria while in eukaryotic cells it is present on the inner membrane of mitochondria. Therefore, inhibitory actions of membrane targeting AMPs may be attributable to their interactions with membrane bound ATP synthase. Similarly in tumor cells the presence of the F1 sector of ATP synthase on the plasma membrane [56].
AMPs have become potential sources of compounds with useful pharmacological properties and medical utility in antimicrobial [160–162] and anticancer [163] applications. One probable drawback of the usefulness of these molecules is that they seem to be effective only at very high doses [164]. This may be related to the observed high concentrations in vivo, but may possibly be improved for pharmacological purposes by modifications of amino acid sequences or functional groups, based on molecular modeling studies, as has been observed recently with polyphenolic compounds [100] and peptides [Z. Ahmad and T. Laughlin unpublished results]. Synergistic effects with AMPs among different α-helical peptides have also been observed [165] and may be related to the large number of different isoforms found in several species [166]. This suggests that the evaluation of potential ATP synthase inhibitory activity by AMPs may be enhanced by combinatorial studies.
A number of experimental studies described below used α-helical peptide to induce inhibition of ATP synthase. α-helical peptides used to inhibit ATP synthase have been derived from a variety of organisms, including insects, yeasts, and frogs Apparently the sterochemical interactions of α-helical peptides with the β-DELSEED-motif of ATP synthase are more compatible than other secondary structures.
Melittin, the primary component of honey bee venom (A. mellifera), is an α-helical basic peptide composed of 26 residues and is known to have inhibitory effects on the ATPase activity of F1-ATP synthase [2, 57–59]. The peptide is a potent inhibitor of both E. coli and bovine ATP synthase (IC50~5µM) [58, 59], and may have an effect similar to that of other known α-helical peptide inhibitors, IF1, Wild-type yeast cytochrome oxidase, and synthetic Syn-A2 [57, 58, 167–171].
Fig. (1B) shows the x-ray crystallographic structure of anionic βDELSEED-loop (residue numbers β380–386) of ATP synthase, while some select cationic α-helical peptides with potential therapeutic properties are shown in Fig. (2). Indirect experimental evidence on protection against the inhibition of quinacrine mustard by melittin suggested a common βDELSEED binding site for peptides. To date, several peptides that form basic amphiphilic α-helical structures have been shown to bind at the βDELSEED-loop of E. coli ATP synthase. [57, 58].
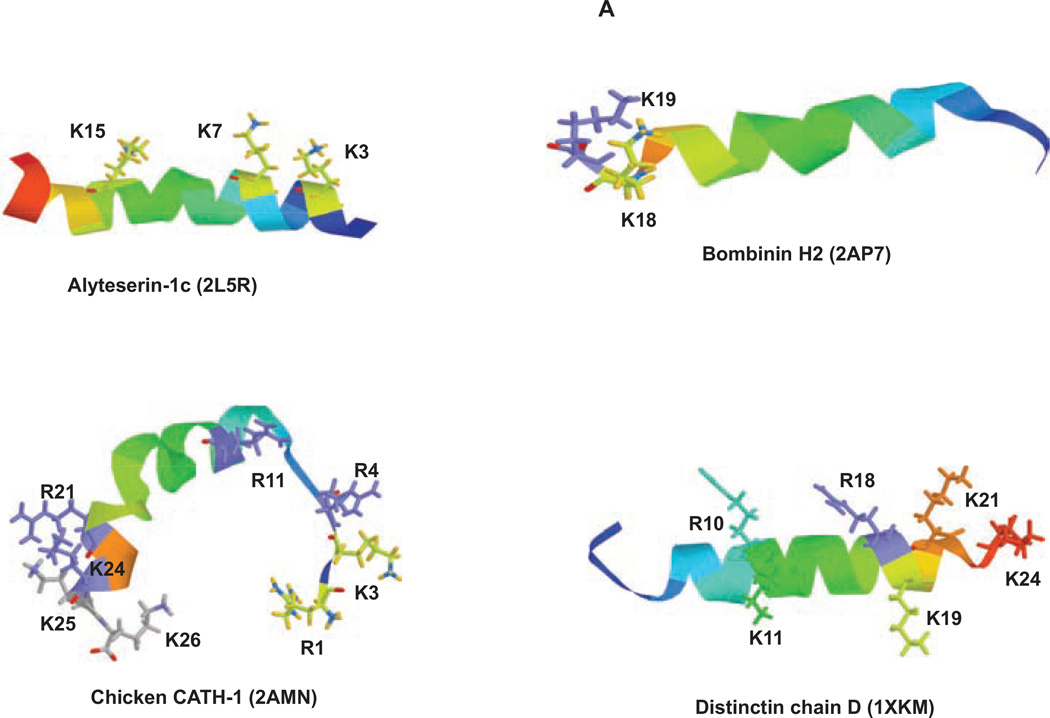
Fig. (2). X-ray crystallographic structures of select peptides.
(A & B) found to have only antimicrobial properties, (C) with antibacterial and anticancer properties, (D) with antibacterial and anti fungal properties, and (E) with antibacterial, anti cancer, and anti-fungal properties. Associated positively charged residues are identified for each peptide. RasMol [179] was used to generate these figure using PDB files 2LRR [180], 2AP7 Zangger, K., Jilek, A., Khatai, L. “Solution structure and orientation of bombinin H2 and H4 in a membrane-mimetic environment”,2AMN [181], 1XKM [182], 1OT0 Lee, K.H., Lee, D.G., Park, Y., Hahm, K.-S., Kim, Y. “Structure of Antimicrobial Peptide, HP (2–20) and its Analogues Derived from Helicobacter pylori, as Determined by 1H NMR Spectroscopy”, 1T51 [183], 2RLG [184], 2JQ0 [185], 1Z64 [186], 2KET Yang, S., Jung, H., Kim, J. “solution structure of BMAP-27”, 1YTR [187], 2JMY [188], 1ZRV [189], 2LMF [190], 2PCO [191], 1FRY [192], 1DUM [193], 2JOS [194], 2MLT [168].
Previous studies indicated that during conditions of high gradients and low ATP concentrations, the c-terminal α-helical domain of the ε-subunit of F1-ATPase undergoes large conformational changes and interacts with the α3β3 hexagon ring, where it comes in close proximity to the βDELSEED-loop. Electrostatic interactions between basic residues of the ε-subunit and the acidic residues of βDEL-SEED-motif cause inhibition of ATPase activity [172–174]. ATP synthase activity is also affected by mitochondrial if1 [175, 176]. if1 is a natural regulatory peptide of 56–87 residues in length that inhibits the ATPase activity of ATP synthase in a manner that is both reversible and noncompetitive [177]. A crystal structure of if1 with the F1- subunit shows the N-terminal domain of IF1 to be bound at the α and βF1 interface [178].
We expect AMPs with potential inhibitory effects on ATP synthase through binding at the βDELSEED-loop to be relatively short cationic peptides of approximately 10–30 amino acid residues, with α-helical secondary structure, and having previously identified anti-bacterial or anti-cancer effects. Candidate AMPs for anti ATP synthase activity and anti-microbial activity (Table I and Table II) were selected from the APD based on an α-helical secondary structure, a net positive charge, a total length of 10–30 residues, and identified antibacterial, antiviral, anticancer, or antifungal activity. Laughlin and Ahmad [59] tested fifteen peptides for ATP synthase inhibitory activity based on the previously identified ATP synthase inhibition by the melittin peptide [57, 58]. It was shown that MRP and MRP-amide strongly inhibited the ATPase activity of ATP synthase. However, magainin II, magainin II-amide, and caerin 1.9 only partially inhibited ATPase activity. Other peptides exerting partial inhibition of E. coli ATP synthase, but not shown in Fig. (2), were ascaphin-8, aurein 2.2, aurein 2.3, citropin 1.1, and maculatin 1.1. The presence of an amide group at the c-terminal end of MRP and magainin II caused an additional ~20–40% inhibition. All the above amphibian AMPs had varying degrees of effect on E. coli cell growth. Ascaphin-8, aurein 2.2, aurein 2.3, caerin 1.9, citropin 1.1, dermaseptin, magainin II-NH2, MRP, or MRP-NH2 resulted in significant inhibition of cell growth, which was interpreted as a possible result of anti ATP synthase activity [2, 59].
Table 1.
