Summary
There is accumulating evidence from behavioural, neurophysiological and neuroimaging studies that the acquisition of motor skills involves both perceptual and motor learning. Perceptual learning alters movements, motor learning and motor networks of the brain. Motor learning changes perceptual function and the brain’s sensory circuits. Here we review studies of both human limb movement and speech which indicate that plasticity in sensory and motor systems is reciprocally linked. Taken together, this points to an approach to motor learning in which perceptual learning and sensory plasticity play a fundamental role.
Keywords: human motor learning, somatosensory plasticity, perceptual learning
Perceptual change and human motor learning
There has been recent interest in the idea that perceptual and motor learning do not occur in isolation but rather that motor learning changes sensory systems and sensory networks in the brain and, likewise that perceptual learning changes movements and the brain’s motor areas. This review will present evidence in support of both of these ideas, drawing on examples from human arm movement and speech motor learning. We suggest that perceptual learning is an integral part of motor learning and contributes in several ways. Perceptual learning results in changes to motor networks in the brain and in this way participates directly in motor learning. Perceptual learning is also associated with plasticity in sensory systems that is dependent on both afferent inputs from the periphery and on cortico-cortical projections from motor areas. We will propose that perceptual learning, and associated changes to sensory systems, play a fundamental role in human motor learning and that in this context, the two generally occur together.
Neuroanatomical basis for reciprocal plasticity in sensory and motor networks
The efferent and afferent pathways linking the spinal cord with sensorimotor cortex and cerebellum are well known (see [1–3] for reviews). There are also extensive neuroanatomical connections between cortical motor and somatosensory areas that could drive plasticity in either direction, on the basis of use or experience. The connections extend from those between primary motor and somatosensory cortices to more distant connections linking premotor and prefrontal cortex with second somatosensory (SII) and parietal cortex (Table 1).
Table 1.
Anatomical connections between somatosensory cortex and frontal motor areas. M1 (primary motor cortex), PMC (premotor cortex), SMA (supplemental motor area), CMA (cingulate motor area), PF, PFG, SII (second somatosensory cortex), PE, PMV (ventral premotor cortex), PMD (dorsal premotor cortex). The data are for macaques unless otherwise indicated.
| Source/Origin | Target | |
|---|---|---|
|
| ||
| Core Sensorimotor Network | Frontal Motor Areas | Somatosensory Cortex |
| M1 | 1 [4] | |
| M1, PMC | 2 [5] | |
| SMA, CMA | 3a [6] | |
| PMC | PV [7] | |
| M1, SMA | 3a (marmosets) [8] | |
| M1 | 3b (squirrel monkeys) [9] | |
|
| ||
| Somatosensory Cortex | Frontal Motor Areas | |
| 1, 2, 3a, 5 | M1 [6, 10] | |
| 1 | M1, SMA [11] | |
| 2 | SMA, PMC [11] | |
| 3a, 3b, 1, 2, SII, PV | M1 (squirrel monkeys) [12] | |
| 3a, 1, 2, SII, PV, 5 | PMV (owl monkeys) [13] | |
|
| ||
| Extended Network | Parietal Cortex | Frontal Cortex |
| PF, PFG, SII | PMV, 46v [14 – 15] | |
| PE | PMD, SMA [16] | |
Somatosensory receptive fields are present in primary motor cortex and dorsal and ventral premotor cortices [17 – 18] and there are both visual and auditory receptive fields in ventral premotor cortex [19 – 20]. Neurons in ventral premotor cortex, SMA, and even ventrolateral prefrontal cortex are involved in perceptual decision making [21–22]. Accordingly one would expect that plasticity in the frontal motor networks should occur in conjunction with sensory processing and in particular from the extended and systematic nature of inputs related to perceptual learning.
Motor learning results in changes to sensory function
In work on human arm movement, both somatosensory and visual perceptual change have been observed to accompany sensorimotor adaptation. The changes are obtained in the context of force-field learning [23 – 26], visuomotor adaptation [27 – 29] and prismatic adaptation [30 – 35]. In each, there are systematic shifts in the somatosensory perceptual boundary (the felt position of the limb) and these occur over the same time period as adaptation [26]. There are also changes in visual motion processing in relation to force-field learning and prism adaptation [33 – 36] and changes to auditory localization following visuomotor adaptation [37]. The magnitude of the perceptual change ranges from about 20% to as much as 50% of the observed change in movement associated with adaptation. This is true even for force-field learning if average rather than maximum movement deviation is used as a behavioral measure of learning. The somatosensory shifts are in the direction of the perturbation. Thus, if the limb is deflected to the right, the sensed position of the limb likewise shifts rightward.
The perceptual change that occurs in conjunction with adaptation is durable. In studies to date, the magnitude of perceptual change is little altered in the period from immediately following training to 24 hours later [24, 38]. In work with prisms, it was shown that initial changes in sensed limb position initially decreased and then recover and are present up to seven days later [39]. The other notable features are that subjects that show greater motor adaptation likewise show greater perceptual change [24] and similarly, larger experimental perturbations result in larger perceptual changes [40].
The perceptual alteration that is observed in these studies is primarily in the perceptual boundary rather than in perceptual acuity. In functional terms the perceptual boundary shift seems to be central to the phenomenon. For example, in visuomotor adaptation, the altered visual input creates a sensory mismatch between visual and somatosensory information. The resulting somatosensory perceptual shift is in the direction of the external perturbation and would seem to be required to keep the senses in register. This same notion has been advanced to explain both somatosensory and visual changes in prism adaptation although in this case the visual and proprioceptive shifts are in opposite directions ([30 – 32], see [41] for review). It is noteworthy that in force-field adaptation studies, which are normally conducted without introducing a mismatch between visual feedback and limb position, adaptation is nevertheless accompanied by systematic changes to somatosensory function and to somatosensory brain areas.
