Abstract
Objective
A growing body of literature supports the view that essential tremor (ET) involves alteration of cerebellar-thalamo-cortical networks which can result in working memory and executive deficits. In this study we tested the hypothesis that individuals with ET would exhibit worse performance on memory tasks requiring more intrinsic organization and structuring (i.e., word lists) relative to those with fewer ‘executive’ demands (i.e., stories), similar to that previously observed in individuals with Parkinson’s disease (PD).
Method
Participants included a convenience sample of 68 ET patients and 68 idiopathic PD patients, retrospectively matched based on age, education, and sex. All patients underwent routine neuropsychological evaluation assessing recent memory, auditory attention/working memory, language, and executive function. Memory measures included the Hopkins Verbal Learning Test-R and WMS-III Logical Memory.
Results
Both ET and PD patients performed significantly worse on word list than story memory recall tasks. The magnitude of the difference between these two memory tasks was similar for ET and PD patients. In both patient groups, performance on measures of executive function and auditory attention/working memory was not distinctly correlated with word list vs. story recall.
Conclusions
These findings suggest that frontal-executive dysfunction in both ET and PD may negatively influence performance on memory tests that are not inherently organized. Although the pathophysiology of these two ‘movement disorders’ are quite distinct, both have downstream effects on thalamo-frontal circuitry which may provide a common pathway for a similar memory phenotype. Findings are discussed in terms of neuroimaging evidence, conceptual models, and best practice.
Keywords: essential tremor, Parkinson disease, memory, cognition, cerebellum
Introduction
Essential tremor (ET) and Parkinson’s disease (PD) are two of the most common movement disorders in the world (Tanner & Goldman, 1994). The prevalence of each disorder increases with age, though ET is as much as 20 times more common than PD, with between 4 and 50 cases observed per 1000 persons (deLau & Breteler, 2006; Louis, Ottman, & Hauser, 1998). While development of tremor is a prominent feature in both ET and PD, each disorder is distinct with regard to presentation, course of illness, and pathophysiology. In contrast to the resting, 4-7 Hz “pill-rolling” tremor seen in PD, individuals with ET present with 5-12 Hz tremors during purposeful (action) movement and static posing (Baumann, 2012; Louis, 2001). Tremor in PD typically first develops unilaterally, while in ET, bilateral onset is more common (Deuschl, Bain, & Brin, 1998). Other motoric differences include the presence of bradykinesia and rigidity in PD (Sian, Gerlach, Youdim, & Riederer, 1999) and the development of head tremor in ET (Louis & Dogu, 2009).
With respect to onset, PD is typically diagnosed in the 5th or 6th decade of life and has been associated with both genetic and environmental etiologies (Semchuk, Love, & Lee, 1993). While the course of functional decline is variable, deterioration is often seen as more rapid in the early phase of the disease and more gradual as pathology progresses (Jankovic & Kapadia, 2001; Lang, 2007; Post, Merkus, & de Haan, 2007). In contrast, ET typically manifests as a slowly progressive disorder, with both age and disease duration independently associated with tremor severity; however, the course of the disease can vary considerably across individuals (Elble, 2000; Louis, Jurewicz, & Watner, 2003; Putzke, Whaley, Baba, Wszolek, & Uitti, 2006). Epidemiological studies have observed a bimodal age of onset, with younger onset (2nd or 3rd decade) associated with familial etiology and older onset (7th or 8th decade) associated with sporadic/environmental genesis (Brin & Koller, 1998; Lou & Jankovic, 1991; Tanner et al., 2001), though the timeline of development remains a topic of ongoing debate (Louis, Clark, & Ottman, 2015).
The prevailing view regarding the pathophysiological development of PD implicates the sequential accumulation of alpha-synuclein (the principal structural component of Lewy bodies), beginning in the peripheral autonomic nervous system, and extending to subcortical areas and the cortex in mid- and late stages of the disease, respectively (Braak et al., 2003). Degeneration of dopamine producing neurons in the substantia nigra (prompted by Lewy body pathology) results in dopaminergic depletion in parallel basal ganglia-thalamocortical circuits that exert influence on specific areas of the frontal lobes (Alexander, DeLong, & Strick, 1986; Rodriguez-Oroz et al., 2009). Disruption of circuits that involve the motor cortex results in typical motor symptoms, while disruption of circuits affecting dorsolateral and orbitofrontal regions results in a cognitive phenotype that includes cognitive slowing, forgetfulness, and difficulties with set-shifting, multi-tasking, and working memory (Levin & Katzen, 2005; Zgaljardic, Borod, Foldi, & Mattis, 2003).
A second view highlights the notion that PD neurodegeneration extends beyond the depletion of nigrostriatal dopamine, such that cognitive disturbances may be attributed to the alteration of multiple neurotransmitter systems. The basal forebrain complex, which provides primary cholinergic input to the cortex, is known to accumulate alpha-synuclein deposition in PD, resulting in a loss of cholinergic neurotransmission and subsequent cognitive dysfunction (Bohnen & Albin, 2011). Cholinergic depletion leads to a cognitive profile with memory and visuospatial deficits and is thought to result in a more rapid transition to dementia, in contrast to the relatively mild cognitive deficits resulting from dopamine depletion (Svenningsson, Westman, Ballard & Aarsland, 2012; Williams-Gray, Foltynie, Lewis, & Barker, 2006).
By comparison, the pathophysiological basis of ET is less clear. Converging data from imaging and post-mortem studies suggests that the characteristic ‘action’ tremor in ET results from degenerative pathological processes in the cerebellum (e.g., Purkinje cell abnormalities) and its inflow (ponto-cerebellar) or outflow pathways from cerebellar to thalamic to frontal motor regions (Grimaldi & Manto, 2013; Louis et al., 2006). Alternative views argue against a degenerative basis of ET and propose that motor manifestations are related to abnormal neuronal oscillation within cerebello-thalamo-cortical networks caused by brain-based biochemical abnormalities (e.g.,, altered GABA function) or metabolic dysfunction (Deuschl & Elble, 2009; Rajput, Adler, Shill, & Rajput, 2012). However, despite variability in pathological studies of degenerative changes, findings supporting non-degenerative hypotheses do not necessarily preclude a degenerative contribution to disease onset and progression. In fact, the clinical heterogeneity observed in ET may well reflect the diversity of underlying pathophysiological mechanisms.
