Abstract
The renin-angiotensin system (RAS) plays a major role in liver fibrosis. Recently, a homolog of angiotensin-converting-enzyme 1 (ACE1), termed ACE2, has been identified that appears to be a negative regulator of the RAS by degrading Ang II to Ang1–7. The aim of this study was to characterize the long-term effects of gene deletion of ACE2 in the liver, to define the role of ACE2 in acute and chronic liver disease, and to characterize the role of Ang1–7 in hepatic stellate cell (HSC) activation. Ace2 knockout (KO) mice and wild-type (wt) littermates underwent different models of acute and chronic liver injury. Liver pathology was analyzed by histology, immunohistochemistry, alpha smooth muscle actin (α-SMA) immunoblotting, and quantitative polymerase chain reaction (qPCR). Murine HSCs were isolated by collagenase-pronase-perfusion, and density gradient centrifugation. One-year-old ace2 KO mice spontaneously developed an inflammatory cell infiltration and mild hepatic fibrosis that was prevented by treatment with irbesartan. Ace2 KO mice showed increased liver fibrosis following bile duct ligation for 21 days or chronic carbon tetrachloride (CCl4) treatment. In contrast, ace2 KO mice subjected to acute liver injury models did not differ from wt littermates. Treatment with recombinant ACE2 attenuated experimental fibrosis in the course of cholestatic and toxic liver injury. HSCs express the Ang1–7 receptor Mas and Ang1–7 inhibited Ang II-induced phosphorylation of extracellular signal-regulated kinase (ERK)-1/2 in cultured HSCs.
Conclusion
ACE2 is a key negative regulator of the RAS and functions to limit fibrosis through the degradation of Ang II and the formation of Ang1–7. Whereas loss of ACE2 activity worsens liver fibrosis in chronic liver injury models, administration of recombinant ACE2 shows therapeutic potential.
The renin-angiotensin system (RAS) is a master regulator of human physiology that plays a key role in maintaining blood pressure homeostasis, as well as fluid and salt balance in mammals through coordinated effects on the heart, blood vessels, and kidneys.1 In the classic pathway of the RAS, renin is secreted by juxtaglomerular cells at the renal afferent arterioles and cleaves the liver-derived precursor peptide angiotensinogen into the decapeptide angiotensin I (Ang I). Ang I is further hydrolyzed into the octapeptide Ang II by the angiotensin converting enzyme (ACE), which represents the main effector peptide of the RAS.
The discovery of the ACE homolog ACE2 adds a new complexity to the RAS. ACE2 degrades Ang II to Ang1–7, which exerts effects opposite to those of Ang II through its receptor, Mas.2,3 Accordingly, ace2 knockout (KO) mice display elevated serum and tissue levels of Ang II4 and develop spontaneous glomerulosclerosis with increased deposition of fibrillar collagen, which can be prevented by treatment with an AT1 receptor antagonist.5 Furthermore, ACE2 is up-regulated in response to tissue injury and hypoxia,6,7 protects from severe acute lung failure8 and diabetic kidney injury,9 and prevents cardiac hypertrophy and myocardial fibrosis caused by Ang II infusion.10 Although a recent study reported that ACE2 expression and activity is increased following experimental liver injury and in patients with cirrhosis, the functional and mechanistic role of ACE2 in liver fibrosis remains elusive.7
The role of ACE2 in liver disease is of special interest as several lines of evidence suggest that the RAS also participates in the regulation of hepatic inflammation, tissue remodeling, and fibrosis after liver injury analogous to other organs. The RAS is activated in patients with cirrhosis accompanied by elevated serum Ang I and II levels.11 In addition, an intrahepatic RAS is expressed in livers undergoing tissue remodeling, increasing local levels of Ang II.12 Activated hepatic stellate cells (HSCs) express all components of the RAS including AT1 receptors, rendering them susceptible to the fibrogenic, inflammatory, and oxidative effects of Ang II.13,14 The most compelling evidence supporting a major role for RAS in liver fibrosis is the finding that blocking the generation of Ang II or its binding to AT1 receptors markedly attenuates experimental liver fibrosis in several models of liver injury.15–24
The aim of this study was to characterize the long-term consequences of gene deletion of ACE2 in the liver, to define the role of ACE2 in acute and chronic liver disease, and to analyze the function of Ang1–7 on HSCs.
Materials and Methods
Animal Models of Liver Injury
Ace2-deficient mice have been described.4 Liver fibrosis was induced by bile duct ligation (BDL) or carbon tetrachloride (CCl4) treatment as described.25 To test if recombinant ACE2 attenuates cholestatic or toxic liver injury, fibrosis-susceptible Balb/c mice were subjected to BDL for 14 days or four injections of CCl4 and received recombinant ACE2 (2 mg/kg) daily intraperitoneally starting the first day after initial treatment. As a third model of liver injury mice were subjected to ischemia reperfusion injury.
Cell Isolation, Purification, and Culture
Mouse HSCs were isolated from normal and fibrotic livers.26 Cell fractions of livers were isolated as described.27
RNA isolation and quantitative polymerase chain reaction (qPCR) RNA was extracted and differential gene expression was evaluated by real-time qPCR using commercially available primer-probe sets (Applied Biosystems, Foster City, CA). A detailed list of the primers used will be provided upon request.
Quantification of Hepatic Collagen Content
Collagen content was assessed both by morphometric analysis of Sirius Red and Masson Trichrome staining of liver sections and by hydroxyproline concentration as described.14,25
Measurement of intracellular reactive oxygen species (ROS) was measured using the redox-sensitive dye DCFDA as described.14
Western Blot Analysis
Protein samples of HSCs and liver samples were prepared by standard protocols and protein concentration was determined using a BCA assay (Pierce). Western blotting was performed using standard methods.
Immunohistochemstry
Immunohistochemistry was performed using standard protocols, which will be provided upon request.
Recombinant ACE2
Please see the Supporting Materials and Methods for a detailed description of generation of recombinant ACE2.
Results
Aged Ace2 KO Mice Display Collagen Deposition
Ace2 KO mice spontaneously develop hepatocellular degeneration, accumulation of inflammatory cells, and collagen deposition around portal tracts at 12 months of age (Fig. 1A). Immunoblot analysis showed that several mitogen-activated protein kinase (MAPK) pathways, including the p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK) pathway, were activated in ace2 KO mice compared to age-matched wt littermates (Fig. 1B). Furthermore, qPCR showed that messenger RNA (mRNA) levels of collagen α1(I), alpha smooth muscle actin (αSMA), as well as profibrogenic mediators transforming growth factor beta 1 (TGFβ1) and tissue inhibitor of metalloproteinase 1 (TIMP1) and the proinflammatory cytokine tumor necrosis factor alpha (TNFα) were significantly up-regulated in livers of ace2 KO mice compared to wt littermates (Fig. 1C). Treatment of ace2 KO with the angiotensin receptor blocker (ARB) irbesartan reversed this phenotype (Fig. 1A,C). Thus, genetic disruption of ace2 results in hepatic inflammation and collagen deposition in an Ang II-dependent manner, paralleling our previous results from Ang II-perfused rats.28
Fig. 1.
