Summary
Objective
Evidence for the efficacy and safety of adjunctive lacosamide in the treatment of partial‐onset seizures (POS) was gained during placebo‐controlled clinical trials in patients with treatment‐resistant seizures who were taking one to three concomitant antiepileptic drugs (AEDs). The VITOBA study (NCT01098162) evaluated the effectiveness and tolerability of adjunctive lacosamide added to one baseline AED in real‐world clinical practice.
Methods
We conducted a 6‐month observational study at 112 sites across Germany. Adult patients (≥16 years) with POS received lacosamide adjunctive to only one baseline AED. Seizure frequency reduction at the end of the observation period was compared with a 3‐month retrospective baseline period.
Results
Five hundred seventy‐one patients received lacosamide at least once (Safety Set [SS]); 520 provided evaluable seizure records (Full Analysis Set [FAS]); and 499 took in‐label dosages of lacosamide (up to 400 mg) and were evaluated for effectiveness (modified FAS). Median baseline seizure frequency was 2.0 per 28 days: 47.1% of patients (235/499, mFAS) took a concomitant sodium channel–blocking (SCB) AED; 38.1% (190/499) had only one lifetime AED; and 18.4% (92/499) were aged ≥65 years (mFAS). At the final visit, 72.5% (358/494) of patients showed a ≥50% reduction in seizure frequency from baseline, 63.8% (315/494) showed a ≥75% reduction, and 45.5% (225/494) were seizure‐free. Seizure freedom rates were higher in patients aged ≥65 years (56.7%) compared with patients aged <65 years (43.1%), in patients with ≤5 years epilepsy duration (52.5%) versus >5 years duration (41.0%), and when added to first monotherapy (60.5%) rather than as a later therapy option. Treatment‐emergent adverse events (TEAEs) were reported by 48.5% (277/571) of patients (SS), with a profile similar to that observed in pivotal trials; 466 of patients (81.6%, SS) continued lacosamide therapy after the trial.
Significance
These results suggest that lacosamide use, added to one concomitant AED, was effective at improving seizure control and was well tolerated in patients treated in routine clinical practice.
Keywords: Real‐world, Adjunctive, Open‐label, Treatment, Safety, Antiepileptic drug
Key Points.
This prospective real‐world study demonstrated the effectiveness, safety, and tolerability of adjunctive lacosamide when added to one concomitant AED in patients with POS
During the last 3 months, 45.5% of patients achieved seizure freedom and 72.5% were ≥50% responders
In patients aged ≥65 years, 56.7% were seizure‐free and 81.1% were 50% responders during the last 3 months with related rates of adverse events comparable to those of younger patients
Similar outcomes were observed when patients were grouped according to concomitant use of sodium channel–blocker [SCB(+)] AED or no sodium channel–blocker [SCB(−)] AED use
The addition of lacosamide therapy in patients from an early treatment population in this study was generally well tolerated
After an epilepsy diagnosis, the goal of antiepileptic drug (AED) therapy is seizure freedom with few or no adverse drug reactions (ADRs).1, 2 The challenge is to select one AED or AED combinations that best attain seizure freedom while minimizing ADRs. Many newly diagnosed patients are successfully treated with their first AED monotherapy.3, 4 However, >50% of patients will require changes to their initial AED regimen to further reduce seizure frequency or eliminate intolerable ADRs. Such changes include switching monotherapy regimens or initiating AED polytherapy.4
Lacosamide is a third‐generation AED that selectively enhances sodium‐channel slow inactivation.5, 6 It is indicated at doses up to 400 mg/day as monotherapy and adjunctive therapy in patients (≥17 years) with partial‐onset seizures (POS) in the United States7 and as adjunctive therapy for adolescents and adults (≥16 years) with POS in the European Union.8 The efficacy, safety, and tolerability of adjunctive lacosamide was established in three randomized, double‐blind, placebo‐controlled clinical trials comprising 1,308 patients with POS.9, 10, 11, 12 Addition of lacosamide to one to three baseline AEDs resulted in significant reductions in seizure frequency compared to placebo, with significantly higher proportions of patients experiencing a ≥50% reduction in seizure frequency from baseline.9, 10, 11 In a pooled efficacy analysis of the three pivotal trials, 44.3% and 21.9% of patients (modified intent‐to‐treat [ITTm] population) randomized to lacosamide 400 mg/day achieved a ≥50% or ≥75% reduction in seizure frequency. Moreover, 3.3% of the patients taking 400 mg/day lacosamide and completing the maintenance phase of the pivotal studies experienced seizure freedom.13 The majority of treatment‐emergent adverse events (TEAEs) were mild‐to‐moderate in intensity, with the most common dose‐related AEs being dizziness, nausea, vomiting, fatigue, ataxia, and diplopia.9, 10, 11
Randomized, double‐blinded, placebo‐controlled clinical studies are designed to evaluate the efficacy and safety of new drugs in an idealized setting, with minimal influence from external factors, such as patient or physician preference and placebo effects.14 The results of such clinical studies may be difficult to extrapolate to patients in real‐world practice settings, since cohorts may not be representative of the true patient population and treatment regimens are often less flexible than those used in daily practice.15 Observational studies involving patients in clinical practice complement randomized clinical studies by evaluating the effectiveness of medications in a broader range of patient types, use of flexible dosing, and physician treatment preferences.16 Therefore, observational studies inform more about the effect of drug treatment, which includes all these external factors, while randomized clinical studies focus on the drug effect itself by minimizing the external factors. The three pivotal double‐blind trials compared the efficacy of lacosamide to placebo within stringent parameters, including fixed‐dose titration and maintenance schedules.9, 10, 11 The majority of patients enrolled in these studies had treatment‐refractory epilepsy with a mean time since diagnosis of 24 years and a median baseline seizure frequency per 28 days of 11.5 seizures, despite treatment with two (62%) or three (22%) concomitant AEDs. Moreover, 77% of patients enrolled in these studies had been treated with four or more AEDs prior to study start, with 45% having tried more than seven AEDs, and 82% of patients having been treated with therapy combinations that included at least one traditional sodium channel–blocking AED [SCB(+)].9, 10, 11
In contrast to the pivotal trials and as observed in daily practice, lacosamide therapy is used in patients with less treatment‐refractory epilepsy, often adjunctive to only one concomitant AED. In addition, flexible dosing of lacosamide is considered an effective method for achieving seizure control while minimizing AEs, according to the needs of the patient. The objective of this real‐world analysis in a clinical practice setting was to evaluate the seizure control and tolerability of lacosamide in adults with POS receiving only one concomitant AED.
