Abstract
Background and aims
Osteoarthritic patients treated with high doses of chondroitin sulfate (CS) have a lower incidence of coronary heart disease – but the mechanistic aspects of these beneficial effects of CS remain undefined. We examined how CS treatment affects the formation of atheroma via interaction with endothelial cells and monocytes.
Methods
We characterized arterial atheromatous plaques by multiphoton microscopy and serum pro-inflammatory cytokines by immunoenzymatic techniques in obese mice receiving CS (1g/Kg/day, i.p.) or vehicle for 6 days. Effects of CS on signaling pathways, cytokine secretion and macrophage migration were evaluated in cultures of human coronary endothelial cells and in a monocyte cell line stimulated with TNF-α by Western blot, immunoenzymatic techniques and transwell migration assays.
Results
Treatment of obese mice with CS reduced the extension of foam cell coverage in atheromatous plaques of arterial bifurcations by 62.5%, the serum concentration of IL1β by 70%, TNF-α by 82% and selected chemokines by 25–35%. Cultures of coronary endothelial cells and monocytes stimulated with TNF-α secreted less pro-inflammatory cytokines in the presence of CS (P<0.01). CS reduced the activation of the TNF-α signaling pathway in endothelial cells (pErk 36% of reduction, and NFκB 33% of reduction), and the migration of activated monocytes to inflamed endothelial cells in transwells (81±6 vs.13±2, P<0.001).
Conclusions
CS interferes with the pro-inflammatory activation of monocytes and endothelial cells driven by TNF-α thus reducing the propagation of inflammation and preventing the formation of atherosclerotic plaques.
Keywords: Inflammation, extracellular matrix, immunology, vascular biology, atherosclerosis
Introduction
Obesity and atherosclerosis are chronic inflammatory processes very closely integrated, characterized by activation of immune system and endothelium where TNF-α plays a pivotal role.1–4 Circulating monocytes adhere to inflamed endothelium, infiltrate atherosclerotic lesions, differentiate into macrophages5–6, and deliver pro-inflammatory mediators such as TNF-α which participate decisively in the development and exacerbation of atherosclerosis.7–8
Glycosaminoglycans are large linear polysaccharides constructed of repeating disaccharide units. Among them, heparan sulfate has been suggested as critical to regulation of vascular repair after injury and promoting atherogenesis.9–10 The glycosaminoglycan chondroitin sulfate (CS) has traditionally being associated to the prevention of cardiac events, but its role in atherosclerosis has not been completely elucidated as yet. In the early 70s, 60 patients with coronary heart disease treated with commercial CS showed a seven-fold lower incidence of coronary events compared to the control group11. After 6 years of follow-up, only 10% of those CS-treated patients presented an acute cardiac event, of which four died, compared with 70% of which 14 died in the control group.12 Few years later, a different clinical trial showed similar beneficial effects of CS therapy in mortality on atherosclerotic subjects.13 However, since then, the therapeutic use of CS has been focused mainly to the treatment of osteoarthritis as CS is present in the extracellular matrix (ECM) especially in the cartilage, skin, blood vessels, ligaments and tendons.14 Recently, new evidences raised in patients with osteoarthritis and CS therapy. Interestingly, osteoarthritic patients treated with CS showed a reduction of seven-fold in the incidence of coronary events.15 Animal studies have demonstrated the anti-inflammatory potential of CS inhibiting hind paw oedema, synovitis and destruction of the articular cartilage in a dose-dependent manner.16 In advanced atherosclerosis, there is now a notion that there is a decrease in CS 4S (mainly composed of glucuronic acid and galactosamine residues sulfated in position 4; the disaccharides are Δdi-4S) and/or CS 6S (mainly composed of Δdi-6S), with a concomitant increase in arterial walls of dermatan sulfate (CS with the glucuronic acid moiety is replaced by its C-5 epimer, the iduronic acid).14 Administration of CS 6S has been described to interfere with the proinflammatory response of activated murine macrophages17, but no studies have described the potential immunomodulatory effects of CS in atherogenesis. We therefore aimed to investigate whether CS could modulate inflammation in activated monocytes and endothelial cells to prevent atherogenesis in obese mice.