There have been a small number of recent studies in which perceptual acuity rather than the perceptual boundary is found to change with learning. These are notable because neither is an adaptation study. In one report [42] subjects learned to make movements in a restricted part of the workspace. This resulted in changes in proprioceptive acuity that were spatially localized. Improvements in somatosensory acuity were likewise observed when movement training was paired with reinforcement [43].
A number of studies have presented data that speak to the robustness of the perceptual adaptation result. In particular, in visuomotor adaptation the perceptual change is observed regardless of whether visual feedback of the hand is rotated or translated [28]. It likewise occurs and is roughly similar in magnitude when perceptual testing involves either active or passive movement. Finally, it occurs regardless of whether the perturbation is introduced abruptly [24, 44] or gradually [28].
The pattern of generalization for perceptual change is similar to that observed for motor learning. Thus for example, in recent work on visuomotor adaptation there was no evidence of inter-manual transfer of proprioceptive re-calibration [45]. Limited interlimb generalization has been previously reported for force field learning [46]. Note however, that others have reported small amounts of inter-limb transfer following force-field adaptation. In a more recent study, generalization of proprioceptive recalibration to positions nearer than the training target but not further has been reported [47], which is likewise consistent with work on force-field learning [48].
Perceptual shifts are similarly observed in association with speech motor adaptation to altered auditory and altered somatosensory feedback. Auditory perceptual shifts have been reported following adaptation to altered vowel sounds [49]. It is shown that the perceptual change is related to the sounds that subjects have to produce in order to compensate for the acoustical perturbation rather than what the subjects hear as a perturbed utterance. Auditory perceptual change in speech is also observed in the context of adaptation to altered somatosensory feedback even in cases where there is no measurable auditory perturbation [50]. In one further study of the effects of adaptation to altered auditory feedback on speech perception, auditory perturbation of consonant sounds resulted in a perceptual boundary shift in the identification of consonants that is in a direction opposite to the applied perturbation [51]. That is, if the auditory perturbation made a consonant sequence sound more like “sh” than “s”, subjects were more likely following adaptation to classify subsequent sounds on this continuum as sounding “sh”-like.
Motor learning results in changes to sensory areas of the brain
There is a range of electrophysiological evidence indicating that sensory processing changes with motor learning. Changes to activity in orofacial somatosensory cortex are observed in conjunction with orofacial motor learning [52]. In this study, monkeys were trained in a novel tongue protrusion task. Rapid and long-lasting changes to M1 and S1 were observed in parallel. In both cases, there are increases in the proportion of task modulated neurons, and reductions across trials in firing rate variability. In humans there is also electrophysiological data showing sensory change following learning. In particular there are changes to somatosensory evoked potentials following force-field learning [53], following learning an arm muscle timing task [54], and a repetitive typing task [55].
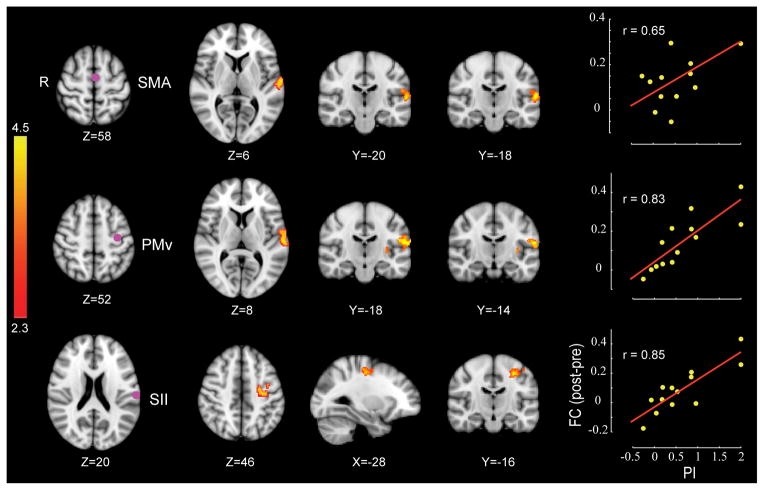
There are likewise neuroimaging data showing changes in functional connectivity following force-field adaptation in networks related to both perceptual change and motor learning [25]. Changes in resting-state networks that are related to the perceptual changes that occur in conjunction with force-field adaptation can be seen in patterns of altered functional connectivity between second somatosensory cortex, supplementary motor area and ventral premotor cortex (Figure 1). The results are noteworthy in that these same areas have been implicated in tasks involving the transient storage of somatosensory information and somatosensory decision-making [21 – 22]. Other examples of changes to sensory systems in conjunction with motor learning come from tasks involving sequence learning and from long duration training tasks. In particular, daily practice of a sequential movement task involving the fingers and thumb of the left hand resulted in clusters of activity in postcentral gyrus and supramarginal gyrus that were related to learning [56]. Jugglers were found to have increases in gray matter in V5/MT following extended periods of practice [57 – 58] and musicians show greater gray matter concentration in right planum temporale (auditory cortex) than non-musicians [59]. A somewhat different pattern is seen in prism adaptation, where the main changes in activity during adaptation are in parietal multisensory areas and cerebellum [60].
Figure 1.