Spontaneous inflammation and collagen deposition in 1-year-old ace2 KO mice. Livers of 1-year-old ace2 KO mice (n = 10), wt littermates (n = 9), and ace2 KO mice treated with irbesartan (n = 8) were evaluated by Masson Trichrome staining (A). Activation of p38, c-Jun, and ERK was analyzed by immunoblotting using phospho-specific antibodies (B). mRNA expression of collagen α1(I), αSMA, TGFβ1, TIMP1, and TNFα was evaluated by qPCR. Data are presented as mean ± standard error of the mean (SEM); *P < 0.05, **P < 0.01.
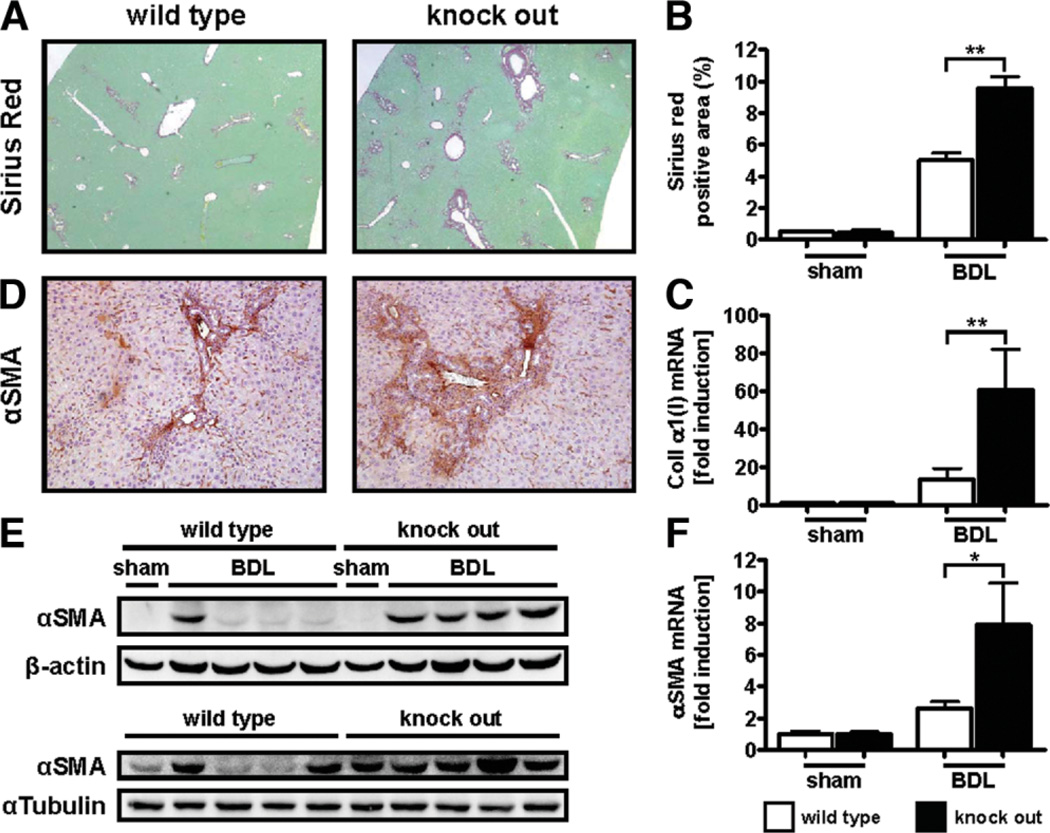
Loss of Ace2 Exacerbates Experimental Biliary Fibrosis
Loss of ace2 results in increased serum and tissue levels of Ang II and accelerates diabetic kidney injury.4,9 To test if ace2 KO mice are susceptible to liver fibrosis, ace2 KO mice were subjected to BDL. Livers of ace2 KO mice displayed a 2-fold increase in fibrillar collagen deposition compared to wt littermates as evaluated by Sirius Red staining (percent positive area: 9.58 ± 2.03 versus 5.01 ± 1.31, P < 0.01; Fig. 2B). These results were further supported by qPCR data showing that ace2 KO mice displayed a significantly higher induction of collagen α1(I) mRNA as compared to their wt littermates (P < 0.01; Fig. 2C). Ace2 KO mice also displayed an increased number of activated myofibroblasts as evaluated by immunohistochemistry (Fig. 2D) and immunoblotting for αSMA (densitometry of αSMA/β-actin or α-tubulin bands: 0.86 ± 0.11 versus 0.51 ± 0.07, P < 0.05; Fig. 2D,E). Similar results were obtained for αSMA mRNA levels as evaluated by qPCR (Fig. 2F). Livers of ace2 KO mice were also characterized by significantly increased inflammatory cell infiltration (290.4 ± 56.57 versus 129.5 ± 7.38; P < 0.05) as evaluated by immunohistochemistry for CD43. We also observed a small increase in staining for HNE in ace2 KO mice compared to wt littermates following bile duct ligation (percent positive area: 4.00 ± 0.39 versus 2.98 ± 0.34, P = 0.0982; Supporting Fig. 1). Ace2 KO mice did not differ from wt littermates with regard to liver injury as evaluated by serum alanine aminotransferase ALT levels, mortality, or body weight loss following BDL (Supporting Fig. 2). We also did not observe any differences between ace2 KO mice and wt littermates in terms of serum bilirubin (59.85 ± 7.99 versus 63.92 ± 5.33, P = 0.6862) or alkaline phosphatase levels (1,075 ± 113.9 versus 898.4 ± 60.65, P = 0.0927; Supporting Fig. 3). Furthermore, we also evaluated bile duct proliferation in ace2 KO mice and wt littermates by immunohistochemistry for CK19 but did not observe any differences (percent positive area: 2.57 ± 0.21 versus 2.67 ± 0.16, P = 0.7061; Supporting Fig. 3). These results indicate that ace2 KO mice are susceptible to cholestatic liver injury independent of liver injury, cholestasis, or compensatory activation and proliferation of bile ducts.
Fig. 2.
Loss of ace2 exacerbates experimental biliary fibrosis. Ace2 KO mice (n = 8) and wt littermates (n = 8) were subjected to BDL for 21 days. Fibrosis was evaluated by morphometric analysis of Sirius Red-stained sections (A,B), by qPCR for collagen α1(I) and αSMA (C,F), by immunohistochemistry (IHC) and immunoblotting for αSMA (D,E). Ace2 KO mice displayed significantly increased deposition of fibrillar collagen, mRNA levels of collagen α1(I) and αSMA, and αSMA-positive cells. αSMA immunoblots for two independent cohorts of mice are shown (E). Data are presented as mean ± SEM; *P < 0.05, **P < 0.01.
