Abstract
♦ Background:
Older in-center hemodialysis patients have a high burden of functional disability. However, little is known about patients on home chronic peritoneal dialysis (PD). As patients opting for home dialysis are expected to play a greater role in their own dialysis care, we hypothesized that a relatively low number of PD patients would require help with basic self-care tasks (ADL) and instrumental activities of daily living (IADL).
♦ Methods:
We used a cross-sectional study design to measure the proportion of patients aged 65 years and older undergoing outpatient PD who needed help with day-to-day activities. Patients living in nursing homes were excluded from the study. Functional dependence in ADL and IADL tasks were measured by the Barthel and Lawton Scales. Physical performance measures used included the timed up-and-go (TUG) test, chair stands and Folstein mini-mental score (MMSE).
♦ Results:
A total of 74 of 76 (97%) eligible PD patients participated. Patients had a mean age of 76.2 ± 7.5 years. Thirty-six percent had impaired MMSE scores, 69% were unable to stand from a chair without the use of their arms and 51% had abnormal TUG scores. Only 8 patients (11%) were fully independent for both ADL and IADL activities. Dependence in one or more ADL activity was reported by 64% of participants, while 89% reported dependence in one or more IADL.
♦ Conclusions:
Impaired physical and functional performance is common in older patients maintained on PD. Collaborative geriatric-renal programs may be beneficial within the dialysis community.
Keywords: Cross-sectional, disability, elderly, functional independence, observational, peritoneal dialysis
Peritoneal dialysis (PD) is currently used as a chronic life-sustaining treatment by approximately 11% of the global dialysis population, an equivalent of 197,000 end-stage renal disease (ESRD) patients (1). Many are older and have high levels of comorbidity, physical frailty, or sensory impairment. These factors contribute to modality selection and may be perceived as barriers to providing PD care (2–6). Amongst the broader population of all older individuals (and not just those with renal disease), the presence of functional dependency is predictive of a variety of significant clinical outcomes, including hospitalization, the need for increased levels of care, and mortality (7–16). In studies of hemodialysis (HD) patients, disability in basic self-care tasks has been shown to be highly prevalent (17–22). In a single-center cross-sectional study, 95% of patients aged over 65 years of age reported requiring assistance to manage higher-level tasks (shopping, transportation, medication management, housekeeping, meal preparation, laundry, and finances) and over 50% needed help with basic tasks (stair use, bathing, walking, transferring, dressing, grooming, eating, toileting, bowel control, bladder control) (17). Results from the Dialysis Outcomes and Practices Patterns study suggest that functional dependency is seen in HD patients of all ages, but that those of older ages have a higher need for personal care (23). However, the functional status of older individuals on PD has not yet been well-studied. As PD is primarily a self-care dialysis modality that requires basic functional skills to be undertaken independently, we hypothesized that older patients on PD had higher levels of self-care independence compared to patients on HD. The objective of this study was to assess the functional status of older patients on PD.
Materials and Methods
Study Population
This study reports data that were collected as part of a longitudinal cohort study examining the burden of falls in older patients undergoing chronic dialysis in 2003-2004. All patients who were aged 65 years or older and undergoing home PD therapy as an outpatient at the University Health Network, Toronto, Canada, were eligible. Patients were excluded if they lived in an institutional care setting (e.g. in long-term care facilities, nursing homes, or equivalent) or were unable or unwilling to provide informed consent. Both patients performing dialysis exchanges independently and those getting assistance with dialysis exchanges were considered eligible. Patients with amputations or with limited mobility were not excluded as they were at risk of falls while transferring from one position to another. Ethics approval was granted by the University Health Network Research Ethics Board (REB # 02-0237-E).
Baseline Data Collection
As part of a fall risk assessment, patients underwent a full baseline comprehensive geriatric assessment using previously published standardized protocols (17). Electronic chart records were used to obtain the medical history, cause of ESRD, comorbid conditions and laboratory values at the time of recruitment. Complete medication history of each subject was recorded. All patients were asked to participate in structured interviews to determine living status, functional independence, years of education, and history of falls in the previous 12 months.