Like PD, ET is now known to involve mild cognitive changes. Such changes include poor performance on frontal-executive tasks assessing cognitive inhibition, speeded verbal fluency, set-shifting, and working memory (Benito-Leon, Louis, & Bermejo-Pareja, 2006; Duane & Vermilion, 2002; Gasparini et al., 2001; Janicki, Cosentino, & Louis, 2013; Higginson et al., 2008; Kim et al., 2009; Lacritz, Dewey, Giller, & Cullum, 2002; Lombardi, Woolston, Roberts, & Gross, 2001; Sahin et al., 2006; Thawani, Schupf, & Louis, 2009; Troster et al., 2002). Two primary hypotheses have been put forward to account for these cognitive changes. One hypothesis relates to the cognitively sedating effects of medications used to treat tremors, such as primidone and propranolol. However, Sahin et al. (2006) found that 16 medication-naïve ET patients also exhibited mild fronto-executive disturbances, arguing against a purely medication-related interpretation as the sole source of cognitive difficulties.
A second hypothesis attributes cognitive disturbances to alterations in cerebellar-thalamic-prefrontal/parietal circuitry which in turn influence performance on frontally-mediated cognitive tasks (Troster et al., 2002). Indeed, functional magnetic resonance imaging (fMRI) studies examining cognitive performance in ET have demonstrated overactivation of the dorsolateral prefrontal and inferior parietal cortices and reduced task-related functional connectivity between these areas and regions within the cerebellum consistently implicated in cognitive functioning (Cerasa et al., 2010; Passamonti et al., 2011; Stoodley, Valera, & Schmahmann, 2012). Studies using diffusion tensor imaging (DTI) have revealed microstructural changes (e.g., reduced fractional anisotropy) in white matter outflow pathways from the dentate nucleus of the cerebellum, the ventral portion of which projects to prefrontal and parietal areas via the thalamus (Nicoletti et al., 2010; Shin, Han, Kim, & Lee, 2008). Such findings align with histological studies documenting anatomically distinct cerebellar motor and cognitive loops, much like the segregated basal ganglia-thalamocortical circuits affected in PD (Middleton & Strick, 2000; Schmahmann & Pandya, 1995).
In light of the mild frontal-executive difficulties described in previous ET investigations, the question arises whether these difficulties will influence other cognitive domains such as the ability to learn and retrieve verbal information. For example, several studies have pointed to differences in memory profiles of individuals with frontal-subcortical vs. mesial temporal lobe abnormalities, with the former having difficulties retrieving information due to poor use of organizational strategies during encoding and the latter exhibiting abnormal forgetting and retention (Cummings, 1990; Panegyres, 2004; Perry & Hodges, 1996). This distinction is most elegantly highlighted in studies finding performance discrepancies on serial list-learning (‘word list’) and paragraph recall (‘story’) tasks (Helmstaedter, Witzke, & Lutz, 2009; Randolph et al., 1994; Wicklund, Johnson, Rademaker, Weitner, & Weintraub, 2006). While delayed retrieval on word list tasks is benefitted by the mental reorganization of words into semantically related categories during encoding, story tasks are typically pre-organized into semantically meaningful narratives, which likely facilitates subsequent retrieval. Accordingly, focal frontal lobe damage and executive dysfunction are associated with worse memory performance on unstructured word list tasks relative to story tasks delivered in narrative prose (Brooks, Weaver, & Scialfa, 2006; Kopelman & Stanhope, 1998; Tremont, Halpert, Javorsky, & Stern, 2000).
In support of this dissociation in memory task performance, Zahodne et al. (2011) found that individuals with PD exhibited worse performance on a memory task requiring more intrinsic organization and structuring (Hopkins Verbal Learning Test-R) relative to one with fewer ‘executive’ demands (WMS-III Logical Memory). Furthermore, performance on the word list task, but not the story task, was associated with composite measures of executive functioning and working memory. This pattern of findings was interpreted as reflecting frontal-subcortical dysfunction secondary to dopamine deficiency within striatal-thalamo-cortical circuits in PD. While ET is generally described as pathophysiologically distinct from PD, both disorders have similar cognitive profiles (frontal-executive dysfunction). Therefore, it is possible that similar alterations in thalamo-cortical circuitry among these disorders result in a comparable memory dissociation. Drawing such parallels between ET and PD may have implications for the clinical management of ET patients, such as underscoring the need for early detection of cognitive impairments and the refinement of pharmacological, neurorehabilitative, and surgical interventions.
The current study sought to test the hypothesis that both ET and PD patients would exhibit reduced performance on a word list memory task relative to a story memory task as a consequence of compromised executive abilities. To address this hypothesis, we retrospectively examined a matched sample of ET and PD patients who underwent neuropsychological evaluation as part of a standard clinical workup through our Movement Disorders Center. Specifically, we predicted that ET patients would perform worse on a word list memory task than a story memory task. This pattern was also predicted for PD patients. In accordance with this prediction, we aimed to determine whether performance on a word list task, but not a story task, would correlate with traditional neuropsychological measures of executive function and auditory attention/working memory, in line with that previously reported by Zahodne et al. (2011) in PD patients.
Methods
Participants
Participants included a retrospective sample of 68 ET patients and 68 PD patients, between 60 and 80 years of age, who were drawn from a larger convenience sample of patients from the INFORM database at the University of Florida Center for Movement Disorders and Neurorestoration (CMDNR). Informed consent to participate in this research was obtained following University of Florida Institutional Review Board (IRB) guidelines.