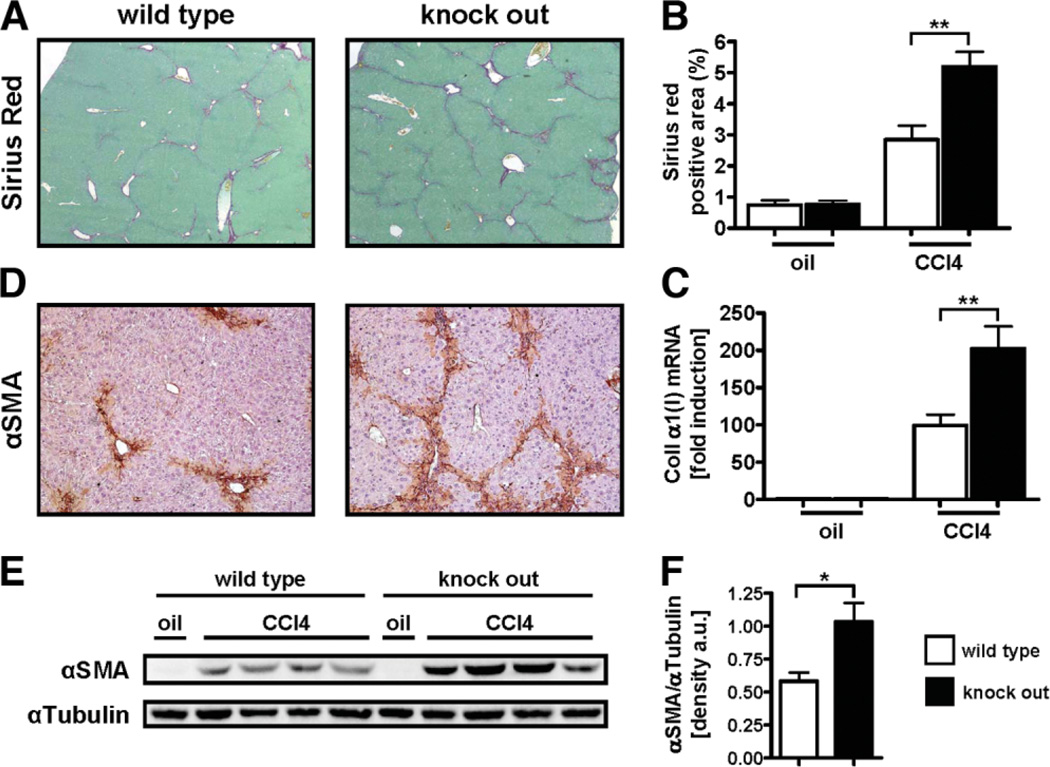
Loss of Ace2 Exacerbates Toxic Liver Injury
As an alternative model of chronic liver injury, ace2 KO mice were subjected to treatment with CCl4. Ace2 KO mice showed significantly more fibrosis compared to wt littermates as evaluated by Sirius Red staining (percent positive area 5.20 ± 1.64 versus 2.85 ± 1.82, P < 0.01; Fig. 3A,B). Immunohistochemistry demonstrated an increased number of αSMA positive myofibroblasts in ace2 KO mice compared to wt littermates (Fig. 3D). Protein levels of αSMA were also increased in ace2 KO mice compared to wt littermates (densitometry of αSMA/αTubulin bands: 1.03 ± 0.14 versus 0.58 ± 0.07, P < 0.05; Fig. 3E,F). Livers of ace2 KO mice were also characterized by increased inflammatory cell infiltration (516.2 ± 27.56 versus 200.2 ± 48.76; P < 0.01) similar to results obtained from ace2 KO mice undergoing BDL. Ace2 KO mice did no differ from wt littermates with regard to liver injury as evaluated by serum ALT levels. No difference with regard to body weight was observed between ace2 KO mice and wt littermates at baseline before the first injection of CCl4. However, ace2 KO mice weighed significantly less over the course of chronic CCl4 treatment and at the time of sacrificing (Supporting Fig. 4). Collectively, these data indicate that loss of ace2 exacerbates liver fibrosis in two different models of chronic liver injury and that ACE2 might be a protective factor in the course of persisting liver injury.
Fig. 3.
Loss of ace2 exacerbates toxic liver injury. Ace2 KO mice (n = 12) and wt littermates (n = 16) received eight injections of CCl4 (0.5 µL/g every 4 days) and were analyzed 48 hours after the last injection. Fibrosis was evaluated by morphometric analysis of Sirius Red-stained sections (A,B), by qPCR for collagen α1(I) (C), by IHC and immunoblotting for αSMA (D,E). Immunoblots were quantified by densitometry (F). Ace2 KO mice displayed significantly increased deposition of fibrillar collagen, mRNA levels of collagen α1(I), and αSMA-positive cells. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01.
