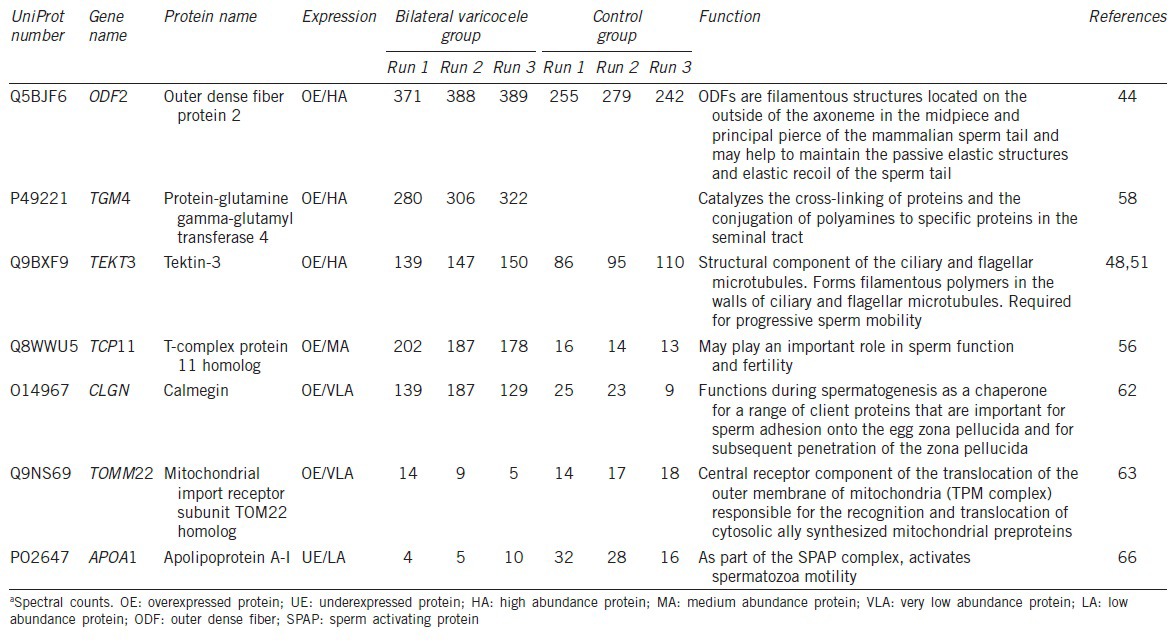
Abstract
Among infertile men, a diagnosis of unilateral varicocele is made in 90% of varicocele cases and bilateral in the remaining varicocele cases. However, there are reports of under-diagnosis of bilateral varicocele among infertile men and that its prevalence is greater than 10%. In this prospective study, we aimed to examine the differentially expressed proteins (DEP) extracted from spermatozoa cells of patients with bilateral varicocele and fertile donors. Subjects consisted of 17 men diagnosed with bilateral varicocele and 10 proven fertile men as healthy controls. Using the LTQ-orbitrap elite hybrid mass spectrometry system, proteomic analysis was done on pooled samples from 3 patients with bilateral varicocele and 5 fertile men. From these samples, 73 DEP were identified of which 58 proteins were differentially expressed, with 7 proteins unique to the bilateral varicocele group and 8 proteins to the fertile control group. Majority of the DEPs were observed to be associated with metabolic processes, stress responses, oxidoreductase activity, enzyme regulation, and immune system processes. Seven DEP were involved in sperm function such as capacitation, motility, and sperm-zona binding. Proteins TEKT3 and TCP11 were validated by Western blot analysis and may serve as potential biomarkers for bilateral varicocele. In this study, we have demonstrated for the first time the presence of DEP and identified proteins with distinct reproductive functions which are altered in infertile men with bilateral varicocele. Functional proteomic profiling provides insight into the mechanistic implications of bilateral varicocele-associated male infertility.
Keywords: bilateral varicocele, bioinformatics, male infertility, proteomics, spermatozoa proteins, varicocele
INTRODUCTION
Varicoceles are the abnormal dilations and tortuosity of the pampiniform venous plexus within the spermatic cord.1 Varicoceles are diagnosed in about 15% of the adult male population and are present in almost 40% of infertile males.2 The prevalence of varicocele is 35% in men with primary infertility but increases to 81% in men with secondary infertility, implicating varicocele as a cause of progressive decline in fertility.3
Among adult males, approximately 90% of varicoceles occur unilaterally on the left side (only 0.4% occurs on the right side); while 10% occurs bilaterally.4 In infertile patients, a palpable unilateral varicocele is diagnosed on the left side in 85% to 90% of cases. However, a palpable right varicocele is detected mostly in cases of bilateral varicocele in the remaining 10% of infertile patients, and is hardly discovered on its own.5 Thus, it seems that the incidence of bilateral varicocele is underestimated among infertile men.6,7
Despite extensive research, the exact cause of infertility in men having varicocele remains unknown. Among possible contributory mechanisms to the pathogenesis of varicocele and potential factors that could affect testicular function are: (1) venous stasis leading to testicular hypoxia, (2) testicular heat stress, (3) oxidative stress, (4) hormonal imbalances, and (5) exogenous toxicants.1,8,9 Molecular and genetic differences among men with varicoceles may determine whether or not varicocele is associated with male infertility.1
Men with varicocele may retain normal fertility or may present with infertility. Additionally, semen analysis of infertile men with varicocele could result in normal or abnormal sperm parameters, the reasons of which remain unclear.10 Varicocele repair is associated with improved semen parameters and has resulted in successful pregnancies.11 Cases of unilateral varicocelectomy yielded significantly higher spontaneous pregnancy rates compared to bilateral varicocelectomy (49% vs 39% respectively).12 However, varicocele repair showed greater significant improvement of semen parameters in patients with bilateral varicocelectomy compared to unilateral varicocelectomy.12 Patients undergoing unilateral varicocelectomy may obtain sub-optimal results when a contralateral disease is present.11 While surgical repair eliminates varicocele in the majority of the cases, its impact on infertility remains unclear.
Proteomics involves the large-scale analysis of proteins, and it contributes greatly to our understanding of gene function in the postgenomic era. Proteomic studies have examined the sperm proteome in both normozoospermic and abnormal spermatozoa.13,14,15 Prior studies on the human sperm or seminal plasma proteome have either compared the proteomic profiles of adult or adolescent varicocele patients respectively with those of healthy fertile males,16,17,18 or investigated the sperm or seminal plasma proteome before and after varicocelectomy.19,20 Selected proteins highlighted in these studies may be the key proteins that could help predict the success of varicocele repair and/or shed some light on the main proteins involved in fertilization.17
To date, there are no proteomic studies comparing the sperm protein profile of infertile men with bilateral varicocele with that of fertile men. Our current preliminary study uniquely examines bilateral varicocele-related male infertility and how it may affect the differential expression of key spermatozoal proteins. We have attempted to link these findings with testicular dysfunction and impaired male fertility potential in the study subjects. We also validated two differentially expressed proteins (DEP) which have reproductive function. We have attempted to identify potential biomarkers of bilateral varicocele in order to determine the severity of the disease as this may help clinicians in the effective management of varicocele.
MATERIALS AND METHODS
Patient enrollment and sample collection
Following approval of the study by the Institutional Review Board of Cleveland Clinic, semen samples were collected from infertile patients with bilateral varicocele (n = 17) seeking investigation for fertility and healthy male volunteers with proven fertility (n = 10). All patients had provided written consent for enrollment in this prospective study. Varicocele was diagnosed by clinical analysis, including scrotal palpation in a temperature-controlled room (23.8°C) with adequate illumination. Varicocele was graded according to the criteria of Dubin and Amelar, as described earlier.21
Inclusion-exclusion criteria
Patients between 20 and 40 years of age who were referred to the Glickman Urology and Kidney Institute of Cleveland Clinic from March 2012 to April 2014 were included in this study. All patients included in the study were nonsmokers with a normal body mass index. None of these patients had been exposed to environmental stressors, including radiation or chemicals. All female partners of these infertile men had undergone gynecologic evaluation and had normal results on a fertility workup. Patients were excluded from the study if they had a recurring fever during the 90-day period prior to semen analysis with evidence of urogenital infection, or any other reproductive or urological diseases diagnosed by andrological examination, genetic defects, and/or occupational exposure to spermatogenetic-toxic chemicals. Similarly, men with azoospermia and a sperm concentration of <10 million sperm ml−1 were excluded from the present study.