To be included, participants had to have undergone a neuropsychological evaluation and received diagnoses of ET or idiopathic PD by fellowship-trained movement disorders neurologists according to the Louis criteria or UK Brain Bank criteria, respectively (Hughes, Ben-Shlomo, Daniel, & Lees, 1992; Louis, Ford, Lee, Andrews, & Cameron, 1998). Exclusion criteria included: a) history of brain surgery, b) neurological conditions other than ET or PD, c) severe psychiatric disability (e.g., schizophrenia, psychosis, current active major depression), and d) evidence of cognitive disturbance based on scores below 130 on the Dementia Rating Scale-2 (DRS-2). The majority of participants were seen to determine their candidacy for deep brain stimulation surgery. As part of the standard clinical workup at the CMDNR, all were screened for psychiatric disturbance by neuropsychiatrists using a standard diagnostic clinical interview, based on DSM-IV criteria. Additionally, all participants received mood questionnaires including the Beck Depression Inventory-II (Beck, Steer, & Brown, 1996), the State-Trait Anxiety Inventory (Spielberger & Sydeman, 1994) and the Apathy Scale (Marin, Biedrzycki, & Firinciogullari, 1991), and neuropsychological exams. Clinical measures of PD severity included the Unified Parkinson’s Disease Rating Scale (UPDRS; Fahn & Elton, 1987) and Hoehn-Yahr staging (Hoehn & Yahr, 1967). The average “on” score from Part III (motor) of the UPDRS was used, where higher scores indicate greater motor severity. The Hoehn-Yahr scale defines broad stages (from 1 to 5) of symptom progression in PD, with higher scores reflecting worse severity. Table 1 lists the total number of PD participants classified under each stage. The levodopa equivalency dose (LED), which measures the amount of dopaminergic medication used by each PD patient, was also obtained (Tomlinson et al., 2010).
Table 1.
Sample Characteristics
| Essential Tremor (N = 68) | Parkinson’s (N = 68) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Demographics | ||||
| Age (years) | 69.4 | 5.6 | 69.4 | 5.6 |
| Education (years) | 14.3 | 2.8 | 14.3 | 2.5 |
| Gender (m/f) | 43/25 | - | 43/25 | - |
| Disease | ||||
| Duration (years) | 24.4* | 17.5 | 10.2* | 6.1 |
| UPDRS-III “on” | - | - | 25.5 | 10.1 |
| Hoehn and Yahr Stage (# at each stage) |
- | - | 1.5 (n = 1) 2.0 (n = 41) 2.5 (n = 7) 3.0 (n = 8) 4.0 (n = 3) |
- |
| LED | - | - | 736.2 | 549.2 |
| Mood Measures | ||||
| BDI-II | 9.5 | 8.3 | 9.7 | 7.4 |
| Apathy Scale | 11.2 | 5.9 | 11.7 | 6.1 |
| STAI percentile (state) |
59.2 | 28.6 | 59.2 | 30.4 |
| STAI percentile (trait) |
51.9 | 31.9 | 54.9 | 31.8 |
| General Cognitive | ||||
| DRS-2 | 136.6 | 3.4 | 137.5 | 3.8 |
SD = Standard deviation; UPDRS = Unified Parkinson Disease Rating Scale; LED = Levodopa Equivalent Dose; BDI-II = Beck Depression Inventory-II, STAI= State-Trait Anxiety Inventory; DRS-2 = Dementia Rating Scale-2;
Significant difference (p < .05)
The ET group was retrospectively matched to a subset of PD patients extracted from a larger available sample. This was done, as follows, by an individual blinded to cognitive scores. Each patient in the ET group was matched to an identical counterpart in the PD group based on age, education, and gender. When identical matches across all three variables were unavailable, patients were matched first on age and then as closely as possible on education. Sample characteristics are presented in Table 1. Men outnumbered women at an approximate 2:1 ratio in both diagnostic groups. The overall sample was well educated. The groups did not significantly differ with respect to age, education, total DRS-2 score, or scores on measures of depression (BDI-II), anxiety (STAI), and apathy (AS). Using the recommended BDI-II clinical cutoff of 14 for individuals with movement disorders (Visser, Leentjens, Marinus, Stiggelbout, & van Hilten, J., 2006), 12 individuals with ET and 16 with PD scored in the clinically elevated range. The specific cognitive measures administered are listed in the subsequent sections.
Memory Measures
Hopkins Verbal Learning Test-R (HVLT)
This list-learning test involves the oral presentation of 12 words, four in each of three semantically related categories (Benedict, Schretlen, Groninger, & Brandt, 1998). Patients are asked to immediately recall the list after each of three consecutive recitations, and again after a 20-25 minute delay. Patients are not told they will be tested after the delay. The dependent variable for the immediate recall trial is total correct words recalled following the three consecutive list recitations. The dependent variable for the delayed recall trial is total correct words recalled after the delay.
Logical Memory subtest of the Wechsler Memory Scale-Third Edition (Stories)
This subtest assesses narrative memory under two different conditions: immediate and delayed free recall (Wechsler, 1997). Two brief stories are presented orally and patients are asked to recall the stories both immediately and after a 25-35 minute delay. Patients are informed that they will be asked to recall the stories after the delay. The dependent variable for the immediate recall trial is total story units recalled from each story following one presentation of the first story and two presentations of the second story. The dependent variable for the delayed recall trial is total story units recalled from each story after the delay.
Other Cognitive Measures
Composite scores were created to reflect three neurocognitive domains: executive function, auditory attention/working memory, and language. The executive function composite consisted of the Controlled Oral Word Association Test (COWA) and the Stroop Test (Color-Word trial, Golden Version). The auditory attention/working memory composite consisted of the Digit Span Forward and Backward from the WAIS-III. The language composite consisted of the Boston Naming Test (BNT) and the Vocabulary subtest from the WAIS-III. Scores from individual measures were normed using test-specific manuals or previously published norms and were converted to Z-score metric. Composite scores reflect an average of the Z-scores of tests within a given cognitive domain.