Physical performance measures were performed within 2 weeks of recruitment at a time convenient to the patient. Functional mobility was tested using the timed up-and-go (TUG) test (24). In the TUG test, subjects are asked to stand from a chair, walk 10 feet (3 m), and return to the seated position. Completion of the task is timed, in seconds, and scores < 10 s are considered normal. Subjects with scores between 10–15 s were considered as having ‘slowed mobility’ and those > 15 seconds as ‘impaired’ mobility (25–28). Lower extremity power was evaluated by asking the subject to rise independently from a chair of standard height and seat depth without the use of their arms or an aid within 3 s (chair stands) (16). For each task, patients were given a total of 3 attempts and the best performance was recorded. Patients unable to attempt or complete the task were said to have impaired physical performance on that test.
The Barthel Index (29) for activities of daily living (ADL) and the Lawton-Brody Instrumental Activities of Daily Living Scale (30) (IADL) questionnaires were administered by structured interview by the study nurse. These are well validated questionnaires that evaluate the individual's ability to perform key activities of daily living (stair use, bathing, ambulation (50 yards), toileting, chair/bed transfer, dressing, bladder continence, bowel continence, feeding, grooming; minimum and maximum scores 0 and 100, respectively, with 100 being fully independent) and instrumental activities of daily living (shopping, housework, laundry, meal preparation, transportation, financial management, medication management, telephone use; minimum and maximum scores 0 and 24, respectively, with 24 being fully independent). In all activities, subjects were considered to be ‘dependent’ for a given function if they needed supervision or assistance with or were unable to complete the task. These have been previously used in the dialysis population (17). The Folstein mini-mental score (MMSE) test was used for cognitive assessment (31). Scores could range from 0 to 30 with a maximum possible score of 30/30. Patients with scores < 24 were defined as having cognitive impairment. Depression was assessed using the validated single item depression scale “How much of the time over the past month have you felt downhearted and sad?” (32).
Analysis
Descriptive data were reported using mean and standard deviation or median and quartiles as appropriate (continuous variables) or as a frequency for ordinal or nominal data. Modeling was not performed due to the small sample of patients with full independence. Differences between those on continuous ambulatory PD (CAPD) and continuous cycler PD (CCPD), between genders and those with diabetes were sought using Fishers exact test, chi-square and the Kolmogorov–Smirnov test as appropriate. Differences in functional burden in those with normal, impaired or not attempted TUG tests were evaluated using the Fisher's exact test due to the small sample size. Analyses were performed using SPSS Version 17.0 (SPSS Inc. 2008, Chicago, IL, USA).
Results
Study Population
A total of 74 of 76 (97%) eligible PD patients who were older than 65 at the time of study agreed to participate. Participant characteristics are presented in Table 1. Fifty-five percent of patients were male, with a mean age of 76.2 ± 7.5 years. Hypertension was the most common cause of ESRD, and although diabetes as a cause of ESRD was uncommon, almost one-third of patients had concomitant diabetes. Nineteen percent of patients lived alone; of those living with others 75% lived with a spouse, while 25% lived with children, relatives and/or non-related caregivers. Most patients (n = 59, 78.7%) were managed with CAPD, while the remainder were using cycler dialysis. Three patients (4%) were using icodextrin on a regular basis.
TABLE 1.
Baseline Characteristics of Study Participants
Patients had a high burden of age-related morbidity such as depression, cognitive impairment, functional loss and reduced mobility. Sixteen percent of patients had depressive symptoms, while 36% were found to have cognitive impairment on the MMSE. Most patients demonstrated multiple deficits in the physical performance measures. A total of 69% were unable to stand from a chair without the use of their arms or an aid, while 51% were unable to perform the TUG mobility test within the normal range of 0–10 s.