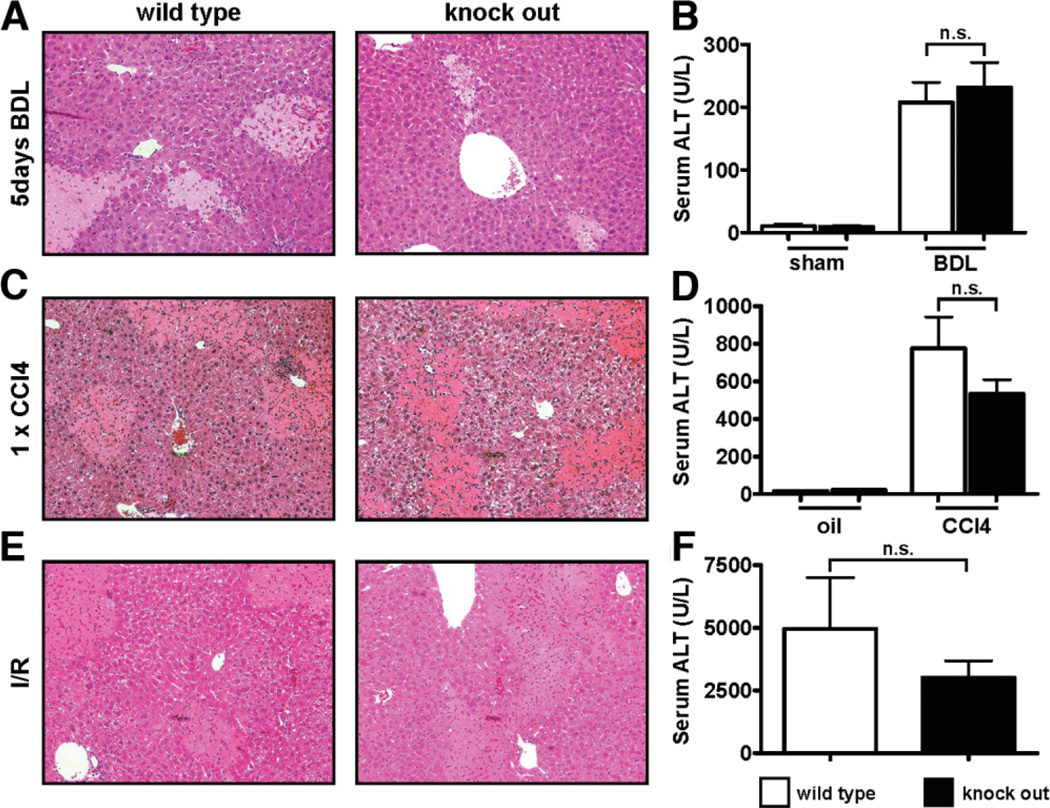
ACE2 Does Not Play a Role in Acute Liver Injury
Loss of ace2 results in increased serum levels of Ang II(4) and predisposes to severe acute lung failure.8 To evaluate the role of ACE2 in acute liver injury, ace2 KO mice were subjected to three different injury models: BDL for 5 days (acute cholestatic injury), single injection of CCl4 (acute toxic injury), and ischemia-reperfusion injury (Fig. 4). No differences with respect to serum ALT levels and histological extent of necrosis were observed between ace2 KO mice and wt littermates in any of the models used. Ace2 KO mice also did not differ from wt littermates with respect to collagen α1(I), αSMA, TIMP1, TGFβ1 mRNA levels, or mRNA levels of proinflammatory mediators such as monocyte chemoattractant protein 1 (MCP-1) or TNFα after BDL (Supporting Fig. 5) or CCl4 treatment (Supporting Fig. 6). Thus, endogenous ACE2 activity plays a role in chronic but not acute liver injury.
Fig. 4.
Ace2 does not play a role in acute hepatocellular injury. Ace2 KO mice and wt littermates were subjected to different models of acute liver injury: BDL for 5 days (A,B), single injection of CCl4 (C,D), and ischemia-reperfusion injury (E,F). No differences were observed between ace2 KO mice and wt littermates as evaluated by hematoxylin and eosin staining (A,C,E) and serum ALT levels (B,D,F). Data are presented as mean ± SEM; n.s. indicates not significant.
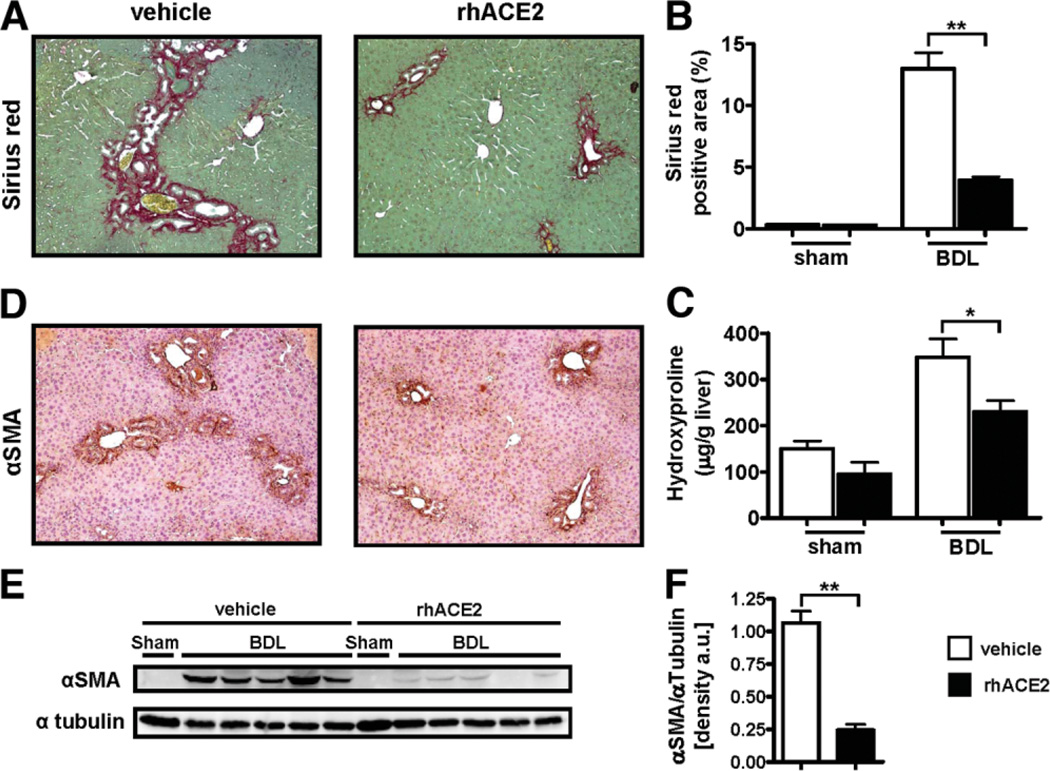
Recombinant ACE2 Attenuates Experimental Biliary Fibrosis
To test whether recombinant ACE2 attenuates experimental fibrosis, fibrosis-susceptible Balb/c mice were subjected to BDL. Mice treated with recombinant human ACE2 (rhACE2) showed significantly reduced liver fibrosis as evaluated by Sirius Red staining (12.99 ± 4.08 versus 3.93 ± 0.92% positive area, P < 0.01; Fig. 5A,B) and determination of liver hydroxyproline content (347.7 ± 119.1 versus 230.4 ± 78.6 µg/g liver, P < 0.05; Fig. 5C). Immunohistochemistry and immunoblot analysis demonstrated that mice treated with rhACE2 also showed reduced expression of αSMA compared to vehicle-treated mice following BDL (densitometry of αSMA/αTubulin bands: 0.24 ± 0.04 versus 1.07 ± 0.09, P < 0.01; Fig. 5D–F). Mice treated with rhACE2 did not differ from vehicle-treated controls with regard to serum ALT levels (264.1 ± 45.67 versus 471.6 ± 72.30, P = 0.1206), mortality, or body weight loss following BDL (Supporting Fig. 7). Thus, treatment with recombinant ACE2 attenuates biliary fibrosis.
Fig. 5.
Recombinant ACE2 attenuates experimental biliary fibrosis. Balb/c mice were subjected to BDL for 14 days. Treatment with rhACE2 (2 mg/kg, daily, intraperitoneally) or vehicle was started the first day after surgery. Fibrosis was evaluated by morphometric analysis of Sirius Red-stained sections (A,B), by hepatic hydroxyproline content, and by IHC and immunoblotting for αSMA (D,E). Immunoblots were quantified by densitometry (F). Mice receiving rhACE2 (n = 11) showed significantly reduced deposition of fibrillar collagen, hepatic hydroxyproline content, and expression of αSMA as compared to the vehicle-treated group (n = 10). Data are presented as mean ± SEM; *P < 0.05, **P < 0.01.