In order to ensure sufficient sperm protein concentration for proteomic analysis in this study, we employed the pooling of samples for the patient and fertile groups respectively. From the samples collected, those that had a sperm concentration of >20 million ml−1 and a semen volume of >2.5 ml were selected for analysis. Thus, 5 samples from 10 fertile men made up the fertile pool while 3 samples from the 17 patients with bilateral varicocele made up the patient pool.
To assure a heterogeneous mixture of samples from various subjects in each of the groups, each sample for pooling was normalized according to protein concentration, in which each sample in the pool contributed an equal amount of protein from an equal number of spermatozoa. Therefore, about 75 μl aliquot of sample with a protein concentration of about 1.5 mg ml−1 is ideal and is obtained from about 80 to 100 million sperm ml−1. For global proteomics analysis, each sample from control and patient group was run in triplicate.
Semen analysis
All specimens were collected by masturbation at the Andrology Laboratory after 48–72 h of sexual abstinence. Samples were allowed to liquefy completely for 15–30 min at 37°C before further processing. Following liquefaction, manual semen analysis was performed using a MicroCell counting chamber (Vitrolife, San Diego, CA, USA) to determine sperm concentration and motility. Smears of the raw semen were stained with a Diff-Quik kit (Baxter Healthcare Corporation, Inc., McGaw Park, IL, USA) for assessment of sperm morphology according to WHO criteria.22 Sample was tested for leukoctyospermia, i.e., >1 million WBC ml−1 of semen when the round cell concentration was >1 million ml−1. This was confirmed by the peroxidase or the Endtz test.23 No further processing was done to remove the round cells from the ejaculates for proteomic analysis.
Measurement of reactive oxygen species
ROS formation was measured by chemiluminescence assay using luminol (5-amino-2, 3-dihydro-1, 4-phthalazinedione) as the probe. Chemiluminescence was measured for 15 min using a Berthold luminometer (Autolumat Plus 953, Oakridge, TN, USA). Results were expressed as relative light units (RLU) per s per million sperm.24
Measurement of total antioxidant capacity
Total antioxidant capacity (TAC) was measured in the seminal plasma using the antioxidant assay kit (Cayman Chemical, Ann Arbor, Mich, USA). All seminal plasma samples were diluted 1:10 with the assay buffer before assaying to avoid variability due to interference by the plasma proteins or sample dilution. Trolox standards and reagent were prepared as per the manufacturer's instructions at the time of the assay. Absorbance was monitored at 750 nm using ELx800 Absorbance Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA). Results were expressed as micromoles of Trolox.
Measurement of sperm DNA fragmentation
Sperm DNA fragmentation was evaluated using a terminal deoxynucleotidyl transferase–mediated fluorescein end labeling (TUNEL) assay with an Apo-Direct kit (Pharmingen, San Diego, CA, USA). Positive and negative kit controls provided by the manufacturer and internal controls (specimens from donors and patients with known DNA damage) were included for each run. The staining solution contained terminal deoxytransferase (TdT) enzyme, TdT reaction buffer, fluorescein isothiocynate-tagged deoxyuridine triphosphate nucleotides (FITC-dUTP) and distilled water. All fluorescence signals of labeled-spermatozoa were analyzed by the flow cytometer FacScan (Becton Dickinson, San Jose, CA, USA). Ten thousand spermatozoa were examined for each assay at a flow rate of 100 cells s−1. The excitation wavelength was 488 nm supplied by an argon laser at 15 mW. The percentage of positive cells (TUNEL-positive) was calculated on a 1023-channel scale using the flow cytometer software FlowJoMac version 8.2.4 (FlowJo, LLC, Ashland, OR, USA).25
Preparation of samples for proteomic analysis
Equal numbers of spermatozoa from the individual samples were pooled and washed with PBS 3 times. Once the supernatant was removed, the spermatozoa were solubilized in radio-immunoprecipitation assay (RIPA) lysis buffer (Sigma-Aldrich, St. Louis, MO, USA) containing the proteinase inhibitor cocktail (Roche, Indianapolis, IN, USA). After complete lysis of the spermatozoa, protein concentration was determined using a bicinchoninic acid (BCA) kit (Thermo, Rockford, IL, USA) and equal amounts of protein was fractionated using SDS PAGE 1-D gel electrophoresis.
Proteomic analysis
Global proteomic analysis was done in triplicate and quantified using the label-free spectral counting method. A 15 μg aliquot of each sample was boiled, and a standard SDS-PAGE was run on a 12.5% Tris-HCl 1-D gel with constant voltage of 150V for 35 min. For the protein digestion, the entire gel lane was cut and divided into 6 smaller pieces. The bands were reduced with dithiothreitol (DTT) and alkylated with iodoacetamide prior to the in-gel digestion. All bands were digested in-gel using trypsin by adding 5 μl of 10 ng μl−1 trypsin in 50 mmol l−1 ammonium bicarbonate. After overnight incubation at room temperature to achieve complete digestion, the resulting peptides were extracted from the polyacrylamide in two aliquots of 30 μl 50% acetonitrile with 5% formic acid. Gels from the bilateral varicocele and fertile group were run in triplicate to examine the reproducibility of the assay.
Liquid chromotography mass spectrometer analysis (LC-MS)
The extracts were combined and evaporated to <10 μl in Speedvac and then resuspended in 1% acetic acid to make a final volume of ~30 μl for LC-MS analysis. The LC-MS system was a Finnigan LTQ-Orbitrap Elite hybrid mass spectrometer system. The HPLC column was a Dionex 15 cm by 75 μm internal diameter Acclaim Pepmap C18, 2 μm, 100 Å reversed phase capillary chromatography column. A 5 μl aliquot of the extract was injected, and the peptides eluted from the column by an acetonitrile per 0.1% formic acid gradient at a flow rate of 0.25 μl min−1. They were introduced into the source of the mass spectrometer on-line. The microelectrospray ion source was operated at 2.0 kV. The digest was analyzed using the data dependent multitask capability of the instrument acquiring full scan mass spectra to determine peptide molecular weights and tandem mass spectra (MS/MS) to determine amino acid sequence in successive instrument scans.
Data analysis
For semen parameters, the Kruskal-Wallis test was used to compare the fertile group to the bilateral varicocele group. The nonparametric Kruskal–Wallis tests comparing pairs of groups were performed at individual significance levels of <0.05 without adjustment for multiple comparisons.
Database searching
Tandem mass spectra were extracted by Proteome Discoverer version 1.4.1.288. Charge state deconvolution and de-isotoping were not performed. All MS/MS samples were analyzed using Mascot (Matrix Science, London, UK; version 2.3.02), SEQUEST (Thermo Fisher Scientific, San Jose, CA, USA; version 1.4.0.288) and X! Tandem (The GPM, thegpm.org; version CYCLONE (2010.12.01.1). Mascot, Sequest and X! Tandem were set up to search the human reference with database (33 292 entries) assuming trypsin as the digestion enzyme. These searches were performed with a fragment ion mass tolerance of 1.0 Da, and a parent ion tolerance of 10 parts per million (PPM). Carbamidomethylation of cysteine was specified as a fixed modification, and oxidation of methionine was specified as a variable modification.
Criteria for protein identification
To validate MS/MS-based peptide and protein identifications, Scaffold (version 4.0.6.1, Proteome Software Inc., Portland, OR, USA) was used. Peptide identifications were accepted if they could be established at >95.0% probability by the Peptide Prophet algorithm with Scaffold delta-mass correction.26 Protein identifications were accepted if they could be established at >99.0% probability to achieve a false discovery rate (FDR) of <1.0% and contained at least 2 identified peptides. Protein probabilities were assigned by the protein prophet algorithm.27 Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Proteins were annotated with gene ontology (GO) terms from National Center for Biotechnology Information (NCBI) (downloaded Oct 21, 2013).28
Quantitative proteomics
For proteomic analysis, the relative quantity of the proteins was determined by comparing the number of spectra, termed spectral counts (SpCs), used to identify each protein. The total number of mass spectra (SpC) that matched peptides to a particular protein was used to measure the abundance of proteins in the complex mixture. Normalization of spectral counts using the NSAF (normalized spectral abundance factor) approach was applied prior to relative protein quantification.29,30 Differentially expressed proteins (DEP) were obtained by applying different constraints for significance tests and/or fold-change cutoffs based on the average SpC of the protein from multiple runs.