Results
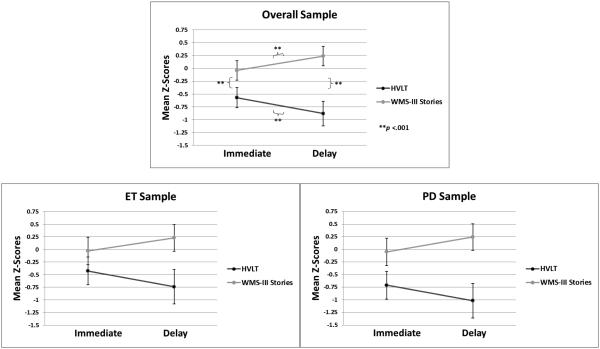
Our comparison of HVLT and Stories performance was analyzed with a mixed-model repeated measures Analysis of Variance (ANOVA). The within-subject factors were Memory Task (HVLT vs. Stories) and Recall Condition (immediate vs. delayed) and the between-subject factor was Group (ET vs. PD). Mean scores on memory tasks are displayed in Table 2 and plotted in Figure 1. The main effect of Memory Task was significant (F(1, 134) = 70.41, p < 0.001; ηp2 = 0.34). Overall, HVLT scores (M = −0.72) were worse than Stories scores (0.10). This effect was qualified by a significant Memory Task × Recall Condition interaction (F(1, 134) = 11.74, p < 0.001; ηp2 < 0.25). Pairwise comparisons revealed the following significant differences: a) immediate recall of HVLT (M = −0.57) was better than delayed recall of HVLT (M = −0.88, p < 0.001), b) immediate recall of Stories (M = −0.04) was worse than delayed recall of Stories (m = 0.24, p < 0.001), c) immediate recall of HVLT was worse than immediate recall of Stories (p < 0.001), and d) delayed recall of HVLT was worse than delayed recall of Stories (p < 0.001). Main effects of Recall Condition (F(1, 134) = 0.09, p = 0.76; ηp2 < 0.01) and Group (F(1, 134) = 14.30, p = 0.39; ηp2 < 0.10) were not significant. No other interactions reached significance (i.e., Memory Task × Recall Condition × Group), suggesting the pattern of memory task performance differences was similar in the ET and PD groups.
Table 2.
Mean Z-scores Across Cognitive Domains/Measures
| Essential Tremor (N=68) | Parkinson’s (N=68) | |
|---|---|---|
| Mean Z-score (SD) | Mean Z-score (SD) | |
| HVLT Word List | ||
| Immediate Total Recall | −0.43 (1.05) | −0.71 (1.21) |
| 20’ Delayed Recall | −0.74 (1.30) | −1.01 (1.50) |
| WMS-III Logical Memory (Stories) | ||
| Immediate Total Recall | −0.03 (1.06) | −0.05 (1.18) |
| 30’ Delayed Recall | 0.23 (1.03) | 0.25 (1.15) |
| Auditory Attention/ Working Memory | ||
| Digit Span Total Score (WMS-III) | 0.08 (0.91) | 0.24 (1.00) |
| Language | ||
| BNT | 0.04 (1.05)* | 0.44 (1.15)* |
| Vocabulary (WASI) | 0.38 (0.80) | 0.61 (0.91) |
| Composite Score | 0.21 (0.74) | 0.53 (0.92) |
| Executive Function | ||
| COWA (letter fluency) | −0.74 (1.00)* | −0.37 (1.16)* |
| Stroop Color-Word, 3rd trial | −0.41 (0.99) | −0.17 (1.07) |
| Composite Score | −0.57 (0.77)* | −0.26 (0.92)* |
For a normative sample, the Z-score mean is 0 and standard deviation (SD) is 1;
Significant difference (p < .05)
HVLT = Hopkins Verbal Learning Test; WMS-III = Wechsler Memory Scale, 3rd edition; BNT = Boston Naming Test; WASI = Wechsler Abbreviated Scale of Intelligence; COWA = Controlled Oral Word Association Test; Stroop Color Word Test (Golden version)
Note: normative scores for both the HVLT and WMS-III are based on age, but not sex or education; Normative scores for remaining tasks based on age, education and sex.
Figure 1. Mean memory scores of overall sample and individual patient groups.
ET = Essential Tremor; PD = Parkinson’s disease; HVLT = Hopkins Verbal Learning Test; WMS-III = Wechsler Memory Scale, 3rd Edition; Error bars represent the 95% confidence interval.
Top panel depicts performance of the overall sample on the word list (HVLT) and story memory (WMS-III) tasks across immediate and delayed recall conditions. The panels below show the scores of the of the ET and Parkinson groups separately,
Our secondary aim was addressed with multiple bivariate Pearson correlations examining associations between performance on memory tasks and separate cognitive domains. Williams’ t-tests were used to evaluate the statistical significance of differences in Pearson correlation magnitudes (Steiger, 1980; Williams, 1959). Mean scores on individual cognitive measures, as well as composite scores are displayed in Table 2. Table 3 shows correlations between memory tasks, other cognitive domains, and mood measures for each group. For both groups, Williams’ t-tests did not reveal significant differences in correlation magnitudes between cognitive domain composites and HVLT or Story task performance.
Table 3.
Pearson correlations between cognitive composites, memory measures, and mood measures.
|
Executive
Function |
Auditory
Attention/Working Memory |
Language | ||||
|---|---|---|---|---|---|---|
| ET | PD | ET | PD | ET | PD | |
| HVLT Word List | ||||||
| Immediate Total Recall | .390** | .408** | .135 | .285* | .398** | .405** |
| 20’ Delayed Recall | .206 | .264** | .058 | .043 | .291 | .266 |
| WMS-III Logical Memory (Stories) | ||||||
| Immediate Total Recall | .258* | .278* | .217 | .372** | .470** | .461** |
| 30’ Delayed Recall | .257* | .328** | .272* | .386** | .380* | .487** |
| Mood Measures | ||||||
| BDI-II | −.150 | −.095 | −.267* | −.114 | .090 | −.143 |
| Apathy Scale | −.107 | −.015 | −.377* | .077 | .206 | −.022 |
| STAI percentile (State) | −.044 | −.075 | −.359** | .117 | −.174 | .039 |
| STAI percentile (Trait) | −.091 | −.029 | −.336** | .036 | −.003 | −.036 |
ET = Essential Tremor; PD = Parkinson’s disease; HVLT = Hopkins Verbal Learning Test; WMS-III = Wechsler Memory Scale, 3rd edition; BDI-II = Beck Depression Inventory-II; STAI = State-Trait Anxiety Inventory;
p<.05;
p<.01.
