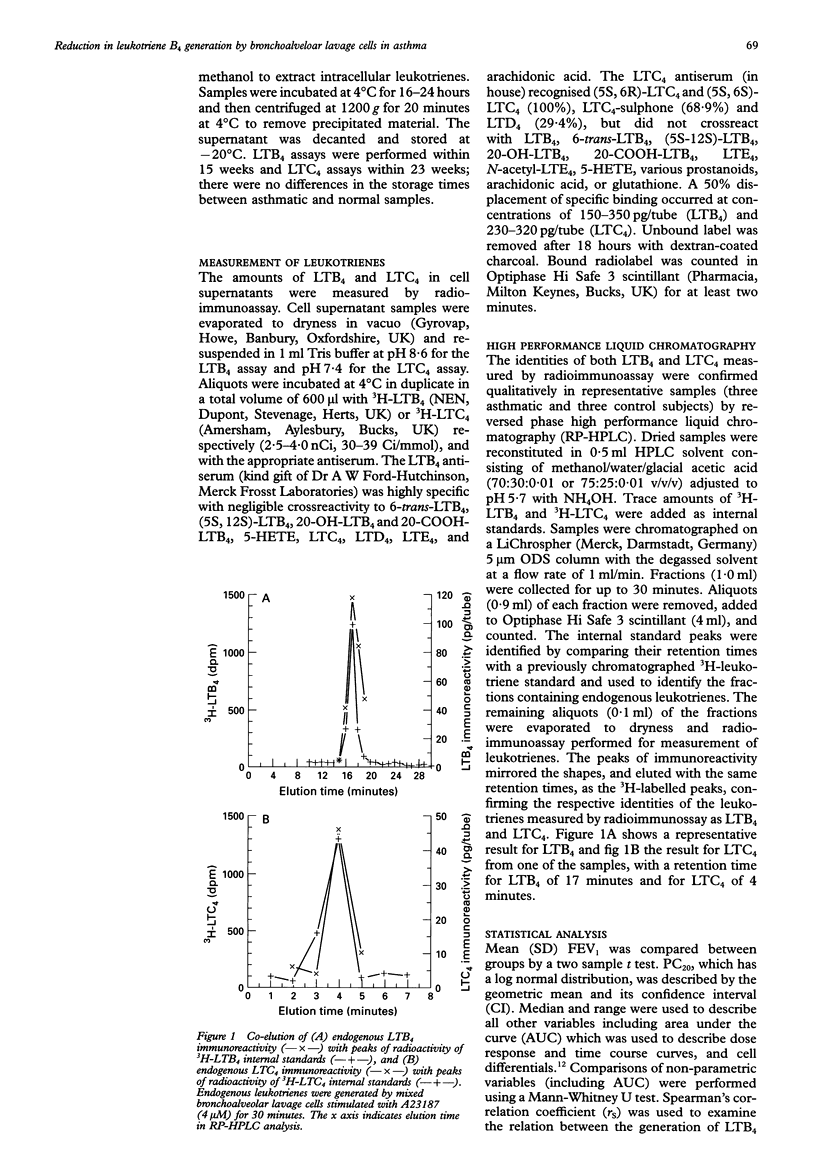
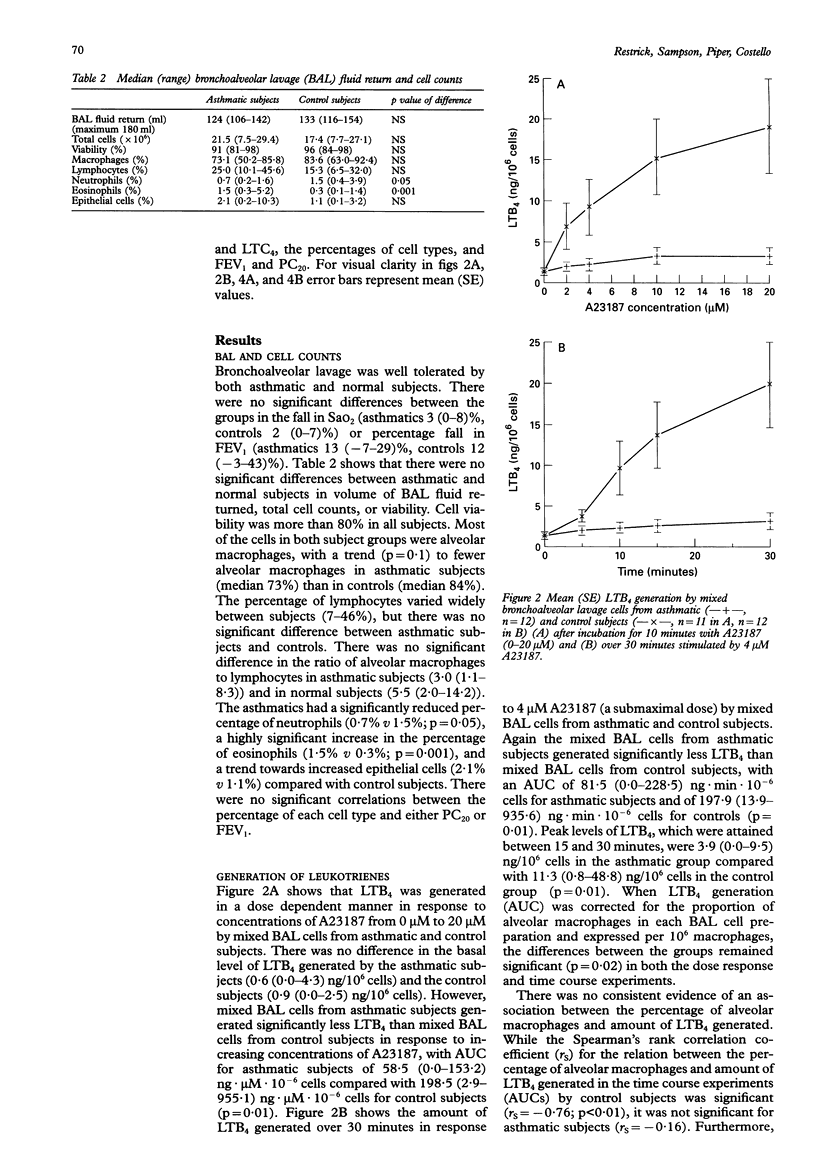
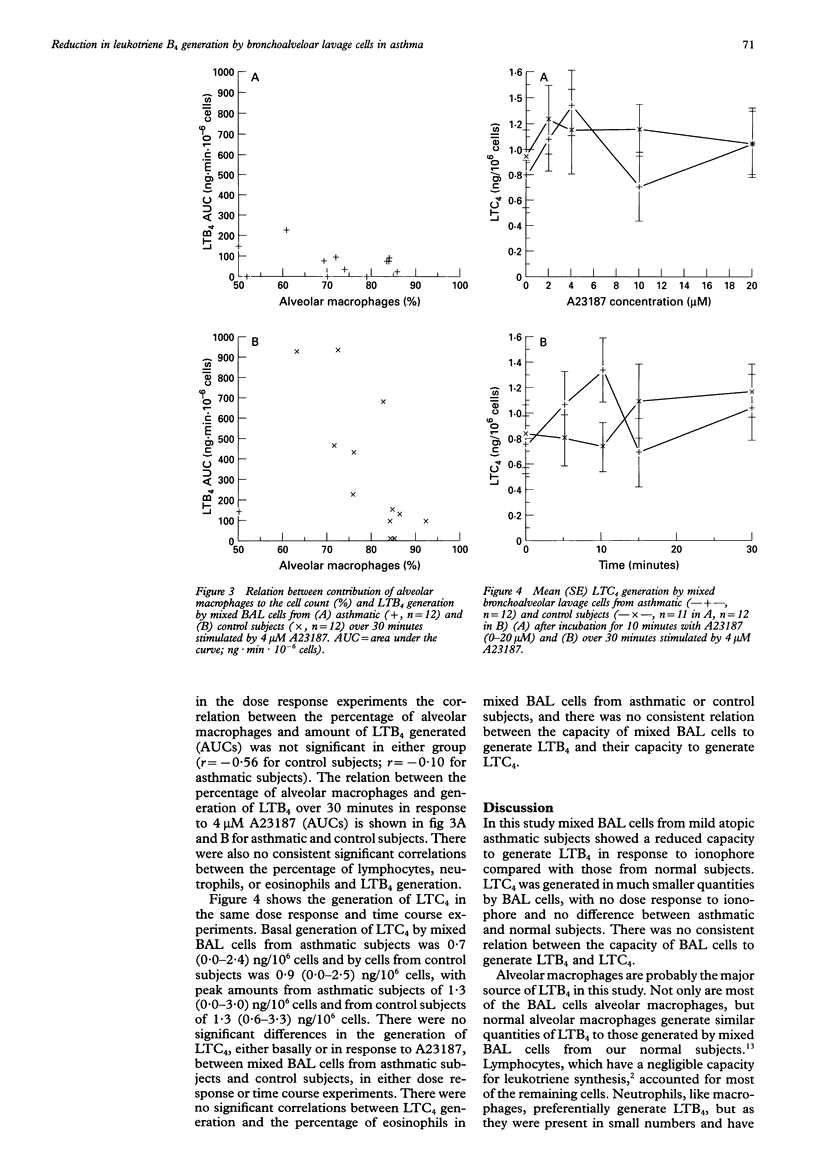
Abstract
BACKGROUND--Leukotrienes are inflammatory mediators implicated in the pathogenesis of asthma. The capacity of inflammatory cells within the airways to generate leukotrienes may be altered in asthma. This hypothesis was tested using bronchoalveolar lavage (BAL) to sample cells within the airways from atopic asthmatic and normal subjects, and by measuring their capacity to generate leukotriene B4 (LTB4) and leukotriene C4 (LTC4) in response to A23187, a potent stimulus of leukotriene generation. METHODS--Bronchoalveolar lavage was performed in 12 mild asymptomatic atopic asthmatic patients and 12 normal subjects. Mixed BAL cell aliquots (approximately 80% alveolar macrophages) were incubated with 0-20 microM A23187 for 10 minutes and with 4 microM A23187 for 0-30 minutes, and leukotrienes were measured by radioimmunoassay and high performance liquid chromatography. RESULTS--Mixed BAL cells from asthmatic subjects generated less LTB4 than cells from normal subjects in dose response and time course experiments (area under the curve 81.5 (0.0-228.5) ng.min.10(-6) cells in asthmatic subjects and 197.9 (13.9-935.6) ng.min.10(-6) cells in normal subjects. There were no differences in LTC4 generation between BAL cells from asthmatic and normal subjects. CONCLUSIONS--Generation of LTB4 by BAL cells from atopic asthmatic subjects in response to A23187 was reduced. As the alveolar macrophage is the major source of LTB4 in BAL cells, these results probably reflect reduced generation of LTB4 by alveolar macrophages from asthmatic patients. This may be a consequence of monocyte migration into the lung, or altered alveolar macrophage function in asthma, or both.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atluru D., Goodwin J. S. Control of polyclonal immunoglobulin production from human lymphocytes by leukotrienes; leukotriene B4 induces an OKT8(+), radiosensitive suppressor cell from resting, human OKT8(-) T cells. J Clin Invest. 