Abstract
Objective
The presence of anti-citrullinated protein antibodies (ACPA) in rheumatoid arthritis (RA) indicates a breach in tolerance. Recent studies indicate that this breach extends to homocitrullination of lysines with the formation of anti-carbamylated protein antibodies (ACarP Ab). We analyzed the clinical and serologic relationships of ACarP in two RA cohorts.
Methods
Circulating levels of IgG ACarP Ab were determined by ELISA in established (Dartmouth) and early (Sherbrooke) cohorts and evaluated for anti-CCP, specific ACPA, and rheumatoid factor (RF) levels using Student's t-test and correlation analysis.
Results
We identified elevated ACarP Ab titers in 47.0% of seropositive patients (Dartmouth, n=164), with relationships to anti-CCP (p<0.0001) and IgM-RF
(p=0.001). Similarly, 38.2% of seropositive patients from Sherbrooke (n=171) had elevated ACarP Ab; titers correlated to anti-CCP (p=0.01) but not IgM-RF (p=0.09). A strong correlation with anti-Sa was observed: 47.9% anti-Sa(+) patients were ACarP Ab(+) versus only 25.4% anti-Sa(−) (Sherbrooke; p=0.0002), and 62.6% anti-Sa(+) patients versus 26.9% anti-Sa(−) were ACarP Ab(+) (Dartmouth; p<0.0001). We found a more variable response for reactivity to citrullinated fibrinogen or to citrullinated peptides from fibrinogen and alpha enolase.
Conclusion
In two North American RA cohorts we observed a high prevalence of ACarP Ab positivity. We also describe a surprising and unexpected association of ACarP with anti-Sa Ab that could not be explained by cross-reactivity. Further, considerable heterogeneity exists between ACarP reactivity and other citrullinated peptide reactivity, raising the question of how the pathogenesis of antibody responses for carbamylated proteins and citrullinated proteins may be linked in vivo.
Keywords: Rheumatoid arthritis, Autoantibodies, cyclic citrullinated peptide, vimentin
Introduction
In addition to the formation of antibodies to citrullinated proteins (anti-citrullinated protein antibodies, or ACPA), recent studies have suggested that the disease-specific breaches in immune tolerance in rheumatoid arthritis (RA) also extends to another post-translational modification, namely homocitrullination of lysines. This modification is similar to the citrullination of arginines with the same functional ureido group. Enzymatic catalysis of arginine to citrulline is mediated by peptidylarginine deiminase (PADI) (1). In contrast, homocitrullination involves chemical carbamylation of the primary amine group of lysine (2) through a reaction with cyanate. Presumably, this process occurs at inflammatory sites by the action of myeloperoxidase (3, 4) although carbamylation can also occur as a result from the spontaneous reversible dissociation of urea (5, 6). Since both PADI 4 and myeloperoxidase are found in the azurophilic granules of neutrophils, it seems likely that these post-translational modifications occur at inflammatory sites. In this regard, fibrinogen has been shown to be a target for both modifications (7).
Humoral responses to homocitrullinated proteins (subsequently referred to as ACarP antibodies; ACarP Ab) have been reported in both patients with early and established seropositive RA, as well as by a proportion of seronegative RA patients (7-9). Indeed, ACarP Ab, like ACPA, can be found in patient sera years before the onset of RA, with a median time of approximately five years from first serologic appearance to the onset of clinical signs and symptoms (8, 10).
Given the similarity of these post-translational modifications of basic amino acids, it is not surprising that some, but not all, ACPA and ACarP Ab in patient sera have been reported to exhibit cross-reactivity (11, 12). It is additionally clear that some ACPA and ACarP Ab demonstrate remarkable fine specificity, being capable of discriminating between citrullinated and homocitrullinated forms of the same protein (7, 10). This is perhaps best demonstrated by the presence of ACarP Ab in ACPA-negative patients (10).
Anti-Sa antibodies represent one subfamily of ACPA that specifically target citrullinated vimentin, with prior research suggesting that they arise following the formation of neutrophil extracellular traps (NETs) and the subsequent breach of self-tolerance that leads to development of RA (13). Present in a subset of approximately 40% of RA patients (14), anti-Sa antibodies are notable in their high specificity (>95%) for RA (15, 16) and strong correlation with poor disease outcomes including radiographic progression, compared to anti-Sa negative patients (17).
In this study, we analyzed the relationships between serum/plasma levels of ACarP Ab and several clinical and serologic parameters, including anti-Sa status. We utilized both an established and an early RA cohort to confirm our findings.
Materials and Methods
Study Population: This study is derived from two North American cohorts of RA patients, based at Dartmouth-Hitchcock Medical Center in Lebanon, NH (USA) and Sherbrooke University Hospital Center in Sherbrooke, QC (Canada) (18). Shared epitope status and X-ray progression were available as previously described (18). A total of 548 RA patients and 65 healthy controls were involved in the analyses. All RA patients fulfilled the American College of Rheumatology 1987 revised criteria for the classification of RA (19). All participants provided written informed consent, and ethical permission was obtained from the Institutional Review Boards of both institutions.