Discussion
As hypothesized, ET patients exhibited significantly worse performance on a word list memory task than a story memory task. This was observed for both immediate and delayed recall conditions. A similar pattern was seen in the PD sample, in line with previous findings of Zahodne et al. (2011). One possible explanation for these findings is that the memory performance discrepancy actually reflects differences in task difficulty among the measures used. For instance, it may be that HVLT, which uses a different normative sample than Stories, is inherently more challenging for the examinee. This possibility was addressed in the Zahodne et al. (2011) study with the addition of a comparable word list task (WMS-III Word List) co-normed with Stories. Indeed, performance on the WMS-III Word List by PD patients still resulted in worse delayed memory than performance on the co-normed WMS-III Stories. Since both tasks came from the same normative sample, this finding provided some support that worse word list memory may not merely be an artifact of different norms. At the time however, Zahodne et al. (2011) found that HVLT delayed recall scores of PD patients were nevertheless worse than WMS-III Word List delayed recall scores. Thus, neither Zahodne et al. (2011) nor the present study can rule out the possibility that the differential deficit between the HVLT and Stories is partially related to differing levels of task difficulty. Future studies can address this concern by prospectively selecting instruments matched on psychometric characteristics (i.e., true-score variance) boosting discriminating power, as advocated by Chapman and Chapman (1978). Alternatively, future studies would benefit from the inclusion of a healthy control group. Similar performance on word list and story memory tasks in a non-patient population may help to clarify the possibility of divergent task demands.
Another related factor potentially influencing discrepant performance on word list and story tasks pertains to basic procedural differences in how the measures are administered. During standard administration of Stories, examinees are explicitly informed after the immediate recall trials that they will be asked to recall stories at a later time. No such orienting instructions are offered during HVLT. This distinction effectively yields intentional and incidental learning contexts, each of which may correspond to a different level of processing during encoding (Craik & Lockhart, 1972; Hyde & Jenkins, 1973). Specifically, the orienting instruction given during Stories could potentially facilitate patients’ encoding (i.e., via intentional mental rehearsal) and subsequent retrieval of information on delayed recall.
The most compelling interpretation for our findings of poor word list recall in PD patients relates to the influence of fronto-subcortical dysfunction. Dopamine depletion in basal ganglia-thalamocortical circuits innervating the prefrontal cortex may negatively influence appropriate mental processes necessary for the categorization of unrelated words into semantically related groups during the encoding phase of word list learning (Bondi, Kaszniak, Bayles, & Vance, 1993; Buytenhuijs et al., 1994; Weingartner, Burns, Diebel, R, & LeWitt, 1984). Although the pathophysiological mechanisms underlying frontal dysfunction in ET are less clear, it may be that analogous disturbances in neurocircuitry result in inefficient mental organization abilities.
Cerebellar degenerative changes (e.g., Purkinje cell loss, axonal swelling, and reduced dendritic complexity) are thought to contribute to motor manifestations of ET, but an emerging line of evidence implicates a role of this pathology in the development of cognitive deficits, such that selective impairments may depend upon the particular corticocerebellar circuits affected by the disorder (Louis et al., 2014; Middleton & Strick, 2000; Troster et al., 2002). As documented in histological studies in primates, a two-stage system links the cerebellum with cortical regions involved in cognitive functioning. Such regions send feedforward projections to the pontine nuclei, which in turn convey information to the posterior lobules of the cerebellum (Schmahmann & Pandya, 1997). The ventral dentate nucleus then sends projections back to the cortex via the thalamus. Within this system, projections distinctively innervate, for instance, prefrontal cortical areas important for planning and working memory (i.e., Brodmann areas 9 and 46) and posterior parietal areas involved in attention and visuospatial functioning (Strick, Dum, & Fiez, 2009). As a consequence of degenerative changes in such circuitry, individuals with ET may present with a frontal-executive cognitive profile similar to that observed in PD. Hence, the shared memory phenotype in this study may reflect analogous downstream effects on thalamo-frontal circuitry resulting from pathology originating in distinct brain structures (i.e., cerebellum vs. basal ganglia).
In contrast to the findings of Zahodne et al. (2011), we were unable to provide support for our hypothesis that performance on a word list task would be distinctly associated with measures of executive functioning and auditory attention/working memory in either patient group. This pattern of results may reflect a high degree of intercorrelation between the neuropsychological measures administered, limiting the interpretability of significant associations among memory and other cognitive tasks. For instance, relative to the executive function composite scores, the language composite scores are more strongly correlated with memory performance across both groups. The most compelling explanation for this finding relates to the fact that the memory and language tasks are all verbally mediated. Another interpretation for these results relates to the tasks used to evaluate executive functioning. For instance, performance on tasks of response inhibition and letter fluency (as measured by Stroop Color-Word and COWA in this study) may not reflect functioning of frontostriatal circuitry involved in the mental organization of verbal material.
While studies attempting to localize specific “executive” abilities to discrete neural areas are highly variable, some general findings have emerged. Inefficient use of semantic clustering strategies during word list learning has been attributed to disturbances in the left dorsolateral prefrontal cortex (Alexander, Stuss, & Fansabedian, 2003; Stuss et al., 1994). In contrast, response inhibition has consistently been shown to rely on activity in the right inferior frontal cortex, while letter fluency is associated with activity in the left inferior gyrus (Aron, Robbins, & Poldrack, 2004; Costafreda et al., 2006). Given that data used in the current study were derived from a convenience sample, we were unable to prospectively select specific tasks in support of our aims. In place of the executive measures used in this study, future studies might incorporate working memory tasks involving the consolidation of related information into fewer units (i.e., “chunking”). Such measures may be more appropriate for comparison with word list and story memory tasks.
Overall, our findings highlight the utility of comparing the neuropsychological profiles of these two disorders in order to shed light on shared pathophysiological mechanisms. This is of particular relevance given the growing evidence of an etiological association between ET and PD. For instance, individuals with ET are, by some estimates, 3-4 times more likely to develop PD than controls (Benito-Leon, Louis, & Bermejo-Pareja, 2009; Simoes et al., 2012; Shahed & Jankovic, 2007; Tan, Lee, Fook-Chong, & Lum, 2008) and relatives of individuals with PD have increased prevalence of familial tremor (Jankovic, Beach, Schwartz, & Contant, 1995; Lang, Kierans, & Blair, 1987). Postmortem studies in ET have shown that a subset of patients has Lewy body pathology confined to the Locus Coeruleus, which raises the possibility that Lewy body pathology spreads to the substantia nigra or associated anatomical regions in individuals with ET who go on to develop PD (Louis et al., 2006; Louis et al., 2007). Mirroring findings in PD, evidence from cross-sectional and prospective studies suggests that ET is associated with increased risk of developing dementia relative to age-matched healthy individuals (Benito-Leon et al., 2006; Bermejo-Pareja, Louis, & Benito-Leon, 2007; Thawani et al., 2009). Given these associations, it may be of considerable prognostic value to monitor the heterogeneous cognitive presentation in these populations. Distinct cognitive profiles may reflect differences in disease onset, progression, and risk of developing dementia.