1984 Oct;74(4):1444–1450. doi: 10.1172/JCI111556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubas P., Cosso B., Godard P., Michel F. B., Clot J. Decreased suppressor cell activity of alveolar macrophages in bronchial asthma. Am Rev Respir Dis. 1984 Nov;130(5):875–878. doi: 10.1164/arrd.1984.130.5.875. [DOI] [PubMed] [Google Scholar]
- Balter M. S., Eschenbacher W. L., Peters-Golden M. Arachidonic acid metabolism in cultured alveolar macrophages from normal, atopic, and asthmatic subjects. Am Rev Respir Dis. 1988 Nov;138(5):1134–1142. doi: 10.1164/ajrccm/138.5.1134. [DOI] [PubMed] [Google Scholar]
- Bigby T. D., Holtzman M. J. Enhanced 5-lipoxygenase activity in lung macrophages compared to monocytes from normal subjects. J Immunol. 1987 Mar 1;138(5):1546–1550. [PubMed] [Google Scholar]
- Chanez P., Bousquet J., Couret I., Cornillac L., Barneon G., Vic P., Michel F. B., Godard P. Increased numbers of hypodense alveolar macrophages in patients with bronchial asthma. Am Rev Respir Dis. 1991 Oct;144(4):923–930. doi: 10.1164/ajrccm/144.4.923. [DOI] [PubMed] [Google Scholar]
- Cluzel M., Damon M., Chanez P., Bousquet J., Crastes de Paulet A., Michel F. B., Godard P. Enhanced alveolar cell luminol-dependent chemiluminescence in asthma. J Allergy Clin Immunol. 1987 Aug;80(2):195–201. doi: 10.1016/0091-6749(87)90129-1. [DOI] [PubMed] [Google Scholar]
- Damon M., Chavis C., Daures J. P., Crastes de Paulet A., Michel F. B., Godard P. Increased generation of the arachidonic metabolites LTB4 and 5-HETE by human alveolar macrophages in patients with asthma: effect in vitro of nedocromil sodium. Eur Respir J. 1989 Mar;2(3):202–209. [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Drazen J. M., Austen K. F. Leukotrienes and airway responses. Am Rev Respir Dis. 1987 Oct;136(4):985–998. doi: 10.1164/ajrccm/136.4.985. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W. Leukotriene B4 in inflammation. Crit Rev Immunol. 1990;10(1):1–12. [PubMed] [Google Scholar]