The Dartmouth cohort is comprised of patients with established arthritis followed in the Rheumatology Clinic at the Dartmouth-Hitchcock Medical Center. This cohort includes 212 RA patients (Table 1). Demographic and serologic data are available on all patients. Serologic status was determined prior to enrollment by nephelometry (IgM RF >14 IU/ml using immunoturbidimetric measurement; Roche Diagnostics) and/or IgG anti-cyclic citrullinated peptide (anti-CCP) >5.0 U/ml by enzyme-linked immunosorbent assay (ELISA) (DiaSorin), with seronegativity being defined as both negative for RF and anti-CCP at all measurements. Subsequently, IgM RF and anti-CCP were measured by ELISA using the serum sample obtained at enrollment within the cohort as outlined below, and reactivity defined as per the manufacturer. This resulted in 3 samples being reclassified as seropositive due to positive RF reactivity.
Table 1.
Features of patients from both RA cohorts
| Dartmouth Established RA Cohort Lebanon, NH, USA | Sherbrooke Early RA Cohort Sherbrooke, QC, Canada | |||
|---|---|---|---|---|
| Seronegative | Seropositive | Seronegative | Seropositive | |
| Patients, % of cohort (n) | 21% (45) | 79% (167) | 49% (165) | 51% (171) |
| Age, mean ± SD years (range) | 57 ± 12 (29-74) | 58 ± 11 (19-91) | 62 ± 16 (19-89) | 57 ± 13 (19-89) |
| Women, % (n) | 67% (30) | 71% (119) | 64% (106) | 60% (103) |
| Years of symptoms, mean ± SD (unavailable for much of Dartmouth cohort) | 9% with <1 year from disease onset | 14% with <1 year from disease onset | 0.44 ± 0.43 | 0.45 ± 0.33 |
| Range of disease duration | From new onset to >20 years | From new onset to >20 years | 0-12 months from disease onset | 0-12 months from disease onset |
| Anti-CarP level, mean ± SD | 15.5 ± 54.1 | 70.2 ± 184.1 | 5.8 ± 12.6 | 34.9 ± 88.0 |
| DAS28-CRP, mean ± SD | Unavailable | Unavailable | 5.1 ± 1.5 | 5.0 ± 1.4 |
| CRP mg/L, mean ± SD | Unavailable | Unavailable | 24.4 ± 33.9 | 26.5 ± 35.7 |
| Anti-CCP units/ml, mean ± SD | N/A | 419 ±337 | N/A | 200 ± 136 |
| IgM RF IU/ml, mean ± SD | N/A | 135 ± 76 | N/A | 170 ± 79 |
| Anti-Sa status, % positive (n) | N/A | 57% (90/158) | 2% (3/134) | 51% (72/142) |
| Total IgG mg/ml, mean ± SD | 16 ± 11.6 | 17.6 ± 10.0 | 9.2 ± 2.5 | 21.1 ± 7.6 |
| CXCL13 pg/ml, mean ± SD | 116 ±76 | 1938 ± 6154 | 313 ± 1460 | 1490 ± 2460 |
| CXCL10 pg/ml, mean ± SD | 164 ± 207 | 392 ± 1121 | 118 ± 308 | 168 ± 307 |
| Shared epitope % positive (n) | 46% (6/13) | 83% (89/107) | 26% (33/128) | 50% (70/141) |
| RA treatment | Wide range from none to biologic therapy | Wide range from none to biologic therapy | Predominantly DMARD and corticosteroid naive | Predominantly DMARD and corticosteroid naive |
In the Dartmouth seronegative patients, only 28 of 45 patients had measured values for total IgG, CXCL13, and CXCL10. When percentages are given but not all patients in that group were tested (i.e. Shared epitope), the “n” is displayed as a fraction representing number of positive over number of those tested. Classification by serostatus and Anti-Sa reactivity was determined using different methods for the two cohorts (see Materials and Methods section).
The Sherbrooke cohort consists of a subset of the patients recruited at the Sherbrooke University Hospital Center, as part of the longitudinal Early Undifferentiated Polyarthritis (EUPA) Cohort. By contrast with the Dartmouth established RA cohort, this cohort represents an early arthritis population, consisting of patients who were largely DMARD- and corticosteroid-naïve at the time of inclusion, and with nearly equivalent numbers of seropositive (n=171) and seronegative (n=165) patients (Table 1). Cohort inclusion criteria included disease duration between 1 and 12 months and swollen joint count of three or more (based on the 66-joint count). For this study, seropositivity was defined as both an IgM-RF titer ≥40 IU/ml measured using RapiTex RF (Dade Behring) and IgG anti-CCP >20 U/ml using QUANTA Lite (Inova Diagnostics), present concurrently at least once. Seronegativity was defined as negative RF and anti-CCP at all visits. This subset of patients was chosen randomly from the Sherbrooke EUPA Cohort, with samples matched only for serologic status. Information gathered at enrollment included demographic details such as age, sex and time since onset of arthritis, as well as clinical details such as DAS28-CRP score, radiographic Sharp/van der Heijde score (SHS) of hand and feet films, and RF and ACPA status.