Such knowledge may also have implications for improved intervention and treatment. Currently, deep brain stimulation (DBS) is a common and effective treatment for ET and PD patients with medication refractory motor symptoms (Zesiewicz et al., 2013). However, DBS is known to induce cognitive, mood, and behavioral changes, and therefore selection of surgery candidates requires careful consideration of preexisting cognitive difficulties. Specifically, patients who are evaluated to have dementia or significant cognitive decline exceeding what is expected for their disorder are likely poor candidates for surgery (Okun et al., 2007). Given the current findings, it is possible that neuropsychological batteries containing only word list memory tasks may inaccurately assess patients as having memory system impairments, while those containing only story tasks may overlook frontally-mediated deficits. Accordingly, memory evaluations employing both word list and story tasks are better suited to identify candidates for surgery. For example, a patient exhibiting impairments on both tasks may be categorized as having a primary memory impairment and deemed less likely to benefit from surgical intervention.
The current study has several additional limitations. Because data for all analyses were drawn from a convenience sample of patients who were seen through a tertiary care specialized movement disorders center, our findings may not generalize to the greater ET/PD population. Rather, they are restricted to a more educated patient group seeking clinical care, and potentially deep brain stimulation, from a specialty provider. Because our sample was relatively well educated and the norms for the memory tasks used in this study are only based on age, it is possible that higher education could inflate scores on these tasks; however, such inflation would be unlikely to affect the discrepancy between word list and story task performance. An additional sample-related limitation relates to the impact of disease duration on cognitive performance among ET and PD patients. Average disease duration was significantly greater in the ET sample, which could presumably signify more advanced disease progression and/or worse cognitive dysfunction in this group. While this cannot be ruled out, basic differences in variability of age of disease onset and rates of functional decline between the two disorders confound our ability to draw such conclusions. Lastly, with the exception of LED for the PD group, information reflecting participants’ medication usage was unavailable. Consequently, we were unable to account for the effects of medications on cognitive performance.
In examining memory discrepancies in ET and PD, we aimed to learn whether there was continued support for the view that these disorders share comparable neuropsychological profiles. Indeed, both disorders are frequently noted to exhibit deficits on tasks of frontal-executive function. The findings from the current study provide additional support for this perspective. Given that disease pathology affects thalamo-cortical circuits in both disorders, it is likely that convergent validity relationships among the disorders are more likely than not. Despite such similarities, there are distinct neuropsychological differences between ET and PD. Perhaps most salient is the cognitive/motor slowing in PD secondary to dopaminergic depletion in fronto-striatal circuits. Indeed, one of the cardinal symptoms of PD is slowness of movement (bradykinesia) and slowness of thought (bradyphrenia). This type slowness is not characteristic of ET.
To appropriately test this ‘divergent’ hypothesis, one needs to move beyond traditional neuropsychological measures of processing speed that often tend to rely heavily on psychomotor indices. This is because the action/intention tremor of ET disrupts performance on fine motor tasks (e.g., Coding, Trail Making). Some ET patients have vocal tremors that can also slow oral responses (e.g., timed baseline trials of the Stroop test). Future studies should address this divergent relationship with processing speed tasks specifically designed to minimize motor involvement (i.e., simple reaction time measures).
Acknowledgements
This study was partially supported by the University of Florida Center for Movement Disorders and Neurorestoration, the UF National Parkinson Foundation Center of Excellence, and the National Institutes of Health (NINDS R21-NS079767). Mr. Lafo was supported by a NINDS predoctoral training grant (T32 NS82168).
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 1986;9(1):357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Stuss DT, Fansabedian N. California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain. 2003;126(6):1493–1503. doi: 10.1093/brain/awg128. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Baumann CR. Epidemiology, diagnosis and differential diagnosis in Parkinson’s disease tremor. Parkinsonism & Related Disorders. 2012;18:90–S92. doi: 10.1016/S1353-8020(11)70029-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- Benito-Leon J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66(1):69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson's disease and parkinsonism in essential tremor: a population based study. Journal of Neurology, Neurosurgery, & Psychiatry. 2009;80(4):423–425. doi: 10.1136/jnnp.2008.147223. [DOI] [PubMed] [Google Scholar]
- Bermejo-Pareja F, Louis ED, Benito-Leon J. Risk of incident dementia in essential tremor: a population-based study. Movement Disorders. 2007;22(11):1573–1580. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behavioural Brain Research. 2011;221(2):564–573. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Kaszniak AW, Bayles KA, Vance KT. Contributions of frontal system dysfunction to memory and perceptual abilities in Parkinson's disease. Neuropsychology. 1993;7(1):89–102. [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brin MF, Koller W. Epidemiology and genetics of essential tremor. Movement Disorders : Official Journal of the Movement Disorder Society. 1998;13(3):55–63. doi: 10.1002/mds.870131310. [DOI] [PubMed] [Google Scholar]
- Brooks BL, Weaver LE, Scialfa CT. Does impaired executive functioning differentially impact verbal memory measures in older adults with suspected dementia? The Clinical Neuropsychologist. 2006;20(2):230–242. doi: 10.1080/13854040590947461. [DOI] [PubMed] [Google Scholar]