Methods
Study design and subjects
This was a 6‐month prospective, noninterventional study conducted at 112 sites in Germany (VITOBA: VImpat added To One Baseline AED; NCT01098162; SP0973). To ensure representation from different treatment settings, specialized epilepsy outpatient units, hospital‐based neurologists, and office‐based neurologists were chosen with the presumption that the majority of patients with less severe disease (i.e., less treatment refractory) would be treated by office‐based neurologists. All patients meeting the inclusion criteria were consecutively enrolled over a period of approximately 2 years.
Adult and adolescent patients (aged ≥16 years) with a POS diagnosis (with and without secondary generalization) and receiving only one baseline AED were eligible for enrollment if a physician's decision to add lacosamide had been reached prior to, and independently of, the decision to include the patient in the study. Lacosamide treatment may have been started up to 2 weeks prior to study inclusion. At inclusion, patients provided seizure frequency for the 3 months prior to the first lacosamide dose (3‐month retrospective baseline). Patients were observed systematically for 6 months or until treatment discontinuation. Patient visits were based on the expected clinical routine, with a visit scheduled at around month 3. A final visit was conducted at around month 6 or study termination. At the final study visit, the seizure frequency was reported for the last 3 months prior to the last visit (i.e., since month 3 visit). Patients reported seizure activity according to usual clinical practice; that is, either through spontaneous reporting or from the use of a seizure diary. Because the study was noninterventional, however, the patients were not required to use a diary. Although approximately half of the patients did so, it is unknown how many of the remaining patients used seizure diaries and how many provided estimates. The study protocol was reviewed by institutional and local ethics committees, and all patients provided signed informed consent for use of their medical data.
Assessments
Outcome variables related to seizure control included ≥50% and ≥75% responder rates, and seizure freedom rates at the final visit of the 6‐month observation period compared with the 3‐month retrospective baseline. Responder rates were based on the difference in seizure frequency between the 3‐month retrospective baseline frequency and the seizure frequency at the final visit; patients who were seizure‐free at baseline were included in the analysis based on their change in seizure frequency and classified as either worsened or unchanged. The physician‐assessed Clinical Global Impression of Change (CGI‐C) was included as an efficacy variable. Safety variables included the incidence of TEAEs, serious TEAEs, and TEAEs leading to lacosamide discontinuation, which were reported spontaneously by the patient or observed by the investigator. Additional predefined and post hoc subgroup analyses were undertaken to detect possible differences in outcomes by age; duration of disease; whether patients were enrolled by specialized epilepsy centers, hospital‐based neurologists, or office‐based neurologists; and type of baseline AEDs (traditional sodium channel blocker: SCB(+) [carbamazepine, lamotrigine, oxcarbazepine, phenytoin] vs. a nontraditional sodium channel blocker: SCB(−) [valproic acid, topiramate, and levetiracetam]).
Analysis
Because of the observational nature of the study design, all statistical analyses were explorative, and therefore only descriptive statistical procedures are reported. The enrolled set comprised all patients included in the study and for whom baseline examination data were available. Seizure control was analyzed using the full analysis set (FAS) comprising all patients who received at least one lacosamide dose and had valid baseline and postbaseline seizure frequency values. A modified FAS (mFAS: patients treated with only in‐label lacosamide doses [up to 400 mg/day] at any time during the study period) was used to assess seizure freedom, change in seizure frequency, and ≥50% and ≥75% responder rates for the final visit of the 6‐month study period. Odds ratios (ORs) were calculated between subgroups for patients achieving ≥50% and ≥75% response to treatment or seizure freedom; ORs were not prespecified in the study analysis plan and are descriptive only. Safety analyses were conducted on the safety set (SS), comprising all subjects who received at least one lacosamide dose. AEs were coded using the MedDRA dictionary.
Results
Patient disposition
A total of 573 patients were enrolled in the study. Two patients did not take a dose of lacosamide and were excluded; the remaining 571 patients were included in the SS. The FAS comprised 520 patients, since 51 patients incurred one or more protocol violations (19 patients took >1 baseline AED when starting lacosamide [e.g., benzodiazepines or pregabalin; n = 16]; 19 patients had no valid post‐baseline seizure frequency assessment). The mFAS comprised 499 patients (patients receiving doses >400 mg/day were excluded). One hundred five enrolled patients (18.3%) terminated the study prematurely. Reasons for discontinuing included AEs (61 patients [10.6%, enrolled set]), patient request (30 patients [5.2%]), lack of efficacy (17 patients [3.0%]), lost to follow‐up (12 patients [2.1%]), and others (three patients [0.5%]). After the study end, 466 patients (81.6%, SS) continued lacosamide therapy.
Demographics and epilepsy characteristics
The baseline demographics and clinical characteristics of patients in this study were similar across all analy‐sis sets (SS, FAS, and mFAS). Patients were recruited from 12 specialized epilepsy outpatient units (n = 103 patients; mFAS), 14 hospital‐based neurologists (n = 51), and 86 office‐based neurologists (n = 345). In the mFAS (N = 499), mean SD; standard deviation patient age was 47.1 years (17.0 years); 51.3% of patients were female. The median frequency of seizures per 28 days at baseline was 2.0 (range 0–504); the mean (SD) frequency of seizures per 28 days at baseline was 8.15 (30.27). Table 1 presents the baseline demographics and clinical characteristics of patients in the mFAS and SS, and compares these with pooled data from patients in the pivotal studies.12 mFAS patients had lower median seizure frequency per 28 days (2.0 vs. 11.5), a shorter median duration of epilepsy (8.0 vs. 24.0 years) at baseline, and tried fewer AEDs (6.2 vs. 44.9% tried ≥7) than patients in the pivotal trials (Table 1). A higher proportion of patients were treated with an SCB(−) baseline AED regimen compared to the pivotal studies (52.9 vs. 17.7%).
Table 1.