Serum/Plasma Analysis
Patient serum and plasma were stored at −80°C (Dartmouth Cohort) or −20°C (Sherbrooke Cohort) until Sirst analysis and then maintained at 4°C while additional assays were performed. Antibodies directed against CarP were detected using carbamylated fetal calf serum (FCS) as previously described (10). FCS was diluted 1:250 in water and then incubated with 1M KCN (Sigma-Aldrich) for 12 h at 37°C prior to 72 h dialysis against water at 4°C. For competition studies shown in Figure 3, this protocol was modified to incubating FCS at a 1:1 ratio with KCN (final 1M concentration) for 12h at 24°C before dialysis against PBS. Successful carbamylation of FCS was confirmed by immunoblot using a rabbit anti-carbamyl-lysine polyclonal antibody (Cell Biolabs, Inc.).
Figure 3.
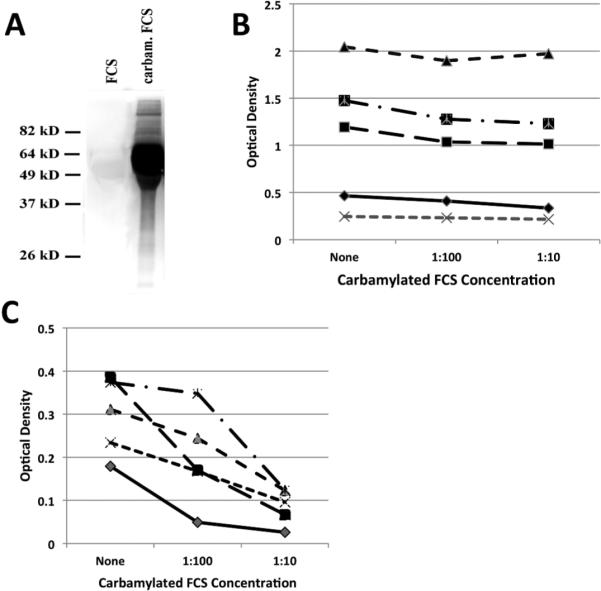
Competition for anti-Sa reactivity. A. Immunoblot analysis of native FCS and carbamylated FCS using rabbit anti-carbamyl lysine antibody confirming extensive carbamylation. B. Five sera were tested for anti-Sa reactivity or (C) CarP reactivity in the absence or presence of varying (1:100 and 1:10) dilutions of carbamylated FCS (0.32-3.2 mg/ml).
96 well plates (R&D Systems) were coated with carbamylated FCS or noncarbamylated FCS at 4°C overnight and then blocked with 1% bovine serum albumin (BSA) (Sigma) in PBS. Human serum or plasma was incubated at 4°C overnight. Bound human IgG antibodies were detected after incubation for 3.5 h at 4°C with horseradish peroxidase-conjugated goat anti-human IgG (H+L) F(ab’)2 antibody fragment (KPL, Kirkegaard & Perry Laboratories, Inc.), at a 1:10,000 dilution, followed by the addition of tetramethylbenzidine (TMB) substrate (R&D Systems) and subsequent administration of stop solution. Absorbance was measured at 450 nm (reference wavelength of 570 nm). Optical density was transformed into arbitrary units (AU/mL) using the titration curve of a strongly ACarP Ab(+) serum sample, whose ACarP Ab concentration was set at 500 AU/mL. In order to analyze specific ACarP Ab reactivity, the background signal with noncarbamylated FCS was subtracted from the carbamylated FCS signal. The cut-off for a positive response was calculated as the mean (4.2 AU/mL) plus two times the SD (9.0 AU/mL) of the specific ACarP reactivity of the healthy controls, corresponding to a level of 22.16 AU/mL.
For both the Dartmouth and the Sherbrooke cohorts, serum levels of anti-Sa IgG were measured by ELISA (EUROIMMUN). For both cohorts, ELISA was utilized to determine IgG levels (Immunology Consultants Laboratory), IgM RF levels (TheraTest Laboratories), and CXCL13 and CXCL10 levels (R&D Systems). In the Dartmouth Cohort, we additionally measured serum levels of IgG ACPA using a human QUANTA Lite CCP3 IgG ELISA kit (Inova Diagnostics).
Competition Studies
The cross-reactivity of ACarP Abs for the Sa antigen (citrullinated vimentin) was examined in sera from the Dartmouth cohort. Sera (n=5) were left untreated or were preincubated (15’ at RT) with carbamylated FCS at a concentration of either 1:10 or 1:100. Samples were then transferred to a 96-well plate coated with Sa antigen or carbamylated FCS, and levels of IgG reactivity were measured by ELISA, as described above.