Appropriate filters were used to identify DEP that were dependent on the overall abundance of the proteins. It has been reported that accurate quantification and determination of real biological change is dependent on the number of SpCs and hence different constraints have to be applied to SpC levels in order to circumvent the biases and maintain a constant false positive rate (FPR) for all proteins.31 The abundance of the proteins was classified as High (H), Medium (M), Low (L), or Very Low (VL) based on their average spectral counts amongst the 3 replicate runs. Different constraints for significance tests (P value) and/or fold-change cutoffs (or NSAF ratio) were applied for these 4 abundance categories, as shown below:
Very Low (VL): spectral count range 1.7–7; P ≤ 0.001 and (NSAF ratio ≥2.5 for overexpressed, ≤0.4 for underexpressed proteins).
Low (L): spectral count range 8–19; P ≤ 0.01 and (NSAF ratio ≥2.5 for overexpressed, ≤0.4 for underexpressed proteins).
Medium (M): spectral count range between 20 and 79; P ≤ 0.05 and (NSAF ratio ≥2.0 for overexpressed, ≥0.5 for underexpressed proteins).
High (H): spectral counts >80; P ≤ 0.05 and (NSAF ratio ≥1.5 for overexpressed, ≤0.67 for underexpressed proteins).
Bioinformatic analysis
Functional annotation and enrichment analysis were performed using publicly available bioinformatic annotation tools and databases such as GO Term Finder, GO Term Mapper, UniProt, Software Tool for Researching Annotations of Proteins (STRAP)32,33 and Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.niaid.nih.gov). Proprietary software package such as IPA (Ingenuity Pathway Analysis) from Ingenuity® Systems was also used to obtain consensus-based, comprehensive functional context for the large list of proteins derived from this proteomic study.
Validation of proteins of interest
Key DEP identified by functional analysis was selected for validation by Western blot analysis. Briefly, 20 μg of protein was loaded in 30 μl, and the SDS-PAGE gel was run at 90 Volts for 120 min. The gel was transferred to the PVDF membrane for 30 min at 18 Volts. The membrane was washed 3 times in TBST. Blocking was done with 5% nonfat milk for 2h at 20°C and washed 4 times in TBST. Incubation was done with the primary antibody. Based on the findings of protein profiling, we selected two differentially expressed proteins Tektin 3 (TEKT3) and T-complex protein 11 (TCP 11) for validation. b-actin was used as loading control in Western blot. After washing 4 times with Tris-Buffered Saline Tween-20 TBST at 25°C, incubation was carried out with secondary antibody for 2h. The membrane was incubated in enhanced chemiluminescent (ECL) substrate for 3 min and exposed. The quantification of the bands was done using the Image J Software (http://imagej.nih.gov/ij/docs/intro.html).
RESULTS
Infertile men with bilateral varicocele were younger than the fertile group (P < 0.025) (Supplementary Table 1 (62.6KB, pdf) ). In the bilateral varicocele group, majority of the cases had a higher grade on the left side compared to the right side. Grade 3 was present on the left side in 12% of the cases; Grade 1 and Grade 2 were more common on the left side while Grade 1 was more common on the right side. Of the 17 patients with bilateral varicocele, 13 of the men (76.4%) had varicoceles of either Grade 1 or 2 (present on either side), and 4 men had varicoceles (23.5%) of Grade 3 present on either side. For the proteomic analysis in this study, 5 samples from the fertile group (n = 10) and 3 samples from the bilateral varicocele group (n = 17) were selected for pooling, such that each pooled sample had over 2.5 ml in seminal volume and contributed >20 million ml−1 sperm to their respective sample pools. The 3 subjects included in the pooling of samples for proteomic analysis presented with varicocele on the left side with Grades 1 or 2 (33.3%) and Grades 3 and 4 (66.7%). Similarly, varicocele on the right side with Grade 1 or 2 was seen in 66.7% respectively. There were no significant differences between the two pooled groups.
Overall semen parameters in fertile and infertile men with bilateral varicocele and those selected for proteomic analysis
SEMEN ANALYSIS
Results for semen analysis between the fertile control group and the infertile bilateral varicocele group (Supplementary Table 1 (62.6KB, pdf) ). With the exception of sperm morphology and ROS levels, all other semen parameters were comparable. The percent normal morphology in the bilateral group was significantly poorer compared to the fertile group (2.25 ± 2.27 vs 8.00 ± 4.10; P = 0.002). Similarly, levels of ROS (median [25th, 75th percentile] RLU s−1 million−1 sperm) were significantly higher than the established ROS reference values of 93 RLU s−1 million−1 sperm in the bilateral varicocele group (894.9 [149.2, 2842]) compared to the fertile group (58.9 [12.0, 110.0]) (P = 0.007).
Proteomic profiling in fertile control group and infertile bilateral varicocele group
For the global proteomic profiling analysis, each pooled sample from the fertile and the bilateral varicocele group was run in triplicate. A total of 1055, 1010 and 1042 proteins from the 3 runs respectively were identified in the fertile control group (Supplementary Tables 2 (404KB, pdf) –4 (382.4KB, pdf) ). Similarly, in the bilateral varicocele group, a total of 1024, 999, 1017 proteins were identified from the 3 runs respectively (Supplementary Tables 5 (379.2KB, pdf) –7 (372KB, pdf) ). Some of the more abundant proteins identified in both the fertile group and the bilateral varicocele group were semenogelin-2 precursor, semenogelin-1 preproprotein, A-kinase anchor protein 4 isoform 2, lactotransferrin isoform 1 precursor, tubulin beta-4B chain and fibronectin isoform 3 preproprotein.
Global proteomic profiling of fertile control group in triplicate – First run
Global proteomic profiling of fertile control group in triplicate - Second run
Global proteomic profiling of fertile control group in triplicate - Third run
Proteomic profile of run 1 of the bilateral varicocele group proteins showing peptide number and peptide coverage
Proteomic profile of run 2 of the bilateral varicocele group proteins showing peptide number and peptide coverage.
Proteomic profile of run 3 of the bilateral varicocele group proteins showing peptide number and peptide coverage
Identification of differentially expressed proteins among the fertile control group and bilateral varicocele groups
A total of 73 differentially expressed proteins (DEP) were identified after the proteomic comparison of the fertile control and bilateral varicocele groups as shown in the Venn diagram (Figure 1a). These are the DEP's that fit the filtering criteria discussed in the Methods section (Quantitative Proteomics). Of the 8 proteins unique to the fertile control group, 3 (37.5%) showed low abundance, and 5 (62.5%) displayed very low abundance. Among the 7 proteins unique to the bilateral varicocele group, 2 (28.6%) presented with medium abundance; 4 (57.1%) presented with low abundance, and 1 (14.3%) presented with very low abundance. When compared to the control group, 33 proteins were overexpressed in the bilateral varicocele group; of these 16 (48.5%) proteins showed high abundance; 15 (45.5%) medium abundance and 2 (6.1%) low abundance. Similarly, compared to the control group, 25 proteins were underexpressed in the bilateral varicocele group, of which 2 (8.0%) showed high abundance; 6 (24.0%) medium abundance; 5 (20.0%) low abundance; and 12 (48.0%) very low abundance (Figure 1b). The distribution of high, medium, low and very low proteins in the fertile and the bilateral varicocele groups are shown in Figure 1c.
Figure 1.