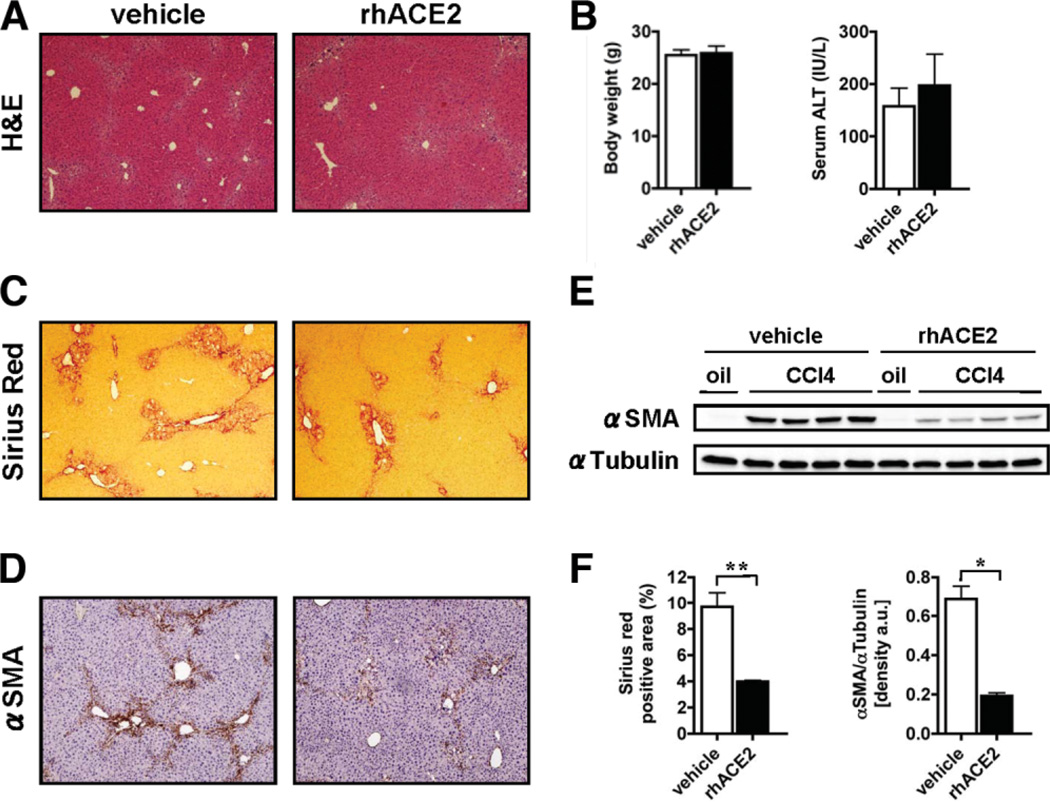
As an alternative model of chronic liver injury, Balb/c mice were subjected to CCl4 treatment. In parallel to the results obtained from BDL, mice receiving rhACE2 showed significantly reduced fibrosis compared to control mice as evaluated by Sirius Red staining (percent positive area: 3.99 ± 0.09 versus 9.70 ± 1.12, P < 0.01; Fig. 6C,F). Immunohistochemistry and immunoblot analysis furthermore demonstrated that mice treated with rhACE2 also showed reduced expression of αSMA compared to control mice (densitometry ofαSMA/αTubulin bands: 0.19 ± 0.02 versus 0.69 ± 0.06, P < 0.05; Fig. 6D–F). Mice treated with rhACE2 did not differ from vehicle-treated controls with regard to serum ALT levels (197.2 ± 59.90 versus 157.6 ± 34.69, P = 0.5830) or body weight (Fig. 6A,B).
Fig. 6.
Recombinant ACE2 attenuates fibrosis caused by toxic liver injury. Balb/c mice received four injections of CCl4. Treatment with rhACE2 (2 mg/kg, daily, intraperitoneally) or vehicle was started the first day after the first injection of CCl4. Fibrosis was evaluated by morphometric analysis of Sirius Red-stained sections (C,F), by IHC, and immunoblotting for αSMA (D,E). Immunoblots were quantified by densitometry (F). Mice receiving rhACE2 (n = 5) showed significantly reduced deposition of fibrillar collagen and expression of αSMA as compared to control mice (n = 5). Data are presented as mean ± SEM; *P < 0.05, **P < 0.01.
Ang1–7 Interferes with ERK Phosphorylation Induced by Ang II
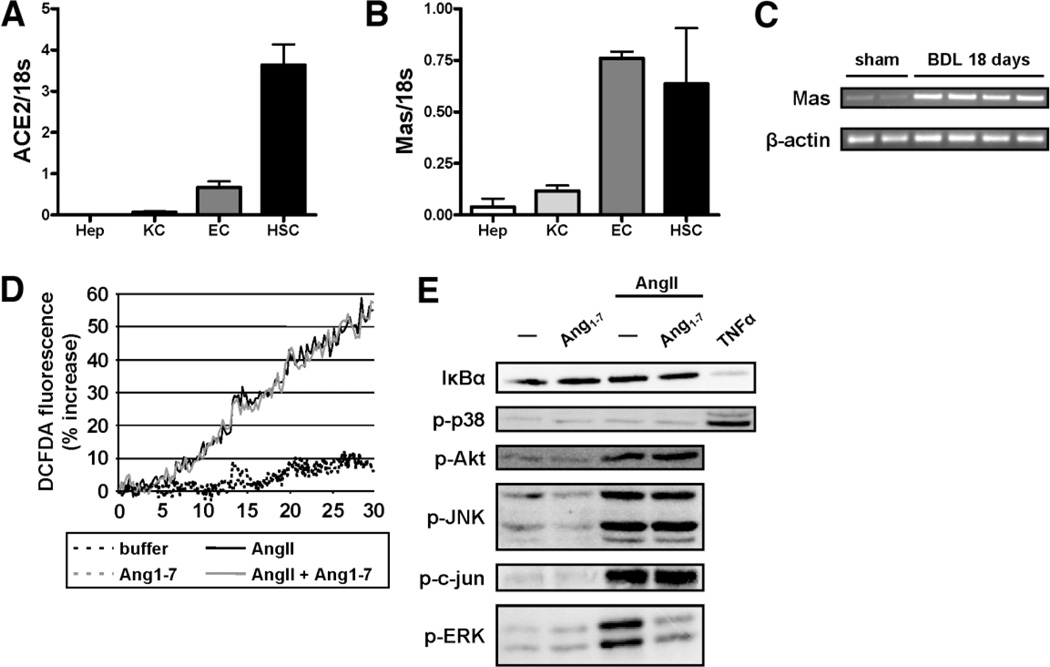
Our data indicate that ACE2 is a physiological regulator of chronic liver injury and that administration of ACE2 attenuates liver injury. We therefore asked if this effect is mediated by the degradation of Ang II or if Ang1–7 generated by ACE2 represents a biologic effective peptide in HSC activation. To address which cells in the liver express ACE2 and the Ang1–7 receptor Mas, we isolated hepatocytes, Kupffer cells (KCs), endothelial cells (ECs), and HSCs and analyzed mRNA expression of all major components of the RAS (ACE1 and ACE2, AT1a, and Mas) by qPCR. Although ACE1 was mainly expressed by EC, but also by KC and HSCs, HSCs represent the main cell population in the liver expressing mRNAs for the AT1a receptor as well as ACE2 (Fig. 7A; Supporting Fig. 8). The Ang1–7 receptor Mas was expressed by both ECs and HSCs (Fig. 7B), and Mas expression increased following BDL (Fig. 7C). In vivo activated HSCs isolated from mice subjected to chronic CCl4 treatment or BDL also expressed Mas, suggesting that HSCs are a target of Ang1–7 (Supporting Figs. 9, 10).