Multiplex Assay
A subset of the Dartmouth cohort was analyzed for reactivity to citrullinated native fibrinogen and to synthetic citrullinated peptides using a bead-based multiplex array platform. Specified peptides were synthesized with C- and N-terminal cysteines to cyclize the peptide that include an N-terminal biotin. Microsphere beads (LumAvidin, Luminex, Inc.) were coated with a >10-fold molar excess of each type of biotinylated antigen (citrullinated, native, or other ligand controls), and then blocked with free biotin. A master mixture of different coated microspheres was made. Serum samples were diluted 1:1000 in SM01 buffer (Surmodics, Inc.); then, 200 μL of diluted serum was incubated with 10 μL of the antigen-coated microsphere mixture, representing 2000 beads/analyte. Samples were incubated overnight at 4°C with shaking at 50 rpm, then loaded into individual duplicate wells of a 96-well vacuum filter plate (Millipore). Wells were then washed thrice with PBS-Tween 20 (v/v 0.05%). For detection, 100 μL of PE-conjugated anti-human IgG Fcγ-specific antibody (eBioscience) diluted 1:250 in SM01 buffer was added to each well, and incubated 1 hr at RT, with shaking. After 3 washes, microspheres were resuspended at 150 μL and data collected using a Luminex 200 (Luminex, Inc.). Data represent mean median fluoresence intensity (MFI) values from duplicate wells.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software version 6 (GraphPad Software). Levels of IgG, anti-CCP, CXCL10, CXCL13, hsCRP and anti-Sa were log-transformed in order to normalize the data. Student's t-test for independent samples was used to compare the means of two sets of data. Pearson correlation was used for analysis of log-transformed ACarP Ab and other measures. Chi square analysis was utilized for determination of ACarP Ab(+) relationship to the shared epitope. All p values were two-tailed, with values <0.05 considered significant.
Results
Elevated ACarP Ab titers in seropositive rheumatoid arthritis patients
The Dartmouth RA cohort (n=212) is an established RA cohort, including patients with a broad range of disease duration (Table 1). We analyzed serum/plasma ACarP Ab levels in seropositive and seronegative RA patients relative to a control group of healthy individuals (n=65) to establish baseline values. Among the healthy cohort, the mean ACarP Ab level was measured at 4.2 AU/mL, with over fifty percent of donors having no detectable signal. We established the cut-off for ACarP Ab positivity at 22.16 AU/mL, corresponding to the mean plus two times the standard deviation (9.0 AU/mL). Three healthy donors (4.6%) had values above this level; this is similar to levels (6.3%) previously described (8).
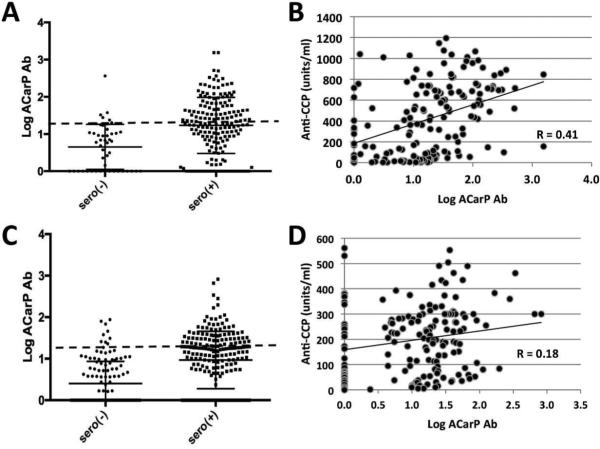
Among seropositive RA patients (n=167), 46.1% exhibited elevated levels of ACarP Ab (Figure 1A). With a median of 18.9 AU/mL, mean of 70.2 AU/mL, and an interquartile range of 6.8-58.7 AU/mL, the distribution of ACarP Ab concentrations was strongly positively skewed, with observed levels ranging as high as 1550 AU/mL. Due to the non-normal nature of the distribution, ACarP Ab levels were log-transformed for analysis. Among seronegative patients (n=45), 8.9% were ACarP Ab(+), and ACarP Ab levels fell within a more narrow range, with an interquartile range (IQR) of 5.9-11.0 AU/mL. An ACarP Ab titer greater than 40 AU/mL was observed in only one patient (2.2%) while this value was exceeded in nearly one-third (31.1%) of seropositive patients. This patient had been seronegative for ACPA and RF on multiple determinations over >10 years. Overall, the mean ACarP Ab level in seropositive RA patients was significantly higher than in otherwise seronegative patients (70.2 AU/mL vs. 7.6 AU/mL; p < 0.0001; Figure 1A).
Figure 1.
Relationships of anti-carbamylated protein antibody (ACarP Ab) titer to seropositivity and anti-cyclic citrullinated peptide antibody (anti-CCP). Panels (A) and (B) represent the Dartmouth established RA cohort, and panels (C) and (D) represent the Sherbrooke EUPA cohort. A, C. Log-transformed ACarP Ab is higher in seropositive than in seronegative RA patients in both the Dartmouth cohort (seronegative: n = 45, mean (SEM) = 0.66 (0.08); seropositive: n = 167, mean (SEM) = 1.23 (0.05); p<0.0001) and the Sherbrooke cohort patients (seronegative: n = 165, mean (SEM) = 0.40 (0.05); seropositive: n = 171, mean (SEM) = 0.97 (0.05); p< 0.0001). B, D. Among seropositive samples, a positive correlation is observed between ACarP Ab titer and anti-CCP in both cohorts (p< 0.0001, p= 0.01, respectively). Diagonal lines represent lines of best fit.
ACarP Ab relationships with other serologic and clinical features
Among seropositive RA patients in this cohort, we observed a significant positive correlation between levels of ACarP Ab and anti-CCP (R = 0.41, p < 0.0001; Figure 1B). Among this group, ACarP Ab(+) patients had a median ACPA of 660 units/mL (IQR: 373 - 788) versus a level of 143 units/mL (IQR: 27 - 450) among ACarP Ab(−) patients (p < 0.0001). ACarP Ab titers among seropositive patients also demonstrated a direct but weaker relationship with IgM RF (R = 0.25, p = 0.001, Supp. Figure 1A). Additional analyses of this cohort demonstrated a lack of relationship between ACarP Ab titer and total serum IgG levels in the seropositive patients (R = 0.15, p = 0.06, Supp. Figure 1B).