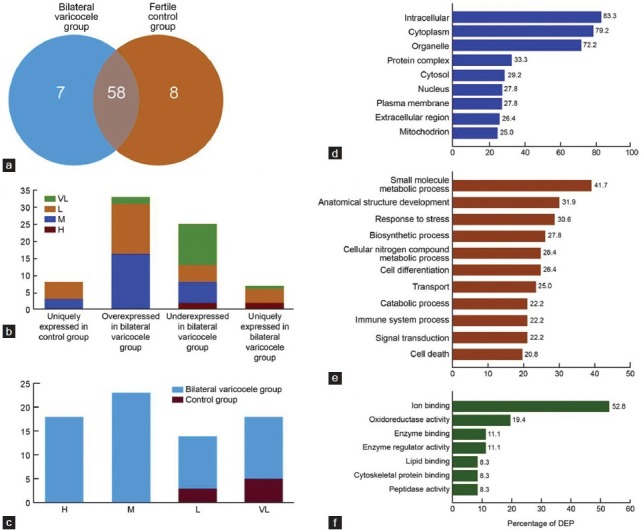
Distribution of differentially expressed proteins (DEP) in bilateral varicocele group (a) Venn Diagram of DEP. Eight proteins are uniquely expressed in the fertile control group, 7 proteins are uniquely expressed in bilateral varicocele group while 58 proteins are commonly expressed in both groups (b) Protein abundance of DEP that are overexpressed, underexpressed or uniquely expressed either in the fertile control versus bilateral varicocele group (c) Comparison of high, medium, low or very low abundance of DEP in fertile control and bilateral varicocele group based on the normalized spectral counts obtained from the proteomic profile and Gene Ontology annotations for DEP for (d) Location (e) Biological processes and (f) Molecular functional processes.
Classification of the differentially expressed proteins based on their cellular location, molecular functions and role in biological processes
GO Term Mapper was used to classify the DEP to identify their involvement in major cellular and molecular functions, biological processes as well as analyze their cellular distribution. The majority of DEP were seen distributed in the intracellular (60/72), cytoplasmic (57/72), organelle (52/72) compartments (Figure 1d). Among the biological processes, the majority of the DEP were involved in small molecule and metabolic processes (30/72), anatomical and structural development (23/72), response to stress (22/72) (Figure 1e). The molecular functions associated with a majority of the DEP were ion binding (38/72), oxidoreductase activity (14/72), and enzyme regulator activity (8/72) (Figure 1f).
Major pathways of DEP identified by the Reactome database
Of the 73 DEP, only 37 were assigned to the Reactome database. The major pathways identified included the metabolism (20/37), immune system (9/37), and disease (8/37). The percent distribution of DEP in these pathways is also shown in Figure 2a.
Figure 2.
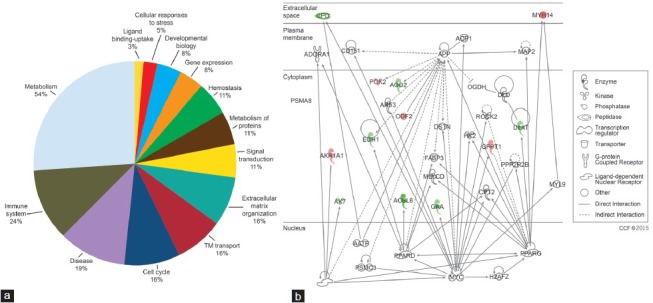
Major pathways identified by the reactome database (a) DEP participating in metabolism, immune system, disease, signal transduction and other pathways and (b) Top disease and function networks and involvement of DEP in lipid metabolism. Green color shows that these DEP were underexpressed and red color shows overexpression of DEP in the fertile control group compared to the bilateral varicocele group respectively. Gradation of color reflects their intensity/abundance of expression (e.g., brighter the red, the greater the protein expression). DEP: differentially expressed protein.
Participation of DEP in the top networks and pathways
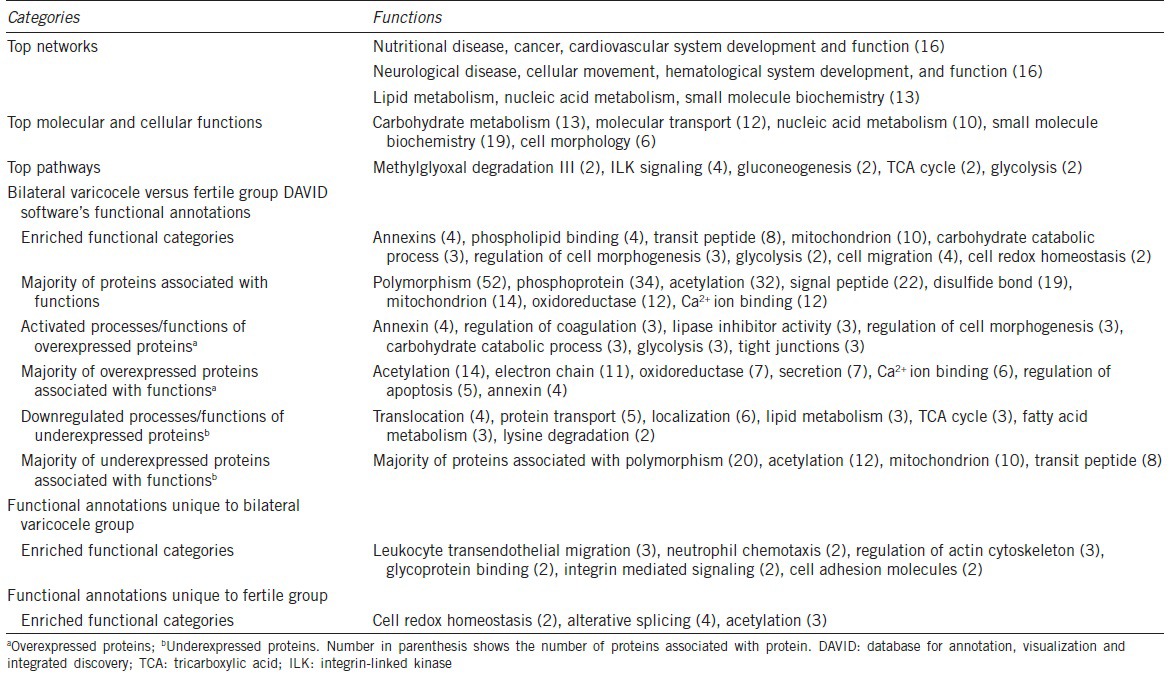
The top networks involving the DEP that have been identified by IPA and DAVID software’ functional annotations are shown (Table 1). Networks of interactions for some of the DEP identified in our study and their possible roles in the various functions as well as their subcellular localization are shown in Figure 2b.
Table 1.
Ingenuity pathway analysis showing top networks, top molecular and cellular functions and top pathways; functional annotations analyzed by DAVID's software showing different categories and functions
Twelve of the DEP were involved in core functions of “lipid metabolism”, “nucleic acid metabolism,” and “small molecule biochemistry” (Figure 2b). The overexpressed proteins are shown in green color and proteins that are underexpressed are in red color respectively. The intensity of the color reflects the level of expression. The majority of the DEP were localized in the cytoplasm and only 2 (MYH14 and CPD) were identified to be present in the extracellular space.
Similar distributions of DEP, with respect to functions, networks and locations were depicted by DAVID functional annotations (Table 1). This table also shows the enriched functional categories, activated processes/functions, proteins associated with functions of acetylation, electron chain, oxidoreductase, calcium ion binding, and regulation of apoptosis, and processes/functions that are down-regulated.
Identification of differentially expressed proteins relevant to spermatogenesis
UniProt analysis identified 12 DEP annotated with distinct functional categories. Of these, only 7 were related to reproduction and/or spermatogenesis. The proteins that possibly play a role in bilateral varicocele or male infertility-related functions, as obtained from UniProt database, and STRAP annotation tool are shown in Tables 2 and 3. The details of the UniProt number, gene name, protein name and the spermatogenic function of the DEP in the bilateral varicocele group compared to the fertile control group is shown in Tables 2 and 3.
Table 2.
Differentially expressed proteins involved in reproductive functions showing the number of peptides and the peptide coverage
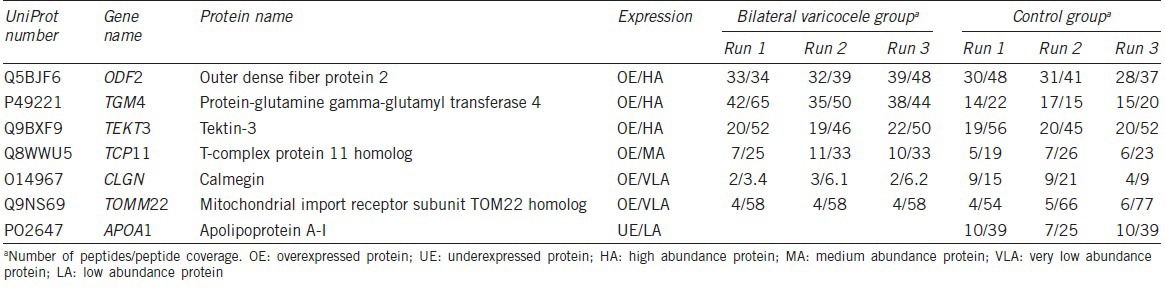
Table 3.