Motor learning increases functional connectivity in sensory networks in the brain. Each row shows a seed region (left) and those clusters of voxels whose correlation with the seed regions are strengthened in proportion to the perceptual change produced by motor learning (PI). The bar plots show functional connectivity (FC) before and after force-field training. The scatter plots show that subjects that show greater perceptual change (PI) likewise show greater changes in connectivity between each specific seed and the clusters shown to the right. It is seen that motor learning results in increases connectivity in proportion to perceptual change in a network comprised of SII, PMv and SMA.
Taken together, the studies summarized above indicate that motor adaptation involves concurrent and durable changes to motor, visual and somatosensory systems. In neuroimaging studies it is seen that sensorimotor adaptation also results in changes in connectivity in sensory networks of the brain. In adaptation studies the changes appear to keep sensory and motor systems in register, whereas more limited results from studies focused on skill acquisition suggest that in some cases improvements are focused on acuity rather than alignment. We expect that future studies will reveal further instances of perceptual change in the context of motor learning, each matched to the specific role of the sensory system in the acquired motor behavior. While the relative contribution to sensory plasticity of cortico-cortical and afferent factors are unresolved, the evidence to date is consistent with the conclusion that perceptual learning and motor adaptation are part and parcel of human motor skill learning.
Source of sensory effects in motor learning: Are they efferent or afferent?
What causes sensory change during motor learning? Is it due to motor outflow, sensory inflow or the two in combination? There is an extensive literature documenting sensory plasticity produced by manipulating afferent input alone. For example, there is reorganization of somatosensory cortex following digit amputation in raccoons and monkeys [61 – 62] and following peripheral nerve stimulation in adult cats [63]. There are changes to primary auditory cortex following frequency discrimination training in adult monkeys [64] and human cortical plasticity in primary and second somatosensory cortex has been shown using fMRI following somatosensory stimulation applied passively to the fingertip [54].
There is also evidence that plasticity in the sensorimotor network arises from cortico-cortical connections. Evidence in sensory systems is obtained in work on crossmodal plasticity in which the loss of input to one sensory modality results in cortical reorganization in other sensory systems. For example, in response to somatosensory stimulation, individuals with early loss of vision show significant activation in early visual areas that is not seen when the same stimulation is applied in sighted control subjects [65]. Concordantly, stimulation of visual cortex in early blind subjects using TMS disrupts tactile perception but this disruption does not occur in sighted controls [66]. In animal studies, crossmodal reorganization in the posterior auditory field of deaf cats results in enhanced visual localization of peripheral stimuli [67]. Evidence for plasticity induced in motor areas was reported in recent studies in which mice learned to discriminate textures using facial whiskers. It was found that the fraction of whisker-touch activated neurons in M1, which receive projections from S1 neurons, increases over the course of training and the increase persists during post training recordings [68].
In studies that have attempted to distinguish sensory changes associated with motor learning that are due to afferent input, from changes related to the efferent outflow, the results are mixed. In one study, the magnitude of perceptual recalibration and motor adaptation was similar when the hand was moved passively during the training phase, rather than actively [69]. However in another, no perceptual change was observed following passive control experiments in which limb kinematics were matched to those which occurred during learning, but in the absence of voluntary movement [24]. In experiments on speech motor learning, it appears that sensory change is dominated by motor outflow. Specifically, perceptual change is associated with the sounds the subject produces rather than what the subject hears during adaptation to altered auditory feedback [49].
One potential account of sensory change that occurs in conjunction with learning is that it is a by-product of efference copy during voluntary movement. This is certainly a possibility. Somatosensory evoked potentials (SEPs) are reduced in conjunction with finger and limb movement [70 – 72]. However these changes are transient, on a time scale much shorter than the sensory and perceptual changes observed in association with motor learning. Similarly, an SEP reduction occurs early in visuomotor learning but dissipates quickly such that SEPs are back to baseline levels well before the end of training [73]. Efference copy might well contribute to perceptual changes in the early stages of learning but sensory and perceptual change persists long after the end of any ongoing motor signals (and associated efference copy).
A number of lines of evidence suggest that somatosensory change is not an epiphenomenon but is tied to the motor system. Circumstantial evidence is that movements follow new perceptual boundaries [24]. A more direct test involves manipulating the somatosensory system (for example, the auditory system in speech) and observing systematic changes in motor behaviour and learning (as in [74 – 76]. In studies of force-field and visuo-motor adaptation, and in adaptation to altered auditory feedback during speech, correlations are often observed between the magnitude of adaptation and the associated perceptual change [24, 28]. In other studies, while changes are observed in both sensory and motor function, there is no indication that the magnitudes of adaptation are correlated [40, 48]. It has been suggested that the latter constitutes evidence that the adaptation processes involved are independent, although they occur simultaneously [40].
Perceptual learning changes movement and the brain’s motor areas
The preceding sections focus on the idea that motor learning drives sensory plasticity and changes perceptual systems. In this section we consider evidence that the relationship is reciprocal, that is, perceptual learning produces changes in motor function and motor areas of the brain. Indeed, perceptual learning, particularly in the somatosensory system, may well play an essential role in motor learning, particularly in the initial stages of learning when the somatosensory targets of movement are frequently unknown. In situations such as learning the feel of a good tennis serve or learning to speak in a foreign language, perceptual and motor learning occur together. Studies that have attempted to separate perceptual from motor learning provide the possibility of better assessing the relative balance of these factors in the broader undertaking of motor learning.