Disability in Daily Functional Activities
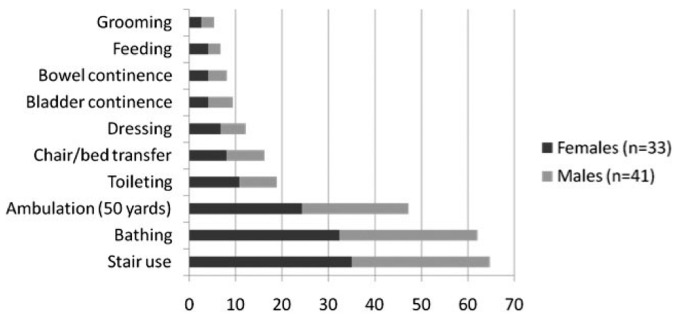
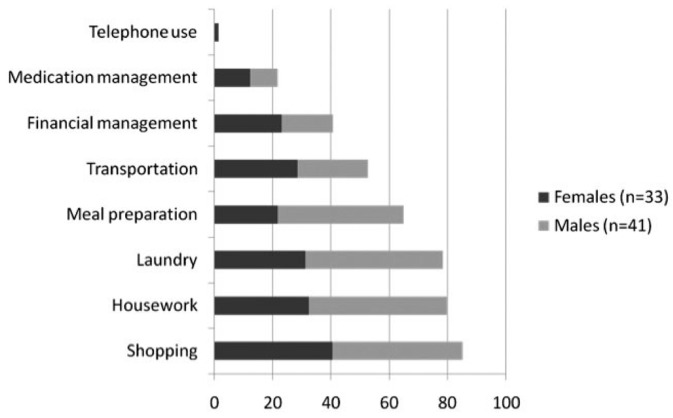
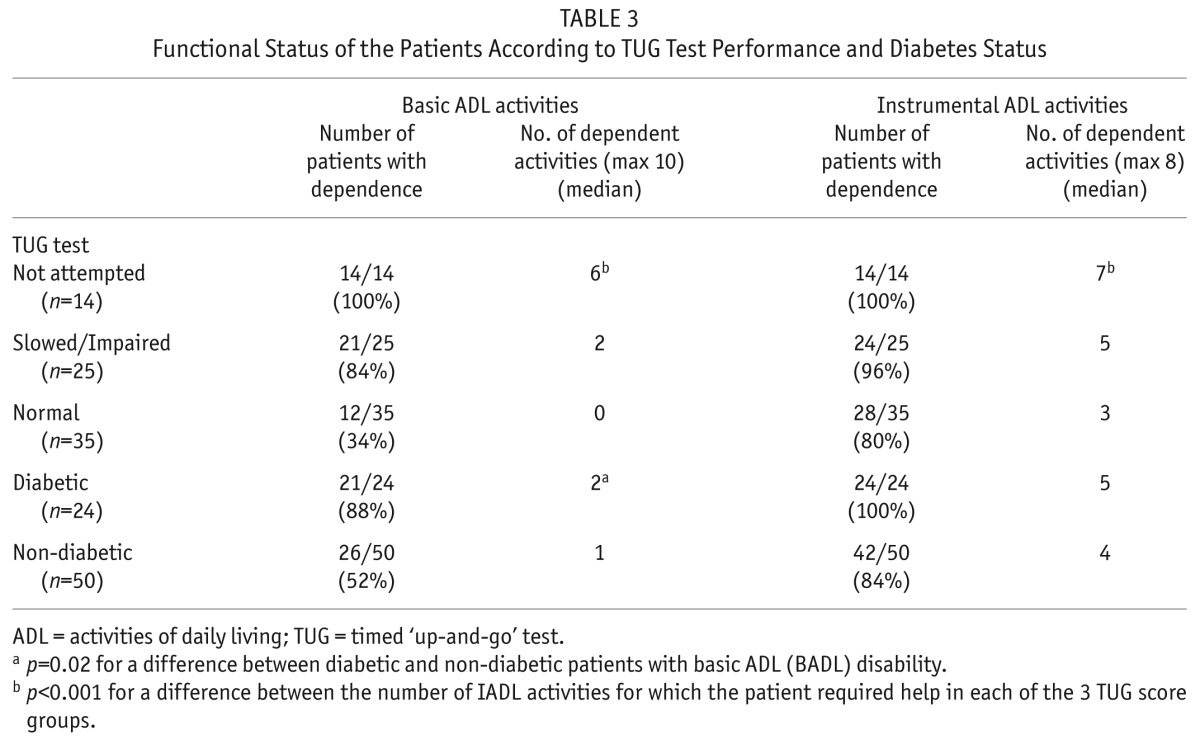
Only 8 patients (11%) were fully independent for both ADL and IADL activities. Dependence in one or more ADL activity was reported by 64% of participants (Figure 1), while 89% reported dependence in one or more IADL (Figure 2). All patients who reported ADL dependence also experienced IADL dependence. The most common tasks where patients reported requiring help or supervision were shopping (85%), housework (80%), laundry (78%), stair use (66%), meal preparation (65%), bathing (61%), transportation (53%), and outdoor ambulation (49%). Patients required regular assistance from family members or caregivers for a median of 1 ADL and 4 IADL (Table 2). Dependence appeared to associate with decreased gait speed as measured by the TUG score (p < 0.001 for both ADL and IADL disability), and the presence of diabetes (p = 0.03 for ADL burden, Table 3). No relationship with gender and dialysis prescription (CAPD vs CCPD) was found.
Figure 1 —
Percentage of study population showing ADL dependence across 10 aspects of personal care measured using the Barthel Index. ADL = activities of daily living.
Figure 2 —
Percentage of study population dependent in 8 activities of daily living measured using the Lawton Brody IADL scale. IADL = instrumental activities of daily living.
TABLE 2.
Results of Barthel (ADL) and Lawton Brody IADL Scoresa
TABLE 3.
Functional Status of the Patients According to TUG Test Performance and Diabetes Status
Discussion