Fig. 7.
Ang1–7 inhibits ERK phosphorylation induced by AngII in HSC. mRNA was isolated from hepatocytes (Hep), KCs, ECs, and HSCs and expression of ace2 and Mas was evaluated by qPCR (A,B). RT-PCR for Mas was performed using mRNA isolated from whole liver from balb/c mice subjected to BDL for 18 days (C). Mouse HSCs were cultured for 5 days and then serum starved for 24 hours. ROS production was evaluated by DCFD fluorescence of mouse HSCs stimulated with AngII (black solid line) and preincubated with Ang1–7 (gray solid line) (D). Cells were stimulated with AngII (10−7 M) in the presence or absence of Ang1–7 (10−7 M) for 15 minutes. Activation of signaling pathways was analyzed by immunoblotting for IκBα and using phospho-specific antibodies for p38, Akt, JNK, c-Jun, and ERK. Equal loading was evaluated by Ponceau S staining (not shown). Data are representative of three independent experiments.
Ang II mediates its effect on HSC activation through nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase-mediated generation of ROS.14 To analyze if Ang1–7 interferes with this early event of HSC activation, we performed DCFDA measurement of HSC stimulated with Ang II. Although Ang II led to a robust increase in ROS formation, preincubation with Ang1–7 had no effect on spontaneous ROS formation nor on ROS formation by Ang II (Fig. 7D). Ang II has been reported to activate the PI3 kinase signaling pathway as well as several MAPK pathways and the nuclear factor kappa B (NFκB) pathway. Interestingly, preincubation with Ang1–7 reduced Ang II induced ERK phosphorylation (Fig. 7E). We also tested if preincubation with Ang1–7 interferes with Ang II or platelet-derived growth factor (PDGF)-stimulated proliferation of primary mouse HSC as well as immortalized human HSC. Ang1–7 did not abrogate HSC proliferation induced by Ang II or PDGF (data not shown). These data suggest that Ang1–7 does not interfere with Ang II-mediated HSC activation in a global manner, but rather specifically abrogates ERK activation.
Discussion
RAS is a key regulator of the human body, maintaining and controlling multiple essential functions such as blood pressure homeostasis and fluid and salt balance, cell differentiation, and inflammation. The need for a tightly controlled regulation of this system is highlighted by the detrimental role of RAS in tissue remodeling and scarring observed in multiple organs, including the liver. The recent discovery of ACE2 has provided significant insight into how this system is turned off.
Our study provides evidence that ACE2 serves as an endogenous negative regulator and therefore as a “brake” of RAS in the course of chronic liver injury. Using two complementary models of liver injury, we show that genetic disruption of ace2 exacerbates liver fibrosis (Figs. 2, 3). No differences in mortality and liver injury were observed between ace2 KO mice and wt littermates, suggesting that increased fibrosis in ace2 KO mice represent a direct consequence of genetic disruption of ace2 and cannot be attributed to increased liver injury in these mice.
In contrast, when we challenged ace2 KO mice with a single injection of CCl4 or BDL for only 5 days (reflecting acute toxic and cholestatic liver injury), we did not find any difference by means of histology, serum ALT levels, or mRNA levels of markers for fibrosis or inflammation between KO mice and wt littermates (Fig. 4; Supporting Figs. 5, 6). In contrast to the heart, the lungs, and the kidneys, baseline expression of ACE2 is low in the liver3 (and data not shown), explaining why the genetic deletion of ACE2 does not have a detrimental consequence in acute liver injury as compared to acute lung injury.8
In the liver the consequences of loss of ace2 become apparent only after chronic injury when ACE2 expression and activity increases. Indeed, we observed that mRNA levels of ACE2 increase only minimally after 3 days of BDL or one injection of CCl4. In contrast, ACE1 is already ≈7-fold increased after 3 days of BDL and ≈4-fold increased after a single injection of CCl4. However, with persistent liver injury ACE2 mRNA levels and ACE2 activity gradually increase over time, as reported by others.29 We speculate that ACE1 activity and formation of Ang II predominates acute phases of liver injury and that ACE2 is up-regulated in chronic liver injury in order to limit fibrogenesis through the degradation of Ang II, thereby providing negative regulation for the RAS. To our knowledge, ACE2 represents the first negative regulator of a hormone/peptide system to be discovered in liver fibrosis.
Based on our data, we predicted that administration of recombinant ACE2 would have therapeutic potential for the treatment of liver fibrosis. Administration of rhACE2 reduced all critical features of liver fibrosis in a cholestatic liver injury model in mice: reduced Sirius Red-positive area, reduced hydroxyproline content, and reduced activated/αSMA-positive myofibroblasts (Fig. 5). Similar results were obtained for mice subjected to CCl4 treatment and treated with rhACE2 (Fig. 6). This intervention might be especially beneficial during early phases of liver injury when endogenous ACE2 levels in the liver are low. In addition to the degradation of fibrogenic Ang II, part of this protective effect of ACE2 might come through the generation of Ang1–7.