Cationic α-helical Peptides Having Antibacterial, Anticancer, Antifungal, and Antiviral Properties. + Equals Activity Against. Peptide Data Taken From Antimicrobial Peptide Database [147]
| Name | Length | Charge | Bacteria | Cancer Cells | Fungi | Viruses |
|---|---|---|---|---|---|---|
| Alyteserin-1c | 23 | 3 | + | |||
| Ascaphin-8 | 19 | 4 | + | + | + | + |
| Aurein 2.2 | 16 | 1 | + | + | + | + |
| Bactrocerin-1 | 20 | 6 | + | + | ||
| BMAP-27 | 27 | 10 | + | + | + | + |
| Bombinin H2 | 20 | 3 | + | |||
| Bombinin-like peptide 1 | 27 | 3 | + | |||
| Brevinin-1BYa | 24 | 4 | + | + | + | |
| Buforin II | 21 | 6 | + | + | + | |
| Caerin 1.6 | 24 | 2 | + | + | ||
| Cathelicidin-BF | 30 | 11 | + | + | ||
| Ceratotoxin A | 29 | 6 | + | |||
| Chicken CATH-1 | 26 | 8 | + | |||
| Chrysophsin-1 | 25 | 6 | + | + | ||
| Ci-MAM-A24 | 24 | 7 | + | + | ||
| Citropin 1.1 | 16 | 2 | + | + | + | |
| Clavanin A | 23 | 1 | + | |||
| Clavaspirin | 23 | 1 | + | |||
| CM15 | 15 | 5 | + | + | ||
| CM-3 | 18 | 6 | + | + | ||
| Cryptonin | 24 | 8 | + | + | ||
| Decoralin | 11 | 2 | + | + | ||
| Dermaseptin-S3 | 30 | 5 | + | + | ||
| Dicynthaurin | 30 | 4 | + | |||
| Distinctin | 25 | 4 | + | |||
| Eumenitin | 15 | 3 | + | |||
| Fallaxidin 4.1 | 21 | 1 | + | |||
| FK-13 | 13 | 5 | + | + | + | |
| Frenatin 3 | 14 | 4 | + | |||
| Halictine 1 | 12 | 3 | + | + | + | |
| Halocyntin | 26 | 2 | + | |||
| Hedistin | 22 | 3 | + | |||
| HFIAP-3 | 30 | 8 | + | |||
| HP 2–20 | 19 | 5 | + | |||
| Human Histatin 5 | 24 | 5 | + | + | + | |
| Human KR-20 | 20 | 4 | + | + | ||
| Human KS-30 | 30 | 6 | + | |||
| Human LL-23 | 23 | 5 | + | + | ||
| Hylaseptin P1 | 14 | 1 | + | + | ||
| Hylin a1 | 18 | 2 | + | + | ||
| IsCT | 13 | 2 | + | |||
| Japonicin-1CDYa | 14 | 3 | + | |||
| Japonicin-1Npa | 14 | 2 | + | |||
| Kassinatuerin-1 | 21 | 2 | + | + | ||
| L5K5W | 11 | 5 | + | |||
| Lasiocepsin | 27 | 9 | + | + | ||
| Lasioglossin LL-I | 15 | 5 | + | + | ||
| Latarcin 1 | 26 | 10 | + | + | ||
| Lycotoxin I | 25 | 5 | + | |||
| Magainin 2 | 23 | 3 | + | + | + | + |
| Mastoparan B | 14 | 4 | + | + | ||
| Maximin 4 | 27 | 3 | + | + | + | + |
| MB-21 | 15 | 4 | + | + | ||
| Melectin | 18 | 4 | + | + | ||
| Melittin | 26 | 6 | + | + | + | + |
| Meucin-13 | 13 | 2 | + | + | ||
| Misgurin | 21 | 7 | + | + | ||
| Moronecidin | 22 | 3 | + | |||
| MUC7 20-Mer | 20 | 7 | + | + | ||
| Nigrocin-2 | 21 | 3 | + | + | ||
| Ocellatin-F1 | 25 | 3 | + | |||
| Odorranain-B1 | 20 | 5 | + | + | ||
| Oxt 4a | 30 | 9 | + | |||
| P-18 | 18 | 7 | + | + | ||
| Pandinin 2 | 24 | 3 | + | + | ||
| Parasin I | 19 | 8 | + | + | ||
| Parkerin | 20 | 2 | + | + | ||
| Pd_mastoparan PDD-A | 14 | 4 | + | |||
| Pep27 | 27 | 4 | + | + | ||
| PGLa | 21 | 5 | + | |||
| Phylloseptin-H1 | 19 | 2 | + | |||
| Phylloxin-B1 | 19 | 1 | + | |||
| Piscidin 1 | 22 | 3 | + | + | + | + |
| Plantaricin A | 26 | 6 | + | + | ||
| Pleurocidin | 25 | 4 | + | |||
| PMAP-23 | 23 | 6 | + | |||
| Polybia-MP-I | 14 | 2 | + | + | ||
| Ponericin G1 | 30 | 7 | + | + | ||
| PP13 | 22 | 6 | + | |||
| Pseudin-1 | 24 | 2 | + | + | ||
| Ranalexin | 20 | 3 | + | + | ||
| RP-1 | 17 | 8 | + | |||
| SMAP-29 | 29 | 9 | + | + | ||
| Spinigerin | 25 | 5 | + | + | + | |
| Styelin A | 19 | 5 | + | |||
| Substance P | 11 | 3 | + | + | ||
| Temporin A | 13 | 1 | + | + | ||
| The K4 peptide | 14 | 4 | + | |||
| Uperin 3.6 | 17 | 2 | + | + | ||
| WLBU2 | 24 | 13 | + | |||
| XT-7 | 18 | 3 | + | + | + |
Table 2.
Sequence and Origin of Table I Peptides. Peptide Data Taken From Antimicrobial Peptide Database [147]
| Name | Sequence | Source |
|---|---|---|
| Alyteserin-1c | GLKEIFKAGLGSLVKGIAAHVAS | Amphibian |
| Ascaphin-8 | GFKDLLKGAAKALVKTVLF | Amphibian |
| Aurein 2.2 | GLFDIVKKVVGALGSL | Amphibian |
| Bactrocerin-1 | VGKTWIKVIRGIGKSKIKWQ | Insect |
| BMAP-27 | GRFKRFRKKFKKLFKKLSPVIPLLHLG | Mammal |
| Bombinin H2 | IIGPVLGLVGSALGGLLKKI | Amphibian |
| Bombinin-like peptide 1 | GIGASILSAGKSALKGLAKGLAEHFAN | Amphibian |
| Brevinin-1BYa | FLPILASLAAKFGPKLFCLVTKKC | Amphibian |
| Buforin II | TRSSRAGLQFPVGRVHRLLRK | Amphibian |
| Caerin 1.6 | GLFSVLGAVAKHVLPHVVPVIAEK | Amphibian |
| Cathelicidin-BF | KFFRKLKKSVKKRAKEFFKKPRVIGVSIPF | Reptile |
| Ceratotoxin A | SIGSALKKALPVAKKIGKIALPIAKAALP | Insect |
| Chicken CATH-1 | RVKRVWPLVIRTVIAGYNLYRAIKKK | Bird |
| Chrysophsin-1 | FFGWLIKGAIHAGKAIHGLIHRRRH | Fish |
| Ci-MAM-A24 | WRSLGRTLLRLSHALKPLARRSGW | Urochordate |
| Citropin 1.1 | GLFDVIKKVASVIGGL | Amphibian |
| Clavanin A | VFQFLGKIIHHVGNFVHGFSHVF | Urochordate |
| Clavaspirin | FLRFIGSVIHGIGHLVHHIGVAL | Urochordate |
| CM15 | KWKLFKKIGAVLKVL | Synthetic |
| CM-3 | ALKAALLAILKIVRVIKK | Synthetic |
| Cryptonin | GLLNGLALRLGKRALKKIIKRLCR | Insect |
| Decoralin | SLLSLIRKLIT | Insect |
| Dermaseptin-S3 | ALWKNMLKGIGKLAGKAALGAVKKLVGAES | Amphibian |
| Dicynthaurin | ILQKAVLDCLKAAGSSLSKAAITAIYNKIT | Urochordate |
| Distinctin | NLVSGLIEARKYLEQLHRKLKNCKV | Amphibian |
| Eumenitin | LNLKGIFKKVASLLT | Insect |
| Fallaxidin 4.1 | GLLSFLPKVIGVIGHLIHPPS | Amphibian |
| FK-13 | FKRIVQRIKDFLR | Synthetic |
| Frenatin 3 | GLMSVLGHAVGNVLGGLFKS | Synthetic |
| Halictine 1 | GMWSKILGHLIR | Insect |
| Halocyntin | FWGHIWNAVKRVGANALHGAVTGALS | Urochordate |
| Hedistin | LGAWLAGKVAGTVATYAWNRYV | Annelid |
| HFIAP-3 | GWFKKAWRKVKNAGRRVLKGVGIHYGVGLI | Hagfish |
| HP 2–20 | AKKVFKRLEKLFSKIQNDK | Synthetic |
| Human Histatin 5 | DSHAKRHHGYKRKFHEKHHSHRGY | Human |
| Human KR-20 | KRIVQRIKDFLRNLVPRTES | Human |
| Human KS-30 | KSKEKIGKEFKRIVQRIKDFLRNLVPRTES | Human |
| Human LL-23 | LLGDFFRKSKEKIGKEFKRIVQR | Human |
| Hylaseptin P1 | GILDAIKAIAKAAG | Amphibian |
| Hylin a1 | IFGAILPLALGALKNLIK | Amphibian |
| IsCT | ILGKIWEGIKSLF | Arachnid |
| Japonicin-1CDYa | FFPLALLCKVFKKC | Amphibian |
| Japonicin-1Npa | FLLFPLMCKIQGKC | Amphibian |
| Kassinatuerin-1 | GFMKYIGPLIPHAVKAISDLI | Amphibian |
| L5K5W | KKLLKWLKKLL | Snythetic/amphibian |
| Lasiocepsin | GLPRKILCAIAKKKGKCKGPLKLVCKC | Insect |
| Lasioglossin LL-I | ILGKLLSTAAGLLSNL | Insect |
| Latarcin 1 | SMWSGMWRRKLKKLRNALKKKLKGEK | Arachnid |
| Lycotoxin I | IWLTALKFLGKHAAKHLAKQQLSKL | Arachnid |
| Magainin 2 | GIGKFLHSAKKFGKAFVGEIMNS | Amphibian |
| Mastoparan B | LKLKSIVSWAKKVL | Insect |
| Maximin 4 | GIGGVLLSAGKAALKGLAKVLAEKYAN | Amphibian |
| MB-21 | FASLLGKALKALAKQ | Synthetic |
| Melectin | GFLSILKKVLPKVMAHMK | Insect |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | Insect |
| Meucin-13 | IFGAIAGLLKNIF | Arachnid |
| Misgurin | RQRVEELSKFSKKGAAARRRK | Fish |
| Moronecidin | FFHHIFRGIVHVGKTIHKLVTG | Fish |
| MUC7 20-Mer | LAHQKPFIRKSYKCLHKRCR | Human |
| Nigrocin-2 | GLLSKVLGVGKKVLCGVSGLC | Amphibian |
| Ocellatin-F1 | GVVDILKGAAKDIAGHLASKVMNKL | Amphibian |
| Odorranain-B1 | AALKGCWTKSIPPKPCFGKR | Amphibian |
| Oxt 4a | GIRCPKSWKCKAFKQRVLKRLLAMLRQHAF | Arachnid |
| P-18 | KWKLFKKIPKFLHLAKKF | Synthetic |
| Pandinin 2 | FWGALAKGALKLIPSLFSSFSKKD | Arachnid |
| Parasin I | KGRGKQGGKVRAKAKTRSS | Fish |
| Parkerin | GWANTLKNVAGGLCKITGAA | Amphibian |
| Pd_mastoparan PDD-A | INWKKIFEKVKNLV | Insect |
| Pep27 | MRKEFHNVLSSGQLLADKRPARDYNRK | Bacteria |
| PGLa | GMASKAGAIAGKIAKVALKAL | Amphibian |
| Phylloseptin-H1 | FLSLIPHAINAVSAIAKHN | Amphibian |
| Phylloxin-B1 | GWMSKIASGIGTFLSGMQQ | Amphibian |
| Piscidin 1 | FFHHIFRGIVHVGKTIHRLVTG | Fish |
| Plantaricin A | KSSAYSLQMGATAIKQVKKLFKKWGW | Bacteria |
| Pleurocidin | GWGSFFKKAAHVGKHVGKAALTHYL | Fish |
| PMAP-23 | RIIDLLWRVRRPQKPKFVTVWVR | Mammal |
| Polybia-MP-I | IDWKKLLDAAKQIL | Insect |
| Ponericin G1 | GWKDWAKKAGGWLKKKGPGMAKAALKAAMQ | Insect |