The data presented here suggest that geriatric syndromes such as functional dependence, polypharmacy, muscular dysfunction (as evidenced by impaired TUG scores and impaired chair stands), and cognitive impairment were common amongst older PD patients living in the community at the time of the study. Both impaired cognitive function (33–35) and reduced physical performance measures (2,20,36) have been previously reported in patients established on PD. However, previous studies have not included data regarding functional independence.
Prior to the analysis, we had anticipated that we would observe a low level of functional dependency based on the clinical impression that patients choosing home dialysis therapies are highly motivated, and often independent. We also expected that caregivers of individuals with functional decline, either at the time of modality selection or at the time of dialysis initiation, would be more likely to encourage patients to choose HD over PD because they may perceive that the increase in the care that patients would require would place a larger burden on them as caregivers. We were therefore surprised at the high level of functional dependency. Although higher than levels seen in the transplant population receiving care at our center, the results of this study suggest disability levels are similar in a PD population to that reported for the population of patients of the same age undergoing HD at our center (17). The data presented in this study are consistent with recent results published in younger patient groups (20). In a recent large systematic review that compared physical well-being (mostly measured using the SF-36 subscales) in mostly younger renal populations across all renal replacement modalities (20), Purnell et al. found that, of those studies that properly adjusted for confounders, 83% showed no difference in physical function activities between PD and HD populations. Purnell et al. also reported that 90% of studies showed that renal transplant patients had a lower burden of physical ill-health, and experienced more freedom from illness (20).