Materials and Methods
Animal studies
Male Diet Induced Obesity (DIO) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All animals were maintained in a temperature-controlled room (22°C) on a 12-h light-dark cycle under institutional and NIH guidelines. After arrival, mice were continuously fed ad libitum with high fat diet (60% of Kcal from fat) until euthanasia. CS (1 g/kg/day from bovine origin with a disaccharide sulfation profile of 63% of 4-sulfated, 31% of 6-sulfated and 6% of O-sulfated; Bioibérica, Barcelona, Spain) or saline solution (Vehicle, VH) was intraperitoneally injected for 6 days. Afterwards animals were sacrificed and blood samples obtained and processed by standard procedure to obtain serum for Multiplex ELISA arrays (Quansys Bioscience Inc., Logan, UT) of the cytokines IL-1β, IL-6, IL-10, TNF-α, MIP-1α, KC, MCP-1, RANTES, TARC. Animal experiments were approved by the Animal Ethics Committee at Massachusetts Institute of Technology, MA, USA.
Whole-mount multiphoton imaging of macrophage presence and angiography in carotid bifurcations
Obese mice (14 weeks old) treated or non-treated with CS were anesthetized with isoflurane, injected with 100 µL of 20 mg/mL 70-kDa Texas red-dextran in PBS into the tail vein and, after 6 hours, euthanized by overexposure to CO2, and intracardially perfused with fluorescein isothiocyanate-labeled dextran (FITC-dextran, MW 2x106 Da., Sigma, St. Louis, MO). Carotid bifurcations and macrophage fluorescence were visualized using a multiphoton intravital microscope (Leica Microsystems, Heerbrugg, Switzerland). The description of this method is expanded in the Online Data Supplement.
Cell culture and live-dead assay
Human coronary artery endothelial cells (HCAEC) were grown on Endothelial Growth Medium-2 (EGM-2, Lonza, Walkersville, MD, USA) and the human monocyte cell line THP-1 on DMEM supplemented with 10% fetal bovine serum. HCAEC or THP-1 were seeded on 6-well plates at a density of 2×105 cells/well. Then, cells were pre-treated with either CS (200 µg/mL) or prednisolone (10 µmol/L) for 24 hours and continuously treated when stimulated with TNF-α (3 ng/ml) for 16 hours. Conditioned media was obtained after additional 24 hours with TNF-α free media and used for multiplex ELISA arrays of cytokines and chemokines (Quansys Biosciences, Logan, UT, USA). Cytotoxicity was tested using a Live/Dead assay (Life Technologies, Grand Island, NY, USA). The description of this method is expanded in the Online Data Supplement.
In vitro migration assay
Migration of the human monocyte cell line (THP-1 cells) to HCAEC, was quantified in 24-well plate Transwell inserts with a 5 µm porous membrane (Corning) with or without PMA (100 ng/mL) in the presence or absence of CS or Prednisolone (200 µg/mL) or in the presence or the absence of TNF-α (3 ng/mL) for 16h. Cell migration was allowed for 16 hours. Nuclei of migratory cells on the lower side of the membrane were stained with 4’,6-diamidino-2-phenylindole (DAPI, Vectashield, Vector laboratories, Burlingame, CA) and quantified in 6 different fields per sextuplicate using the ImageJ software. The description of this method is expanded in the Online Data Supplement.
Western blotting
Cell samples were lysed in RIPA buffer solution (Sigma, St Louis, MO) containing a cocktail of protease inhibitors (Sigma P8340). Whole lysates were used afterwards for the analysis of protein abundance of PhosphoErk/Erk, phosphoJnk/Jnk and NFkB. The description of this method is expanded in the Online Data Supplement.
Gene expression analysis by Real-Time PCR
Briefly, total RNA from HCAEC and THP-1 was extracted using RNeasy kit (Gibco-Invitrogen, Paisley, UK). A 1 µg of total RNA was reverse transcribed using a complementary DNA synthesis kit (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Foster City, California, USA). Taqman primers and probes for gene expression assays were selected from Applied Biosystems for human IL-6, IL-8, TNF-α and IL-1β. The description of this method is expanded in the Online Data Supplement.
Statistical analysis
Data are expressed as mean ± standard error. Statistical analysis of the results was performed by one-way analysis of variance (ANOVA), the Newman-Keuls test, and the unpaired Student’s t test when appropriate. Differences were considered to be significant at a p value of 0.05 or less. Data sampled from Gaussian populations were used for the calculation of Pearson Correlation Coefficient. A Pearson correlation coefficient (r) value of >0.75 was considered to exhibit strong positive correlation between two variables.