Ang1–7 is a biologically active peptide and a potent negative regulator of the RAS: Ang1–7 prevents Ang II-induced cardiac remodeling and attenuates ventricular dysfunction and remodeling after myocardial infarction.30,31 Furthermore, pretreatment of cardiac fibroblasts with Ang1–7 abrogates Ang II-induced increases in collagen synthesis and mRNA expression of endothelin-1.32 Ang1–7 also inhibits serum-stimulated phosphorylation of ERK1/2 in cardiac myocytes mediated by the Mas receptor.33 In addition, Ang1–7 also functions as an endogenous ACE1 inhibitor, while being itself inactivated by the same enzyme.34
Interestingly, the AT1 receptor and the Mas receptor are often coexpressed in cells, including HSCs (Fig. 7B). Ang II is known to activate a large variety of signaling pathways through the AT1 receptor on HSC, most of them being dependent on the activation of the NADPH oxidase complex.14 It is generally believed that Ang II binds to its receptor, which leads to the activation of the PI3 kinase complex, which in turn leads to the generation of ROS by NADPH oxidase. We did not find any effect of Ang1–7 on Ang II-induced Akt phosphorylation, ROS production, or HSC proliferation (Fig. 7D and data not shown). However, Ang1–7 specifically interfered with phosphorylation of ERK1/2 by Ang II (Fig. 7E), suggesting that Ang1–7 might counterbalance the effects of Ang II on HSCs in a more specific way than in proximal tubular cells of the kidney.35 A recent study suggests that Ang1–7 might also play a role in liver fibrosis. Pharmacological inhibition of Ang1–7 aggravated liver fibrosis in rats undergoing BDL with a significant elevation in hepatic hydroxyproline content and TGFβ1 levels.36 Part of this effect might be attributable to the direct inhibitory effects of Ang1–7 on HSCs. The precise mechanisms by which Ang1–7 interferes with HSC activation and the consequences of abrogated ERK phosphorylation remain to be determined. Furthermore, it remains elusive if Ang1–7 interferes with HSC activation by other profibrogenic stimuli like TGFβ1, PDGF, endothelin, or leptin. Future studies are needed to address this question.
In addition to serving as an inducible negative regulator of the RAS by being up-regulated after tissue injury in various organs, ACE2 also appears to have a constitutive function in the RAS that becomes apparent upon aging. Genetic ablation of ace2 in mice results in a cardiac contractility defect, which appears to be caused by increased Ang II levels. Accordingly, ace1/ace2 double KO mice are rescued from this phenotype.4 Ace2 KO mice also develop glomerulosclerosis associated with increased deposition of types I and III collagen and a marked increase in renal lipid peroxidation product formation.5 Pharmacological AT1 receptor blockade prevented both the kidney and the heart phenotype.5,37 Livers from 1-year-old ace2 KO mice displayed a very similar phenotype: spontaneous collagen deposition, inflammatory cell infiltration, up-regulation of markers of fibrosis, and inflammation including collagen, αSMA, TIMP1, and TNF and activation of several MAPK pathways (Fig. 1). We have previously reported that infusion of Ang II in rats causes a very similar phenotype28 and also exacerbates liver fibrosis in bile duct-ligated rats.38 Treatment with an ARB was able to prevent histological features and the increases in fibrosis and inflammation markers in ace2 KO mice, suggesting that increased levels of Ang II account for these findings (Fig. 1).
Finally, ACE2 also functions as a protease for a variety of other peptide mediators including des-Arg9-bradykinin, apelin-13, and apelin-36, neurotensin, dynorphin A and casamorphin.39 Some of these mediators have been reported to be involved in tissue injury and wound healing.40–43 Infusion of bradykinin attenuates liver fibrosis in rats treated with CCl4,44 and treatment of cirrhotic rats with an apelin receptor inhibitor improves liver fibrosis.45 This supports the concept that the RAS and especially its negative regulator ACE2 control a number of different peptide systems involved in liver fibrosis. In this way, ACE2 could serve as a central negative regulator of different peptide systems involved in fibrogenesis, inflammation, angiogenesis, and tissue injury. This might be of special interest with regard to the therapeutic benefits of ACE2 compared to classical ACE inhibitors or ARB. Therefore, ACE2 should be a more effective inhibitor of fibrosis than the previously described ACE inhibitors, ARB or Ang1–7.
Our study provides compelling evidence that up-regulation of ACE2 limits fibrosis and therefore represents a physiological response of the liver that minimizes the results from chronic activation of the RAS. Recombinant ACE2 might represent a novel therapeutic option for the treatment of human liver disease.
Supplementary Material
Acknowledgments
The authors thank Prof. Katsumi Miyai for help and expertise in evaluating sections from aged ace2 KO mice.
C.H.Ö. is a recipient of an Erwin Schrödinger research fellowship kindly provided by the Austrian Science Fund (FWF) and the Irwin A. Arias postdoctoral research fellowship kindly provided by the American Liver Foundation and is now supported by a Fellowship-to-Faculty Transition Award kindly provided by the American Gastroenterology Association. Research in the laboratory of D.A.B. is supported by grants from the National Institutes of Health (NIH). J.M.P. is supported by the Institute of Molecular Biotechnology (IMBA), the Austrian National Bank, the Austrian Ministry of Science and Education, and by a European Union (EU) network grant (EuGeneHeart).
Abbreviations
- α-SMA
alpha smooth muscle actin
- ACE
angiotensin-converting enzyme
- ALT
alanine aminotransferase
- Ang
angiotensin
- ARB
angiotensin receptor blocker
- BDL
bile duct ligation
- EC
endothelial cells
- ERK
extracellular signal-regulated kinase
- HSE
hepatic stellate cell
- JNK
c-Jun N-terminal kinase
- KC
Kupffer cells
- KO
knockout
- MAPK
mitogen-activated protein kinase
- PDGF
platelet-derived growth factor
- qPCR
quantitative polymerase chain reaction
- RAS
renin-angiotensin system
- ROS
reactive oxygen species
- TGFβ1
transforming growth factor beta 1
- TIMP1
tissue inhibitor of metalloproteinase 1
- TNFα
tumor necrosis factor alpha
Footnotes
Potential conflict of interest: M.S. is an employee of Apeiron Biologics. M.S. and J.M.P. hold shares in Apeiron Biologics. Apeiron Biologics is a company that attempts to develop recombinant ACE2 for treatment in humans. All other authors have nothing to disclose.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Inagami T. The renin-angiotensin system. Essays Biochem. 1994;28:147–164. [PubMed] [Google Scholar]
- 2.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 3.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 4.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 5.Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, et al. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168:1808–1820. doi: 10.2353/ajpath.2006.051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. discussion 322–364. [DOI] [PubMed] [Google Scholar]