| PP13 | GAARKSIRLHRLYTWKATIYTR | Insect |
| Pseudin-1 | GLNTLKKVFQGLHEAIKLINNHVQ | Amphibian |
| Ranalexin | FLGGLIKIVPAMICAVTKKC | Amphibian |
| RP-1 | ALYKKFKKKLLKSLKRL | Synthetic |
| SMAP-29 | RGLRRLGRKIAHGVKKYGPTVLRIIRIAG | Mammal |
| Spinigerin | HVDKKVADKVLLLKQLRIMRLLTRL | Insect |
| Styelin A | GFGKAFHSVSNFAKKHKTA | Urochordate |
| Substance P | RPKPQQFFGLM | Human |
| Temporin A | FLPLIGRVLSGIL | Amphibian |
| The K4 peptide | KKKKPLFGLFFGLF | Synthetic |
| Uperin 3.6 | GVIDAAKKVVNVLKNLF | Amphibian |
| WLBU2 | RRWVRRVRRWVRRVVRVVRRWVRR | Synthetic |
| XT-7 | GLLGPLLKIAAKVGSNLL | Amphibian |
It seems probable that there will be variable results from the testing of AMPs for inhibition of ATP synthase or of cell growth for therapeutic applications, depending on the particular target molecules and organisms. We also found that various modifications of naturally occurring AMPs may be used to modulate their effectiveness on a molar scale with regard to both ATP synthase inhibition and cytotoxicity (Z. Ahmad and T.F Laughlin unpublished results). By virtue of the great variability in the structures and potential functions of AMPs, these relatively simple molecules may constitute a rich natural resource of new drug compounds that may be targeted at microorganisms and neoplasms through inhibitory effects on ATP synthase.
ACKNOWLEDGEMENT
This work was supported by the National Institutes of Health Grant GM085771 to ZA.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Senior AE, Nadanaciva S, Weber J. The molecular mechanism of ATP synthesis by F1F0-ATP synthase. Biochim. Biophys. Acta. 2002;1553(3):188–211. doi: 10.1016/s0005-2728(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad Z, Laughlin TF. Medicinal chemistry of ATP synthase: a potential drug target of dietary polyphenols and amphibian antimicrobial peptides. Curr. Med. Chem. 2010;17(25):2822–2836. doi: 10.2174/092986710791859270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad Z, Okafor F, Laughlin TF. Role of Charged Residues in the Catalytic Sites of Escherichia coli ATP Synthase. J Amino Acids. 2011;2011:785741. doi: 10.4061/2011/785741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer PD. The ATP synthase--a splendid molecular machine. Annu. Rev. Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 5.Garcia JJ, Ogilvie I, Robinson BH, Capaldi RA. Structure, functioning, and assembly of the ATP synthase in cells from patients with the T8993G mitochondrial DNA mutation. Comparison with the enzyme in Rho(0) cells completely lacking mtdna. J. Biol. Chem. 2000;275(15):11075–11081. doi: 10.1074/jbc.275.15.11075. [DOI] [PubMed] [Google Scholar]
- 6.Dibrova DV, Galperin MY, Mulkidjanian AY. Characterization of the N-ATPase, a distinct, laterally transferred Na+-translocating form of the bacterial F-type membrane ATPase. Bio-informatics. 2010;26(12):1473–1476. doi: 10.1093/bioinformatics/btq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabaleeswaran V, Puri N, Walker JE, Leslie AG, Mueller DM. Novel features of the rotary catalytic mechanism revealed in the structure of yeast F1 ATPase. EMBO J. 2006;25(22):5433–5442. doi: 10.1038/sj.emboj.7601410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabaleeswaran V, Shen H, Symersky J, Walker JE, Leslie AG, Mueller DM. Asymmetric structure of the yeast F1 ATPase in the absence of bound nucleotides. J. Biol. Chem. 2009;284(16):10546–10551. doi: 10.1074/jbc.M900544200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Walraven HS, Strotmann H, Schwarz O, Rumberg B. The H+/ATP coupling ratio of the ATP synthase from thiol-modulated chloroplasts and two cyanobacterial strains is four. FEBS Lett. 1996;379(3):309–313. doi: 10.1016/0014-5793(95)01536-1. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida M, Muneyuki E, Hisabori T. ATP synthase--a marvellous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2001;2(9):669–677. doi: 10.1038/35089509. [DOI] [PubMed] [Google Scholar]
- 11.Schemidt RA, Qu J, Williams JR, Brusilow WS. Effects of carbon source on expression of F0 genes and on the stoichiometry of the c subunit in the F1F0 ATPase of Escherichia coli. J. Bacteriol. 1998;180(12):3205–3208. doi: 10.1128/jb.180.12.3205-3208.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perzov N, Padler-Karavani V, Nelson H. Nelson, N, Features of V-ATPases that distinguish them from F-ATPases. FEBS Lett. 2001;504(3):223–228. doi: 10.1016/s0014-5793(01)02709-0. [DOI] [PubMed] [Google Scholar]
- 13.Fillingame RH. Membrane sectors of F- and V-type H+- transporting ATPases. Curr. Opin. Struct. Biol. 1996;6(4):491–498. doi: 10.1016/s0959-440x(96)80114-x. [DOI] [PubMed] [Google Scholar]
- 14.Cross RL, Muller V. The evolution of A-, F-, and V-type ATP synthases and ATPases: reversals in function and changes in the H+/ATP coupling ratio. FEBS Lett. 2004;576(1–2):1–4. doi: 10.1016/j.febslet.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 15.Rappas M, Niwa H, Zhang X. Mechanisms of ATPases—a multi-disciplinary approach. Curr. Protein Pept Sci. 2004;5(2):89–105. doi: 10.2174/1389203043486874. [DOI] [PubMed] [Google Scholar]
- 16.Nelson N, Perzov N, Cohen A, Hagai K, Padler V, Nelson H. The cellular biology of proton-motive force generation by V- ATPases. J. Exp. Biol. 2000;203(Pt 1):89–95. doi: 10.1242/jeb.203.1.89. [DOI] [PubMed] [Google Scholar]
- 17.Gogarten JP, Starke T, Kibak H, Fishman J, Taiz L. Evolution and isoforms of V-ATPase subunits. J. Exp. Biol. 1992;172:137–147. doi: 10.1242/jeb.172.1.137. [DOI] [PubMed] [Google Scholar]
- 18.Muller V, Ruppert C, Lemker T. Structure and function of the A1A0-ATPases from methanogenic Archaea. J. Bioenerg. Biomembr. 1999;31(1):15–27. doi: 10.1023/a:1005451311009. [DOI] [PubMed] [Google Scholar]
- 19.Wilms R, Freiberg C, Wegerle E, Meier I, Mayer F, Muller V. Subunit structure and organization of the genes of the A1A0 ATPase from the Archaeon Methanosarcina mazei Go1. J. Biol. Chem. 1996;271(31):18843–18852. doi: 10.1074/jbc.271.31.18843. [DOI] [PubMed] [Google Scholar]
- 20.Toyoshima C, Nakasako M, Nomura H, Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature. 2000;405(6787):647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 21.Stokes DL, Green NM. Structure and function of the calcium. pump. Annu. Rev. Biophys. Biomol. Struct. 2003;32:445–468. doi: 10.1146/annurev.biophys.32.110601.142433. [DOI] [PubMed] [Google Scholar]
- 22.Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 1998;46(1):84–101. doi: 10.1007/pl00006286. [DOI] [PubMed] [Google Scholar]
- 23.Rak M, Gokova S, Tzagoloff A. Modular assembly of yeast mitochondrial ATP synthase. EMBO J. 2011;30(5):920–930. doi: 10.1038/emboj.2010.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senior AE. ATP synthesis by oxidative phosphorylation. Physiol. Rev. 1988;68(1):177–231. doi: 10.1152/physrev.1988.68.1.177. [DOI] [PubMed] [Google Scholar]
- 25.Karrasch S, Walker JE. Novel features in the structure of bovine ATP synthase. J. Mol. Biol. 1999;290(2):379–384. doi: 10.1006/jmbi.1999.2897. [DOI] [PubMed] [Google Scholar]
- 26.Devenish RJ, Prescott M, Roucou X, Nagley P. Insights into ATP synthase assembly and function through the molecular genetic manipulation of subunits of the yeast mitochondrial enzyme complex. Biochim. Biophys. Acta. 2000;1458(2–3):428–442. doi: 10.1016/s0005-2728(00)00092-x. [DOI] [PubMed] [Google Scholar]