Results
Administration of CS ameliorates the inflammatory profile and the extension of atherosclerotic plaques in obese mice
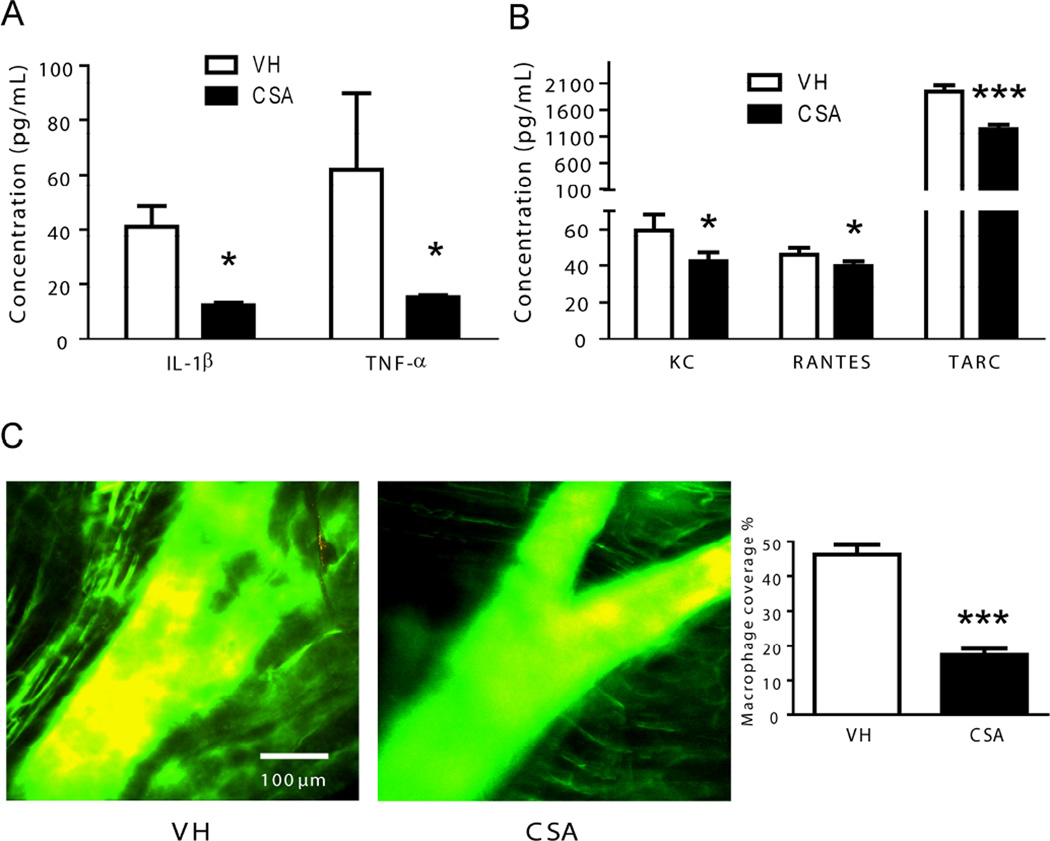
DIO obese mice treated with CS showed lower serum levels of the mediators of the inflammatory response IL-1β and TNF-α than animals receiving vehicle (Figure 1A). These cytokines are mainly released by activated monocytes and macrophages.8 Moreover we also found a significant reduction of the serum concentration of the chemotactic factors KC, RANTES and TARC in animals treated with CS as compared with control animals (Figure 1B). Those factors are mainly released by endothelium in response to inflammatory stimuli.8 The anti-inflammatory effects of CS in obese mice were also associated with a drastic reduction in the extension of the macrophage presence in the layers of arterial bifurcations (Figure 1C).
Figure 1. Effects of CS treatment on the profile of pro-inflammatory cytokines in obese mice.
Ten serum samples from diet-induced obesity (DIO) mice receiving vehicle (VH) and ten serum samples from mice receiving chondroitin sulfate A (CS) (1000 mg/kg/day) for 6 days were used to test a panel of inflammatory cytokines A and chemokines B. *p<0.01 and ***p<0.0001 vs. VH samples. C, Effects of CS treatment in the number of macrophages in atherosclerotic plaques in “in situ” carotid arterial bifurcations of obese mice. Representative images of multiphoton microscopy from animals receiving vehicle (VH, n=8) or chondroitin sulfate A (CS, n=8). Quantification of macrophage area coverage is shown to the right. Mice were perfused first with dextran-Texas red in order to induce phagocytosis of macrophages and after with dextran-FITC to stain arteries. Macrophages are shown in yellow. Original magnification, ×25; ***, p<0.0001 vs. VH samples.