Differentially expressed proteins involved in reproductive functions showing the spectral counts and the function of the identified proteins
Validation of proteins
We have validated two of the identified DEP from one individual sample both in the fertile control and the bilateral varicocele groups respectively, due to the lack of protein from the samples collected (Figure 3a–3d). The selected DEP associated with reproductive functions were TEKT3 and TCP11 (Figure 3a). Lane 1 is representative of the fertile group, and Lane 2 represents the bilateral varicocele group. β-Actin was used as the in-house protein, and its presence was demonstrated in all the lanes (Figure 3b). The relative presence of DEP in fertile control and bilateral varicocele group is shown in Figure 3c and 3d. The quantification of TEKT3 and TCP11 is shown in Figure 3c and 3d. Overexpression of TEK3 and TCP11 was quantified and confirmed to be in the bilateral varicocele group.
Figure 3.
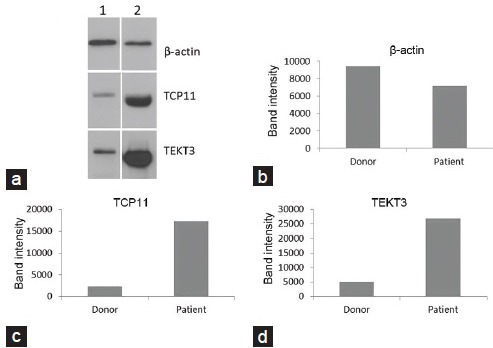
Validation of proteins by Western blot analysis. (a) Lane 1 is control and Lane 2 represents sample from bilateral varicocele group. (b) β-actin was used as an in-house protein. TEKT3 and TCP11 overexpression in men with bilateral varicocele was validated by Western blot analysis. (c and d) shows the quantification of the overexpressed proteins TEKT3 and TCP11 in the bilateral varicocele group by measuring the protein band intensity.
DISCUSSION
In the present study, males with bilateral varicocele were significantly younger in age compared to fertile donors. The majority of patients presented with either Grade 1 or Grade 2 varicocele (76.4%) compared to Grade 3 varicocele (23.5%). Similarly in the pooled samples used for proteomic analysis, majority of them presented with a Grade 3 or 4 varicocele on the left (66.7%) and Grade 1 or 2 on the right side (66.7%). However, we did not compare the patients’ varicocele grades to their semen quality or to the differential expression of protein (DEP) in their samples due to the small sample size (n = 3) of the proteomic pool.
In the current study, although bilateral varicocele patients presented with lower values for sperm concentration, motility or presence of round cells compared to fertile control, there was no significant differences in these parameters between both groups respectively. Bilateral varicocele patients presented with significantly poor sperm morphology compared to fertile males, as assessed by Kruger's strict criteria. A Kruger's score of <4% is an indicator of poor prognosis of the fertilizing ability of spermatozoa both in vivo and in vitro.34
However, according to the new WHO guidelines, values of 4% are representative of the 5th percentile and are debatable.22 In any case, sperm morphology patterns are a good indicator to assess the overall fertility potential of an individual. Unlike those that are genetically determined, abnormal sperm morphology resulting from repeated physiological and environmental stresses is reversible. However, in men with bilateral varicocele, the testis is potentially under recurring heat stress and/or oxidative stress, and it is likely that the testis may fail to fully recuperate, resulting in a permanent decrease of sperm with normal morphology.
In our study, significantly higher levels of ROS present in men with bilateral varicoceles alludes to oxidative stress being an underlying cause of varicocele. Other studies have also reported of high ROS levels in men with either unilateral or bilateral varicocele.9,35,36,37,38 In our study, the extent of DNA fragmentation varied from 7% to 48.3% in men with bilateral varicocele compared to 8.5%–18.2% in fertile men. Men with bilateral varicocele have been reported to have a progressive decline in DNA integrity, which could be improved by varicocelectomy.4,39,40
Besides improved DNA integrity, the significant decline in oxidative stress levels in men after unilateral or bilateral varicocelectomy, alludes to the substantial negative impact of oxidative stress in these men.35 However, measurement of ROS levels and DNA damage do not explain the pathogenic and molecular mechanism responsible for the etiology of varicocele.8,9,38 As seen from the Supplementary Table 1 (62.6KB, pdf) , which contains only five specimens from fertile group and three from bilateral varicocele group that were evaluated for proteomic analysis, there were no significant differences between the two groups; this could be explained by the small sample size and/or the large standard deviation especially in ROS and sperm DNA fragmentation parameter. Despite this, we have demonstrated significant differences in the differentially expressed proteins in the bilateral group. This shows that proteomics provides significant information into the underlying pathology of the disease reflected in the altered protein expression. This type of information at the molecular level cannot be given by a routine semen analysis or even the advanced tests such as reactive oxygen species, oxidative stress, or DNA fragmentation.
The impact of the disease severity in men with bilateral varicocele and the extent of protein alteration in these men compared to fertile men have not been examined previously. Therefore, in this pilot study, our goal was to examine and compare infertile men with bilateral varicocele with fertile men to identify the DEP that may provide an insight into the etiology of bilateral varicoceles.
In an effort to highlight likely protein biomarkers that may contribute to the etiology of varicocele, previous studies have compared the protein profiles in men with and without varicocele as well as in men undergoing varicocelectomy.16,17,20 Comparing the DEP in men with and without varicoceles, Hossenifer and colleagues identified 15 DEP-of which most were down regulated (CLU, PARK7, KLK3, SOD1, SEMG2, SEMG2pre, ATP5D, HSPA5), while others were either downregulated/absent (PIP) or upregulated (ACPP).16 The group's subsequent study reported of increase in HSPA5, SOD1 and ATP5D in varicocele Grade 3 patients after varicocelectomy.20
Similarly, Chan et al.17 reported the differential expression of protein profiles in men with and without varicocele and highlighted the upregulation of HSP70 and HSP90 in infertile men with varicoceles. Current literature suggests that while causes of varicocele are multifactorial, contemporary management of varicocele cannot accurately predict the fertility benefit of varicocele repair. Ferlin and colleagues demonstrated increased expression of heat shock proteins/factors HSP90, HSPA4, HSF1 and HSF2 in men with varicocele and in men with oligozoospermia, while HSFY was shown to be upregulated only in the presence of varicocele and particularly in men with normozoospermia.41
Using genome-wide profiling in the present study, we found > 1000 proteins in the fertile group and in the bilateral varicocele group respectively. We identified 73 DEP, of which 7 were unique to the bilateral group, and 58 were differentially expressed (overexpressed or underexpressed) in either group. Proteins were further classified according to their abundance (high, medium, low and very low) (Figure 1b and c). The abundance (both high and medium) of proteins seen in the bilateral varicocele group demonstrates their likely involvement in the etiology of the disease.
Most of the DEP that we identified for the first time were intracellular proteins (Figure 1d), which reflects the purity of the sample. It also indicates that the identified proteins were largely contributed by the spermatozoa, along with a very negligible contribution by the seminal plasma proteins. The majority of the DEP were involved in various biological processes such as small molecule metabolic processes, response to stress, transport, catabolic processes signal transduction and cell death (Figure 1e), which could be expected in case of oxidative stress and DNA damage. The major molecular functions of the DEP involved ion binding, oxidoreductase activity, enzyme binding and enzyme regulator activity (Figure 1f). Most of the DEP play a role in enzyme activity, and this was found to be altered in bilateral varicocele patients. In our earlier study, we had demonstrated the presence of precursor proteins in seminal ejaculates exhibiting high ROS levels.42
Additionally, altered proteins responsible for ion binding and oxidoreductase activity could significantly influence changes in the cellular defense against oxidative stress in men with bilateral varicocele. Overexpression of acetylation, oxidoreductase, calcium ion binding, regulation of apoptosis proteins and down regulation of mitochondrial and transit peptides reflect the altered functions of the DEP in the bilateral varicocele group.