- 27.Diez M, Zimmermann B, Borsch M, Konig M, Schwein-berger E, Steigmiller S, Reuter R, Felekyan S, Kudryavtsev V, Seidel CAM, Graber P. Proton-powered subunit rotation in single membrane-bound F0F1-ATP synthase. Nat. Struct. Mol. Biol. 2004;11(2):135–141. doi: 10.1038/nsmb718. [DOI] [PubMed] [Google Scholar]
- 28.Itoh H, Takahashi A, Adachi K, Noji H, Yasuda R, Yoshida M, Kinosita K. Mechanically driven ATP synthesis by F1-ATPase. Nature. 2004;427(6973):465–468. doi: 10.1038/nature02212. [DOI] [PubMed] [Google Scholar]
- 29.Weber J. ATP synthase: subunit-subunit interactions in the stator stalk. Biochim. Biophys. Acta. 2006;1757(9–10):1162–1170. doi: 10.1016/j.bbabio.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber J, Senior AE. ATP synthase: what we know about ATP hydrolysis and what we do not know about ATP synthesis. Biochim. Biophys. Acta. 2000;1458(2–3):300–309. doi: 10.1016/s0005-2728(00)00082-7. [DOI] [PubMed] [Google Scholar]
- 31.Senior AE, Nadanaciva S, Weber J. Rate acceleration of ATP hydrolysis by F(1)F(o)-ATP synthase. J. Exp. Biol. 2000;203(Pt 1):35–40. doi: 10.1242/jeb.203.1.35. [DOI] [PubMed] [Google Scholar]
- 32.Frasch WD. The participation of metals in the mechanism of the F(1)-ATPase. Biochim. Biophys. Acta. 2000;1458(2–3):310–325. doi: 10.1016/s0005-2728(00)00083-9. [DOI] [PubMed] [Google Scholar]
- 33.Nakamoto RK, Ketchum CJ, al-Shawi MK. Rotational coupling in the F0F1 ATP synthase. Annu. Rev. Biophys. Biomol Struct. 1999;28:205–234. doi: 10.1146/annurev.biophys.28.1.205. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen PL. Transport ATPases into the year 2008: a brief overview related to types, structures, functions and roles in health and disease. J. Bioenerg. Biomembr. 2007;39(5–6):349–355. doi: 10.1007/s10863-007-9123-9. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad Z, Senior AE. Identification of phosphate binding residues of Escherichia coli ATP synthase. J. Bioenerg. Biomembr. 2005;37(6):437–440. doi: 10.1007/s10863-005-9486-8. [DOI] [PubMed] [Google Scholar]
- 36.Senior AE. ATP synthase: motoring to the finish line. Cell. 2007;130(2):220–221. doi: 10.1016/j.cell.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Noji H, Yoshida M. The rotary machine in the cell, ATP synthase. J. Biol. Chem. 2001;276(3):1665–1668. doi: 10.1074/jbc.R000021200. [DOI] [PubMed] [Google Scholar]
- 38.Weber J, Senior AE. ATP synthesis driven by proton transport in F1F0-ATP synthase. FEBS Lett. 2003;545(1):61–70. doi: 10.1016/s0014-5793(03)00394-6. [DOI] [PubMed] [Google Scholar]
- 39.Khan S. Rotary chemiosmotic machines. Biochim. Biophys. Acta. 1997;1322(2–3):86–105. doi: 10.1016/s0005-2728(97)00075-3. [DOI] [PubMed] [Google Scholar]
- 40.Ren H, Allison WS. On what makes the gamma subunit spin during ATP hydrolysis by F(1) Biochim. Biophys. Acta. 2000;1458(2–3):221–233. doi: 10.1016/s0005-2728(00)00075-x. [DOI] [PubMed] [Google Scholar]
- 41.Senior AE. Two ATPases. J. Biol. Chem. 2012;287(36):30049–30062. doi: 10.1074/jbc.X112.402313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber J. Structural biology: Toward the ATP synthase mechanism. Nat. Chem. Biol. 2010;6(11):794–795. doi: 10.1038/nchembio.458. [DOI] [PubMed] [Google Scholar]
- 43.Leslie AG, Walker JE. Structural model of F1-ATPase and the implications for rotary catalysis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2000;355(1396):465–471. doi: 10.1098/rstb.2000.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menz RI, Walker JE, Leslie AG. Structure of bovine mitochondrial F(1)-ATPase with nucleotide bound to all three catalytic sites: implications for the mechanism of rotary catalysis. Cell. 2001;106(3):331–341. doi: 10.1016/s0092-8674(01)00452-4. [DOI] [PubMed] [Google Scholar]
- 45.Boyer PD. A perspective of the binding change mechanism for ATP synthesis. FASEB J. 1989;3(10):2164–2178. doi: 10.1096/fasebj.3.10.2526771. [DOI] [PubMed] [Google Scholar]
- 46.Boyer PD. A research journey with ATP synthase. J. Biol. Chem. 2002;277(42):39045–39061. doi: 10.1074/jbc.X200001200. [DOI] [PubMed] [Google Scholar]
- 47.Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Direct observation of the rotation of F1-ATPase. Nature. 1997;386(6622):299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 48.Kinosita K, Jr, Yasuda R, Noji H, Ishiwata S, Yoshida M. F1-ATPase: a rotary motor made of a single molecule. Cell. 1998;93(1):21–24. doi: 10.1016/s0092-8674(00)81142-3. [DOI] [PubMed] [Google Scholar]
- 49.Nishizaka T, Oiwa K, Noji H, Kimura S, Muneyuki E, Yoshida M, Kinosita K., Jr Chemomechanical coupling in F1-ATPase revealed by simultaneous observation of nucleotide kinetics and rotation. Nat. Struct. Mol. Biol. 2004;11(2):142–148. doi: 10.1038/nsmb721. [DOI] [PubMed] [Google Scholar]
- 50.Senior AE, Weber J. Happy motoring with ATP synthase. Nat. Struct. Mol. Biol. 2004;11(2):110–112. doi: 10.1038/nsmb0204-110. [DOI] [PubMed] [Google Scholar]
- 51.Yasuda R, Noji H, Kinosita K, Jr, Yoshida M. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120 degree steps. Cell. 1998;93(7):1117–1124. doi: 10.1016/s0092-8674(00)81456-7. [DOI] [PubMed] [Google Scholar]
- 52.Whitesides GM. The ‘right’ size in nanobiotechnology. Nat. Biotechnol. 2003;21(10):1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 53.Li W, Brudecki LE, Senior AE, Ahmad Z. Role of {alpha}-subunit VISIT-DG sequence residues Ser-347 and Gly-351 in the catalytic sites of Escherichia coli ATP synthase. J. Biol. Chem. 2009;284(16):10747–10754. doi: 10.1074/jbc.M809209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ernst S, Duser MG, Zarrabi N, Dunn SD, Borsch M. Elastic deformations of the rotary double motor of single F(o)F(1)-ATP synthases detected in real time by Forster resonance energy transfer. Biochim. Biophys. Acta. 2012;1817(10):1722–1731. doi: 10.1016/j.bbabio.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 55.Khataee A, Khataee HR. Advances in F0F1-ATP synthase Biological Protein Nanomotor: from Mechanisms and Strategies to Potential Applications NANO. 2009;4(2):55–67. [Google Scholar]
- 56.Hong S, Pedersen PL. ATP Synthase and the Actions of Inhibitors Utilized To Study Its Roles in Human Health, Disease, and Other Scientific Areas. Microbiol. Mol. Biol. Rev. 2008;72(4):590–641. doi: 10.1128/MMBR.00016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gledhill JR, Walker JE. Inhibition sites in F1-ATPase from bovine heart mitochondria. Biochem. J. 2005;386(Pt 3):591–598. doi: 10.1042/BJ20041513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bullough DA, Ceccarelli EA, Roise D, Allison WS. Inhibition of the bovine-heart mitochondrial F1-ATPase by cationic dyes and amphipathic peptides. Biochim. Biophys. Acta. 1989;975(3):377–383. doi: 10.1016/s0005-2728(89)80346-9. [DOI] [PubMed] [Google Scholar]
- 59.Laughlin TF, Ahmad Z. Inhibition of Escherichia coli ATP synthase by amphibian antimicrobial peptides. Int. J. Biol. Macromol. 2010;46(3):367–374. doi: 10.1016/j.ijbiomac.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber J, Wilke-Mounts S, Lee RS, Grell E, Senior AE. Specific placement of tryptophan in the catalytic sites of Escherichia coli F1-ATPase provides a direct probe of nucleotide binding: maximal ATP hydrolysis occurs with three sites occupied. J. Biol. Chem. 1993;268(27):20126–20133. [PubMed] [Google Scholar]
- 61.Lobau S, Weber J, Senior AE. Catalytic site nucleotide binding and hydrolysis in F1F0-ATP synthase. Biochemistry (Mosc) 1998;37(30):10846–10853. doi: 10.1021/bi9807153. [DOI] [PubMed] [Google Scholar]