CS treatment disrupts the TNF-α-induced release of leucocyte activators and chemokines in HCAEC via Erk and NFκB signaling
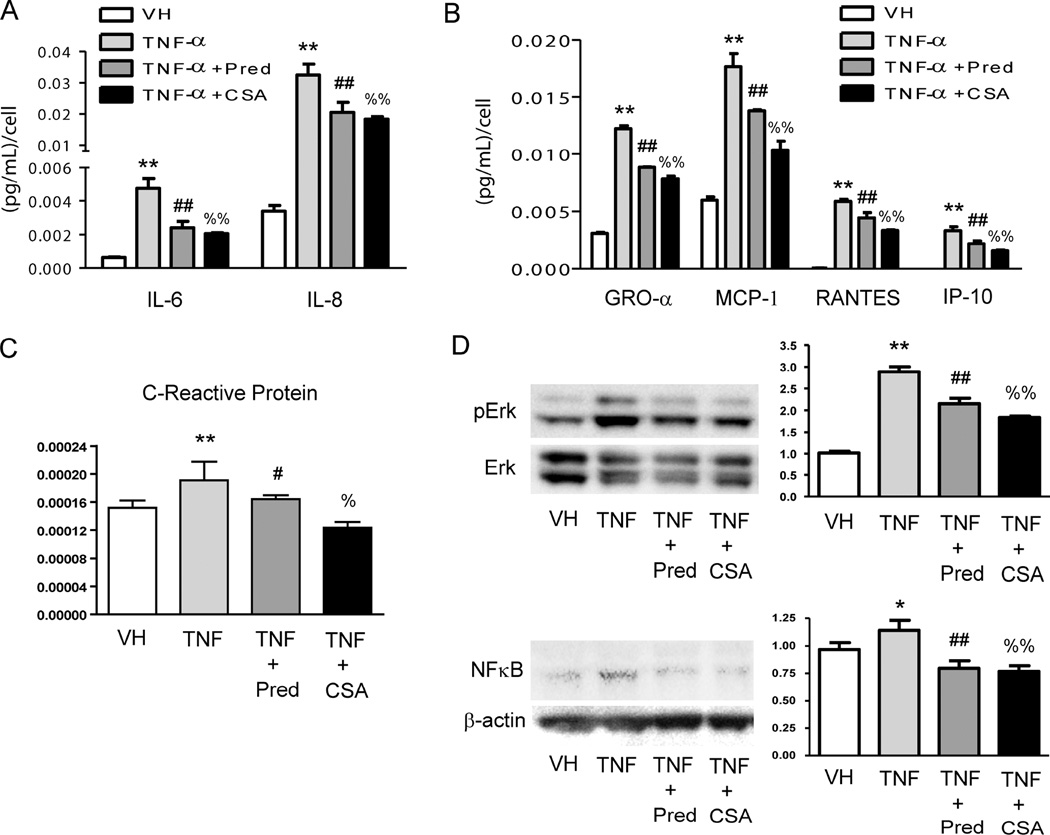
Incubation of HCAEC with TNF-α produced an increase in the release of phagocyte activators IL-6 and IL-8 (Figure 2A), and also a proportional raise of both mRNA levels (Supplementary Figure 1). These increased values were attenuated when HCAEC were treated either with prednisolone or CS (Figure 2A and Supplementary Figure 1). Likewise, incubation of inflamed HCAEC with CS partially prevented the release of the chemokines GRO- α, MCP-1, RANTES and IP-10 showing similar outcomes to the anti-inflammatory drug prednisolone (Figure 2B). Treatment of inflamed HCAEC with CS also prevented the increase of the biomarker of inflammation C-reactive protein (Figure 2C). The anti-inflammatory effects of CS in inflamed HCAEC were related to the disruption of both the phosphorylation of the protein extracellular signal-regulated kinase (Erk) and the downstream activation of NFκB (Figure 2D). No toxic effects of any of the treatments were observed in HCAEC (Supplementary Figure 2).
Figure 2. Effects of CS treatment on the profile of release of pro-inflammatory cytokines in inflamed human coronary artery endothelial cells (HCAEC).
Cell supernatants from non-inflamed HCAEC (VH), inflamed with TNF-α (3 ng/mL, 16 h) or inflamed and treated with Prednisolone (Pred, 10 µmol/L) or CS (200 µg/mL) were used to test a panel of human inflammatory cytokines A, chemokines B and C-reactive protein C by using ELISA arrays. D, Analysis of the effects of CS in the phosphorylation and subsequent activation of extracellular signal-regulated kinases (Erk) and NFκB in the TNF-α signaling pathway in HCAEC. Protein lysates were obtained from non-inflamed HCAEC (VH), inflamed with TNF-α (3 ng/mL, 16 h) or inflamed and treated with Prednisolone (Pred, 10 µmol/L) or CS (200 µg/mL) to perform western Blotting and densitometric analysis of protein bands. Non-phosphorylated Erk or β-actin was used as a loading control. *, p<0.05 and **, p<0.01 vs. VH samples; #, p<0.05 and ##, p<0.01 vs. TNF-α samples; %, p<0.05 and %%, p<0.01 vs. TNF-α samples. Experiments were carried out per sextuplicate in two independent experiments.