This study suffers from common limitations that are associated with studies using single-center observational, cross-sectional designs. These include a potential for survival bias that may have resulted in an underestimation of the burden of disability, inability to generalize across all populations, and a lack of longitudinal outcome data. Several studies have noted that patients on dialysis (both HD and PD) have a high burden of cognitive impairment (34,37,38). Through our choice of MMSE as a cognitive screening test, we have likely not identified several patients who also had changes in executive brain functioning that may have impacted their ability to perform home-based PD therapy or to function within their own home. This again would have led to the potential underreporting of the burden of cognitive impairment in our study population. In addition, the statistical associations with clinical characteristics, such as gait speed and diabetes, must be interpreted with caution due to the small sample size and high chance of statistical error. The study does, however, have several strengths. The data are directly comparable to other studies across both the elderly HD population and the prevalent transplant population that have used identical protocols for recruitment and assessment (17). The high recruitment rate seen in our study limits any bias arising from the observation that often the sickest patients do not participate in studies, and the inclusion of objective measures of physical performance allows cross-validation of the self-report questionnaires.
This study is likely to be an underestimate of the burden of functional disability now seen in many units. Since these data were collected, our unit, like many others across Canada, has developed community care programs that offer in-home assistance with PD (39). Nurses visit up to 3 times daily and help with set up, PD connections and fluid assessments. As a result, many more patients with disabilities that would have precluded undergoing dialysis in their personal home, are now maintained in the community. The data presented here are of importance, particularly in those units offering assisted care, as they show there is a greater need for assistance with personal care than what was previously appreciated. When designing the study, we did not include measures of the type nor how much caregiving support was available to patients, as we did not anticipate PD patients would experience such a high number of geriatric syndromes. This study cannot address several issues. It is unclear if upper arm dysfunction plays any significant role in disability. Nor can these data answer whether there is any relationship between functional disability and PD technique survival. There is a strong literature showing that caregivers of patients undergoing chronic dialysis treatment have a high burden of physical and mental stress from the caregiving role. One can only speculate as to the effect on the overall success of PD therapy. Several studies have captured the negative emotional, social, physical and financial impact of living with and supporting individuals on dialysis, while a few have described positive effects such as an increased self-esteem and satisfaction, and an improved sense of meaning in life (40). While purely speculative, we question whether caregivers, under certain circumstances where they have experienced a gradual increase in the amount of care-giving required or feel overly burdened by the care needs, may advocate against patients returning to PD after, for example, a peritonitis requiring catheter removal or an acute illness requiring temporary cessation of PD. Data from ongoing studies, such as PD outcomes and practice patterns study may help answer some of these questions, in particular whether the observed gradual increase in the number of patients with functional disability who now are managed using chronic PD, correlate with a gradual increase in PD technique failure rates, such as that seen over the last decade (21,41–45).
In conclusion, we have demonstrated a high level of geriatric syndromes in a prevalent PD population that is of concern. We speculate that the burden of geriatric symptoms may, in the long term, affect technique failure and caregiver satisfaction, and suggest that further work is required to evaluate if support programs such as respite care, geriatric dialysis rehabilitation programs and caregiver appreciation nights can improve outcomes.
