Abstract
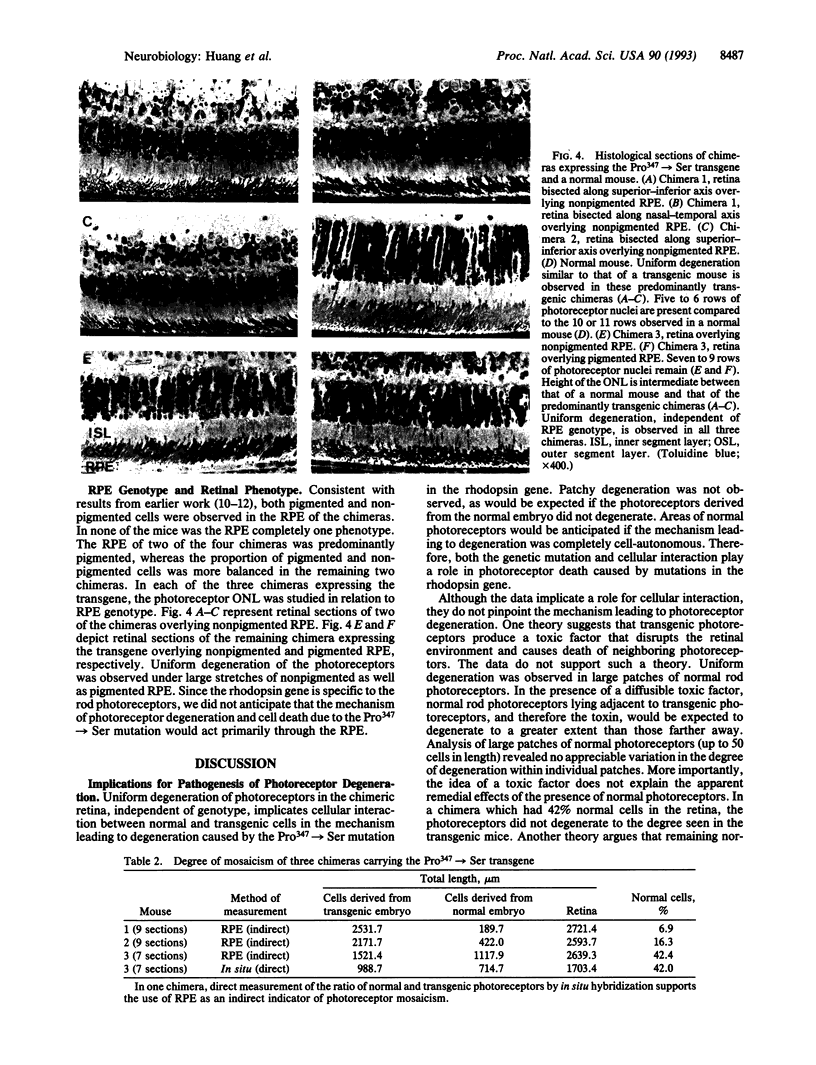
Photoreceptors of transgenic mice expressing a mutant rhodopsin gene (Pro347-->Ser) slowly degenerate. The mechanism of degeneration was studied by aggregation of embryos of normal and transgenic mice to form chimeras. In these chimeras, mosaicism was observed in the coat color, retinal pigment epithelium, and retina. In the retina, the genotype of adjacent patches of normal and transgenic photoreceptors was determined by in situ hybridization with a transgene-specific RNA probe. Photoreceptors in the chimeric retina degenerated uniformly, independent of the genotype and similar to the photoreceptors in transgenic mice. However, the chimeric retinas showed varying proportions of normal and transgenic cells. The chimeric retina with a nearly even proportion of normal and transgenic photoreceptors displayed uniform but slower degeneration than that observed in a transgenic mouse of the same age. Our results demonstrate non-autonomy of gene action for the mutated rhodopsin gene and imply that cellular interactions between photoreceptors in the retina probably play a role in degeneration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berson E. L. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993 Apr;34(5):1659–1676. [PubMed] [Google Scholar]
- Blumberg D. D. Creating a ribonuclease-free environment. Methods Enzymol. 1987;152:20–24. doi: 10.1016/0076-6879(87)52005-5. [DOI] [PubMed] [Google Scholar]
- Boughman J. A., Fishman G. A. A genetic analysis of retinitis pigmentosa. Br J Ophthalmol. 1983 Jul;67(7):449–454. doi: 10.1136/bjo.67.7.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes C., Li T., Danciger M., Baxter L. C., Applebury M. L., Farber D. B. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990 Oct 18;347(6294):677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Young W. S., 3rd Localization and quantitation of opsin and transducin mRNAs in bovine retina by in situ hybridization histochemistry. FEBS Lett. 1986 May 12;200(2):275–278. doi: 10.1016/0014-5793(86)81151-6. [DOI] [PubMed] [Google Scholar]
- Connell G., Bascom R., Molday L., Reid D., McInnes R. R., Molday R. S. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):723–726. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Hahn L. B., Cowley G. S., McGee T. L., Berson E. L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Hahn L. B., Cowley G. S., Olsson J. E., Reichel E., Sandberg M. A., Berson E. L. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990 Nov 8;323(19):1302–1307. doi: 10.1056/NEJM199011083231903. [DOI] [PubMed] [Google Scholar]
- LaVail M. M., Mullen R. J. Role of the pigment epithelium in inherited retinal degeneration analyzed with experimental mouse chimeras. Exp Eye Res. 1976 Aug;23(2):227–245. doi: 10.1016/0014-4835(76)90206-2. [DOI] [PubMed] [Google Scholar]
- LaVail M. M., Unoki K., Yasumura D., Matthes M. T., Yancopoulos G. D., Steinberg R. H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. I. In situ hybridization of messenger RNA sequences. Histochem J. 1989 Aug;21(8):435–440. doi: 10.1007/BF01845792. [DOI] [PubMed] [Google Scholar]
- McLaughlin M. E., Sandberg M. A., Berson E. L., Dryja T. P. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993 Jun;4(2):130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- Naash M. I., Hollyfield J. G., al-Ubaidi M. R., Baehr W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5499–5503. doi: 10.1073/pnas.90.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J. E., Gordon J. W., Pawlyk B. S., Roof D., Hayes A., Molday R. S., Mukai S., Cowley G. S., Berson E. L., Dryja T. P. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992 Nov;9(5):815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- Petters R. M., Mettus R. V. Survival rate to term of chimeric morulae produced by aggregation of five to nine embryos in the mouse, Mus musculus. Theriogenology. 1984 Aug;22(2):167–174. doi: 10.1016/0093-691x(84)90429-1. [DOI] [PubMed] [Google Scholar]
- Pittler S. J., Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S., Hawkins R. K., Zeilmaker G. H. Development and degeneration of retina in rds mutant mice: analysis of interphotoreceptor matrix staining in chimaeric retina. Curr Eye Res. 1988 Dec;7(12):1183–1190. doi: 10.3109/02713688809033222. [DOI] [PubMed] [Google Scholar]
- Sanyal S., Zeilmaker G. H. Development and degeneration of retina in rds mutant mice: light and electron microscopic observations in experimental chimaeras. Exp Eye Res. 1984 Aug;39(2):231–246. doi: 10.1016/0014-4835(84)90011-3. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis G. H., Sutcliffe J. G., Bok D. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991 Jan;6(1):61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]
- Treisman J. E., Morabito M. A., Barnstable C. J. Opsin expression in the rat retina is developmentally regulated by transcriptional activation. Mol Cell Biol. 1988 Apr;8(4):1570–1579. doi: 10.1128/mcb.8.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]