We have previously identified a strong relationship to the B cell chemokine CXCL13 and autoantibody status (18). We evaluated the relationship of ACarP titer with CXCL13 as well as CXCL10, another chemokine strongly associated with RA. We did identify correlations in seropositive RA patients between ACarP Ab titer and the levels of the chemokines CXCL13 (R = 0.21, p = 0.007, Supp. Figure 1C) and CXCL10 (R = 0.18, p = 0.03, Supp. Figure 1D), but no relationships were identified with age (R= −0.01, p = 0.87, Supp. Figure 1E) or gender (p = 0.08, Supp. Figure 1F). Similarly, ACarP positivity was not more commonly associated with inheritance of those MHC II alleles associated with the shared epitope that are the strongest set of genetic susceptibility factors for RA (p = 0.61; n=107; data not shown).
Relationships between ACarP Ab and antibody levels in an early rheumatoid arthritis cohort
We confirmed these results in validation studies of the Sherbrooke EUPA cohort, a cohort of predominantly DMARD- and corticosteroid-naïve patients with early RA (Table 1). Seropositive patients in this cohort (n=171) displayed similar trends as their Dartmouth counterparts with established RA; 38.2% had elevated ACarP Ab levels (p<0.0001, Figure 1C). Once again, the distribution of ACarP Ab concentrations was not normally distributed, with a median of 17.0 AU/mL, mean of 34.9 AU/mL, interquartile range of 5.0-31.3 AU/mL and a maximal value of 821 AU/mL. As with the Dartmouth cohort, the ACarP Ab levels had a positive correlation with anti-CCP levels (R = 0.2, p = 0.01) (Figure 1D); however, no relationship was seen with IgM RF (R = 0.13, p = 0.09) or serum total IgG (R = 0.097, p = 0.2) (Supp. Figures 2A,B). When results from seropositive patients from the Dartmouth and Sherbrooke cohorts were pooled, ACarP Ab levels maintained a direct relationship with ACPA levels (R = 0.35, p < 0.0001, Supp. Figure 4A) with a weaker relationship to IgM RF (R = 0.16, p = 0.004, Supp. Figure 4B).
Among seronegative patients (n=165), 6.1% were ACarP Ab(+), and the distribution of ACarP Ab levels were within a relatively narrow range, with an interquartile range (IQR) of 0.0-7.3 AU/mL. An ACarP Ab titer greater than 40 AU/mL was observed in four patients (2.4%) compared to 31 of 173 (18%) seropositive patients. Furthermore, akin to the Dartmouth cohort, the mean ACarP Ab level in seropositive RA patients was significantly higher than in seronegative patients (34.7 vs. 5.8 AU/ml; p < 0.0001).
As with the Dartmouth cohort, no relationships between ACarP Ab levels and age or gender were observed among seropositive Sherbrooke patients (Supp. Figures 2E,F). In this cohort we also have clinical variables such as DAS28-CRP, CRP levels, and erosions, yet none exhibited any relationships to ACarP Ab levels (Supp. Figure 3A-C). We were able to evaluate ACarP reactivity in relation to being a non-smoker or to being a current or former smoker, and found that there is a relationship observed, with a mean ACarP Ab level of 24.1 and 40.3 AU/ml, respectively (p = 0.02, Supp. Figure 3D). There were no correlations with CXCL13 (R=0.08, p=0.31) or CXCL10 (R=0.09, p=0.26) (Supp. Figures 2C,D), and no relationship with the shared epitope was seen (p = 0.3, n=141; data not shown).
Relationship between ACarP Ab and anti-Sa antibody levels
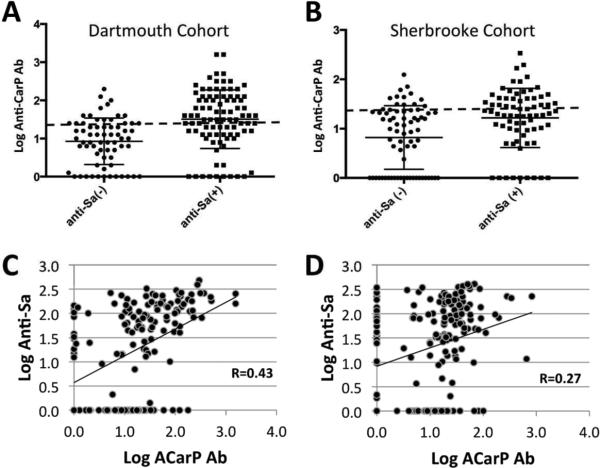
The Sherbrooke cohort has previously been evaluated for anti-Sa status, which has been shown to be a biomarker for RA disease and severity (21). In the current studies, we found that ACarP-positivity was more highly represented in the anti-Sa(+) patients (47.9%) compared to only 25.4% percent of anti-Sa(−) patients (Figure 2B; p = 0.0002). Moreover, anti-Sa(+) patients in this cohort had higher ACarP Ab levels than anti-Sa(−) patients (37.6 AU/mL and 14.6 AU/mL, respectively). Intrigued by this association, we tested seropositive samples from the Dartmouth cohort for anti-Sa antibody level (n=158). Once again, anti-Sa status had a strong relationship with ACarP Ab status (p <0.0001), with 62.6% of anti-Sa(+) patients also ACarP(+) versus only 26.9% of anti-Sa(−) patients (Figure 2A). Similarly, the mean ACarP Ab titer was higher among anti-Sa(+) patients (112.5 AU/mL) relative to the anti-Sa(−) wherein the mean titer (19.1 AU/mL) was below the threshold for positivity. Correlation analysis of ACarP Ab titers and anti-Sa titers also yields a significant relationship in both the Dartmouth cohort (R = 0.43, p < 0.0001; Figure 2C) and the Sherbrooke cohort (R=0.27, p = 0.0002; Figure 2D). 133/165 seronegative patients from the Sherbrooke cohort and 4/45 from the Dartmouth cohort were also tested for their anti-Sa status. Among these samples, 14 donors were ACarP Ab(+), 4 were anti-Sa Ab(+), but only one was positive for both ACarP and anti-Sa Ab.