- 62.Weber J, Hammond ST, Wilke-Mounts S, Senior AE. Mg2+ coordination in catalytic sites of F1-ATPase. Biochemistry (Mosc) 1998;37(2):608–614. doi: 10.1021/bi972370e. [DOI] [PubMed] [Google Scholar]
- 63.Dou C, Fortes PA, Allison WS. The alpha 3(beta Y341W)3 gamma subcomplex of the F1-ATPase from the thermophilic Bacillus PS3 fails to dissociate ADP when MgATP is hydrolyzed at a single catalytic site and attains maximal velocity when three catalytic sites are saturated with MgATP. Biochemistry (Mosc) 1998;37(47):16757–16764. doi: 10.1021/bi981717q. [DOI] [PubMed] [Google Scholar]
- 64.al-Shawi MK, Senior AE. Effects of dimethyl sulfoxide on catalysis in Escherichia coli F1-ATPase. Biochemistry (Mosc) 1992;31(3):886–891. doi: 10.1021/bi00118a034. [DOI] [PubMed] [Google Scholar]
- 65.Al-Shawi MK, Ketchum CJ, Nakamoto RK. The Escherichia coli FOF1 gammaM23K uncoupling mutant has a higher K0.5 for Pi. Transition state analysis of this mutant and others reveals that synthesis and hydrolysis utilize the same kinetic pathway. Biochemistry (Mosc) 1997;36(42):12961–12969. doi: 10.1021/bi971478r. [DOI] [PubMed] [Google Scholar]
- 66.Rastogi VK, Girvin ME. Structural changes linked to proton translocation by subunit c of the ATP synthase. Nature. 1999;402(6759):263–268. doi: 10.1038/46224. [DOI] [PubMed] [Google Scholar]
- 67.Gibbons C, Montgomery MG, Leslie AG, Walker JE. The structure of the central stalk in bovine F(1)-ATPase at 2.4 A resolution. Nat. Struct. Biol. 2000;7(11):1055–1061. doi: 10.1038/80981. [DOI] [PubMed] [Google Scholar]
- 68.Dadi PK, Ahmad M, Ahmad Z. Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int. J. Biol. Macromol. 2009;45(1):72–79. doi: 10.1016/j.ijbiomac.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Chinnam N, Dadi PK, Sabri SA, Ahmad M, Kabir MA, Ahmad Z. Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol. 2010;46(5):478–486. doi: 10.1016/j.ijbiomac.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braig K, Menz RI, Montgomery MG, Leslie AG, Walker JE. Structure of bovine mitochondrial F(1)-ATPase inhibited by Mg(2+) ADP and aluminium fluoride. Structure. 2000;8(6):567–573. doi: 10.1016/s0969-2126(00)00145-3. [DOI] [PubMed] [Google Scholar]
- 71.Orriss GL, Leslie AG, Braig K, Walker JE. Bovine F1-ATPase covalently inhibited with 4-chloro-7-nitrobenzofurazan: the structure provides further support for a rotary catalytic mechanism. Structure. 1998;6(7):831–837. doi: 10.1016/s0969-2126(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 72.Perez JA, Greenfield AJ, Sutton R, Ferguson SJ. Characterisation of phosphate binding to mitochondrial and bacterial membrane-bound ATP synthase by studies of inhibition with 4-chloro-7-nitrobenzofurazan. FEBS Lett. 1986;198(1):113–118. doi: 10.1016/0014-5793(86)81195-4. [DOI] [PubMed] [Google Scholar]
- 73.Ahmad Z, Senior AE. Mutagenesis of residue betaArg-246 in the phosphate-binding subdomain of catalytic sites of Escherichia coli F1-ATPase. J. Biol. Chem. 2004;279(30):31505–31513. doi: 10.1074/jbc.M404621200. [DOI] [PubMed] [Google Scholar]
- 74.Ahmad Z, Senior AE. Role of betaAsn-243 in the phosphate-binding subdomain of catalytic sites of Escherichia coli F(1)-ATPase. J. Biol. Chem. 2004;279(44):46057–46064. doi: 10.1074/jbc.M407608200. [DOI] [PubMed] [Google Scholar]
- 75.Ahmad Z, Senior AE. Involvement of ATP synthase residues alphaArg-376, betaArg-182, and betaLys-155 in Pi binding. FEBS Lett. 2005;579(2):523–528. doi: 10.1016/j.febslet.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 76.Ahmad Z, Senior AE. Modulation of charge in the phosphate binding site of Escherichia coli ATP synthase. J. Biol. Chem. 2005;280(30):27981–27989. doi: 10.1074/jbc.M503955200. [DOI] [PubMed] [Google Scholar]
- 77.Ahmad Z, Senior AE. Inhibition of the ATPase activity of Escherichia coli ATP synthase by magnesium fluoride. FEBS Lett. 2006;580(2):517–520. doi: 10.1016/j.febslet.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 78.Brudecki LE, Grindstaff JJ, Ahmad Z. Role of alphaPhe-291 residue in the phosphate-binding subdomain of catalytic sites of Escherichia coli ATP synthase. Arch. Biochem. Biophys. 2008;471(2):168–175. doi: 10.1016/j.abb.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 79.Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl. Acad. Sci. U. S. A. 2007;104(34):13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson JA, Ogbi M. Targeting the F1Fo ATP Synthase: modulation of the body’s powerhouse and its implications for human disease. Curr. Med. Chem. 2011;18(30):4684–4714. doi: 10.2174/092986711797379177. [DOI] [PubMed] [Google Scholar]
- 81.de Vries DD, van Engelen BG, Gabreels FJ, Ruitenbeek W, van Oost BA. A second missense mutation in the mitochondrial ATPase 6 gene in Leigh’s syndrome. Ann. Neurol. 1993;34(3):410–412. doi: 10.1002/ana.410340319. [DOI] [PubMed] [Google Scholar]
- 82.Osanai T, Magota K, Tanaka M, Shimada M, Murakami R, Sasaki S, Tomita H, Maeda N, Okumura K. Intracellular signaling for vasoconstrictor coupling factor 6: novel function of beta-subunit of ATP synthase as receptor. Hypertension. 2005;46(5):1140–1146. doi: 10.1161/01.HYP.0000186483.86750.85. [DOI] [PubMed] [Google Scholar]
- 83.Osanai T, Okada S, Sirato K, Nakano T, Saitoh M, Magota K, Okumura K. Mitochondrial coupling factor 6 is present on the surface of human vascular endothelial cells and is released by shear stress. Circulation. 2001;104(25):3132–3136. doi: 10.1161/hc5001.100832. [DOI] [PubMed] [Google Scholar]
- 84.Arakaki N, Kita T, Shibata H, Higuti T. Cell-surface H+-ATP synthase as a potential molecular target for anti-obesity drugs. FEBS Lett. 2007;581(18):3405–3409. doi: 10.1016/j.febslet.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 85.Arakaki N, Nagao T, Niki R, Toyofuku A, Tanaka H, Kuramoto Y, Emoto Y, Shibata H, Magota K, Higuti T. Possible role of cell surface H+ -ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol. Cancer Res. 2003;1(13):931–939. [PubMed] [Google Scholar]
- 86.Berger K, Winzell MS, Mei J, Erlanson-Albertsson C. En-terostatin and its target mechanisms during regulation of fat intake. Physiol. Behav. 2004;83(4):623–630. doi: 10.1016/j.physbeh.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 87.Champagne E, Martinez LO, Collet X, Barbaras R. Ecto-F1Fo ATP synthase/F1 ATPase: metabolic and immunological functions. Curr. Opin. Lipidol. 2006;17(3):279–284. doi: 10.1097/01.mol.0000226120.27931.76. [DOI] [PubMed] [Google Scholar]
- 88.Kenan DJ, Wahl ML. Ectopic localization of mitochondrial ATP synthase: a target for anti-angiogenesis intervention? J. Bioenerg. Biomembr. 2005;37(6):461–465. doi: 10.1007/s10863-005-9492-x. [DOI] [PubMed] [Google Scholar]
- 89.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc. Natl. Acad. Sci. U. S. A. 2001;98(12):6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang WJ, Ma Z, Liu YW, He YQ, Wang YZ, Yang CX, Du Y, Zhou MQ, Gao F. A monoclonal antibody (Mc178-Ab) targeted to the ecto-ATP synthase beta-subunit-induced cell apoptosis via a mechanism involving the MAPKase and Akt pathways. Clin. Exp. Med. 2012;12(1):3–12. doi: 10.1007/s10238-011-0133-x. [DOI] [PubMed] [Google Scholar]