The Reactome software demonstrated that the majority of the pathways related to the bilateral varicocele group involved metabolism, immune system, disease, transmembrane (TM) transport, extracellular matrix organization and signal transduction activities (Figure 2a). IPA and DAVID software’ functional annotations indicated that lipid metabolism, small molecule biochemistry, and nucleic acid metabolism were affected as a result of bilateral varicocele. Similarly methylglyoxal degradation, ILK signaling, gluconeogenesis, TCA cycle, and glycolysis were the major pathways affected in the bilateral varicocele group. The proteins in the molecular interaction network showed that the majority of the DEP were of intracellular origin (Figure 2b).
In our study, 7 out of the 73 DEP identified were involved in reproductive functions such as sperm motility, sperm-egg recognition, and subsequent penetration of the zona pellucida (Tables 2 and 3). Outer dense fiber protein 2 (ODF2), Protein-glutamine gamma-glutamyltransferase 4 (TGM4), Tektin-3 (TEKT3) and T-complex protein 11 homolog (TCP11) were all found to be overexpressed in the bilateral varicocele group.
Outer dense fibers comprise the main cytoskeletal structure surrounding the axoneme in the mid and principal piece of the spermatozoa tail. These fibers maintain the elastic structure and recoil of the sperm tail and protect the tail from shear forces during epididymal transport and ejaculation.43,44 Developmental defects of outer dense fibers result in severe abnormalities of the spermatozoa tail, leading to abnormal motility, morphology, and infertility.45,46 Another family of proteins found in the sperm tail structure and therefore implicated in sperm motility is tektin proteins. Tektins are thought to provide stability in the axonemal structure and microtubule in the sperm tail, and assist in ciliary movement.47 Tektin 3 is required for progressive sperm motility,48 while the lack or absence of other tektin proteins (e.g., Tektin 1, Tektin 4, Tektin-t) is related to asthenozoospermia.47,49,50
In mice, TEKT3 is expressed in flagellar accessory structures predominantly in late pachytene spermatocytes and early round spermatids,48,51 while in humans, transcription of ODF2 was demonstrated in the cytoplasm of round spermatids.52 The overexpression of both TEKT3 and ODF2 with high abundance suggests the increased presence of immature spermatozoa in bilateral varicocele patients compared to fertile males. These findings are suggestive of disrupted spermatogenesis or maturation arrest in varicocele patients, which was also indicated by increased sperm with abnormal morphology in the bilateral varicocele patients.
Previous studies have reported of increased expression of ODF 2 protein in the sperm proteome of a patient with failed fertilization in IVF53 and in asthenozoospermic patients.54 However, sperm motility did not differ in our varicocele patient group compared to fertile control. It has been suggested that the inhibition of sperm motility could be due to the modulation of not just one single protein, but instead a cascade of proteins which eventually will manifest as poor sperm motility.49
The human TCP11 gene expression is exclusive to the male germ cells in the fertile adult testis, which suggests that the TCP11 protein may play an important role in spermatogenesis and sperm function.55 TCP11 is localized in the head and tail of the human mature sperm,56 where it may have a role in sperm capacitation and acrosome reaction, as seen in mouse sperm.57 TCP11 was found to bind with outer dense fibers (mainly ODF1) in human sperm, which alludes that it contributes to the morphology and function of the sperm tail.56 In this study, TCP11 overexpressed with medium abundance in bilateral varicocele patients may have contributed to poor morphology of sperm from these men.
TGM4 was overexpressed in high abundance in spermatozoa from patients with bilateral varicocele compared to fertile control group. Also known as prostate-specific transglutaminase, TGM4 catalyzes the cross-linking of proteins and the conjugation of polyamines to sperm membrane and specific proteins in the seminal tract, which aids in sperm maturation.58,59 A previous study reported that TGM4 levels were increased in pooled seminal plasma of adult varicocele patients with surgical indication, but TGM4 levels reduced following bilateral varicocelectomy.19 However, seminal plasma TGM4 expression in adolescents with varicocele was found only in the post-bilateral varicocele repair samples.60 Our group has previously reported an increased differential expression of TGM4 in sperm protein from ROS-positive samples (comprising mostly of men with primary infertility and varicocele) compared to samples with normal ROS levels.42 Thus in the present study, increased expression of TGM4 in high abundance in spermatozoa from bilateral varicocele patients correlates with the presence of high ROS levels in these patients.
Two proteins that were underexpressed in very low abundance in bilateral varicocele patients compared to fertile controls are the calmegin (CLGN) and the mitochondrial import receptor subunit TOM22 homolog (TOMM22). CLGN is a testis-specific endoplasmic reticulum chaperone protein for a range of sperm surface proteins that mediate sperm adhesion onto the egg zona pellucida and for subsequent penetration of the zona pellucida. In human males, CLGN may play a role in spermatogenesis and unexplained infertility.61,62 The under expression of these proteins in bilateral varicocele patients may contribute to the infertility experienced by some varicocele patients.
TOMM22, the central receptor component of the preprotein translocase of the outer membrane of mitochondria (TOM complex), along with TOMM20, acts as the transit peptide receptor which facilitates the transition of preproteins into the translocation pore.63,64 Low expression of TOMM22 seen in varicocele patients may be a result of oxidative stress, which could negatively impact mitochondrial protein levels.65
In the current study, one DEP that was uniquely expressed in low abundance in fertile males was the Apolipoprotein A-1 (APOA1). APOA1 is part of the sperm activating protein (SPAP) complex and it activates spermatozoa motility66 and also plays an important role in capacitation.67 APOA1 is the main apolipoprotein of the high-density lipoprotein (HDL) involved in cholesterol transport. HDL acts as an antioxidant which can prevent oxidative stress and has potent anti-inflammatory and anti-apoptotic properties.68 The unique presence of APOA1 in fertile males suggests that fertile males are better protected against oxidative stress and inflammation, which are conditions associated with varicocele.
Among the limitations of our study (1) round cells were not completely removed from the samples and it may influence the differences in the proteins although to a smaller extent and (2) lack of adequate protein concentration from bilateral varicocele patient samples was another limitation. Although 17 patients were recruited for the study, however, only three samples fit the pooling criteria. The low protein concentration in these samples was a potential limitation. These samples were used to extract for proteins and for proteomic profiling only after conducting routine semen analysis, ROS measurement as well as DNA measurement. This has limited the availability of adequate number of spermatozoa for normalizing the protein concentration in specimens pooled for proteomic analysis especially in the bilateral varicocele samples. Thus in this study, we were only able to validate TEKT3 and TCP11 expression in spermatozoa from patients with bilateral varicocele using Western blot analysis. Sample limitations prevented the validation of other DEP of interest, and additional studies are necessary to validate the expression of other major reproductive proteins identified in our study.
In conclusion, we have demonstrated for the first time significantly poor sperm morphology and elevated levels of ROS in the bilateral varicocele group. These abnormalities translate into significant alteration of DEP, such as overexpression of ODF2, TEKT3, TCP11, TGM4 and underexpression of CLMGN and TOM22 that may be responsible for the severity of the disease and sperm dysfunction in these bilateral varicocele patients. We have also successfully validated the overexpression of TEKT3 and TCP11 in the bilateral varicocele group.
Alterations in the sperm proteome of varicocele patients in studies such as this one could potentially shed light on the underlying mechanism as to why certain men with varicocele have impaired sperm quality while other may not, or why some varicocele patients have compromised fertility potential but others do not. In addition, these DEP may serve as useful biomarkers in the identification of the bilateral varicocele and could act as a useful noninvasive tool that aids the clinician in identifying better clinical management options for infertile men with bilateral varicocele. However, further extensive studies of the sperm proteome in infertile patients with varicocele are required to further expand on the findings of this preliminary study and confirm the presence of these and other potential biomarkers in infertile men with bilateral varicocele.