There is a substantial literature documenting benefits to movement that originate in sensory systems. The focus of much of this work is on plasticity in sensory systems which is due to stimulation or sensory loss and subsequent changes to movement that occur as a result (see [76] for a recent review). There is also evidence involving human limb movement showing that somatosensory training acts directly on the motor system and improves motor learning, increases motor cortex excitability (as reflected in changes to MEPs), and increases activity in frontal motor areas during passive movement. Thus for example, it has been shown that passive limb movement paired with visual input improved the learning of complex hand trajectories [42]. It has also been shown that perceptual training involving somatosensory discrimination with feedback resulted in changes to motor learning in a task requiring rapid thumb abduction and changes to motor evoked potentials in vibrated hand muscles [78]. Similarly, passive wrist movement over a 28-day period resulted in increases in activity in primary motor cortex and supplementary motor area in response to passive movement conducted in the MRI scanner [79]. Finally, it has been demonstrated that proprioceptive stimulation resulting from passive movement of the wrist leads to increases in the evoked response to a constant cortical stimulus produced using TMS [80].
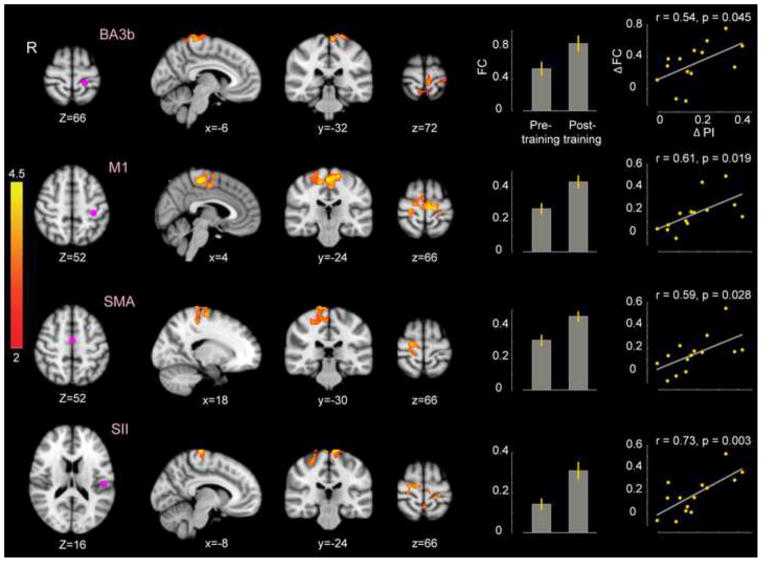
When passive somatosensory training is paired with reinforcement, changes are observed in perceptual function, movement and in motor networks of the brain. In these studies, participants hold the handle of a robot that moves the arm along one of a number of trajectories. The participant is required to indicate the direction of limb displacement. Binary feedback regarding the accuracy of judgement is provided. Perceptual training conducted in this fashion results in improvements in perceptual acuity and to changes in judgments regarding the position of the limb. It also produces improvements in the rate and extent of subsequent force-field adaptation, which persist for at least a day [74]. This same perceptual training procedure, involving passive movement paired with reinforcement, alters functional connectivity in both sensory and motor networks of the brain. Reliable changes in connectivity related to perceptual learning are observed in the links between SMA and M1, PMd and cerebellar cortex, and also bilaterally in M1 ([75], Figure 2).
Figure 2.
Somatosensory perceptual learning strengthens functional connectivity in motor networks in the brain. It is seen that perceptual learning results in changes in connectivity that strengthens networks in M1, S1, SMA and dorsal premotor cortex. Layout as in Figure 1.
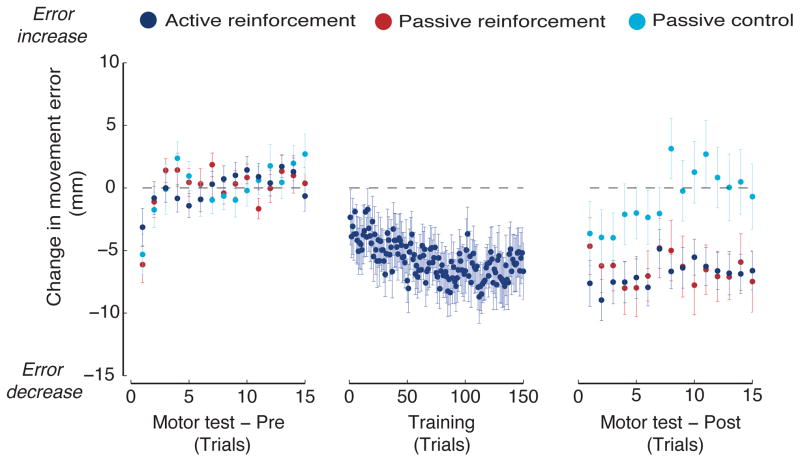
Recent work using a similar procedure ([43], Figure 3) suggests that somatosensory inputs may drive the initial stages of human motor learning. In these studies, participants make movements to uncertain target locations in the absence of either visual feedback or vision of the limb. When a movement successfully lands within an unseen target zone, binary reinforcement is provided. Subjects who train using active movement show improvements to movement accuracy that persist for at least one week. Participants in a matched passive condition that have their arm moved along the same trajectories and receive the same reinforcement as subjects in the active movement condition, show just as great a benefit to subsequent movement as subjects that train actively, and like active subjects, the benefits persist at one week retest. Participants that experience the same passive movements, but in the absence of feedback or reinforcement, show little benefit from the perceptual training.
Figure 3.