- 91.Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 1999;96(6):2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Percival RS, Devine DA, Duggal MS, Chartron S, Marsh PD. The effect of cocoa polyphenols on the growth, metabolism, and biofilm formation by Streptococcus mutans and Streptococcus sanguinis. Eur. J. Oral Sci. 2006;114(4):343–348. doi: 10.1111/j.1600-0722.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 93.Duarte S, Gregoire S, Singh AP, Vorsa N, Schaich K, Bowen WH, Koo H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol. Lett. 2006;257(1):50–56. doi: 10.1111/j.1574-6968.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 94.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. A di-arylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307(5707):223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 95.Cole ST, Alzari PM. MICROBIOLOGY: Enhanced: TB-A New Target, a New Drug. Science. 2005;307(5707):214–215. doi: 10.1126/science.1108379. [DOI] [PubMed] [Google Scholar]
- 96.van Raaij MJ, Abrahams JP, Leslie AG, Walker JE. The structure of bovine F1-ATPase complexed with the antibiotic inhibitor aurovertin B. Proc. Natl. Acad. Sci. U. S. A. 1996;93(14):6913–6917. doi: 10.1073/pnas.93.14.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abrahams JP, Buchanan SK, Van Raaij MJ, Fearnley IM, Leslie AG, Walker JE. The structure of bovine F1-ATPase complexed with the peptide antibiotic efrapeptin. Proc. Natl. Acad. Sci. U. S. A. 1996;93(18):9420–9424. doi: 10.1073/pnas.93.18.9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolvetang EJ, Johnson KL, Krauer K, Ralph SJ, Linnane AW. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994;339(1–2):40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]
- 99.Mills KI, Woodgate LJ, Gilkes AF, Walsh V, Sweeney MC, Brown G, Burnett AK. Inhibition of mitochondrial function in HL60 cells is associated with an increased apoptosis and expression of CD14. Biochem. Biophys. Res. Commun. 1999;263(2):294–300. doi: 10.1006/bbrc.1999.1356. [DOI] [PubMed] [Google Scholar]
- 100.Ahmad Z, Ahmad M, Okafor F, Jones J, Abunameh A, Cheniya RP, Kady IO. Effect of structural modulation of poly-phenolic compounds on the inhibition of Escherichia coli ATP synthase. Int. J. Biol. Macromol. 2012;50(3):476–486. doi: 10.1016/j.ijbiomac.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weber J, Senior AE. Effects of the inhibitors azide, dicyclohexylcarbodiimide, and aurovertin on nucleotide binding to the three F1-ATPase catalytic sites measured using specific tryptophan probes. J. Biol. Chem. 1998;273(50):33210–33215. doi: 10.1074/jbc.273.50.33210. [DOI] [PubMed] [Google Scholar]
- 102.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000;130(5):1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Firoz A, Malik A, Joplin KH, Ahmad Z, Jha V, Ahmad S. Residue propensities, discrimination and binding site prediction of adenine and guanine phosphates. BMC Biochem. 2011;12:20. doi: 10.1186/1471-2091-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmad Z, Dadi PK, Elord J, Kady IO. Synthesis and evaluation of diphenol aldimines as inhibitors of Escherichia coli ATPase and cell growth. Advances in Biological Chemistry. 2012;2:160–166. [Google Scholar]
- 105.Frasch AC, Cazzulo JJ, Stoppani AO. Solubilization and some properties of the Mg2+-activated adenosine triphosphatase from Trypanosoma cruzi. Comp. Biochem. Physiol. B. 1978;61(2):207–212. doi: 10.1016/0305-0491(78)90162-1. [DOI] [PubMed] [Google Scholar]
- 106.Bowler MW, Montgomery MG, Leslie AG, Walker JE. How azide inhibits ATP hydrolysis by the F-ATPases. Proc. Natl. Acad. Sci. U. S. A. 2006;103(23):8646–8649. doi: 10.1073/pnas.0602915103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zharova TV, Vinogradov AD. Energy-dependent transformation of F0.F1-ATPase in Paracoccus denitrificans plasma membranes. J. Biol. Chem. 2004;279(13):12319–12324. doi: 10.1074/jbc.M311397200. [DOI] [PubMed] [Google Scholar]
- 108.Yoshida M, Allison WS, Esch FS, Futai M. The specificity of carboxyl group modification during the inactivation of the Escherichia coli F1-ATPase with dicyclohexyl[14C]carbodiimide. J. Biol. Chem. 1982;257(17):10033–10037. [PubMed] [Google Scholar]
- 109.Hermolin J, Fillingame RH. H+-ATPase activity of Escherichia coli F1F0 is blocked after reaction of dicyclohexylcarbodiimide with a single proteolipid (subunit c) of the F0 complex. J. Biol. Chem. 1989;264(7):3896–3903. [PubMed] [Google Scholar]
- 110.Tommasino M, Capaldi RA. Effect of dicyclohexylcarbodiimide on unisite and multisite catalytic activities of the adenosinetriphosphatase of Escherichia coli. Biochemistry (Mosc) 1985;24(15):3972–3976. doi: 10.1021/bi00336a026. [DOI] [PubMed] [Google Scholar]
- 111.Lardy H, Reed P, Lin CH. Antibiotic inhibitors of mitochondrial ATP synthesis. Fed Proc. 1975;34(8):1707–1710. [PubMed] [Google Scholar]
- 112.Yarlett N, Lloyd D. Effects of inhibitors on mitochondrial adenosine triphosphatase of Crithidia fasciculata: an unusual pattern of specificities. Mol. Biochem. Parasitol. 1981;3(1):13–17. doi: 10.1016/0166-6851(81)90073-6. [DOI] [PubMed] [Google Scholar]
- 113.Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011;50(3):586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 114.Colin D, Limagne E, Jeanningros S, Jacquel A, Lizard G, Athias A, Gambert P, Hichami A, Latruffe N, Solary E, Delmas D. Endocytosis of resveratrol via lipid rafts and activation of downstream signaling pathways in cancer cells. Cancer Prev Res (Phila) 2011;4(7):1095–1106. doi: 10.1158/1940-6207.CAPR-10-0274. [DOI] [PubMed] [Google Scholar]
- 115.Kim M, Song E. Iron transport by proteoliposomes containing mitochondrial F(1)F(0) ATP synthase isolated from rat heart. Biochimie. 2010;92(4):333–342. doi: 10.1016/j.biochi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 116.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17(14):1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 117.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009;486(2):95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3’-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5(5):1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 119.Sekiya M, Nakamoto RK, Nakanishi-Matsui M, Futai M. Binding of phytopolyphenol piceatannol disrupts beta/gamma subunit interactions and rate-limiting step of steady-state rotational catalysis in Escherichia coli F1-ATPase. J. Biol. Chem. 2012;287(27):22771–22780. doi: 10.1074/jbc.M112.374868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martini S, Bonechi C, Rossi C, Figura N. Increased susceptibility to resveratrol of Helicobacter pylori strains isolated from patients with gastric carcinoma. J. Nat. Prod. 2011;74(10):2257–2260. doi: 10.1021/np100761u. [DOI] [PubMed] [Google Scholar]
- 121.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283(5407):1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 122.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walle Cvd. Peptide and protein delivery. 1st ed. London; San Diego, CA: Academic Press; 2011. [Google Scholar]
- 124.Hancock RE. Peptide antibiotics. Lancet. 1997;349(9049):418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 125.Yeung AT, Gellatly SL, Hancock RE. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011;68(13):2161–2176. doi: 10.1007/s00018-011-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vaara M. New approaches in peptide antibiotics. Curr Opin Pharmacol. 2009;9(5):571–576. doi: 10.1016/j.coph.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 127.Marr AK, Gooderham WJ, Hancock RE. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006;6(5):468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 128.Langel Ü. Introduction to peptides and proteins. Boca Raton: CRC Press/Taylor & Francis; 2010. [Google Scholar]