CS treatment interferes with the release of inflammatory cytokines in activated monocytes and macrophages
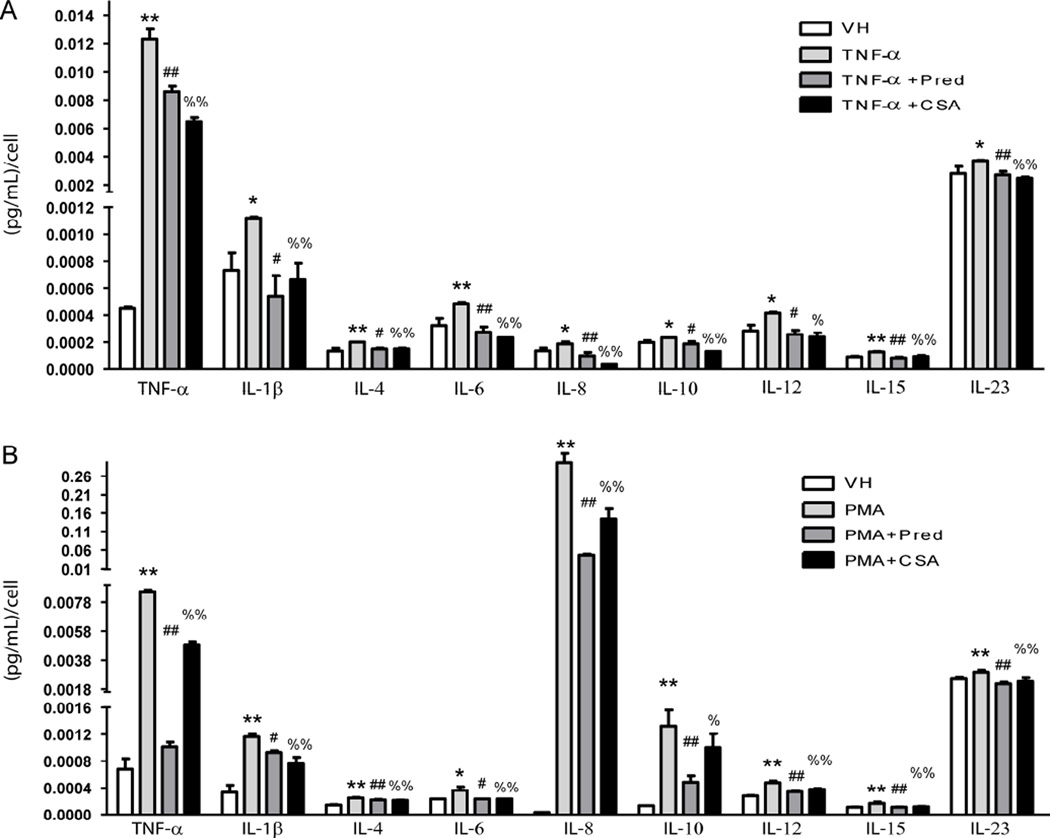
We activated the human cell line of monocytes THP-1 with TNF-α and analyzed the profile of the cytokines released by these cells in contact with CS, and identified higher release of TNF-α, IL-1β, IL-6 and IL-12 when compared with vehicle - an increase that was partially or totally abolished with the use of CS or Prednisolone (Figure 3A). To a lesser extent, IL-4, IL-8, IL-10, IL-15 and IL-23 levels also rose significantly with the presence of TNF-α and dropped to normal when these cells were incubated with CS or prednisolone (Figure 3A). Accordingly, TNF-α up-regulated the mRNA expression of TNF-α, IL-1β, IL-6 and IL-8, and the treatment with CS or Prednisolone obliterated the expression of these cytokines (Supplementary Figure 3A). To investigate whether CS could also prevent the pro-inflammatory profile of macrophages, we induced the differentiation of monocytes to macrophages with PMA for 2 days and analyzed their profile of cytokine release. Similar to monocytes activated by TNF-α, macrophages displayed a higher release of TNF-α, IL-1β, IL-6, IL-12 and also IL-10 than monocytes treated with vehicle in a manner partially or totally obliterated with the use of CS or Prednisolone (Figure 3B). Interestingly, the levels of IL-8 released by macrophages were more than 1000 fold-higher than in monocytes receiving vehicle (Figure 3B) or than monocytes treated with TNF-α. The levels of all of these cytokines were reduced with the treatment of CS or Prednisolone. To a lesser extent, IL-4, IL-15 and IL-23 levels also rose significantly in macrophages and dropped when these cells were incubated with CS or Prednisolone (Figure 3B). Accordingly, macrophages displayed higher mRNA expression of TNF-α, IL-1β, IL-6 and IL-8 than monocytes, and the treatment with CS or Prednisolone significantly reduced the expression of these cytokines (Supplementary Figure 3B).