- Buytenhuijs EL, Berger HK, Van Spaendonck KP, Horstink MW, Borm GF, Cools AR. Memory and learning strategies in patients with Parkinson’s disease. Neuropsychologia. 1994;32(3):335–342. doi: 10.1016/0028-3932(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Passamonti L, Novellino F, Salsone M, Gioia MC, Morelli M, Quattrone A. Fronto-parietal overactivation in patients with essential tremor during Stroop task. Neuroreport. 2010;21(2):148–151. doi: 10.1097/WNR.0b013e328335b42c. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. Journal of Psychiatric Research. 1978;14(1-4):303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CHY, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Human Brain Mapping. 2006;27(10):799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11(6):671–684. [Google Scholar]
- Cummings JL. Subcortical dementia. Oxford University Press; New York: 1990. [Google Scholar]
- deLau LML, Breteler MMB. Epidemiology of Parkinson’s disease. The Lancet Neurology. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Elble R. Essential tremor--neurodegenerative or nondegenerative disease towards a working definition of ET. Movement Disorders. 2009;24(14):2033–2041. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]
- Duane DD, Vermilion KJ. Cognitive deficits in patients with essential tremor. Neurology. 2002;58(11):1706. doi: 10.1212/wnl.58.11.1706. [DOI] [PubMed] [Google Scholar]
- Elble RJ. Diagnostic criteria for essential tremor and differential diagnosis. Neurology. 2000;54(11 Suppl 4):2–6. [PubMed] [Google Scholar]
- Fahn S, Elton R, Members of the UPDRS Development Committee . Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Holstein N, editors. Recent developments in Parkinson’s disease. Vol. 2. Macmillian Healthcare Information; Plurham Park, N.J.: 1987. pp. 153–63. [Google Scholar]
- Gasparini M, Bonifati V, Fabrizio E, Fabbrini G, Brusa L, Lenzi GL, Meco G. Frontal lobe dysfunction in essential tremor: a preliminary study. Journal of Neurology. 2001;248(5):399–402. doi: 10.1007/s004150170181. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Movement Disorders. 2013;28(13):1759–61. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Wietzke J, Lutz MT. Unique and shared validity of the "Wechsler logical memory test", the "California verbal learning test", and the "verbal learning and memory test" in patients with epilepsy. Epilepsy Research. 2009;87(2-3):203–212. doi: 10.1016/j.eplepsyres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Higginson CI, Wheelock VL, Levine D, King DS, Pappas CT, Sigvardt KA. Cognitive deficits in essential tremor consistent with frontosubcortical dysfunction. Journal of Clinical and Experimental Neuropsychology. 2008;30(7):760–765. doi: 10.1080/13803390701754738. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 2001;57(10 Suppl 3):11–26. 1967. [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 2001;57(10 Suppl 3):S34–38. [PubMed] [Google Scholar]
- Hyde TS, Jenkins JJ. Recall for words as a function of semantic, graphic, and syntactic orienting tasks. Journal of Verbal Learning and Verbal Behavior. 1973;12(5):471–480. [Google Scholar]
- Janicki SC, Cosentino S, Louis ED. The cognitive side of essential tremor: what are the therapeutic implications? Therapeutic Advances in Neurological Disorders. 2013;6(6):353–368. doi: 10.1177/1756285613489591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Archives of Neurology. 2001;58(10):1611–1615. doi: 10.1001/archneur.58.10.1611. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Beach J, Schwartz K, Contant C. Tremor and longevity in relatives of patients with Parkinson's disease, essential tremor, and control subjects. Neurology. 1995;45(4):645–648. doi: 10.1212/wnl.45.4.645. [DOI] [PubMed] [Google Scholar]
- Kim JS, Song IU, Shim YS, Park JW, Yoo JY, Kim YI, Lee KS. Cognitive Impairment in Essential Tremor without Dementia. Journal of Clinical Neurology. 2009;5(2):81–84. doi: 10.3988/jcn.2009.5.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Stanhope N. Recall and recognition memory in patients with focal frontal, temporal lobe and diencephalic lesions. Neuropsychologia. 1998;36(8):785–795. doi: 10.1016/s0028-3932(97)00167-x. [DOI] [PubMed] [Google Scholar]
- Lacritz LH, Dewey R, Jr., Giller C, Cullum CM. Cognitive functioning in individuals with "benign" essential tremor. Journal of the International Neuropsychological Society. 2002;8(1):125–129. doi: 10.1017/s1355617702001121. [DOI] [PubMed] [Google Scholar]
- Lang AE. The progression of Parkinson disease: a hypothesis. Neurology. 2007;68(12):948–952. doi: 10.1212/01.wnl.0000257110.91041.5d. [DOI] [PubMed] [Google Scholar]
- Lang AE, Kierans C, Blair RD. Family history of tremor in Parkinson's disease compared with those of controls and patients with idiopathic dystonia. Advances in Neurology. 1987;45:313–316. [PubMed] [Google Scholar]
- Levin BE, Katzen HL. Early cognitive changes and nondementing behavioral abnormalities in Parkinson's disease. Advances in Neurology. 2005;96:84–94. [PubMed] [Google Scholar]
- Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology. 2001;57(5):785–790. doi: 10.1212/wnl.57.5.785. [DOI] [PubMed] [Google Scholar]
- Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology. 1991;41(2):234–238. doi: 10.1212/wnl.41.2_part_1.234. [DOI] [PubMed] [Google Scholar]
- Louis ED. Essential tremor. The New England Journal of Medicine. 2001;345(12):887–891. doi: 10.1056/NEJMcp010928. [DOI] [PubMed] [Google Scholar]
- Louis ED, Clark LN, Ottman R. Familial versus sporadic essential tremor: What patterns can one decipher in age of onset? Neuroepidemiology. 2015;44(3):166–172. doi: 10.1159/000381807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Dogu O. Isolated head tremor: part of the clinical spectrum of essential tremor? Data from population-based and clinic-based case samples. Movement Disorders : Official Journal of the Movement Disorder Society. 2009;24(15):2281–2285. doi: 10.1002/mds.22777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, Hernandez N. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(12):3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ford B, Lee H, Andrews H, Cameron G. Diagnostic criteria for essential tremor: a population perspective. Archives of Neurology. 1998;55(6):823–828. doi: 10.1001/archneur.55.6.823. [DOI] [PubMed] [Google Scholar]
- Louis ED, Jurewicz EC, Watner D. Community-based data on associations of disease duration and age with severity of essential tremor: implications for disease pathophysiology. Movement Disorders. 2003;18(1):90–93. doi: 10.1002/mds.10302. [DOI] [PubMed] [Google Scholar]