- Gant V., Cluzel M., Shakoor Z., Rees P. J., Lee T. H., Hamblin A. S. Alveolar macrophage accessory cell function in bronchial asthma. Am Rev Respir Dis. 1992 Oct;146(4):900–904. doi: 10.1164/ajrccm/146.4.900. [DOI] [PubMed] [Google Scholar]
- Godard P., Chaintreuil J., Damon M., Coupe M., Flandre O., Crastes de Paulet A., Michel F. B. Functional assessment of alveolar macrophages: comparison of cells from asthmatics and normal subjects. J Allergy Clin Immunol. 1982 Aug;70(2):88–93. doi: 10.1016/0091-6749(82)90234-2. [DOI] [PubMed] [Google Scholar]
- Hui K. P., Barnes N. C. Lung function improvement in asthma with a cysteinyl-leukotriene receptor antagonist. Lancet. 1991 May 4;337(8749):1062–1063. doi: 10.1016/0140-6736(91)91709-4. [DOI] [PubMed] [Google Scholar]
- Israel E., Dermarkarian R., Rosenberg M., Sperling R., Taylor G., Rubin P., Drazen J. M. The effects of a 5-lipoxygenase inhibitor on asthma induced by cold, dry air. N Engl J Med. 1990 Dec 20;323(25):1740–1744. doi: 10.1056/NEJM199012203232505. [DOI] [PubMed] [Google Scholar]
- Juniper E. F., Frith P. A., Dunnett C., Cockcroft D. W., Hargreave F. E. Reproducibility and comparison of responses to inhaled histamine and methacholine. Thorax. 1978 Dec;33(6):705–710. doi: 10.1136/thx.33.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzan S., Nolan R. D., Fournier T., Bignon J., Eling T. E., Brody A. R. Stimulation of arachidonic acid metabolism by adherence of alveolar macrophages to a plastic substrate. Modulation by fetal bovine serum. Am Rev Respir Dis. 1988 Jan;137(1):38–43. doi: 10.1164/ajrccm/137.1.38. [DOI] [PubMed] [Google Scholar]
- Laviolette M., Coulombe R., Picard S., Braquet P., Borgeat P. Decreased leukotriene B4 synthesis in smokers' alveolar macrophages in vitro. J Clin Invest. 1986 Jan;77(1):54–60. doi: 10.1172/JCI112301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. J., Watson R. M., Margolskee D. J., Williams V. C., Schwartz J. I., O'Byrne P. M. Inhibition of exercise-induced bronchoconstriction by MK-571, a potent leukotriene D4-receptor antagonist. N Engl J Med. 1990 Dec 20;323(25):1736–1739. doi: 10.1056/NEJM199012203232504. [DOI] [PubMed] [Google Scholar]