Figure 3. Effects of CS treatment on the profile of secreted pro-inflammatory cytokines in the human monocytic cell line THP-1 activated with TNF-α.
A, Cell supernatants from non-activated THP-1 (VH), inflamed with TNF-α (3 ng/mL, 16 h) or inflamed and treated with CS (200 µg/mL) or Prednisolone (Pred, 10 µmol/L) were used to test a panel of human inflammatory mediators (TNF-α, IL-1β, IL-4, IL6, IL-8, IL-10, IL-12, IL-15 and IL-23) by ELISA arrays. B, Cell supernatants from non-differentiated THP-1 (VH), differentiated into macrophages with phorbol myristate acetate (PMA, 100 ng/mL for 2 days) or differentiated with PMA and treated with CS (200 µg/mL) or Prednisolone (Pred, 10 µmol/L) were used to test a panel of human inflammatory mediators as above by ELISA arrays; *, p<0.05 and **, p<0.01 vs. VH samples; #, p<0.05 and ##, p<0.01 vs. TNF-α samples; %, p<0.05 and %%, p<0.01 vs. TNF-α samples. Experiments were carried out per sextuplicate in two independent experiments.
CS plays a dual role reducing the migration of macrophages to inflamed HAECs
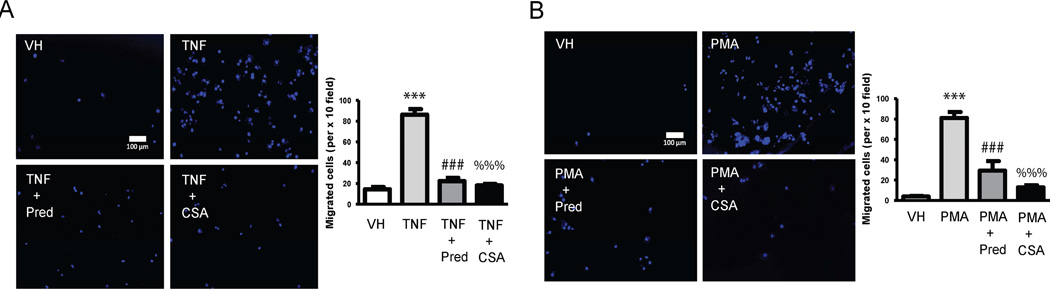
We incubated Transwell inserts containing macrophages differentiated by PMA with HAEC in the presence or absence of TNF and CS or Prednisolone. Macrophages migrated through the other side of the membrane at a higher rate when HAEC were pre-stimulated with TNF-α and that effect was drastically prevented when HAEC were also treated with CS or Prednisolone (Figure 4A). These migration outcomes are strongly positively correlated (r>0.75, Pearson) with the profile of cytokines and chemokines released by inflamed HAEC in the presence of CS (Supplementary Table 1). Likewise THP-1 human monocytes in Transwell inserts non treated or treated with PMA in the presence or absence of CS or Prednisolone were incubated with HAEC inflamed with TNF-α. CS, but also prednisolone, considerably reduced the number of monocytes differentiating and transmigrating to the side of the membrane in contact with inflamed HCAECs (Figure 4B). These migration outcomes strongly positively correlated with the profile of cytokines secreted by activated monocytes in the presence of CS (Supplementary Table 2).
Figure 4. CS effects on migration of activated monocytes and macrophages.
A, Migration assay was performed in THP-1 monocytes incubated with PBS (VH), inflamed with TNF-α (3 ng/mL, 16 h) or inflamed and treated with CS (200 µg/mL) or Prednisolone (Pred, 10 µmol/L). Representative images show cell nuclei of migrated THP-1 macrophages stained with 4',6-diamidino-2-phenylindole (DAPI). Nuclei were detected by epifluorescence microscopy (original magnification, ×100). Quantification was carried out by counting fluorescent cells and is shown on the right. B, Representative images of the migration assay from non-differentiated THP-1 (VH), differentiated into macrophages with phorbol myristate acetate (PMA, 100 ng/mL for 2 days) or differentiated with PMA and treated with CS (200 µg/mL) or Prednisolone (Pred, 10 µmol/L). Quantification was carried out as above and shown on the right. ***, p<0.0001 vs. VH samples; ###, p<0.0001 vs. TNF-α samples; %%%, p<0.0001 vs. TNF-α samples. Experiments were carried out per sextuplicate in two independent experiments.