AUTHOR CONTRIBUTIONS
AA conceived the idea, supervised the study, and edited the article for submission. RS and DD helped with the writing, reviewing and editing of the manuscript. DD, ZC, AAy and SG conducted the study and helped with the data collection and management of this study. BW and BG helped with proteomic and bioinformatic data. ES helped with the editing of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
ACKNOWLEDGMENTS
The authors are grateful to the Andrology Center technologists Debbie Garlak, Carmen Carballo and Larry Harmych for scheduling the study subjects and Jeff Hammel, senior biostatistician, for his help with data analysis. This work was partially supported by an internal grant from the Research Program Committee of the Cleveland Clinic (No. 2012-1013) and by financial support from the Center for Reproductive Medicine. The Orbitrap Elite mass spectrometer used in this study was purchased with funds from an NIH shared instrument grant 1S10RR031537-01 (BW).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Sheehan MM, Ramasamy R, Lamb DJ. Molecular mechanisms involved in varicocele-associated infertility. J Assist Reprod Genet. 2014;31:521–6. doi: 10.1007/s10815-014-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmar JL. The pathophysiology of varicoceles in the light of current molecular and genetic information. Hum Reprod Update. 2001;7:461–72. doi: 10.1093/humupd/7.5.461. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–6. [PubMed] [Google Scholar]
- 4.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Skoog SJ, Roberts KP, Goldstein M, Pryor JL. The adolescent varicocele: what's new with an old problem in young patients? Pediatrics. 1997;100:112–21. doi: 10.1542/peds.100.1.112. [DOI] [PubMed] [Google Scholar]
- 6.McClure RD, Hricak H. Scrotal ultrasound in the infertile man: detection of subclinical unilateral and bilateral varicoceles. J Urol. 1986;135:711–5. doi: 10.1016/s0022-5347(17)45827-7. [DOI] [PubMed] [Google Scholar]
- 7.Gat Y, Bachar GN, Zukerman Z, Belenky A, Gornish M. Varicocele: a bilateral disease. Fertil Steril. 2004;81:424–9. doi: 10.1016/j.fertnstert.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Bertolla RP, Cedenho AP, Hassun Filho PA, Lima SB, Ortiz V, et al. Sperm nuclear DNA fragmentation in adolescents with varicocele. Fertil Steril. 2006;85:625–8. doi: 10.1016/j.fertnstert.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 9.Blumer CG, Fariello RM, Restelli AE, Spaine DM, Bertolla RP, et al. Sperm nuclear DNA fragmentation and mitochondrial activity in men with varicocele. Fertil Steril. 2008;90:1716–22. doi: 10.1016/j.fertnstert.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 11.Trussell JC, Haas GP, Wojtowycz A, Landas S, Blank W. High prevalence of bilateral varicoceles confirmed with ultrasonography. Int Urol Nephrol. 2003;35:115–8. doi: 10.1023/a:1025905908378. [DOI] [PubMed] [Google Scholar]
- 12.Kim ED, Leibman BB, Grinblat DM, Lipshultz LI. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol. 1999;162:737–40. doi: 10.1097/00005392-199909010-00031. [DOI] [PubMed] [Google Scholar]
- 13.Azpiazu R, Amaral A, Castillo J, Estanyol JM, Guimerà M, et al. High-throughput sperm differential proteomics suggests that epigenetic alterations contribute to failed assisted reproduction. Hum Reprod. 2014;29:1225–37. doi: 10.1093/humrep/deu073. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, Hu H, Wang Z, Chen X, Yang F, et al. Proteomic characteristics of spermatozoa in normozoospermic patients with infertility. J Proteomics. 2012;75:5426–36. doi: 10.1016/j.jprot.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Heredia J, Estanyol JM, Ballescà JL, Oliva R. Proteomic identification of human sperm proteins. Proteomics. 2006;6:4356–69. doi: 10.1002/pmic.200600094. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinifar H, Gourabi H, Salekdeh GH, Alikhani M, Mirshahvaladi S, et al. Study of sperm protein profile in men with and without varicocele using two-dimensional gel electrophoresis. Urology. 2013;81:293–300. doi: 10.1016/j.urology.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Chan CC, Sun GH, Shui HA, Wu GJ. Differential spermatozoal protein expression profiles in men with varicocele compared to control subjects: upregulation of heat shock proteins 70 and 90 in varicocele. Urology. 2013;81:1379.e1–8. doi: 10.1016/j.urology.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Zylbersztejn DS, Andreoni C, Del Giudice PT, Spaine DM, Borsari L, et al. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil Steril. 2013;99:92–8. doi: 10.1016/j.fertnstert.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 19.Camargo M, Intasqui Lopes P, Del Giudice PT, Carvalho VM, Cardozo KH, et al. Unbiased label-free quantitative proteomic profiling and enriched proteomic pathways in seminal plasma of adult men before and after varicocelectomy. Hum Reprod. 2013;28:33–46. doi: 10.1093/humrep/des357. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinifar H, Sabbaghian M, Nasrabadi D, Modarresi T, Dizaj AV, et al. Study of the effect of varicocelectomy on sperm proteins expression in patients with varicocele and poor sperm quality by using two-dimensional gel electrophoresis. J Assist Reprod Genet. 2014;31:725–9. doi: 10.1007/s10815-014-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–9. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 22.Geneva, Switzerland: WHO Press; 2010. WHO. World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 23.Sharma R, Agarwal A, Mohanty G, Du Plessis SS, Gopalan B, et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol. 2013;11:85. doi: 10.1186/1477-7827-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashou AH, Sharma R, Agarwal A. Assessment of oxidative stress in sperm and semen. Methods Mol Biol. 2013;927:351–61. doi: 10.1007/978-1-62703-038-0_30. [DOI] [PubMed] [Google Scholar]
- 25.Sharma RK, Sabanegh E, Mahfouz R, Gupta S, Thiyagarajan A, et al. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology. 2010;76:1380–6. doi: 10.1016/j.urology.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 26.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 27.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner M. A biologist's view of the Drosophila genome annotation assessment project. Genome Res. 2000;10:391–3. doi: 10.1101/gr.10.4.391. [DOI] [PubMed] [Google Scholar]
- 29.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–24. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wen Z, Washburn MP, Florens L. Refinements to label free proteome quantitation: how to deal with peptides shared by multiple proteins. Anal Chem. 2010;82:2272–81. doi: 10.1021/ac9023999. [DOI] [PubMed] [Google Scholar]
- 31.Gokce E, Shuford CM, Franck WL, Dean RA, Muddiman DC. Evaluation of normalization methods on GeLC-MS/MS label-free spectral counting data to correct for variation during proteomic workflows. J Am Soc Mass Spectrom. 2011;22:2199–208. doi: 10.1007/s13361-011-0237-2. [DOI] [PubMed] [Google Scholar]
- 32.Boyle EI, Weng S, Gollub J, Jin H, Botstein D, et al. GO: TermFinder - open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–5. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatia VN, Perlman DH, Costello CE, McComb ME. Software tool for researching annotations of proteins: open-source protein annotation software with data visualization. Anal Chem. 2009;81:9819–23. doi: 10.1021/ac901335x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, et al. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–7. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 35.Y, Ishikawa T, Kondo Y, Yamaguchi K, Fujisawa M. The assessment of oxidative stress in infertile patients with varicocele. BJU Int. 2008;101:1547–52. doi: 10.1111/j.1464-410X.2008.07517.x. [DOI] [PubMed] [Google Scholar]
- 36.Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002;23:737–52. [PubMed] [Google Scholar]
- 37.Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ, Jr, Agarwal A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999;14:2801–7. doi: 10.1093/humrep/14.11.2801. [DOI] [PubMed] [Google Scholar]
- 38.Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Jr, Agarwal A. Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999;161:1831–4. [PubMed] [Google Scholar]