Baseline demographics and clinical characteristics and comparison with the pooled pivotal trial populationa
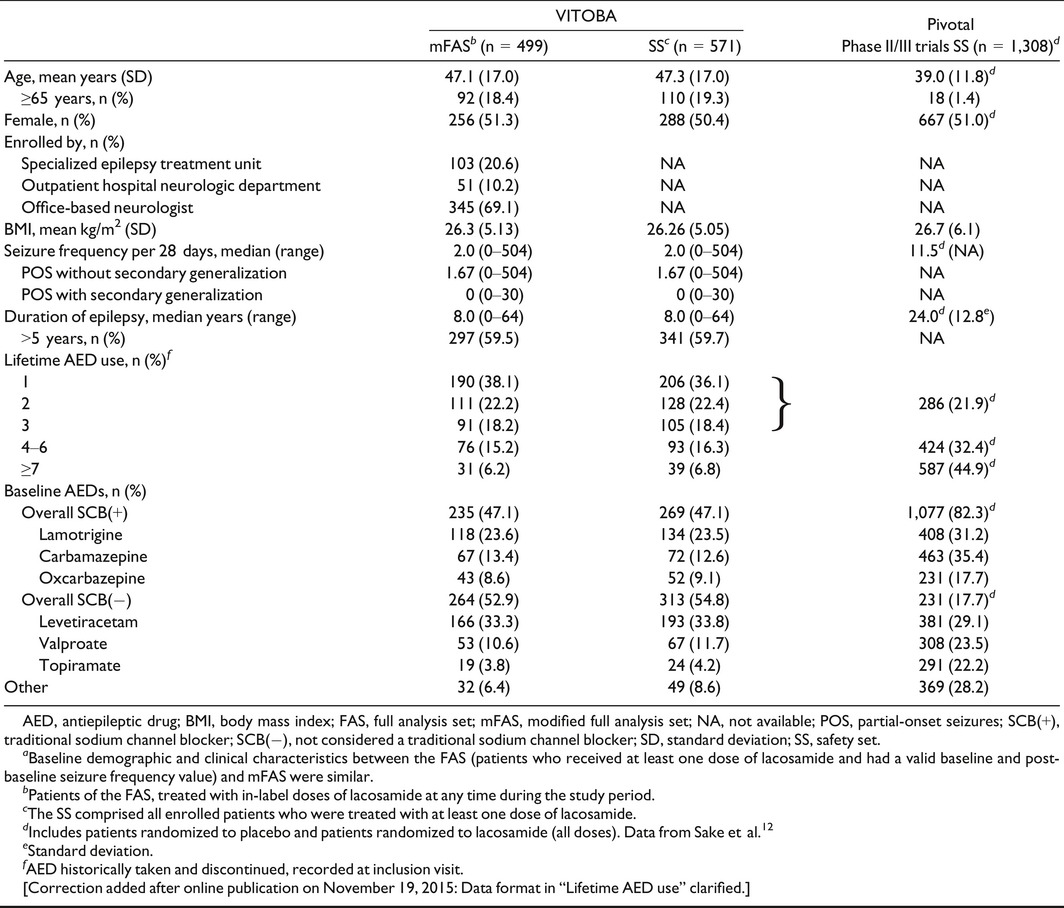
In total, 110 (19.3%) of 571 patients in the SS and 92 (18.4%) of 499 patients in the mFAS were aged ≥65 years. This mFAS subpopulation had a similar median seizure frequency per 28 days at baseline compared with patients aged <65 years (2.00 vs. 2.33), but presented with a shorter median duration of epilepsy (4.0 vs. 9.5 years) and a lower number of lifetime AEDs (71.7 vs. 57.7% had only 1–2 lifetime AEDs) compared to patients aged <65 years. A higher proportion of older patients took concomitant non‐AEDs (78.2 vs. 45.8%, SS).
The mean (SD) lacosamide dosage during the maintenance phase (mFAS) was 279.2 (101.3) mg/day; the median dosage during the maintenance phase (mFAS) was 300 mg/day (range 50–400 mg/day). The median daily dose (300 mg/day; range 50–400 mg/day) was similar when patients were grouped by baseline AED and by SCB(+) or SCB(−). Additional dosing information is shown in Table 2.
Table 2.
Maintenance phase lacosamide dosing for overall study population and age group subanalysis (mFAS)
| Dose | Subjects n (%) |
|---|---|
|
Overall mFAS (n = 416): mean (SD) dosage 279.2 (101.3) mg/day; median dose 300 mg/day (range 50–400 mg/day) |
|
| <200 mg/day | 44 (10.6) |
| 200–300 mg/day | 229 (55.0) |
| >300–400 mg/day | 143 (34.4) |
| Age <65 years (n = 337): mean (SD) dosage 283.7 (99.7) mg/day; median (range) dose 300 (50–400) mg/day | |
| <200 mg/day | 29 (8.6) |
| 200–300 mg/day | 187 (55.5) |
| >300–400 mg/day | 121 (35.9) |
| Age ≥65 years (n = 79): mean (SD) dosage 260.1 (106.6) mg/day; median dose 250 (range 50–400) mg/day. | |
| <200 mg/day | 15 (19.0) |
| 200–300 mg/day | 42 (53.2) |
| >300–400 mg/day | 22 (27.8) |
mFAS, modified full analysis set; SD, standard deviation.
Effectiveness
The proportion of patients achieving a ≥50% or ≥75% responder rate increased from the 3‐month interim visit to the final visit. Similar ≥50% and ≥75% responder rates were observed for the FAS and mFAS (Fig. 1A). Seizure‐freedom rates for the FAS were 44.3%, and 45.5% for the mFAS. Seizure freedom and ≥50% or ≥75% responder rates were 39.3%, 61.1%, and 54.5% for POS without secondary generalization and 36.9%, 42.1% and 39.7% for POS with secondary generalized seizures (mFAS). Of 238 patients who were seizure‐free at the end of the study, 225 had become seizure‐free under treatment with lacosamide compared to the 3‐month retrospective baseline. At study end, patients in the FAS and mFAS showed an identical median reduction from baseline in combined seizure frequency of 1.33 seizures. Similar results were observed when seizure control was analyzed by use of individual concomitant AEDs (Fig. 1B). Slightly higher ≥50% responder rates were observed in patients adding lacosamide to valproate (80.6%) than in patients adding lacosamide to other AEDs. Seizure freedom rates were lower in patients receiving lacosamide and oxcarbazepine relative to the other AEDs (Fig. 1B), which may have been due to the higher median baseline seizure frequency of patients initially treated with oxcarbazepine (3.33 [range 0.3–217.7]). Patients grouped by concomitant SCB(−) AED use showed similar responder rates than those adding lacosamide to a SCB(+) AED. Subgroup analyses showed that improved seizure control was achieved in patients who were prescribed one lifetime baseline AED (mFAS, Fig. 2A) versus those who were prescribed more. The odds of becoming a responder decreased with increased number of lifetime AEDs with respect to the groups of patients treated with one lifetime AED (Fig. S1). For example, OR for seizure freedom between patients with one versus six or more lifetime AEDs was 0.23 (95% confidence interval [CI] 0.08–0.56).