Discussion
Cardiovascular disease is the leading cause of death in many industrialized countries, and atherosclerosis is the most important underlying pathology.8 Atherosclerosis is characterized by accumulation of lipids and an inflammatory response in the arterial intima, resulting in the formation of plaque that is susceptible to rupture and cause an acute coronary event.18 TNF-α, a pleiotropic cytokine released by adipose tissue in obesity, exerts major modulatory effects in the pathogenesis of atherosclerosis.2–3,19 The non-genetically modified DIO animal model (high fat diet) resembles the chronic inflammation and metabolic disorders that lead to acute and chronic vascular injury in atherosclerosis.20–21 Our in vivo outcomes showing reduction of both number of macrophages in arteries and serum inflammatory cytokines in CS-treated obese mice pointed to a possible role of CS interfering with the inflammatory response of endothelium and monocytes in obesity. For that reason we analyzed the effects of the treatment of CS in inflamed HCAEC and monocytes THP-1.
We describe herein for the first time, to our knowledge, the main cellular mechanism by which CS controls the atherogenic milieu. CS interacts with the main local cells involved in the atherosclerotic process, that is, endothelial cells and monocytes. CS interaction with TNF-α-inflamed coronary endothelial cells or monocytes reduces the secretion of IL-6, IL-8, C-Reactive protein and chemokines by endothelial cells and the secretion of IL1-β and TNF-α by monocytes and macrophages. This explains part of the in vivo effects of CS on the cytokine profile in obese mice. Interestingly, CS also interferes with the TNF-α-induced secretion of IL-6 and IL-8 in monocytes and macrophages. These two locally secreted cytokines are directly related to some adipokines relevant in modulating atherosclerosis, and they are also a risk factor in future coronary artery disease in healthy population.22–26 Furthermore, they can act synergistically in association with C-reactive protein27 and other cytokines (e.g. IL-4, IL-10, IL-12, IL-15, IL-23) and markedly amplify their activity either inducing their expression or regulating the expression of cytokine receptors.8 This together with the fact that we showed how CS treatment prevents the activation of monocytes to differentiate and migrate to the inflamed endothelium supports the hypothesis that CS treatment reduces atherogenesis via a dual way of action: CS interacts with endothelial cell matrix to prevent TNF-α-mediated pro-inflammatory effects; and CS interacts with cell matrix of monocytes and macrophages preventing their activation and migration. The data presented here give insight into how CS reduces cardiovascular events by interfering with the promotion of atherogenesis. CS appears as a candidate to be used in the prevention of atherosclerotic cardiovascular events in obese population.
Limitations and Strengths
This report provides a new insight into the role of CS in the activation of pro-inflammatory pathways that are shared between obesity and atherogenesis. However, this study provides a proof of concept of beneficial effects of CS only at the level of prevention of mild atherosclerotic plaque formation. In clinical trials CS demonstrated to protect from cardiovascular events. Therefore animal experiments using models of advanced atherosclerosis and cardiovascular disease are needed to assess the effects of CS in unstable plaques and in mortality due to cardiovascular events.
Supplementary Material
Highlights.
-
-
CS therapy reduces macrophage presence in arterial plaques in obese mice.
-
-
CS therapy reduces pro-inflammatory cytokines in obese mice.
-
-
CS interacts with monocytes and endothelial cells reducing TNF-α effects.
-
-
CS reduces migration of monocytes to inflamed endothelial cells.
-
-
CS offers a totally safe strategy to reduce the risk of atherosclerosis in obesity.
Acknowledgments
We acknowledge support provided by the David H. Koch Institute for Integrative Cancer Research at the Massachusetts Institute of Technology for providing access to multiphoton microscopy used for this study.
Sources of Funding
This work was supported by Bioibérica, by Fundacio Empreses IQS, and a grant from Spanish Ministerium of Economy, SAF2013-43302-R. P.M-L was supported by a post-doctoral fellowship from the Fundacion Alfonso Martin Escudero program 2012 and then by the Beatriu de Pinós Program, Modalitat-A awarded by AGAUR (fellowship number: 2013 BP_A 00051). ERE is funded in part by a grant from the National Institutes of Health (R01 GM49039).
Abbreviation list
- CS
Chondoitin sulfate
- HCAEC
human coronary artery endothelial cells
- EC
Endothelial cell
- MCP-1
monocyte chemotactic protein 1
- PMA
Phorbol-12-myristate-13-acetate
- TNF-α
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts to disclose.