Disclosures
SVJ has received an honorarium from Amgen for giving Grand rounds at an academic institution within the past 12 months. No other disclosures. Grant Funding: Supported by a grant from the Physicians Services Foundation Inc. The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012. March; 23(3):533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int 2010. December; 78(11):1164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oliver MJ, Garg AX, Blake PG, Johnson JF, Verrelli M, Zacharias JM, et al. Impact of contraindications, barriers to self-care and support on incident peritoneal dialysis utilization. Nephrol Dial Transplant 2010. August; 25(8):2737–44. [DOI] [PubMed] [Google Scholar]
- 4. Oliver MJ, Quinn RR. Is the decline of peritoneal dialysis in the elderly a breakdown in the process of care? Perit Dial Int 2008. Sep-Oct; 28(5):452–6. [PubMed] [Google Scholar]
- 5. Brown EA, Johansson L, Farrington K, Gallagher H, Sensky T, Gordon F, et al. Broadening Options for Long-term Dialysis in the Elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant 2010; 25(11):3755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown EA. How to address barriers to peritoneal dialysis in the elderly. Perit Dial Int 2011. March; 31(Suppl 2):S83–5. [DOI] [PubMed] [Google Scholar]
- 7. Sands LP, Xu H, Craig BA, Eng C, Covinsky KE. Predicting change in functional status over quarterly intervals for older adults enrolled in the PACE community-based long-term care program. Aging Clin Exp Res 2008. October; 20(5):419–27. [DOI] [PubMed] [Google Scholar]
- 8. Carey EC, Covinsky KE, Lui LY, Eng C, Sands LP, Walter LC. Prediction of mortality in community-living frail elderly people with long-term care needs. J Am Geriatr Soc 2008. January; 56(1):68–75. [DOI] [PubMed] [Google Scholar]
- 9. Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med 2004. October; 19(10):1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc 2003. April; 51(4):451–8. [DOI] [PubMed] [Google Scholar]
- 11. Mehta KM, Yaffe K, Covinsky KE. Cognitive impairment, depressive symptoms, and functional decline in older people. J Am Geriatr Soc 2002. June; 50(6):1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walter LC, Brand RJ, Counsell SR, Palmer RM, Landefeld CS, Fortinsky RH, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA 2001. June 20; 285(23):2987–94. [DOI] [PubMed] [Google Scholar]
- 13. Gill T, Allore H, Holford T, Guo Z. The development of insidious disability in activities of daily living among community-living older persons. Am J Med 2004; 117(7):484–91. [DOI] [PubMed] [Google Scholar]
- 14. Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc 2002. September; 50(9):1492–7. [DOI] [PubMed] [Google Scholar]
- 15. Gill TM, Kurland B. The burden and patterns of disability in activities of daily living among community-living older persons. J Gerontol A Biol Sci Med Sci 2003. January; 58(1):70–5. [DOI] [PubMed] [Google Scholar]
- 16. Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci 2005. January; 60(1):74–9. [DOI] [PubMed] [Google Scholar]
- 17. Cook WL, Jassal SV. Functional dependencies among the elderly on hemodialysis. Kidney Int 2008. June; 73(11):1289–95. [DOI] [PubMed] [Google Scholar]
- 18. Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009. October 15; 361(16):1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mansilla Francisco JJ, Diez De los Rios Cuenca F, Cabrera Azana S, Cortes Torres J, Macias Lopez MJ, Gonzalez Castillo JA, et al. Impact of incident comorbidity on functional loss in elderly chronic kidney disease patients undergoing hemodialysis. CANNT J 2012. Jan-Mar; 22(1):25–9. [PubMed] [Google Scholar]
- 20. Purnell TS, Auguste P, Crews DC, Lamprea-Montealegre J, Olufade T, Greer R, et al. Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: a systematic review. Am J Kidney Dis 2013. November; 62(5):953–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belasco A, Barbosa D, Bettencourt AR, Diccini S, Sesso R. Quality of life of family caregivers of elderly patients on hemodialysis and peritoneal dialysis. Am J Kidney Dis 2006. December; 48(6):955–63. [DOI] [PubMed] [Google Scholar]
- 22. Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. New Engl J Med 2009. October 15; 361(16):1612–3. [DOI] [PubMed] [Google Scholar]