Passive movement paired with reinforcement (red circles) results in improvements in movement following training as great as those obtained for subjects who train with active movement and receive the same reinforcement (dark blue circles). The benefits of passive movement without reinforcement (light blue circles) are transient. The left panel shows baseline movements in the absence of feedback. The dashed line shows baseline performance prior to training. The middle panel shows training movements produced by subjects in the active reinforcement condition. Subjects in the other two groups experience the same movements passively (produced under position servo-control by a robot arm). The right panel shows movements following training, also in the absence of feedback or vision of the arm.
Concluding remarks
Changes to both sensory and motor systems are observed in the context of sensorimotor adaptation and motor skill acquisition. Perceptual changes occur in each of the widely studied motor adaptation tasks — force-field learning, visuomotor and prism adaptation and they also occur in adaptation to altered auditory feedback in speech. Perceptual change occurs concurrently with motor adaptation, it is durable and is often correlated in magnitude with behavioural measures of motor adaptation. In neuroimaging studies, changes to sensory networks in the brain are seen in conjunction with sensorimotor adaptation. When perceptual learning is directly manipulated, it results in systematic changes to motor adaptation and also alters motor networks of the brain. Indeed, in some cases, skill acquisition is substantially driven by somatosensory inflow. These observations are consistent with the idea that perceptual learning and sensory plasticity are fundamental to sensorimotor adaptation and motor skill acquisition. If we are to improve models of skill acquisition or develop new therapeutic interventions we need a better understanding of how skill acquisition is determined by plasticity in both sensory and motor systems.
Box 1. Motor Learning and Sensorimotor Adaptation.
The studies reviewed here primarily involve adaptation to experimentally imposed perturbations. The main examples come from the literature on force-field learning which involves the introduction of novel force loads, visuomotor and prism adaptation in which the correspondence between vision and proprioception is manipulated and adaptation to altered auditory feedback in speech. The subject is required to move to a target, perturbations are delivered, and the resulting movement error is corrected by changes to motor commands. Although termed adaptation, these manipulations typically result in changes to behaviors and brain networks that are normally associated with learning. Adaptation is generally distinguished from motor learning in that adaptation is associated with corrections that occur when perturbations are delivered to well learned motor behaviors. The corrections or compensation bring the behavior back towards its unperturbed form. Learning on the other hand is not dependent on perturbation and results in persistent changes to brain and behavior. The studies reviewed here have many of the features of learning. The correction, like the original learning, is often incomplete and the changes to movement are durable in the sense that when subjects are retested after considerable delay, there are substantial savings, both in the rate of re-adaptation and in changes to perceptual systems. Moreover, adaptation results in changes to both motor and sensory networks of the brain, a finding that would not be expected if adaptation acted simply to return the system to its unperturbed state.
Trends.
Sensorimotor adaptation results in changes to sensory systems and sensory networks in the brain.
Perceptual learning modifies sensory systems and directly alters the brain’s motor networks.
Perceptual changes associated with sensorimotor adaptation are durable and occur in parallel with motor learning.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development R01 HD075740, the National Institute on Deafness and Other Communication Disorders R01 DC012502, Les Fonds Québécois de la Recherche sur la Nature et les Technologies, Québec (FQRNT) and the Natural Sciences and Engineering Research Council of Canada (NSERC).
Glossary
- Force-field learning
Predictable mechanical loads are applied to the arm during movement or to the jaw in speech, in both cases typically using a robotic device. The perturbations initially alter the movement path which gradually returns to normal as subjects learn to counteract the load. A negative after-effect (movement in the opposite direction) occurs when the perturbation is removed. The after-effect provides a measure of the compensation learned by subjects to produce straight movement in the presence of load.
- Visuomotor adaptation
Predictable displacements of a visual target are applied during reaching movement, typically by changing the mapping between the position of the hand and the position of a cursor on a display screen. The pattern during adaptation is similar to that seen in force-field learning, namely, an initial error in the direction of the perturbation is gradually corrected over a series of subsequent movements. A negative-after effect follows the removal of the perturbation. In visuomotor adaptation, participants tolerate proprioceptive error in order to have movements appear visually correct.
- Prismatic adaptation
The earliest motor adaptation studies were done using prisms. Prisms shift the entire visual scene rather than just a single point (as in visuomotor adaptation). The compensatory pattern is similar to that in visuomotor adaptation. Prism adaptation is associated with both visual and proprioceptive perceptual change.
- Adaptation to altered auditory feedback
Participants read words aloud that are presented on a computer screen. The acoustical speech signal is altered in real-time and played back to the participant through headphones. As in other adaptation procedures, participants learn to shift their vocal output in a direction opposite to the applied acoustical shift. As in visuomotor adaptation, participants tolerate proprioceptive error, in this case, to have their speech sound correct.
- Perceptual boundary
In these studies, subjects typically make binary judgements to classify perceptual stimuli. For somatosensory judgements, limb position is systematically varied. For auditory judgements, participants classify sounds. In vision, the stimulus position is varied. The set of actual positions and participant’s judgments are fit with psychometric function. The 50% point serves as an estimate of the perceptual boundary.