- 129.Fjell CD, Hiss JA, Hancock REW, Schneider G. Designing antimicribial peptides: form follows function. Nat. Reviews-drug Discov. 2012;11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 130.Okumura K. Cathelicidins-Therpeutic antimicrobial and antitumor host defence peptides for oral diseases. Japanese Dental Sci. Review. 2011;47:67–81. [Google Scholar]
- 131.de la Fuente-Nunez C, Korolik V, Bains M, Nguyen U, Breidenstein EB, Horsman S, Lewenza S, Burrows L, Hancock RE. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012;56(5):2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brandenburg K, Andra J, Garidel P, Gutsmann T. Peptide-based treatment of sepsis. Appl. Microbiol. Biotechnol. 2011;90(3):799–808. doi: 10.1007/s00253-011-3185-7. [DOI] [PubMed] [Google Scholar]
- 133.Achtman AH, Pilat S, Law CW, Lynn DJ, Janot L, Mayer ML, Ma S, Kindrachuk J, Finlay BB, Brinkman FS, Smyth GK, Hancock RE, Schofield L. Effective adjunctive therapy by an innate defense regulatory peptide in a preclinical model of severe malaria. Sci. Transl. Med. 2012;4(135):135ra164. doi: 10.1126/scitranslmed.3003515. [DOI] [PubMed] [Google Scholar]
- 134.Fitzgerald-Hughes D, Devocelle M, Humphreys H. Beyond conventional antibiotics for the future treatment of methicillin-resistant Staphylococcus aureus infections: two novel alternatives. FEMS Immunol. Med. Microbiol. 2012;65(3):399–412. doi: 10.1111/j.1574-695X.2012.00954.x. [DOI] [PubMed] [Google Scholar]
- 135.Butler MS, Cooper MA. Antibiotics in the clinical pipeline in 2011. J. Antibiot. (Tokyo) 2011;64(6):413–425. doi: 10.1038/ja.2011.44. [DOI] [PubMed] [Google Scholar]
- 136.da Rocha Pitta MG, Galdino SL. Development of novel therapeutic drugs in humans from plant antimicrobial peptides. Curr Protein Pept. Sci. 2010;11(3):236–247. doi: 10.2174/138920310791112066. [DOI] [PubMed] [Google Scholar]
- 137.Reddy KV, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int. J. Antimicrob. Agents. 2004;24(6):536–547. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 138.Powers JP, Hancock RE. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24(11):1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 139.Jakubke H-D, Sewald N. Peptides from A to Z : a concise encyclopedia. Weinheim: Wiley-VCH; 2008. [Google Scholar]
- 140.Hultmark D, Steiner H, Rasmuson T, Boman HG. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 141.Steiner H, Hultmark D, Engstrom A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292(5820):246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 142.Boman HG, Nilsson I, Rasmuson B. Inducible antibacterial defence system in Drosophila. Nature. 1972;237(5352):232–235. doi: 10.1038/237232a0. [DOI] [PubMed] [Google Scholar]
- 143.Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta. 2008;1778(2):357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosenfeld Y, Papo N, Shai Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J. Biol. Chem. 2006;281(3):1636–1643. doi: 10.1074/jbc.M504327200. [DOI] [PubMed] [Google Scholar]
- 145.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 146.Mangoni ML, Shai Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim. Biophys. Acta. 2009;1788(8):1610–1619. doi: 10.1016/j.bbamem.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 147.Wang Z, Wang G. APD: the Antimicrobial Peptide Database. Nucleic Acids Res. 2004;32:D590–D592. doi: 10.1093/nar/gkh025. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Piotto SP, Sessa L, Concilio S, Iannelli P. YADAMP: yet another database of antimicrobial peptides. Int. J. Antimicrob. Agents. 2012;39(4):346–351. doi: 10.1016/j.ijantimicag.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 149.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. U. S. A. 1987;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Erspamer V, Falconieri Erspamer G, Cei JM. Active peptides in the skins of two hundred and thirty American amphibian species. Comp. Biochem. Physiol. C. 1986;85(1):125–137. doi: 10.1016/0742-8413(86)90063-0. [DOI] [PubMed] [Google Scholar]
- 151.Erspamer V. Half a century of comparative research on biogenic amines and active peptides in amphibian skin and molluscan tissues. Comp. Biochem. Physiol. C. 1984;79(1):1–7. doi: 10.1016/0742-8413(84)90153-1. [DOI] [PubMed] [Google Scholar]
- 152.Davidson C, Benard MF, Shaffer HB, Parker JM, O’Leary C, Conlon JM, Rollins-Smith LA. Effects of chytrid and carbaryl exposure on survival, growth and skin peptide defenses in foothill yellow-legged frogs. Environ. Sci. Technol. 2007;41(5):1771–1776. doi: 10.1021/es0611947. [DOI] [PubMed] [Google Scholar]
- 153.Amiche M, Ladram A, Nicolas P. A consistent nomenclature of antimicrobial peptides isolated from frogs of the subfamily Phyllomedusinae. Peptides. 2008;29(11):2074–2082. doi: 10.1016/j.peptides.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 154.Mangoni ML. Temporins, anti-infective peptides with expanding properties. Cell. Mol. Life Sci. 2006;63(9):1060–1069. doi: 10.1007/s00018-005-5536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oren Z, Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998;47(6):451–463. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 156.Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. Membrane pores induced by magainin. Biochemistry (Mosc) 1996;35(43):13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- 157.Matsuzaki K, Murase O, Fujii N, Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry (Mosc) 1996;35(35):11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 158.Matsuzaki K, Nakamura A, Murase O, Sugishita K, Fujii N, Miyajima K. Modulation of magainin 2-lipid bilayer interactions by peptide charge. Biochemistry (Mosc) 1997;36(8):2104–2111. doi: 10.1021/bi961870p. [DOI] [PubMed] [Google Scholar]
- 159.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66(4):236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 160.Conlon JM, Al-Ghaferi N, Abraham B, Leprince J. Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods. 2007;42(4):349–357. doi: 10.1016/j.ymeth.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 161.Conlon JM, Kolodziejek J, Nowotny N. Antimicrobial peptides from ranid frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim. Biophys. Acta. 2004;1696(1):1–14. doi: 10.1016/j.bbapap.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 162.Rinaldi AC. Antimicrobial peptides from amphibian skin: an expanding scenario. Curr. Opin. Chem. Biol. 2002;6(6):799–804. doi: 10.1016/s1367-5931(02)00401-5. [DOI] [PubMed] [Google Scholar]
- 163.Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell. Mol. Life Sci. 2005;62(7–8):784–790. doi: 10.1007/s00018-005-4560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 165.Bowie JH, Separovic F, Tyler MJ. Host-defense peptides of Australian anurans. Part 2. Structure, activity, mechanism of action, and evolutionary significance. Peptides. 2012;37(1):174–188. doi: 10.1016/j.peptides.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 166.Mor A, Hani K, Nicolas P. The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J. Biol. Chem. 1994;269(50):31635–31641. [PubMed] [Google Scholar]
- 167.Roise D, Theiler F, Horvath SJ, Tomich JM, Richards JH, Allison DS, Schatz G. Amphiphilicity is essential for mitochondrial presequence function. EMBO J. 1988;7(3):649–653. doi: 10.1002/j.1460-2075.1988.tb02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Terwilliger TC, Eisenberg D. The structure of melittin. I. Structure determination and partial refinement. J. Biol. Chem. 1982;257(11):6010–6015. doi: 10.2210/pdb1mlt/pdb. [DOI] [PubMed] [Google Scholar]