- 39.Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril. 2011;96:1283–7. doi: 10.1016/j.fertnstert.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Wang YJ, Zhang RQ, Lin YJ, Zhang RG, Zhang WL. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25:307–14. doi: 10.1016/j.rbmo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Ferlin A, Speltra E, Patassini C, Pati MA, Garolla A, et al. Heat shock protein and heat shock factor expression in sperm: relation to oligozoospermia and varicocele. J Urol. 2010;183:1248–52. doi: 10.1016/j.juro.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Sharma R, Agarwal A, Mohanty G, Hamada AJ, Gopalan B, et al. Proteomic analysis of human spermatozoa proteins with oxidative stress. Reprod Biol Endocrinol. 2013;11:48. doi: 10.1186/1477-7827-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindemann CB. Functional significance of the outer dense fibers of mammalian sperm examined by computer simulations with the geometric clutch model. Cell Motil Cytoskeleton. 1996;34:258–70. doi: 10.1002/(SICI)1097-0169(1996)34:4<258::AID-CM1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Hoyer-Fender S, Petersen C, Brohmann H, Rhee K, Wolgemuth DJ. Mouse Odf2 cDNAs consist of evolutionary conserved as well as highly variable sequences and encode outer dense fiber proteins of the sperm tail. Mol Reprod Dev. 1998;51:167–75. doi: 10.1002/(SICI)1098-2795(199810)51:2<167::AID-MRD6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Haidl G, Becker A, Henkel R. Poor development of outer dense fibers as a major cause of tail abnormalities in the spermatozoa of asthenoteratozoospermic men. Hum Reprod. 1991;6:1431–8. doi: 10.1093/oxfordjournals.humrep.a137283. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballescà JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23:783–91. doi: 10.1093/humrep/den024. [DOI] [PubMed] [Google Scholar]
- 47.Iguchi N, Tanaka H, Nakamura Y, Nozaki M, Fujiwara T, et al. Cloning and characterization of the human tektin-t gene. Mol Hum Reprod. 2002;8:525–30. doi: 10.1093/molehr/8.6.525. [DOI] [PubMed] [Google Scholar]
- 48.Roy A, Lin YN, Agno JE, DeMayo FJ, Matzuk MM. Tektin 3 is required for progressive sperm motility in mice. Mol Reprod Dev. 2009;76:453–9. doi: 10.1002/mrd.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siva AB, Kameshwari DB, Singh V, Pavani K, Sundaram CS, et al. Proteomics-based study on asthenozoospermia: differential expression of proteasome alpha complex. Mol Hum Reprod. 2010;16:452–62. doi: 10.1093/molehr/gaq009. [DOI] [PubMed] [Google Scholar]
- 50.Roy A, Lin YN, Agno JE, DeMayo FJ, Matzuk MM. Absence of tektin 4 causes asthenozoospermia and subfertility in male mice. FASEB J. 2007;21:1013–25. doi: 10.1096/fj.06-7035com. [DOI] [PubMed] [Google Scholar]
- 51.Roy A, Yan W, Burns KH, Matzuk MM. Tektin3 encodes an evolutionarily conserved putative testicular microtubules-related protein expressed preferentially in male germ cells. Mol Reprod Dev. 2004;67:295–302. doi: 10.1002/mrd.20025. [DOI] [PubMed] [Google Scholar]
- 52.Petersen C, Füzesi L, Hoyer-Fender S. Outer dense fibre proteins from human sperm tail: molecular cloning and expression analyses of two cDNA transcripts encoding proteins of approximately 70 kDa. Mol Hum Reprod. 1999;5:627–35. doi: 10.1093/molehr/5.7.627. [DOI] [PubMed] [Google Scholar]
- 53.Pixton KL, Deeks ED, Flesch FM, Moseley FL, Björndahl L, et al. Sperm proteome mapping of a patient who experienced failed fertilization at IVF reveals altered expression of at least 20 proteins compared with fertile donors: case report. Hum Reprod. 2004;19:1438–47. doi: 10.1093/humrep/deh224. [DOI] [PubMed] [Google Scholar]
- 54.Zhao C, Huo R, Wang FQ, Lin M, Zhou ZM, et al. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil Steril. 2007;87:436–8. doi: 10.1016/j.fertnstert.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 55.Ma Y, Zhang S, Xia Q, Zhang G, Huang X, et al. Molecular characterization of the TCP11 gene which is the human homologue of the mouse gene encoding the receptor of fertilization promoting peptide. Mol Hum Reprod. 2002;8:24–31. doi: 10.1093/molehr/8.1.24. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Jiang M, Li C, Yang P, Sun H, et al. Human t-complex protein 11 (TCP11), a testis-specific gene product, is a potential determinant of the sperm morphology. Tohoku J Exp Med. 2011;224:111–7. doi: 10.1620/tjem.224.111. [DOI] [PubMed] [Google Scholar]
- 57.Fraser LR, Hosseini R, Hanyalogou A, Talmor A, Dudley RK. TCP-11, the product of a mouse t-complex gene, plays a role in stimulation of capacitation and inhibition of the spontaneous acrosome reaction. Mol Reprod Dev. 1997;48:375–82. doi: 10.1002/(SICI)1098-2795(199711)48:3<375::AID-MRD11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 58.Dubbink HJ, Verkaik NS, Faber PW, Trapman J, Schröder FH, et al. Tissue specific and androgen-regulated expression of human prostate-specific transglutaminase. Biochem J. 1996;315(Pt 3):901–8. doi: 10.1042/bj3150901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porta R, Esposito C, De Santis A, Fusco A, Iannone M, et al. Sperm maturation in human semen: role of transglutaminase-mediated reactions. Biol Reprod. 1986;35:965–70. doi: 10.1095/biolreprod35.4.965. [DOI] [PubMed] [Google Scholar]
- 60.Del Giudice PT, da Silva BF, Lo Turco EG, Fraietta R, Spaine DM, et al. Changes in the seminal plasma proteome of adolescents before and after varicocelectomy. Fertil Steril. 2013;100:667–72. doi: 10.1016/j.fertnstert.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 61.Ikawa M, Wada I, Kominami K, Watanabe D, Toshimori K, et al. The putative chaperone calmegin is required for sperm fertility. Nature. 1997;387:607–11. doi: 10.1038/42484. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka H, Ikawa M, Tsuchida J, Nozaki M, Suzuki M, et al. Cloning and characterization of the human Calmegin gene encoding putative testis-specific chaperone. Gene. 1997;204:159–63. doi: 10.1016/s0378-1119(97)00537-4. [DOI] [PubMed] [Google Scholar]
- 63.Saeki K, Suzuki H, Tsuneoka M, Maeda M, Iwamoto R, et al. Identification of mammalian TOM22 as a subunit of the preprotein translocase of the mitochondrial outer membrane. J Biol Chem. 2000;275:31996–2002. doi: 10.1074/jbc.M004794200. [DOI] [PubMed] [Google Scholar]
- 64.Yano M, Hoogenraad N, Terada K, Mori M. Identification and functional analysis of human Tom22 for protein import into mitochondria. Mol Cell Biol. 2000;20:7205–13. doi: 10.1128/mcb.20.19.7205-7213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright G, Terada K, Yano M, Sergeev I, Mori M. Oxidative stress inhibits the mitochondrial import of preproteins and leads to their degradation. Exp Cell Res. 2001;263:107–17. doi: 10.1006/excr.2000.5096. [DOI] [PubMed] [Google Scholar]
- 66.Akerlöf E, Jörnvall H, Slotte H, Pousette A. Identification of apolipoprotein A1 and immunoglobulin as components of a serum complex that mediates activation of human sperm motility. Biochemistry. 1991;30:8986–90. doi: 10.1021/bi00101a011. [DOI] [PubMed] [Google Scholar]
- 67.Jha KN, Shumilin IA, Digilio LC, Chertihin O, Zheng H, et al. Biochemical and structural characterization of apolipoprotein A-I binding protein, a novel phosphoprotein with a potential role in sperm capacitation. Endocrinology. 2008;149:2108–20. doi: 10.1210/en.2007-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Honda H, Ueda M, Kojima S, Mashiba S, Suzuki H, et al. Oxidized high-density lipoprotein is associated with protein-energy wasting in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1021–8. doi: 10.2215/CJN.06110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overall semen parameters in fertile and infertile men with bilateral varicocele and those selected for proteomic analysis
Global proteomic profiling of fertile control group in triplicate – First run
Global proteomic profiling of fertile control group in triplicate - Second run
Global proteomic profiling of fertile control group in triplicate - Third run
Proteomic profile of run 1 of the bilateral varicocele group proteins showing peptide number and peptide coverage
Proteomic profile of run 2 of the bilateral varicocele group proteins showing peptide number and peptide coverage.
Proteomic profile of run 3 of the bilateral varicocele group proteins showing peptide number and peptide coverage