- Merchant R. K., Schwartz D. A., Helmers R. A., Dayton C. S., Hunninghake G. W. Bronchoalveolar lavage cellularity. The distribution in normal volunteers. Am Rev Respir Dis. 1992 Aug;146(2):448–453. doi: 10.1164/ajrccm/146.2.448. [DOI] [PubMed] [Google Scholar]
- Metzger W. J., Zavala D., Richerson H. B., Moseley P., Iwamota P., Monick M., Sjoerdsma K., Hunninghake G. W. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs. Description of the model and local airway inflammation. Am Rev Respir Dis. 1987 Feb;135(2):433–440. doi: 10.1164/arrd.1987.135.2.433. [DOI] [PubMed] [Google Scholar]
- Mohr C., Davis G. S., Graebner C., Amann S., Hemenway D. R., Gemsa D. Reduced release of leukotrienes B4 and C4 from alveolar macrophages of rats with silicosis. Am J Respir Cell Mol Biol. 1992 Nov;7(5):542–547. doi: 10.1165/ajrcmb/7.5.542. [DOI] [PubMed] [Google Scholar]
- Piacentini G. L., Kaliner M. A. The potential roles of leukotrienes in bronchial asthma. Am Rev Respir Dis. 1991 May;143(5 Pt 2):S96–S99. doi: 10.1164/ajrccm/143.5_Pt_2.S96. [DOI] [PubMed] [Google Scholar]
- Poston R. N., Chanez P., Lacoste J. Y., Litchfield T., Lee T. H., Bousquet J. Immunohistochemical characterization of the cellular infiltration in asthmatic bronchi. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):918–921. doi: 10.1164/ajrccm/145.4_Pt_1.918. [DOI] [PubMed] [Google Scholar]
- Pueringer R. J., Bahns C. C., Hunninghake G. W. Alveolar macrophages have greater amounts of the enzyme 5-lipoxygenase than do monocytes. J Appl Physiol (1985) 1992 Aug;73(2):781–786. doi: 10.1152/jappl.1992.73.2.781. [DOI] [PubMed] [Google Scholar]
- Rankin J. A., Sylvester I., Smith S., Yoshimura T., Leonard E. J. Macrophages cultured in vitro release leukotriene B4 and neutrophil attractant/activation protein (interleukin 8) sequentially in response to stimulation with lipopolysaccharide and zymosan. J Clin Invest. 