Figure 1.
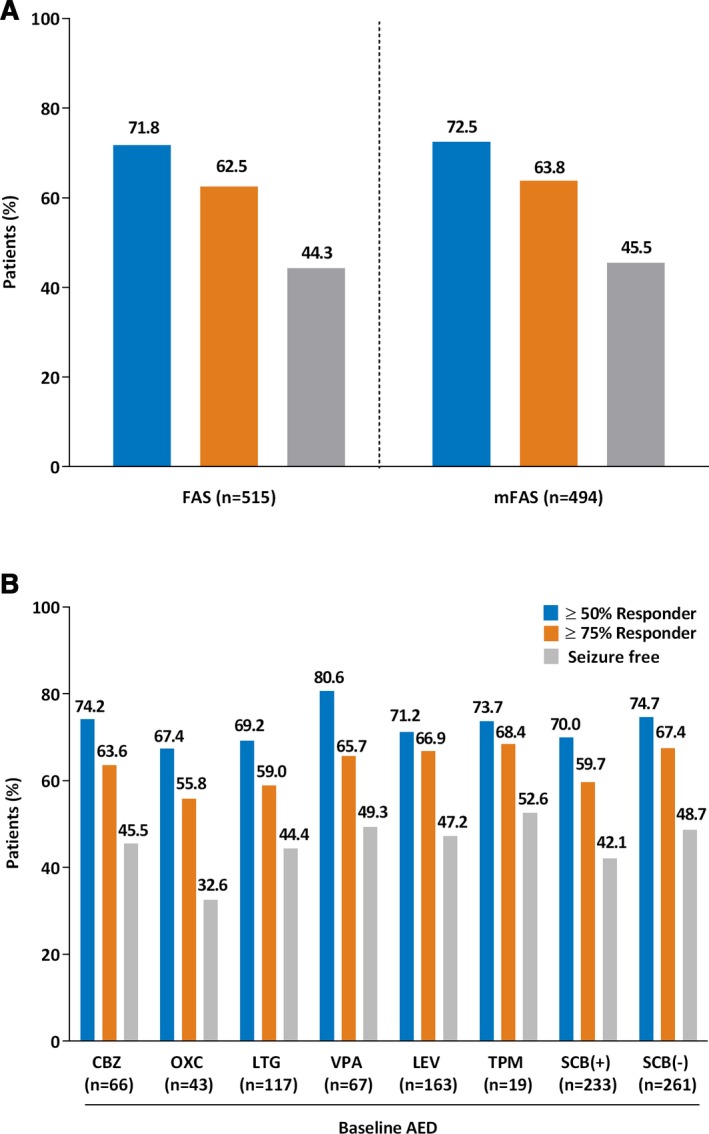
Seizure control and responder rates. Percentage calculations are based on the number of subjects (N) at the final visit. (A) Comparison between FAS and mFAS. (B) By concomitant baseline AED (mFAS). AED, antiepileptic drug. FAS, full analysis set; mFAS, modified full analysis set; CBZ, carbamazepine; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; TPM, topiramate; VPA, valproate. SCB(+), sodium channel blocker; SCB(−), not considered a traditional sodium channel blocker.
Figure 2.
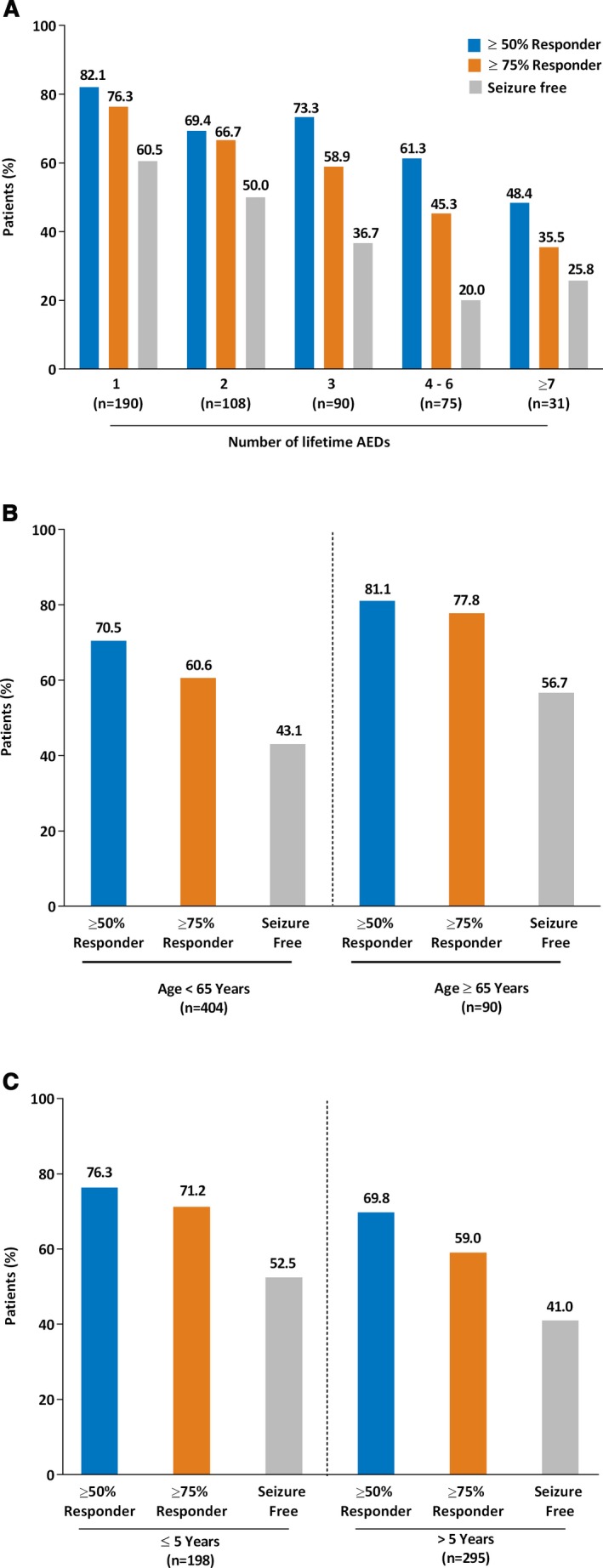
Subgroup responder rate (percentage of patients who experienced a ≥50% and ≥75% reduction in seizure frequency or seizure freedom compared with baseline) analyses at the final visit (mFAS). Percentage calculations are based on the number of subjects (N) at the final 6‐month visit. (A) Number of lifetime AEDs. (B) Age subgroup analysis. (C) Duration of epilepsy. AEDs, antiepileptic drugs; mFAS, modified full analysis set.