Figure 2.
ACarP antibody titer robustly correlates with anti-Sa Ab levels among seropositive RA patients in both the Dartmouth and Sherbrooke cohorts. A. Anti-Sa(+) among seropositive patients in the Dartmouth cohort also exhibit a significantly higher mean ACarP Ab level than anti-Sa(−) among seropositive patients (anti-Sa positive: n = 91, mean (SEM) = 1.51 (0.08); anti-Sa negative: n = 67, mean (SEM) = 0.93 (0.07); p < 0.0001). B. Anti-Sa(+) among seropositive patients in the Sherbrooke cohort have a significantly higher mean ACarP Ab level than anti-Sa(−) among seropositive patients (anti-Sa positive: n = 72, mean (95% CI) = 1.22 (1.15-1.29); anti-Sa negative: n=71, mean (95% CI) = 0.82 (0.74-0.90); p = 0.0002). C. Within the Dartmouth cohort, ACarP Ab is significantly correlated with anti-Sa antibody titer (n=158; p<0.0001). D. A similar relationship is seen with seropositive RA patients from the Sherbrooke cohort (n=169; p=0.0002).
The cross-reactivity of ACarP antibodies with anti-Sa reactivity was evaluated using carbamylated FCS (Figure 3A). Preincubation with carbamylated FCS blocked ACarP reactivity but had no effect on anti-Sa levels (Figure 3B, C). These data are consistent with the interpretation that association of anti-Sa and ACarP reactivity arises from coexpression rather than cross-reactivity.
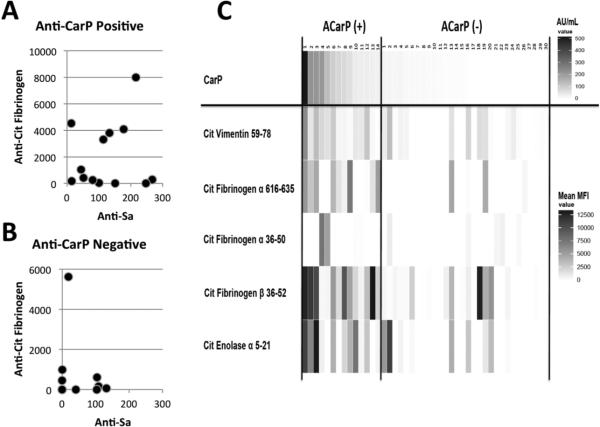
To further evaluate for interactions of ACarP Ab with anti-citrullinated proteins, we analyzed 31 seropositive serum samples for antibody reactivity to CarP (13 ACarP(+); 18 ACarP(−)), Sa antigen and human citrullinated (cit-) full length fibrinogen. ACarP positivity was significantly associated with higher levels of reactivity with Sa (as described above) as well as with cit-fibrinogen relative to ACarP negative sera (p < 0.0001). In contrast, the presence of anti-cit-fibrinogen Abs does not seem linked to the presence of anti-Sa (Figure 4A,B). When specific citrullinated peptide reactivity was tested, a great deal of variability was seen in ACarP (+) sera, although there was less heterogeneity seen with reactivity to citvimentin 59-78 and cit-fibrinogen-β 36-52 (Figure 4C).
Figure 4.
Variability in reactivity to CarP, Sa antigen, citrullinated (cit-) fibrinogen, and cit-peptides in the Dartmouth Cohort. A. ACarP(+) samples, have a stronger relationship to cit-fibrinogen reactivity than in (B) AcarP(−) samples (p< 0.0001). There is no relationship between anti-Sa and cit-fibrinogen values either in the ACarP(+) samples (R= -0.08; p= 0.79; n=13) or in the ACarP(−) samples (R= −0.028; p= 0.91; n=18). C. Using a multiplex fluorescent bead array, antibody reactivity to citrullinated (cit-) vimentin 59-78, cit-fibrinogen-α 616-635, cit-fibrinogen-α 39-50, cit-fibrinogen-β 36-52, and cit-α-enolase 5-21 was measured in ACarP Ab(+) (n=14) and ACarP Ab(−) (n=30) samples. Values displayed represent AU/mL for CarP reactivity while the values for the peptides represent mean MFI (median fluorescence intensity) of reactivity to the citrullinated peptide minus the value obtained from native peptide (corrected value).