1990 Nov;86(5):1556–1564. doi: 10.1172/JCI114875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J. A. The contribution of alveolar macrophages to hyperreactive airway disease. J Allergy Clin Immunol. 1989 Apr;83(4):722–729. doi: 10.1016/0091-6749(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Rich E. A., Tweardy D. J., Fujiwara H., Ellner J. J. Spectrum of immunoregulatory functions and properties of human alveolar macrophages. Am Rev Respir Dis. 1987 Aug;136(2):258–265. doi: 10.1164/ajrccm/136.2.258. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M. Differential effects of leukotriene B4 on T4+ and T8+ lymphocyte phenotype and immunoregulatory functions. J Immunol. 1985 Aug;135(2):1357–1360. [PubMed] [Google Scholar]
- Sampson A. P., Evans J. M., Garland L. G., Piper P. J., Costello J. F. The generation and metabolism of leukotrienes in the ionophore-stimulated blood of normal and asthmatic subjects. Pulm Pharmacol. 1990;3(3):111–119. doi: 10.1016/0952-0600(90)90041-g. [DOI] [PubMed] [Google Scholar]
- Shamsuddin M., Hsueh W., Smith L. J. Production of leukotrienes and thromboxane by resident and activated rat alveolar macrophages: a possible role of protein kinase C. J Lab Clin Med. 1992 Sep;120(3):434–443. [PubMed] [Google Scholar]
- Smith L. J., Shamsuddin M., Houston M. Effect of leukotriene D4 and platelet-activating factor on human alveolar macrophage eicosanoid and PAF synthesis. Am Rev Respir Dis. 1993 Sep;148(3):682–688. doi: 10.1164/ajrccm/148.3.682. [DOI] [PubMed] [Google Scholar]
- Taylor G. W., Taylor I., Black P., Maltby N. H., Turner N., Fuller R. W., Dollery C. T. Urinary leukotriene E4 after antigen challenge and in acute asthma and allergic rhinitis. Lancet. 1989 Mar 18;1(8638):584–588. doi: 10.1016/s0140-6736(89)91611-5. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J., Hay H., Cromwell O., Collins J. V., Kay A. B. Leukotrienes, LTC4 and LTB4, in bronchoalveolar lavage in bronchial asthma and other respiratory diseases. J Allergy Clin Immunol. 1989 Jul;84(1):19–26. doi: 10.1016/0091-6749(89)90173-5. [DOI] [PubMed] [Google Scholar]