- Perceptual acuity
Perceptual classification data are used to estimate acuity, using a measure of the slope of the psychometric function about its midpoint.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 2.Mountcastle VB. The sensory hand. Harvard Univ Press; Cambridge, MA: 2005. [Google Scholar]
- 3.Oscarsson O. Functional organization of the spino- and cuneocerebellar tracts. Physiol Rev. 1965;45:495–522. doi: 10.1152/physrev.1965.45.3.495. [DOI] [PubMed] [Google Scholar]
- 4.Burton H, Fabri M. Ipsilateral intracortical connections of physiologically defined cutaneous representations in areas 3b and 1 of macaque monkeys: Projections in the vicinity of the central sulcus. J Comp Neurol. 1995;355:508–38. doi: 10.1002/cne.903550404. [DOI] [PubMed] [Google Scholar]
- 5.Pons TP, Kaas JH. Corticocortical connections of area 2 of somatosensory cortex in macaque monkeys: a correlative anatomical and electrophysiological study. J Comp Neurol. 1986;248:313–35. doi: 10.1002/cne.902480303. [DOI] [PubMed] [Google Scholar]
- 6.Darian-Smith C, Darian-Smith I, Burman K, Ratcliffe N. Ipsilateral cortical projections to areas 3a, 3b, and 4 in the macaque monkey. J Comp Neurol. 1993;335:200–13. doi: 10.1002/cne.903350205. [DOI] [PubMed] [Google Scholar]
- 7.Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–99. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- 8.Huffman KJ, Krubitzer L. Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb Cortex. 2001;11:849–67. doi: 10.1093/cercor/11.9.849. [DOI] [PubMed] [Google Scholar]
- 9.Liao CC, Gharbawie OA, Qi H, Kaas JH. Cortical connections to single digit representations in area 3b of somatosensory cortex in squirrel monkeys and prosimian galagos. J Comp Neurol. 2013;521:3768–90. doi: 10.1002/cne.23377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh S, Brinkman C, Porter R. A quantitative study of the distribution of neurons projecting to the precentral motor cortex in the monkey (M. fascicularis) J Comp Neurol. 1987;259:424–44. doi: 10.1002/cne.902590309. [DOI] [PubMed] [Google Scholar]
- 11.Vogt BA, Pandya DN. Cortico-cortical connections of somatic sensory cortex (areas 3, 1 and 2) in the rhesus monkey. J Comp Neurol. 1978;77:179–91. doi: 10.1002/cne.901770202. [DOI] [PubMed] [Google Scholar]
- 12.Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M l) of owl monkeys. J Comp Neurol. 1993;330:238–71. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- 13.Stepniewska I, Preuss TM, Kaas JH. Ipsilateral cortical connections of dorsal and ventral premotor areas in New World owl monkeys. J Comp Neurol. 2006;495:691–708. doi: 10.1002/cne.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J Comp Neurol. 2006;498:227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- 15.Preuss TM, Goldman-Rakic PS. Connections of the ventral granular frontal cortex of macaques with perisylvian premotor and somatosensory areas: anatomical evidence for somatic representation in primate frontal association cortex. J Comp Neurol. 1989;282:293–316. doi: 10.1002/cne.902820210. [DOI] [PubMed] [Google Scholar]
- 16.Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–16. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 17.Rosén I, Asanuma H. Peripheral afferent inputs to the forelimb area of the monkey motor cortex: input-output relations. Exp Brain Res. 1972;14:257–73. doi: 10.1007/BF00816162. [DOI] [PubMed] [Google Scholar]
- 18.Wong YC, Kwan HC, MacKay WA, Murphy JT. Spatial organization of precentral cortex in awake primates. I. Somatosensory inputs. J Neurophysiol. 1978;41:1107–19. doi: 10.1152/jn.1978.41.5.1107. [DOI] [PubMed] [Google Scholar]
- 19.Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res. 1988;71:475–90. doi: 10.1007/BF00248741. [DOI] [PubMed] [Google Scholar]
- 20.Kohler E, Keysers C, Umiltà MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: action representation in mirror neurons. Science. 2002;297:846–8. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- 21.Hernández A, Zainos A, Romo R. Temporal evolution of a decision-making process in medial premotor cortex. Neuron. 2002;33:959–72. doi: 10.1016/s0896-6273(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 22.Romo R, Hernández A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron. 2004;41:165–73. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
- 23.Haith A, Jackson CP, Miall RC, Vijayakumar S. Advances in Neural Information Processing Systems. 2008. Unifying the sensory and motor components of sensorimotor adaptation. [Google Scholar]
- 24.Ostry DJ, Darainy M, Mattar AAG, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci. 2010;30:5384–5393. doi: 10.1523/JNEUROSCI.4571-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci. 2011;31:16907–16915. doi: 10.1523/JNEUROSCI.2737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattar AAG, Darainy M, Ostry DJ. Motor learning and its sensory effects: The time course of perceptual change, and its presence with gradual introduction of load. J Neurophysiol. 2013;109:782–91. doi: 10.1152/jn.00734.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malfait N, Henriques DY, Gribble PL. Shape distortion produced by isolated mismatch between vision and proprioception. J Neurophysiol. 2008;99:231–43. doi: 10.1152/jn.00507.2007. [DOI] [PubMed] [Google Scholar]
- 28.Cressman EK, Henriques DY. Sensory recalibration of hand position following visuomotor adaptation. J Neurophysiol. 2009;102:3505–18. doi: 10.1152/jn.00514.2009. [DOI] [PubMed] [Google Scholar]