References
- 1.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6(6):399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tμor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 3.McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009;6(6):410–417. doi: 10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Hansson GK. Inflammation and Immunity in Diseases of the Arterial Tree: Players and Layers. Circ Res. 2015;116(2):307–311. doi: 10.1161/CIRCRESAHA.116.301313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 7.Kevin J. Woollard, Frederic Geissmann. Nat Rev Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86(2):515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 9.Baker AB, Ettenson DS, Jonas M, Nugent MA, Iozzo RV, Edelman ER. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-beta signaling pathway. Circ Res. 2008;103(3):289–297. doi: 10.1161/CIRCRESAHA.108.179465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran-Lundmark K, Tran PK, Paulsson-Berne G, Fridén V, Soininen R, Tryggvason K, Wight TN, Kinsella MG, Borén J, Hedin U. Heparan sulfate in perlecan promotes mouse atherosclerosis: roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ Res. 2008;103(1):43–52. doi: 10.1161/CIRCRESAHA.108.172833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison LM. Response of ischemic heart disease to chondroitin sulfate-A. J Am Geriatr Soc. 1969;17(10):913–923. doi: 10.1111/j.1532-5415.1969.tb02328.x. [DOI] [PubMed] [Google Scholar]
- 12.Morrison LM, Enrick N. Coronary heart disease: reduction of death rate by chondroitin sulfate A. Angiology. 1973;24:269–287. doi: 10.1177/000331977302400503. [DOI] [PubMed] [Google Scholar]
- 13.Nakazawa K, Murata K. Comparative study of the effects of chondroitin sulfate isomers on atherosclerotic subjects. Z Alternsforsch. 1979;34(2):153–159. [PubMed] [Google Scholar]
- 14.du Souich P, García AG, Vergés J, Montell E. Immunomodulatory and anti-inflammatory effects of chondroitin sulphate. J Cell Mol Med. 2009;13(8A):1451–1463. doi: 10.1111/j.1582-4934.2009.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Abajo FJ, Gil MJ, García Poza P, Bryant V, Oliva B, Timoner J, García-Rodríguez LA. Risk of nonfatal acute myocardial infarction associated with non-steroidal antiinflammatory drugs, non-narcotic analgesics and other drugs used in osteoarthritis: a nested case-control study. Pharmacoepidemiol Drug Saf. 2014;23(11):1128–1138. doi: 10.1002/pds.3617. [DOI] [PubMed] [Google Scholar]
- 16.Volpi N. Anti-inflammatory activity of chondroitin sulphate: new functions from an old natural macromolecule. Inflammopharmacology. 2011;19(6):299–306. doi: 10.1007/s10787-011-0098-0. [DOI] [PubMed] [Google Scholar]
- 17.Tan GK, Tabata Y. Chondroitin-6-sulfate attenuates inflammatory responses in murine macrophages via suppression of NF-κB nuclear translocation. Acta Biomater. 2014;10(6):2684–2692. doi: 10.1016/j.actbio.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Koskinas KC, Feldman CL, Chatzizisis YS, Coskun AU, Jonas M, Maynard C, Baker AB, Papafaklis MI, Edelman ER, Stone PH. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: a serial, in vivo intravascular ultrasound study. Circulation. 2010;121(19):2092–2101. doi: 10.1161/CIRCULATIONAHA.109.901678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boesten LS, Zadelaar AS, van Nieuwkoop A, Gijbels MJ, de Winther MP, Havekes LM, van Vlijmen BJ. Tumor necrosis factor-alpha promotes atherosclerotic lesion progression in APOE*3-Leiden transgenic mice. Cardiovasc Res. 2005;66(1):179–185. doi: 10.1016/j.cardiores.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Chen JX, Stinnett A. Critical role of the NADPH oxidase subunit p47phox on vascular TLR expression and neointimal lesion formation in high-fat diet-induced obesity. Lab Invest. 2008;88(12):1316–1328. doi: 10.1038/labinvest.2008.92. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97(12):1245–1252. doi: 10.1161/01.RES.0000194328.57164.36. [DOI] [PubMed] [Google Scholar]
- 23.Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A, Bavendiek U, von Felden J, Divchev D, Kempf T, Wollert KC, Seegert D, Rose-John S, Tietge UJ, Schieffer B, Grote K. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32(2):281–290. doi: 10.1161/ATVBAHA.111.229435. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 25.Boekholdt SM, Peters RJ, Hack CE, Day NE, Luben R, Bingham SA, Wareham NJ, Reitsma PH, Khaw KT. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2004;24(8):1503–1508. doi: 10.1161/01.ATV.0000134294.54422.2e. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.