Higher seizure freedom and responder (≥50% and ≥75%) rates were observed in patients aged ≥65 years compared with those <65 years (Fig. 2B). Odds ratio estimates support a difference in the response between these two subgroups (≥50% response: OR 1.79 [0.99–3.38]; ≥75% response: OR 2.27 [1.30–4.10]; Fig. S1). Patients aged ≥65 years and taking one lifetime AED achieved higher seizure freedom, ≥50%, and ≥75% responder rates (68.6%, 90.2%, and 90.2%) than patients <65 years (57.6%, 79.1%, and 71.2%); this trend continued for the patients taking two or more lifetime AEDs. Numerically higher responder rates were observed for patients with ≤5 years of epilepsy duration versus patients with >5 years of duration achieving a ≥50% (76.3 vs. 69.8%) and ≥75% (71.2 vs. 59.0%) response to lacosamide treatment (Fig. 2C). Seizure freedom rates also were numerically higher in patients with ≤5 years of epilepsy duration than in patients with >5 years of duration (52.5 vs. 41.0%) with an OR (95% CI) of 1.59 (1.09–2.32) (Fig. S1).
A greater proportion of patients achieving ≥50% reduction in seizure frequency from baseline at the final visit (mFAS) was observed in patients treated by office‐based neurologists (80.2%) than those treated by hospital‐based neurologists (60.0%) or epilepsy centers (52.0%). This also was reflected in patients achieving ≥75% responder rates (71.8%, 56.0%, and 40.0%, respectively), and the proportion achieving seizure freedom (50.9%, 38.0%, and 31.0%). OR estimates support the existence of a larger response in patients treated by office‐based neurologists (Fig. S1). Similar ≥50% (75.0 vs. 72.1%) and ≥75% (63.3 vs. 63.8%) responder rates were observed in patients with (n = 60, mFAS) or without (n = 434, mFAS) intelligence impairment; seizure freedom rates were numerically higher in patients without intelligence impairment compared to those with intelligence impairment (46.8 vs. 36.7%).
CGI‐C questionnaire responses showed that 66.4% of patients (328/494; mFAS) were judged by the treating physician to be “much improved” to “very much improved,” and 78.3% of patients (387/494) were “minimally improved” to “very much improved” compared with baseline. Conversely, 5.3% of patients (26/494) were judged to be “minimally worse” to “very much worse” following treatment. “No change” was seen in 14.2% (70/494) of patients.
Safety and tolerability
Overall, 48.5% of patients (277/571, SS) experienced a TEAE (Table 3), of which 28.7% (164/571) of patients experienced a lacosamide‐related TEAE, and 9.8% (56/571) experienced a serious TEAE. The most common (≥2% of patients; N = 571, SS) lacosamide treatment‐related TEAEs included fatigue (10.3%), dizziness (8.8%), nausea (3.0%), and headache (2.6%). A total of eight patients (1.4%) reported cardiac TEAEs, which were thought to be lacosamide‐related in four patients (0.7%). TEAEs led to lacosamide discontinuation in 10.6% (61/573) of enrolled patients. The most common TEAEs (≥1% of patients; SS) that led to discontinuation were dizziness (2.8%), fatigue (1.6%), and nausea (1.2%).
Table 3.
Incidence of any TEAEs and TEAEs related to lacosamide
| N = 571 patients | Any TEAEa n (%) | Lacosamide‐related TEAE n (%) |
|---|---|---|
| Any | 277 (48.5) | 164 (28.7) |
| Fatigue | 71 (12.4) | 59 (10.3) |
| Dizziness | 63 (11.0) | 50 (8.8) |
| Convulsion | 29 (5.1) | 10 (1.8) |
| Headache | 24 (4.2) | 15 (2.6) |
| Nausea | 21 (3.7) | 17 (3.0) |
| Depression | 17 (3.0) | 7 (1.2) |
| Tremor | 15 (2.6) | 9 (1.6) |
| Gait disturbance | 14 (2.5) | 11 (1.9) |
| Somnolence | 12 (2.1) | 11 (1.9) |
TEAE, treatment‐emergent adverse event.
In ≥2% of patients.
The incidences of overall TEAEs were similar in patients aged ≥65 years (45.5%, 50/110) compared with patients aged <65 years (49.2%, 227/461) (Table 4). Overall, the frequency of cardiac TEAEs was 1.8% (2/110, SS) in patients aged ≥65 years and 1.3% (6/461) in patients aged <65 years; none of the cardiac TEAEs were thought to be lacosamide related in patients aged ≥65 years, and 0.9% (4/461) were thought to be treatment related in younger patients. A greater proportion of patients aged ≥65 years reported serious TEAEs (15.5%, 17/110) than patients aged <65 years (8.5%; 39/461), although only a minority of these were considered to be treatment related (2.7 vs. 1.7%, respectively). A similar proportion of patients aged ≥65 years (8.2%, 9/110) and patients aged <65 years (11.3%, 52/461) discontinued lacosamide therapy due to TEAEs.