- 29.Volcic R, Fantoni C, Caudek C, Assad JA, Domini F. Visuomotor adaptation changes stereoscopic depth perception and tactile discrimination. J Neurosci. 2013;33:17081–8. doi: 10.1523/JNEUROSCI.2936-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris CS. Adaptation to displaced vision: visual, motor, or proprioceptive change? Science. 1963;140:812–813. doi: 10.1126/science.140.3568.812. [DOI] [PubMed] [Google Scholar]
- 31.Harris CS. Perceptual adaptation to inverted, reversed, and displaced vision. Psychol Rev. 1965;72:419–444. doi: 10.1037/h0022616. [DOI] [PubMed] [Google Scholar]
- 32.Welch RB. Research on adaptation to rearranged vision: 1966–1974. Perception. 1974;3:367–92. doi: 10.1068/p030367. [DOI] [PubMed] [Google Scholar]
- 33.Wallace B. Stability of Wilkinson’s linear model of prism adaptation over time for various targets. Perception. 1977;6:145–51. doi: 10.1068/p060145. [DOI] [PubMed] [Google Scholar]
- 34.Melamed LE, Beckett PA, Halay M. Individual differences in the visual component of prism adaptation. Perception. 1979;8:699–706. doi: 10.1068/p080699. [DOI] [PubMed] [Google Scholar]
- 35.Beckett PA. Development of the third component in prism adaptation: effects of active and passive movement. J Exp Psychol Hum Percept Perform. 1980;6:433–44. doi: 10.1037//0096-1523.6.3.433. [DOI] [PubMed] [Google Scholar]
- 36.Brown LE, Wilson ET, Goodale MA, Gribble PL. Motor force field learning influences visual processing of target motion. J Neurosci. 2007;27:9975–83. doi: 10.1523/JNEUROSCI.1245-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kagerer FA, Contreras-Vidal JL. Adaptation of sound localization induced by rotated visual feedback in reaching movements. Exp Brain Res. 2009;193:315–21. doi: 10.1007/s00221-008-1630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nourouzpour N1, Salomonczyk D, Cressman EK, Henriques DY. Retention of proprioceptive recalibration following visuomotor adaptation. Exp Brain Res. 2015;233:1019–29. doi: 10.1007/s00221-014-4176-6. [DOI] [PubMed] [Google Scholar]
- 39.Hatada Y, Rossetti Y, Miall RC. Long-lasting aftereffect of a single prism adaptation, shifts in vision and proprioception are independent. Exp Brain Res. 2006;173:415–24. doi: 10.1007/s00221-006-0381-2. [DOI] [PubMed] [Google Scholar]
- 40.Salomonczyk D, Cressman EK, Henriques DY. Proprioceptive recalibration following prolonged training and increasing distortions in visuomotor adaptation. Neuropsychologia. 2011;49:3053–62. doi: 10.1016/j.neuropsychologia.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Redding GM, Rossetti Y, Wallace B. Applications of prism adaptation: a tutorial in theory and method. Neurosci Biobehav Rev. 2005;29:431–44. doi: 10.1016/j.neubiorev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Wong JD, Kistemaker DA, Chin A, Gribble PL. Can proprioceptive training improve motor learning? J Neurophysiol. 2012;108:3313–21. doi: 10.1152/jn.00122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernardi NF, Darainy M, Ostry DJ. Somatosensory contribution to the initial stages of human motor learning. J Neurosci. 2015;35:14316–26. doi: 10.1523/JNEUROSCI.1344-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salomonczyk D, Henriques YP, Cressman EK. Proprioceptive recalibration in the right and left hands following abrupt visuomotor adaptation. Exp Brain Res. 2012;217:187–196. doi: 10.1007/s00221-011-2985-4. [DOI] [PubMed] [Google Scholar]
- 45.Mostafa AA, Salomonczyk D, Cressman EK, Henriques DY. Intermanual transfer and proprioceptive recalibration following training with translated visual feedback of the hand. Exp Brain Res. 2014;232:1639–51. doi: 10.1007/s00221-014-3833-0. [DOI] [PubMed] [Google Scholar]
- 46.Malfait N, Ostry DJ. Is interlimb transfer of force-field adaptation a “cognitive” response to the sudden introduction of load? J Neurosci. 2004;24:8084–8089. doi: 10.1523/JNEUROSCI.1742-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mostafa AA, Kamran-Disfani R, Bahari-Kashani G, Cressman EK, Henriques DY. Generalization of reach adaptation and proprioceptive recalibration at different distances in the workspace. Exp Brain Res. 2015;233:817–27. doi: 10.1007/s00221-014-4157-9. [DOI] [PubMed] [Google Scholar]
- 48.Mattar AA, Ostry DJ. Generalization of dynamics learning across changes in movement amplitude. J Neurophysiol. 2010;104:426–38. doi: 10.1152/jn.00886.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lametti DR, Rochet-Capellan A, Neufeld E, Shiller DM, Ostry DJ. Plasticity in the human speech motor system drives changes in speech perception. J Neurosci. 2014;34:10339–10346. doi: 10.1523/JNEUROSCI.0108-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasir SM, Ostry DJ. Auditory plasticity and speech motor learning. Proc Natl Acad Sci U S A. 2009;106:20470–20475. doi: 10.1073/pnas.0907032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiller DM, Sato M, Gracco VL, Baum SR. Perceptual recalibration of speech sounds following speech motor learning. J Acoust Soc Am. 2009;125:1103–13. doi: 10.1121/1.3058638. [DOI] [PubMed] [Google Scholar]
- 52.Arce-McShane FI, Hatsopoulos NG, Lee JC, Ross CF, Sessle BJ. Modulation dynamics in the orofacial sensorimotor cortex during motor skill acquisition. J Neurosci. 2014;34:5985–97. doi: 10.1523/JNEUROSCI.4367-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nasir SM, Darainy M, Ostry DJ. Sensorimotor adaptation changes the neural coding of somatosensory stimuli. J Neurophysiol. 2013;109:2077–85. doi: 10.1152/jn.00719.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pleger B, Foerster AF, Ragert P, Dinse HR, Schwenkreis P, Malin JP, Nicolas V, Tegenthoff M. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron. 2003;40:643–53. doi: 10.1016/s0896-6273(03)00677-9. [DOI] [PubMed] [Google Scholar]