- 23. Jassal SV, Comment LA, Karaboyas A, Bieber BA, Morgenstern H, Sen A, et al. Functional dependence for activities of daily living in prevalent dialysis patients in the DOPPS study. Abstracts of the 50th ERA-EDTA (European Renal Association-European Dialysis and Transplant Association) Congress. May 18–21, 2013. Istanbul, Turkey. Nephrol Dial Transplant 2013. May; 28(Suppl 1):i1–553 MP8. [PubMed] [Google Scholar]
- 24. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991. February; 39(2):142–8. [DOI] [PubMed] [Google Scholar]
- 25. Botolfsen P, Helbostad JL, Moe-Nilssen R, Wall JC. Reliability and concurrent validity of the Expanded Timed Up-and-Go test in older people with impaired mobility. Physiother Res Int 2008. June; 13(2):94–106. [DOI] [PubMed] [Google Scholar]
- 26. Deathe AB, Miller WC. The L test of functional mobility: measurement properties of a modified version of the timed “up & go” test designed for people with lower-limb amputations. Phys Ther 2005. July; 85(7):626–35. [PubMed] [Google Scholar]
- 27. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther 2002. February; 82(2):128–37. [DOI] [PubMed] [Google Scholar]
- 28. Kornetti DL, Fritz SL, Chiu YP, Light KE, Velozo CA. Rating scale analysis of the Berg Balance Scale. Arch Phys Med Rehabil 2004. July; 85(7):1128–35. [DOI] [PubMed] [Google Scholar]
- 29. Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. MD State Med J 1965. February; 14:61–5. [PubMed] [Google Scholar]
- 30. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9(3):179–86. [PubMed] [Google Scholar]
- 31. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975. November; 12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 32. Pomeroy IM, Clark CR, Philp I. The effectiveness of very short scales for depression screening in elderly medical patients. Int J Geriatr Psychiatry 2001. March; 16(3):321–6. [DOI] [PubMed] [Google Scholar]
- 33. Conde SA, Fernandes N, Santos FR, Chouab A, Mota MM, Bastos MG. Cognitive decline, depression and quality of life in patients at different stages of chronic kidney disease. J Bras Nefrol 2010. Jul-Sep; 32(3):242–8. [PubMed] [Google Scholar]
- 34. Kalirao P, Pederson S, Foley RN, Kolste A, Tupper D, Zaun D, et al. Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis 2011. April; 57(4):612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Radic J, Ljutic D, Radic M, Kovacic V, Sain M, Dodig-Curkovic K. Is there differences in cognitive and motor functioning between hemodialysis and peritoneal dialysis patients? Ren Fail 2011; 33(6):641–9. [DOI] [PubMed] [Google Scholar]
- 36. Alayli G, Ozkaya O, Bek K, Calmasur A, Diren B, Bek Y, et al. Physical function, muscle strength and muscle mass in children on peritoneal dialysis. Pediatr Nephrol 2008. April; 23(4):639–44. [DOI] [PubMed] [Google Scholar]
- 37. Murray AM. Cognitive impairment in hemodialysis patients is common. Neurology 2006; 67(2):216–23. [DOI] [PubMed] [Google Scholar]
- 38. Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol 2010. August; 5(8):1429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ. Home care assistance and the utilization of peritoneal dialysis. Kidney Int 2007; 71(7):673–8. [DOI] [PubMed] [Google Scholar]
- 40. Suri RS, Larive B, Garg AX, Hall YN, Pierratos A, Chertow GM, et al. Burden on caregivers as perceived by hemodialysis patients in the Frequent Hemodialysis Network (FHN) trials. Nephrol Dial Transplant 2011. July; 26(7):2316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fan SLS, Sathick I, McKitty K, Punzalan S. Quality of life of caregivers and patients on peritoneal dialysis. Nephrol Dial Transplant 2008; 23(5):1713–9. [DOI] [PubMed] [Google Scholar]
- 42. Lobbedez T, Verger C, Ryckelynck JP, Fabre E, Evans D. Is assisted peritoneal dialysis associated with technique survival when competing events are considered? Clin J Am Soc Nephrol 2012. April; 7(4):612–8. [DOI] [PubMed] [Google Scholar]
- 43. Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, et al. Dialysis modality preference of patients with CKD and family caregivers: a discrete-choice study. Am J Kidney Dis 2012. July; 60(1):102–11. [DOI] [PubMed] [Google Scholar]
- 44. Juergensen PH, Wuerth DB, Juergensen DM, Finkelstein SH, Steele TE, Kliger AS, et al. Psychosocial factors and clinical outcome on CAPD. Adv Perit Dial 1997; 13:121–4. [PubMed] [Google Scholar]
- 45. Perl J, Wald R, Bargman JM, Na Y, Jassal SV, Jain AK, et al. Changes in patient and technique survival over time among incident peritoneal dialysis patients in Canada. Clin J Am Soc Nephrol 2012. July; 7(7):1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]