Discussion
In the current studies we observed that levels of ACarP Ab are elevated in about half (46.1%) of established seropositive RA patients, while we found a somewhat lower frequency (38.2%) of ACarP Ab in seropositive patients from an early RA cohort. These data are compatible with prior reports of frequencies of 49-73% ACarP Ab positivity among seropositive RA patients in early RA cohorts (9, 10) and 40-55% in established RA cohorts (7). We also found ACarP Ab in only a small percentage (6.1-8.9%) of seronegative RA patients, and our results suggest that most seronegative RA patients with ACarP positivity are low-titer positive, rarely having levels more than 1-2 standard deviations above the determined cutoff for seropositivity. The comparable frequency and titers of the ACarP antibodies in these two cohorts may suggest that ACarP Ab levels remain relatively stable over time. However, additional research is needed to evaluate the stability of ACarP titer over the course of RA treatment. Additionally, despite that these clinical centers used two different methods for determining seropositivity, similar results were documented across both cohorts, which further strengthens the general relevance of these findings.
We also confirmed an association between anti-CCP and ACarP Ab titers in both cohorts, as previously reported by Shi et al (10). Various associations (i.e., with IgM RF, CXCL13, and CXCL10) were observed in the Dartmouth cohort, but not found in the Sherbrooke cohort. The association between ACarP Ab titer and anti-CCP, but absence of an association with the shared epitope, supports previous findings by Jiang et al., and provides further evidence that this immunoreactivity may arise through different pathogenic pathways (9).
An unexpected finding was the high concordance between anti-Sa antibody status and ACarP autoantibody positivity and levels. This association was at least as strong as the association of ACarP and anti-CCP reactivity in the Dartmouth cohort. Anti-Sa represents a type of antibody reactivity with citrullinated vimentin, an intermediate filament cytoplasmic protein. In contrast, ACarP autoantibodies are detected using carbamylated FCS as the source of antigenic ligand, and frequently react with carbamylated fibrinogen (7, 10).
Several lines of evidence suggest ACarP and anti-Sa constitute distinct antibody specificities rather than reflect broadly cross-reactive antibodies. Figure 4A shows specific ACarP positivity using carbamylated FCS was better correlated with reactivity with Sa relative to citrullinated forms of fibrinogen, which is a major target of carbamylation in FCS. Second, anti-Sa reactivity was found in ACarP negative sera (Figure 4B). Finally, competition experiments in patients that contained both ACarP and anti-Sa reactivity did not suggest cross-reactivity (Figure 3). Clarifying this relationship would be enhanced by mapping the neoepitopes in fibrinogen generated by carbamylation that are being recognized in RA patients.
This finding is potentially interesting due to the reports that ACarP antibody positivity is associated with radiographic progression (10). The same relationship has been reported with anti-Sa (17, 22, 23). Our data raises the possibility that the co-expression of ACarP and anti-Sa may be confounding these reports. Alternatively, the presence of both anti-Sa and ACarP might be associated with an improved ability to predict severe erosive RA. Additional evaluation is needed to determine if ACarP has added value to anti-Sa in the determination of disease activity and prognosis. The absence of cross-reactivity between these assays, in contrast to that seen with ACPA and anti-CarP, may make these tests important adjuncts to ACPA testing in predicting disease outcome.
Supplementary Material
Acknowledgments
Sources of Support: This work was supported by National Institutes of Health grant R21 AR-061643 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to WFCR) and the Rheumatology Research Foundation (to WFCR and a separate award to GJS), by the Hitchcock Foundation (to JDJ) and by grants 00/201 and RG06/108 from The Arthritis Society and Canadian Institutes for Health Research grant MOP-110959 (to GB). AJB-F, AM, and GB are members of the Centre de Recherche Clinique Etienne-LeBel at the Centre Hospitalier Universitaire de Sherbrooke, which received a team grant from the Health Research Funds of Quebec. Since 2007, the Sherbrooke EUPA cohort has received financial support from the Canadian ArTHritis CoHort (CATCH) study, designed and implemented by investigators and financially supported initially by Amgen Canada and Pfizer Canada via an unrestricted research grant. As of 2011, further support was provided by Hoffmann-La Roche, Chemicals of Belgium (UCB) Canada, Bristol-Myers Squibb Canada, Abbott Laboratories and Janssen Biotech (a wholly owned subsidiary of Johnson & Johnson).
Abbreviations
- ACarP Ab
Anti-carbamylated protein antibody
- anti-CCP and ACPA
Anti-citrullinated peptide/protein antibody
- CXCL13
C-X-C motif chemokine 13
- DAS28-CRP
Disease Activity Score in 28 joints based on C-reactive protein
- EUPA
Early Undifferentiated Polyarthritis Cohort
- FCS
Fetal calf serum
- hsCRP
High-sensitivity C-reactive protein
- CXCL13
C-X-C motif chemokine 13
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- SEM
Standard error of the mean
- SHS
Sharp/van der Heijde score
Footnotes
Competing interests:
The authors declare that they have no competing interests.