- 55.Andrew D, Haavik H, Dancey E, Yielder P, Murphy B. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin Neurophysiol. 2015;126:575–80. doi: 10.1016/j.clinph.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Ma L, Narayana S, Robin DA, Fox PT, Xiong J. Changes occur in resting state network of motor system during 4 weeks of motor skill learning. Neuroimage. 2011;58:226–33. doi: 10.1016/j.neuroimage.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–2. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 58.Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bermudez P, Lerch JP, Evans AC, Zatorre RJ. Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel-based morphometry. Cereb Cortex. 2009;19:1583–96. doi: 10.1093/cercor/bhn196. [DOI] [PubMed] [Google Scholar]
- 60.Luauté J, Schwartz S, Rossetti Y, Spiridon M, Rode G, Boisson D, Vuilleumier P. Dynamic changes in brain activity during prism adaptation. J Neurosci. 2009;29:169–78. doi: 10.1523/JNEUROSCI.3054-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rasmusson DD. Reorganization of raccoon somatosensory cortex following removal of the fifth digit. J Comp Neurol. 1982;205:313–26. doi: 10.1002/cne.902050402. [DOI] [PubMed] [Google Scholar]
- 62.Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- 63.Recanzone GH, Allard TT, Jenkins WM, Merzenich MM. Receptive-field changes induced by peripheral nerve stimulation in SI of adult cats. J Neurophysiol. 1990;63:1213–25. doi: 10.1152/jn.1990.63.5.1213. [DOI] [PubMed] [Google Scholar]
- 64.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wittenberg GF, Werhahn KJ, Wassermann EM, Herscovitch P, Cohen LG. Functional connectivity between somatosensory and visual cortex in early blind humans. Eur J Neurosci. 2004;20:1923–7. doi: 10.1111/j.1460-9568.2004.03630.x. [DOI] [PubMed] [Google Scholar]
- 66.Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Falz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catalá MD, Hallett M. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–3. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 67.Lomber SG, Meredith MA, Kral A. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci. 2010;13:1421–7. doi: 10.1038/nn.2653. [DOI] [PubMed] [Google Scholar]
- 68.Chen JL, Margolis DJ, Stankov A, Sumanovski LT, Schneider BL, Helmchen F. Pathway-specific reorganization of projection neurons in somatosensory cortex during learning. Nature Neurosci. 2015;18:1101–1108. doi: 10.1038/nn.4046. [DOI] [PubMed] [Google Scholar]
- 69.Cressman EK, Henriques DY. Reach adaptation and proprioceptive recalibration following exposure to misaligned sensory input. J Neurophysiol. 2010;103:1888–95. doi: 10.1152/jn.01002.2009. [DOI] [PubMed] [Google Scholar]
- 70.Papakostopoulos D, Cooper R, Crow HJ. Inhibition of cortical evoked potentials and sensation by self-initiated movement in man. Nature. 1975;258:321–4. doi: 10.1038/258321a0. [DOI] [PubMed] [Google Scholar]
- 71.Rushton DN, Rothwell JC, Craggs MD. Gating of somatosensory evoked potentials during different kinds of movement in man. Brain. 1981;104:465–91. doi: 10.1093/brain/104.3.465. [DOI] [PubMed] [Google Scholar]
- 72.Starr A, Cohen LG. ‘Gating’ of somatosensory evoked potentials begins before the onset of voluntary movement in man. Brain Res. 1985;348:183–6. doi: 10.1016/0006-8993(85)90377-4. [DOI] [PubMed] [Google Scholar]
- 73.Bernier PM, Burle B, Vidal F, Hasbroucq T, Blouin J. Direct evidence for cortical suppression of somatosensory afferents during visuomotor adaptation. Cereb Cortex. 2009;19:2106–2113. doi: 10.1093/cercor/bhn233. [DOI] [PubMed] [Google Scholar]
- 74.Darainy M, Vahdat S, Ostry DJ. Perceptual learning in sensorimotor adaptation. J Neurophysiol. 2013;110:2152–2162. doi: 10.1152/jn.00439.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vahdat S, Darainy M, Ostry DJ. Structure of plasticity in human sensory and motor networks due to perceptual learning. J Neurosci. 2014;34:2451–63. doi: 10.1523/JNEUROSCI.4291-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lametti DR, Krol SA, Shiller DM, Ostry DJ. Brief periods of auditory perceptual training can determine the sensory targets of speech motor learning. Psychol Sci. 2014;25:1325–36. doi: 10.1177/0956797614529978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beste C, Dinse HR. Learning without training. Curr Biol. 2013;23:R489–99. doi: 10.1016/j.cub.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 78.Rosenkranz K, Rothwell JC. Modulation of proprioceptive integration in the motor cortex shapes human motor learning. J Neurosci. 2012;32:9000–6. doi: 10.1523/JNEUROSCI.0120-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carel C, Loubinoux I, Boulanouar K, Manelfe C, Rascol O, Celsis P, Chollet F. Neural substrate for the effects of passive training on sensorimotor cortical representation: a study with functional magnetic resonance imaging in healthy subjects. J Cereb Blood Flow Metab. 2000;20:478–84. doi: 10.1097/00004647-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 80.Lewis GN, Byblow WD. The effects of repetitive proprioceptive stimulation on corticomotor representation in intact and hemiplegic individuals. Clin Neurophysiol. 2004;115:765–73. doi: 10.1016/j.clinph.2003.11.014. [DOI] [PubMed] [Google Scholar]