Table 4.
Incidence of TEAEs by baseline AED, age, and dosing subgroups
| N = 571 patients | Any TEAE n (%) | Serious TEAE n (%) | TEAE leading to discontinuation n (%) |
|---|---|---|---|
| Baseline AED | |||
| SCB(+), n = 269a | 124 (46.1) | 23 (8.6) | 24 (8.9) |
| Lamotrigine, n = 134 | 64 (47.8) | 13 (9.7) | 15 (11.2) |
| Carbamazepine, n = 72 | 30 (41.7) | 4 (5.6) | 6 (8.3) |
| Oxcarbazepine, n = 52 | 26 (50.0) | 5 (9.6) | 3 (5.8) |
| SCB(−), n = 313a | 157 (50.2) | 34 (10.9) | 38 (12.1) |
| Levetiracetam, n = 193 | 100 (51.8) | 20 (10.4) | 18 (9.3) |
| Valproate, n = 82 | 37 (45.1) | 12 (14.6) | 14 (17.1) |
| Topiramate, n = 23 | 12 (52.2) | 1 (4.3) | 4 (17.4) |
| Age | |||
| <65 years, n = 461 | 227 (49.2) | 39 (8.5) | 52 (11.3) |
| ≥65 years, n = 110 | 50 (45.5) | 17 (15.5) | 9 (8.2) |
| Maintenance dose | |||
| <200 mg/day, n = 48 | 24 (50.0) | 6 (12.5) | NAa |
| 200–300 mg/day, n = 240 | 104 (43.3) | 21 (8.8) | NAa |
| >300–400 mg/day, n = 161 | 59 (36.6) | 11 (6.8) | NAa |
| >400 mg/day, n = 17 | 12 (70.6) | 4 (23.5) | NAa |
AED, antiepileptic drug; NA, not available; SCB(+), traditional sodium channel blocker; SCB(−), not considered a traditional sodium channel blocker; TEAE, treatment‐emergent adverse event.
Eleven patients belong to both groups.
The actual maintenance dose was not calculated for subjects who terminated the study prematurely.
Fewer patients enrolled by office‐based neurologists reported TEAEs (41.2%) than patients enrolled at epilepsy centers or by hospital‐based neurologists (64.8% and 64.3%, respectively, SS). The profile of common TEAEs was similar across patient subgroups, except for alopecia, which was reported by seven (5.7%) patients enrolled by epilepsy centers but none enrolled by hospital‐ or office‐based neurologists. Serious TEAEs were reported in 13.1% of patients enrolled at epilepsy centers, 23.2% by hospital‐based neurologists, and 6.9% of patients enrolled by office‐based neurologists. TEAEs led to lacosamide discontinuation in 18.9% patients enrolled at epilepsy centers, 8.9% by hospital‐based neurologists, and 8.4% by office‐based neurologists.
The overall rates of TEAEs reported by patients taking specific concomitant baseline AEDs ranged from 41.7% (carbamazepine) to 52.2% (topiramate) (Table 4). The rate of serious TEAEs ranged from 4.3% (topiramate) to 14.6% (valproate). TEAEs leading to discontinuation ranged from 5.8% (oxcarbazepine) to 17.4% (topiramate) and were highest when lacosamide was added to valproate or topiramate (Table 4). Any and serious TEAEs were reported at similar rates in patients taking a concomitant SCB(+) AEDs and those taking an SCB(−). Lower percentages of patients taking an SCB(+) experienced TEAEs leading to discontinuation than patients taking an SCB(−) (8.9 vs. 12.1%) (Table 4).
Similar rates of any TEAEs were observed across each of the approved dosages for lacosamide during the maintenance phase of the study (Table 4). Patients taking >400 mg/day (nonapproved dose and protocol violation) reported higher frequencies of TEAEs and serious TEAEs than patients taking approved doses of lacosamide (≤400 mg/day).
Discussion
This real‐world study assessed the effectiveness, safety, and tolerability of lacosamide in patients treated with one concomitant AED early in treatment. In this study, 72.5% and 63.8% of the patients achieved a ≥50% and ≥75% reduction in seizure frequency, and 45.5% attained seizure freedom at the final visit of the 6‐month observation period, taking approved doses of lacosamide as a rather early adjunctive treatment with a broad range of baseline AED monotherapies. These observations are consistent with recent observational studies in which the treatment response to lacosamide was greater when added early in treatment (i.e., to one or two previous AEDs) compared to patients later in their treatment (more than three previous AEDs).17, 18 The responder rates observed in our study are higher than those reported from the pooled pivotal trial analysis, where 44.3% of patients in the maintenance phase (ITTm population) taking 400 mg/day lacosamide achieved a ≥50% reduction in seizure frequency from baseline.13 These results and those from the pivotal studies are difficult to compare directly due to differences in baseline patient characteristics and study design. For example, patients in the pivotal trials were more treatment resistant compared to patients in the VITOBA study, which included a high percentage of patients early in their treatment.
The addition of lacosamide in patients from an early population in the present study was generally well tolerated. The most common lacosamide‐related TEAEs consisted of nervous system events, including fatigue (10.3%), dizziness (8.8%), and nausea (3.0%), and these also were identified as common TEAEs in the lacosamide pivotal trials.9, 10, 11 TEAEs led to lacosamide discontinuation in 10.6% of all patients enrolled in the study, in contrast to the pooled analysis of the pivotal trials, with 17–38% of patients discontinuing treatment when adding 200–600 mg/day lacosamide to 1–3 baseline AEDs.13
Epilepsy incidence is higher in earlier and later life.19 Because no upper age limit was specified in the inclusion criteria in the current study, a greater proportion of patients were aged ≥65 years than in the pivotal trials (n = 92/499 [18.4%] vs. 18/1,308 [1.4%] in the pooled trials). Therefore, compared to the pivotal trials, this study provides observational data on the efficacy and tolerability of adjunctive lacosamide in a greater number of elderly patients rather early in their epilepsy treatment. In keeping with the previous evidence that fewer treatment‐refractory patients respond better to early adjunctive therapy, patients aged ≥65 years achieved seizure control more frequently than younger patients. The incidence of TEAEs was similar in patients aged ≥65 years compared with those aged <65 years. Although patients ≥65 years reported a numerically greater number of nonrelated serious TEAEs than patients <65 years, similar numbers of elderly and younger patients reported any TEAE or TEAEs that led to discontinuation. Overall, 1.4% of patients reported cardiac TEAEs during the study. The frequency of lacosamide‐related cardiac TEAEs was low for younger patients (0.9%), and none were reported for older patients. It is worth noting that the mean (SD) lacosamide dosage for the patients aged ≥65 years was 260.1 (106.6) mg/day versus 283.7 (99.7) mg/day for the patients aged <65 years. The differences in dosing notwithstanding, these data indicate that lacosamide may be effective and well tolerated as add‐on therapy in patients aged ≥65 years treated with only one AED.