- 169.Roise D, Horvath SJ, Tomich JM, Richards JH, Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Iwadate M, Asakura T, Williamson MP. The structure of the melittin tetramer at different temperatures--an NOE-based calculation with chemical shift refinement. Eur. J. Biochem. 1998;257(2):479–487. doi: 10.1046/j.1432-1327.1998.2570479.x. [DOI] [PubMed] [Google Scholar]
- 171.Kato-Yamada Y, Bald D, Koike M, Motohashi K, Hisabori T, Yoshida M. Epsilon subunit, an endogenous inhibitor of bacterial F(1)-ATPase, also inhibits F(0)F(1)-ATPase. J. Biol. Chem. 1999;274(48):33991–33994. doi: 10.1074/jbc.274.48.33991. [DOI] [PubMed] [Google Scholar]
- 172.Tsunoda SP, Rodgers AJ, Aggeler R, Wilce MC, Yoshida M, Capaldi RA. Large conformational changes of the epsilon subunit in the bacterial F1F0 ATP synthase provide a ratchet action to regulate this rotary motor enzyme. Proc. Natl. Acad. Sci. U. S. A. 2001;98(12):6560–6564. doi: 10.1073/pnas.111128098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Suzuki T, Murakami T, Iino R, Suzuki J, Ono S, Shirakihara Y, Yoshida M. F0F1-ATPase/synthase is geared to the synthesis mode by conformational rearrangement of epsilon subunit in response to proton motive force and ADP/ATP balance. J. Biol. Chem. 2003;278(47):46840–46846. doi: 10.1074/jbc.M307165200. [DOI] [PubMed] [Google Scholar]
- 174.Hara KY, Kato-Yamada Y, Kikuchi Y, Hisabori T, Yoshida M. The role of the betaDELSEED motif of F1-ATPase: propagation of the inhibitory effect of the epsilon subunit. J. Biol. Chem. 2001;276(26):23969–23973. doi: 10.1074/jbc.M009303200. [DOI] [PubMed] [Google Scholar]
- 175.Lebowitz MS, Pedersen PL. Regulation of the mitochondrial ATP synthase/ATPase complex: cDNA cloning, sequence, overexpression, and secondary structural characterization of a functional protein inhibitor. Arch. Biochem. Biophys. 1993;301(1):64–70. doi: 10.1006/abbi.1993.1115. [DOI] [PubMed] [Google Scholar]
- 176.Lebowitz MS, Pedersen PL. Protein inhibitor of mitochondrial ATP synthase: relationship of inhibitor structure to pH-dependent regulation. Arch. Biochem. Biophys. 1996;330(2):342–354. doi: 10.1006/abbi.1996.0261. [DOI] [PubMed] [Google Scholar]
- 177.Van Heeke G, Deforce L, Schnizer RA, Shaw R, Couton JM, Shaw G, Song PS, Schuster SM. Recombinant bovine heart mitochondrial F1-ATPase inhibitor protein: overproduction in Escherichia coli, purification, and structural studies. Biochemistry (Mosc) 1993;32(38):10140–10149. doi: 10.1021/bi00089a033. [DOI] [PubMed] [Google Scholar]
- 178.Cabezon E, Montgomery MG, Leslie AG, Walker JE. The structure of bovine F1-ATPase in complex with its regulatory protein IF1. Nat. Struct. Biol. 2003;10(9):744–750. doi: 10.1038/nsb966. [DOI] [PubMed] [Google Scholar]
- 179.Sayle RA, Milner-White EJ. RASMOL: biomolecular graphics for all. Trends Biochem. Sci. 1995;20(9):374. doi: 10.1016/s0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]
- 180.Subasinghage AP, O’Flynn D, Conlon JM, Hewage CM. Conformational and membrane interaction studies of the antimicrobial peptide alyteserin-1c and its analogue [E4K]alyteserin-1c. Biochim. Biophys. Acta. 2011;1808(8):1975–1984. doi: 10.1016/j.bbamem.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 181.Xiao Y, Dai H, Bommineni YR, Soulages JL, Gong YX, Prakash O, Zhang G. Structure-activity relationships of fowlicidin-1, a cathelicidin antimicrobial peptide in chicken. FEBS J. 2006;273(12):2581–2593. doi: 10.1111/j.1742-4658.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- 182.Raimondo D, Andreotti G, Saint N, Amodeo P, Renzone G, Sanseverino M, Zocchi I, Molle G, Motta A, Scaloni A. A folding-dependent mechanism of antimicrobial peptide resistance to degradation unveiled by solution structure of distinctin. Proc. Natl. Acad. Sci. U. S. A. 2005;102(18):6309–6314. doi: 10.1073/pnas.0409004102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee K, Shin SY, Kim K, Lim SS, Hahm KS, Kim Y. Antibiotic activity and structural analysis of the scorpion-derived antimicrobial peptide IsCT and its analogs. Biochem. Biophys. Res. Commun. 2004;323(2):712–719. doi: 10.1016/j.bbrc.2004.08.144. [DOI] [PubMed] [Google Scholar]
- 184.Bourbigot S, Dodd E, Horwood C, Cumby N, Fardy L, Welch WH, Ramjan Z, Sharma S, Waring AJ, Yeaman MR, Booth V. Antimicrobial peptide RP-1 structure and interactions with anionic versus zwitterionic micelles. Biopolymers. 2009;91(1):1–13. doi: 10.1002/bip.21071. [DOI] [PubMed] [Google Scholar]
- 185.Resende JM, Moraes CM, Prates MV, Cesar A, Almeida FC, Mundim NC, Valente AP, Bemquerer MP, Pilo-Veloso D, Bechinger B. Solution NMR structures of the antimicrobial peptides phylloseptin-1, −2, and −3 and biological activity: the role of charges and hydrogen bonding interactions in stabilizing helix conformations. Peptides. 2008;29(10):1633–1644. doi: 10.1016/j.peptides.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 186.Syvitski RT, Burton I, Mattatall NR, Douglas SE, Jakeman DL. Structural characterization of the antimicrobial peptide pleurocidin from winter flounder. Biochemistry (Mosc) 2005;44(19):7282–7293. doi: 10.1021/bi0504005. [DOI] [PubMed] [Google Scholar]
- 187.Kristiansen PE, Fimland G, Mantzilas D, Nissen-Meyer J. Structure and mode of action of the membrane-permeabilizing antimicrobial peptide pheromone plantaricin A. J. Biol. Chem. 2005;280(24):22945–22950. doi: 10.1074/jbc.M501620200. [DOI] [PubMed] [Google Scholar]
- 188.Respondek M, Madl T, Gobl C, Golser R, Zangger K. Mapping the orientation of helices in micelle-bound peptides by paramagnetic relaxation waves. J. Am. Chem. Soc. 2007;129(16):5228–5234. doi: 10.1021/ja069004f. [DOI] [PubMed] [Google Scholar]
- 189.Landon C, Meudal H, Boulanger N, Bulet P, Vovelle F. Solution structures of stomoxyn and spinigerin, two insect antimicrobial peptides with an alpha-helical conformation. Biopolymers. 2006;81(2):92–103. doi: 10.1002/bip.20370. [DOI] [PubMed] [Google Scholar]
- 190.Wang G, Elliott M, Cogen AL, Ezell EL, Gallo RL, Hancock RE. Structure, dynamics, and antimicrobial and immune modulatory activities of human LL-23 and its single-residue variants mutated on the basis of homologous primate cathelicidins. Biochemistry (Mosc) 2012;51(2):653–664. doi: 10.1021/bi2016266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dubovskii PV, Volynsky PE, Polyansky AA, Karpunin DV, Chupin VV, Efremov RG, Arseniev AS. Three-dimensional structure/hydrophobicity of latarcins specifies their mode of membrane activity. Biochemistry (Mosc) 2008;47(11):3525–3533. doi: 10.1021/bi702203w. [DOI] [PubMed] [Google Scholar]
- 192.Tack BF, Sawai MV, Kearney WR, Robertson AD, Sherman MA, Wang W, Hong T, Boo LM, Wu H, Waring AJ, Lehrer RI. SMAP-29 has two LPS-binding sites and a central hinge. Eur. J. Biochem. 2002;269(4):1181–1189. doi: 10.1046/j.0014-2956.2002.02751.x. [DOI] [PubMed] [Google Scholar]
- 193.Hara T, Kodama H, Kondo M, Wakamatsu K, Takeda A, Tachi T, Matsuzaki K. Effects of peptide dimerization on pore formation: Antiparallel disulfide-dimerized magainin 2 analogue. Biopolymers. 2001;58(4):437–446. doi: 10.1002/1097-0282(20010405)58:4<437::AID-BIP1019>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 194.Campagna S, Saint N, Molle G, Aumelas A. Structure and mechanism of action of the antimicrobial peptide piscidin. Biochemistry (Mosc) 2007;46(7):1771–1778. doi: 10.1021/bi0620297. [DOI] [PubMed] [Google Scholar]