- 7.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, et al. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, et al. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 11.Arroyo V, Planas R, Gaya J, Deulofeu R, Rimola A, Perez-Ayuso RM, et al. Sympathetic nervous activity, renin-angiotensin system and renal excretion of prostaglandin E2 in cirrhosis. Relationship to functional renal failure and sodium and water excretion. Eur J Clin Invest. 1983;13:271–278. doi: 10.1111/j.1365-2362.1983.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 12.Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667–1676. doi: 10.1053/gast.2002.36561. [DOI] [PubMed] [Google Scholar]
- 13.Bataller R, Sancho-Bru P, Gines P, Lora JM, Al-Garawi A, Sole M, et al. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117–125. doi: 10.1016/s0016-5085(03)00695-4. [DOI] [PubMed] [Google Scholar]
- 14.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurikawa N, Suga M, Kuroda S, Yamada K, Ishikawa H. An angiotensin II type 1 receptor antagonist, olmesartan medoxomil, improves experimental liver fibrosis by suppression of proliferation and collagen synthesis in activated hepatic stellate cells. Br J Pharmacol. 2003;139:1085–1094. doi: 10.1038/sj.bjp.0705339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, et al. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology. 2001;121:148–155. doi: 10.1053/gast.2001.25480. [DOI] [PubMed] [Google Scholar]
- 17.Ohishi T, Saito H, Tsusaka K, Toda K, Inagaki H, Hamada Y, et al. Anti-fibrogenic effect of an angiotensin converting enzyme inhibitor on chronic carbon tetrachloride-induced hepatic fibrosis in rats. Hepatol Res. 2001;21:147–158. doi: 10.1016/s1386-6346(01)00102-4. [DOI] [PubMed] [Google Scholar]
- 18.Paizis G, Gilbert RE, Cooper ME, Murthi P, Schembri JM, Wu LL, et al. Effect of angiotensin II type 1 receptor blockade on experimental hepatic fibrogenesis. J Hepatol. 2001;35:376–385. doi: 10.1016/s0168-8278(01)00146-5. [DOI] [PubMed] [Google Scholar]
- 19.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, et al. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology. 2001;34:745–750. doi: 10.1053/jhep.2001.28231. [DOI] [PubMed] [Google Scholar]
- 20.Wei HS, Lu HM, Li DG, Zhan YT, Wang ZR, Huang X, et al. The regulatory role of AT 1 receptor on activated HSCs in hepatic fibrogenesis: effects of RAS inhibitors on hepatic fibrosis induced by CCl(4) World J Gastroenterol. 2000;6:824–828. doi: 10.3748/wjg.v6.i6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei HS, Li DG, Lu HM, Zhan YT, Wang ZR, Huang X, et al. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl(4) World J Gastroenterol. 2000;6:540–545. doi: 10.3748/wjg.v6.i4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuncer I, Ozbek H, Ugras S, Bayram I. Anti-fibrogenic effects of captopril and candesartan cilexetil on the hepatic fibrosis development in rat. The effect of AT1-R blocker on the hepatic fibrosis. Exp Toxicol Pathol. 2003;55:159–166. doi: 10.1078/0940-2993-00309. [DOI] [PubMed] [Google Scholar]
- 23.Ramalho LN, Ramalho FS, Zucoloto S, Castro-e-Silva O, Jr, Correa FM, Elias J, Jr, et al. Effect of losartan, an angiotensin II antagonist, on secondary biliary cirrhosis. Hepatogastroenterology. 2002;49:1499–1502. [PubMed] [Google Scholar]
- 24.Hirose A, Ono M, Saibara T, Nozaki Y, Masuda K, Yoshioka A, et al. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology. 2007;45:1375–1381. doi: 10.1002/hep.21638. [DOI] [PubMed] [Google Scholar]
- 25.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 26.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, et al. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 28.Bataller R, Gabele E, Schoonhoven R, Morris T, Lehnert M, Yang L, et al. Prolonged infusion of angiotensin II into normal rats induces stellate cell activation and proinflammatory events in liver. Am J Physiol Gastrointest Liver Physiol. 2003;285:G642–G651. doi: 10.1152/ajpgi.00037.2003. [DOI] [PubMed] [Google Scholar]
- 29.Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, et al. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1–7) levels in experimental biliary fibrosis. J Hepatol. 2007;47:387–395. doi: 10.1016/j.jhep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, et al. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7) Am J Physiol Heart Circ Physiol. 2007;292:H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 31.Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, et al. Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105:1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 32.Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, et al. Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol. 2005;289:H2356–H2363. doi: 10.1152/ajpheart.00317.2005. [DOI] [PubMed] [Google Scholar]
- 33.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the Mas receptor. Am J Physiol Heart Circ Physiol. 2005;289:H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- 34.Roks AJ, van Geel PP, Pinto YM, Buikema H, Henning RH, de Zeeuw D, et al. Angiotensin-(1–7) is a modulator of the human renin-angiotensin system. Hypertension. 1999;34:296–301. doi: 10.1161/01.hyp.34.2.296. [DOI] [PubMed] [Google Scholar]
- 35.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69:2212–2218. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- 36.Pereira RM, Dos Santos RA, Teixeira MM, Leite VH, Costa LP, da Costa Dias FL, et al. The renin-angiotensin system in a rat model of hepatic fibrosis: evidence for a protective role of angiotensin-(1–7) J Hepatol. 2007;46:674–681. doi: 10.1016/j.jhep.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, et al. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75:29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Bataller R, Gabele E, Parsons CJ, Morris T, Yang L, Schoonhoven R, et al. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile ductligated rats. Hepatology. 2005;41:1046–1055. doi: 10.1002/hep.20665. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson U, Danilczyk U, Penninger JM. Just the beginning: novel functions for angiotensin-converting enzymes. Curr Biol. 2002;12:R745–R752. doi: 10.1016/s0960-9822(02)01255-1. [DOI] [PubMed] [Google Scholar]
- 40.Souza DG, Lomez ES, Pinho V, Pesquero JB, Bader M, Pesquero JL, et al. Role of bradykinin B2 and B1 receptors in the local, remote, and systemic inflammatory responses that follow intestinal ischemia and reperfusion injury. J Immunol. 2004;172:2542–2548. doi: 10.4049/jimmunol.172.4.2542. [DOI] [PubMed] [Google Scholar]
- 41.Daviaud D, Boucher J, Gesta S, Dray C, Guigne C, Quilliot D, et al. TNFalpha up-regulates apelin expression in human and mouse adipose tissue. FASEB J. 2006;20:1528–1530. doi: 10.1096/fj.05-5243fje. [DOI] [PubMed] [Google Scholar]
- 42.Castagliuolo I, Wang CC, Valenick L, Pasha A, Nikulasson S, Carraway RE, et al. Neurotensin is a proinflammatory neuropeptide in colonic inflammation. J Clin Invest. 1999;103:843–849. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal. 2005;17:415–426. doi: 10.1016/j.cellsig.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Sancho-Bru P, Bataller R, Fernandez-Varo G, Moreno M, Ramalho LN, Colmenero J, et al. Bradykinin attenuates hepatocellular damage and fibrosis in rats with chronic liver injury. Gastroenterology. 2007;133:2019–2028. doi: 10.1053/j.gastro.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Principe A, Melgar-Lesmes P, Fernandez-Varo G, del Arbol LR, Ros J, Morales-Ruiz M, et al. The hepatic apelin system: a new therapeutic target for liver disease. Hepatology. 2008;48:1193–1201. doi: 10.1002/hep.22467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.