Adjunctive lacosamide improved seizure control in a higher proportion of patients enrolled by office‐based neurologists than patients enrolled by hospital‐based neurologists or epilepsy centers. These differences in efficacy and tolerability underscore the dependency of patient baseline demographics and epilepsy disease severity across the different center types on treatment outcomes. TEAEs were reported by fewer patients managed by office‐based neurologists than by patients managed by hospital‐based neurologists or epilepsy centers. The reason for this finding is likely related to the underlying differences in patient characteristics or epilepsy severity; patients enrolled in specialized epilepsy treatment centers would have more serious disease with a longer duration and treatment history. These results should be interpreted with caution due to the relatively small numbers of patients in each subgroup. However, the results of the current study provide important insights for physicians at different treatment sites on the expected outcomes of adjunctive lacosamide treatment based on their patients' treatment profile.
Some studies suggest additive or synergistic effects in efficacy as well as tolerability of the certain AED combinations.12, 18 In this study, lacosamide‐treatment response also was analyzed according to concomitant baseline AED. Some combinations of adjunctive lacosamide therapy with one baseline AED resulted in numerically greater seizure freedom than others (lacosamide+topiramate: 52.6% [10/19 patients] vs. lacosamide+oxcarbazepine: 32.6% [14/43 patients]). Grouping AEDs by mechanism of action [SCB(+) vs. SCB(−)] revealed similar improvements in seizure control (≥50%, ≥75% responder, seizure freedom rates). In general, the study indicates that improvement in seizure control with lacosamide was achieved independent of baseline AED. To confirm if the response to lacosamide is significantly influenced by any baseline AED, large randomized controlled studies with different single baseline AEDs would be necessary.
In the current study, the seizure freedom rates for the lacosamide SCB(−) and SCB(+) combinations were 48.7% versus 42.1%. These seizure freedom rates are in the range of other observational data, such as the LACO‐EXP study.18 The LACO‐EXP study was a retrospective, observational study in patients with POS starting on adjunctive lacosamide at baseline.18 When lacosamide was used early in treatment (as with many VITOBA study patients) as the first and second add‐on, seizure freedom rates were 57.8% and 27.8%, respectively, and response rates were 80.0% and 70.4%. It is important to note that VITOBA was a 6‐month prospective study, whereas LACO‐EXP was a 12‐month retrospective study. In the early add‐on population, Villanueva reported that a greater proportion of patients treated with a lacosamide SCB(−) combination achieved seizure freedom compared to an SCB(+) combination (52.1% vs. 32.7%); the differences were not statistically significant (p = 0.067).18 Although p‐values were not calculated in VITOBA, the differences in the seizure‐freedom rates between the lacosamide SCB(−) and SCB(+) treatment groups (48.7% and 42.1%) are observably smaller than the differences in seizure freedom rates between the SCB(−) and SCB(+) combinations observed in LACO‐EXP.
The incidence of any TEAEs, serious TEAEs, or TEAEs leading to discontinuation in this study were similar in patients receiving an SCB(−) (50.2%, 10.9%, 12.1%) compared to patients receiving an SCB(+) AED (any, 46.1%; serious, 8.6%; discontinuation, 8.9%). In LACO‐EXP, 31.9% of patients treated with an SCB(−) and lacosamide experienced a TEAE compared to 42.5% of patients treated with a lacosamide SCB(+) combination after 12‐months of treatment.18 Overall, these differences may be due to baseline differences in patient characteristics including duration of disease, monthly seizure frequency (indicative of greater disease severity), and being refractory to AED treatment.18 Patients included in our study were recruited earlier in their epilepsy treatment (median 8.0 years) and 58.5% had been taking two or fewer AEDs. In addition, compared with LACO‐EXP, a higher proportion of patients in this study were treated with baseline SCB(−) AEDs (52.9% vs. 27.6%), which may be reflective of the real‐world treatment patterns of patients with epilepsy specific to Germany.18 Taken together, these results suggest that lacosamide, combined with either an SCB(−) or SCB(+), results in a high and similar proportion of patients achieving seizure control and a favorable tolerability outcome.
Several different types of treatment centers and physicians enrolled patients in order to form a patient cohort that mimics the real‐world clinical setting in Germany. Patient enrollment was guided under inclusion criteria defined by the Summary of Product Characteristics for lacosamide8 and the treatment decision was left to the physician's discretion, reflecting daily clinical practice. However, although seizure reporting was consistent between the 3‐month retrospective baseline and during the study treatment period, the possibility of inherent reporting bias due to spontaneous seizure reporting cannot be dismissed. Despite this limitation, the results from this real‐world analysis suggest that the efficacy and tolerability of lacosamide, previously demonstrated in three double‐blind, placebo‐controlled trials, translate into good effectiveness and tolerability in patients with less treatment‐refractory epilepsy treated with only one concomitant AED in daily clinical practice.
In conclusion, this was the largest prospective dataset for lacosamide when added to one baseline AED early in the treatment of patients with POS. When added to any baseline AED, lacosamide demonstrated effectiveness in obtaining numerically higher seizure freedom and responder rates compared to patients in the pivotal clinical trials. Furthermore, this study demonstrated the effectiveness, safety, and tolerability profile of early adjunctive lacosamide when added to one AED in the elderly population.