References
- 1.Sugawara K, Oikawa Y, Ouchi T. Identification and properties of peptidylarginine deiminase from rabbit skeletal muscle. J Biochem. 1982;91:1065–71. doi: 10.1093/oxfordjournals.jbchem.a133755. [DOI] [PubMed] [Google Scholar]
- 2.Stark GR, Stein WH, Moore S. Reactions of the Cyanate Present in Aqueous Urea with Amino Acids and Proteins. J Biol Chem. 1960;235:3177–3181. [Google Scholar]
- 3.Sirpal S. Myeloperoxidase-mediated lipoprotein carbamylation as a mechanistic pathway for atherosclerotic vascular disease. Clin Sci (Lond) 2009;116:681–95. doi: 10.1042/CS20080322. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–84. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 5.Erill S, Calvo R, Carlos R. Plasma protein carbamylation and decreased acidic drug protein binding in uremia. Clin Pharmacol Ther. 1980;27:612–8. doi: 10.1038/clpt.1980.87. [DOI] [PubMed] [Google Scholar]
- 6.Fluckiger R, Harmon W, Meier W, Loo S, Gabbay KH. Hemoglobin carbamylation in uremia. N Engl J Med. 1981;304:823–7. doi: 10.1056/NEJM198104023041406. [DOI] [PubMed] [Google Scholar]
- 7.Scinocca M, Bell DA, Racape M, Joseph R, Shaw G, McCormick JK, et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J Rheumatol. 2014;41:270–9. doi: 10.3899/jrheum.130742. [DOI] [PubMed] [Google Scholar]
- 8.Shi J, van de Stadt LA, Levarht EW, Huizinga TW, Toes RE, Trouw LA, et al. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum. 2013;65:911–5. doi: 10.1002/art.37830. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Trouw LA, van Wesemael TJ, Shi J, Bengtsson C, Kallberg H, et al. Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann Rheum Dis. 2014;73:1761–8. doi: 10.1136/annrheumdis-2013-205109. [DOI] [PubMed] [Google Scholar]
- 10.Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GM, van Veelen PA, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A. 2011;108:17372–7. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mydel P, Wang Z, Brisslert M, Hellvard A, Dahlberg LE, Hazen SL, et al. Carbamylation-dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol. 2010;184:6882–90. doi: 10.4049/jimmunol.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J, Willemze A, Janssen GM, van Veelen PA, Drijfhout JW, Cerami A, et al. Recognition of citrullinated and carbamylated proteins by human antibodies: specificity, cross-reactivity and the 'AMC-Senshu' method. Ann Rheum Dis. 2013;72:148–50. doi: 10.1136/annrheumdis-2012-201559. [DOI] [PubMed] [Google Scholar]
- 13.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vossenaar ER, Despres N, Lapointe E, van der Heijden A, Lora M, Senshu T, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6:R142–50. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hueber W, Hassfeld W, Smolen JS, Steiner G. Sensitivity and specificity of anti-Sa autoantibodies for rheumatoid arthritis. Rheumatology (Oxford) 1999;38:155–9. doi: 10.1093/rheumatology/38.2.155. [DOI] [PubMed] [Google Scholar]
- 16.El-Gabalawy HS, Wilkins JA. Anti-Sa antibodies: prognostic and pathogenetic significance to rheumatoid arthritis. Arthritis Res Ther. 2004;6:86–9. doi: 10.1186/ar1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathsson L, Mullazehi M, Wick MC, Sjoberg O, van Vollenhoven R, Klareskog L, et al. Antibodies against citrullinated vimentin in rheumatoid arthritis: higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008;58:36–45. doi: 10.1002/art.23188. [DOI] [PubMed] [Google Scholar]
- 18.Jones JD, Hamilton BJ, Challener GJ, de Brum-Fernandes AJ, Cossette P, Liang P, et al. Serum C-X-C motif chemokine 13 is elevated in early and established rheumatoid arthritis and correlates with rheumatoid factor levels. Arthritis Res Ther. 2014;16:R103. doi: 10.1186/ar4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Boire G, Cossette P, de Brum-Fernandes AJ, Liang P, Niyonsenga T, Zhou ZJ, et al. Anti-Sa antibodies and antibodies against cyclic citrullinated peptide are not equivalent as predictors of severe outcomes in patients with recent-onset polyarthritis. Arthritis Res Ther. 2005;7:R592–603. doi: 10.1186/ar1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzian MC, Carrier N, Cossette P, de Brum-Fernandes AJ, Liang P, Menard HA, et al. Outcomes in recent-onset inflammatory polyarthritis differ according to initial titers, persistence over time, and specificity of the autoantibodies. Arthritis Care Res (Hoboken) 2010;62:1624–32. doi: 10.1002/acr.20288. [DOI] [PubMed] [Google Scholar]
- 22.Goldbach-Mansky R, Lee J, McCoy A, Hoxworth J, Yarboro C, Smolen JS, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–43. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syversen SW, Goll GL, van der Heijde D, Landewe R, Lie BA, Odegard S, et al. Prediction of radiographic progression in rheumatoid arthritis and the role of antibodies against mutated citrullinated vimentin: results from a 10-year prospective study. Ann Rheum Dis. 2010;69:345–51. doi: 10.1136/ard.2009.113092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.