Conflict of Interest
Uwe Runge has received personal compensation for consulting services from UCB Pharma, Eisai, and Desitin, and financial support for research activities from UCB Pharma; Stephan Arnold has received personal compensation for consulting services from UCB Pharma, Upsher‐Smith, Eisai, and Desitin. Fritjof Reinhardt has no conflicts of interest to report. Frank Kühn has received compensation for participation in UCB studies; no activities with other epilepsy‐related companies were performed in the last 36 months. Christian Brandt has received personal compensation from Otsuka, Eisai, Desitin, Pfizer, and UCB Pharma for serving on scientific advisory boards, for speaking activities, and for congress travel, and financial support for research activities from UCB Pharma and Otsuka. Peter Dedeken, Kathleen Isensee, Thomas Lauterbach, Francisco Ramirez, and Matthias Noack‐Rink are employees of UCB Pharma. Thomas Mayer has received personal compensation from UCB Pharma, Eisai, and Desitin for consulting services. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. Odds ratios and 95% confidence intervals by age groups, duration of epilepsy, center type, and number of lifetime AED use (mFAS). (A) Patients experiencing a ≥50% reduction in seizure frequency. (B) Patients experiencing a ≥75% reduction in seizure frequency. (C) Patients experiencing seizure freedom. AEDs, antiepileptic drugs. mFAS, modified full analysis set.
Acknowledgments
This study was supported by UCB Pharma. Authors acknowledge Florian Hummel, Diplom‐Biologe (UCB Pharma, Monheim am Rhein), Valeska Irrgang, PhD, and Frank Tennigkeit, PhD (UCB Pharma, Monheim am Rhein) for their contribution to the study conduct and analysis. The authors acknowledge Jennifer Bodkin, PhD (Evidence Scientific Solutions, Horsham, United Kingdom) and Richard Fay, PhD, CMPP (Evidence Scientific Solutions, Philadelphia, PA, U.S.A.) for writing support, which was funded by UCB Pharma. The authors also acknowledge Barbara Pelgrims, PhD (UCB Pharma, Brussels, Belgium) for publication coordination. Finally, the authors wish to acknowledge and thank all the patients for their participation in this study.
Biography
Uwe Runge is head of the Epilepsy Center at the University Hospital Greifswald, Germany.

References
- 1. Kwan P, Sperling MR. Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia 2009;50(Suppl. 8):57–62. [DOI] [PubMed] [Google Scholar]
- 2. Sander JW. The natural history of epilepsy in the era of new antiepileptic drugs and surgical treatment. Epilepsia 2003;44(Suppl. 1):17–20. [DOI] [PubMed] [Google Scholar]
- 3. Brodie MJ, Barry SJ, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012;78:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwan P, Brodie MJ. Epilepsy after the first drug fails: substitution or add‐on? Seizure 2000;9:464–468. [DOI] [PubMed] [Google Scholar]
- 5. Cawello W, Stockis A, Andreas J‐O, et al. Advances in epilepsy treatment: lacosamide pharmacokinetic profile. Ann N Y Acad Sci 2014;1329:18–32. [DOI] [PubMed] [Google Scholar]
- 6. Errington AC, Stohr T, Heers C, et al. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage‐gated sodium channels. Mol Pharmacol 2008;73:157–169. [DOI] [PubMed] [Google Scholar]
- 7. UCB Pharma . Vimpat® (lacosamide tablets, injection, oral solution) [U.S. prescribing information]. Smyrna: UCB, Inc; 2015. [Google Scholar]
- 8. UCB Pharma . Vimpat® (lacosamide) EMA summary of product characteristics. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000863/WC500050338.pdf: UCB Pharma; 2014. [Google Scholar]
- 9. Ben‐Menachem E, Biton V, Jatuzis D, et al. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial‐onset seizures. Epilepsia 2007;48:1308–1317. [DOI] [PubMed] [Google Scholar]
- 10. Chung S, Sperling MR, Biton V, et al. Lacosamide as adjunctive therapy for partial‐onset seizures: a randomized controlled trial. Epilepsia 2010;51:958–967. [DOI] [PubMed] [Google Scholar]
- 11. Halász P, Kälviäinen R, Mazurkiewicz‐Beldzińska M, et al. Adjunctive lacosamide for partial‐onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia 2009;50:443–453. [DOI] [PubMed] [Google Scholar]
- 12. Sake JK, Hebert D, Isojarvi J, et al. A pooled analysis of lacosamide clinical trial data grouped by mechanism of action of concomitant antiepileptic drugs. CNS Drugs 2010;24:1055–1068. [DOI] [PubMed] [Google Scholar]
- 13. Chung S, Ben‐Menachem E, Sperling MR, et al. Examining the clinical utility of lacosamide: pooled analyses of three phase II/III clinical trials. CNS Drugs 2010;24:1041–1054. [DOI] [PubMed] [Google Scholar]
- 14. Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005;365:82–93. [DOI] [PubMed] [Google Scholar]
- 15. Ben‐Menachem E, Sander JW, Privitera M, et al. Measuring outcomes of treatment with antiepileptic drugs in clinical trials. Epilepsy Behav 2010;18:24–30. [DOI] [PubMed] [Google Scholar]
- 16. Villanueva V, Lopez‐Gomariz E, Lopez‐Trigo J, et al. Rational polytherapy with lacosamide in clinical practice: results of a Spanish cohort analysis RELACOVA. Epilepsy Behav 2012;23:298–304. [DOI] [PubMed] [Google Scholar]
- 17. Villanueva V, Garces M, Lopez‐Gomariz E, et al. Early add‐on lacosamide in a real‐life setting: results of the REALLY Study. Clin Drug Investig 2015;35:121–131. [DOI] [PubMed] [Google Scholar]
- 18. Villanueva V, Lopez FJ, Serratosa JM, et al. Control of seizures in different stages of partial epilepsy: LACO‐EXP, a Spanish retrospective study of lacosamide. Epilepsy Behav 2013;29:349–356. [DOI] [PubMed] [Google Scholar]
- 19. Banerjee PN, Hauser WA. Epilepsy; a comprehensive textbook. Chapter 5: incidence and prevalence. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Odds ratios and 95% confidence intervals by age groups, duration of epilepsy, center type, and number of lifetime AED use (mFAS). (A) Patients experiencing a ≥50% reduction in seizure frequency. (B) Patients experiencing a ≥75% reduction in seizure frequency. (C) Patients experiencing seizure freedom. AEDs, antiepileptic drugs. mFAS, modified full analysis set.