- Louis ED, Jurewicz EC, Watner D. Community-based data on associations of disease duration and age with severity of essential tremor: implications for disease pathophysiology. Movement Disorders. 2003;18(1):90–93. doi: 10.1002/mds.10302. [DOI] [PubMed] [Google Scholar]
- Louis ED, Lee M, Babij R, Ma K, Cortes E, Vonsattel JP, Faust PL. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain. 2014;137(Pt 12):3142–3148. doi: 10.1093/brain/awu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Movement Disorders. 1998;13(1):5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006;66(11):1756–1759. doi: 10.1212/01.wnl.0000218162.80315.b9. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Research. 1991;38(2):143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research Reviews. 2000;31(2-3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Nicoletti G, Manners D, Novellino F, Condino F, Malucelli E, Barbiroli B, Quattrone A. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74(12):988–994. doi: 10.1212/WNL.0b013e3181d5a460. [DOI] [PubMed] [Google Scholar]
- Okun MS, Rodriguez RL, Mikos A, Miller K, Kellison I, Kirsch-Darrow L, Bowers D. Deep brain stimulation and the role of the neuropsychologist. The Clinical Neuropsychologist. 2007;21(1):162–189. doi: 10.1080/13825580601025940. [DOI] [PubMed] [Google Scholar]
- Panegyres PK. The contribution of the study of neurodegenerative disorders to the understanding of human memory. QJM. 2004;97(9):555–567. doi: 10.1093/qjmed/hch096. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Novellino F, Cerasa A, Chiriaco C, Rocca F, Matina MS, Quattrone A. Altered cortical-cerebellar circuits during verbal working memory in essential tremor. Brain. 2011;134(Pt 8):2274–2286. doi: 10.1093/brain/awr164. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Spectrum of memory dysfunction in degenerative disease. Current Opinion in Neurology. 1996;9(4):281–286. doi: 10.1097/00019052-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Post B, Merkus MP, de Haan RJ, Speelman JD. Prognostic factors for the progression of Parkinson’s disease: a systematic review. Movement Disorders : Official Journal of the Movement Disorder Society. 2007;22(13):1839–1851. doi: 10.1002/mds.21537. [DOI] [PubMed] [Google Scholar]
- Putzke JD, Whaley NR, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: predictors of disease progression in a clinical cohort. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77(11):1235–1237. doi: 10.1136/jnnp.2006.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput AH, Adler CH, Shill HA, Rajput A. Essential tremor is not a neurodegenerative disease. Neurodegenerative Disease Management. 2012;2(3):259–268. doi: 10.2217/nmt.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Gold JM, Kozora E, Cullum CM, Herman BP, Wyler AR. Estimating memory function: disparity of Wechsler Memory Scale – Revised and California Verbal Learning Test indices in clinical and normal samples. The Clinical Neuropsychologist. 1994;8:99–108. [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. The Lancet Neurology. 2009;8(12):1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Sahin HA, Terzi M, Ucak S, Yapici O, Basoglu T, Onar M. Frontal functions in young patients with essential tremor: a case comparison study. Journal of Neuropsychiatry & Clinical Neurosciences. 2006;18(1):64–72. doi: 10.1176/jnp.18.1.64. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neuroscience Letters. 1995;199(3):175–178. doi: 10.1016/0304-3940(95)12056-a. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. International Review of Neurobiology. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson’s disease: a test of the multifactorial etiologic hypothesis. Neurology. 1993;43(6):1173–1180. doi: 10.1212/wnl.43.6.1173. [DOI] [PubMed] [Google Scholar]
- Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson's disease. Parkinsonism & Related Disorders. 2007;13(2):67–76. doi: 10.1016/j.parkreldis.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Shin DH, Han BS, Kim HS, Lee PH. Diffusion tensor imaging in patients with essential tremor. American Journal of Neuroradiology. 2008;29(1):151–153. doi: 10.3174/ajnr.A0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes RM, Constantino A, Gibadullina E, Houghton D, Louis ED, Litvan I. Examining the motor phenotype of patients with both essential tremor and Parkinson's disease. Tremor and Other Hyperkinetic Movements. 2012;2:02–47. doi: 10.7916/D8CN72N0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, Sydeman S. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcome assessment. Lawrence Erlbaum Associates; Hillsdale, NJ: 1994. pp. 292–321. [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59(2):1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Stuss D, Alexander M, Palumbo C, Buckle L, Sayer L, Pogue J. Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology. 1994;8:355–373. [Google Scholar]
- Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. The Lancet. Neurology. 2012;11(8):697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- Tan EK, Lee SS, Fook-Chong S, Lum SY. Evidence of increased odds of essential tremor in Parkinson's disease. Movement Disorders. 2008;23(7):993–997. doi: 10.1002/mds.22005. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Goldman SM, Lyons KE, Aston DA, Tetrud JW, Welsh MD, Koller WC. Essential tremor in twins: an assessment of genetic vs environmental determinants of etiology. Neurology. 2001;57(8):1389–1391. doi: 10.1212/wnl.57.8.1389. [DOI] [PubMed] [Google Scholar]
- Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population-based study in New York. Neurology. 2009;73(8):621–625. doi: 10.1212/WNL.0b013e3181b389f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremont G, Halpert S, Javorsky DJ, Stern RA. Differential impact of executive dysfunction on verbal list learning and story recall. The Clinical Neuropsychologist. 2000;14(3):295–302. doi: 10.1076/1385-4046(200008)14:3;1-P;FT295. [DOI] [PubMed] [Google Scholar]
- Troster AI, Woods SP, Fields JA, Lyons KE, Pahwa R, Higginson CI, Koller WC. Neuropsychological deficits in essential tremor: an expression of cerebello-thalamo-cortical pathophysiology? European Journal of Neurology. 2002;9(2):143–151. doi: 10.1046/j.1468-1331.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Leentjens A, Marinus J, Stiggelbout A, van Hilten J. Reliability and validity of the Beck depression inventory in patients with Parkinson’s disease. Movement Disorders. 2006;21(5):668–672. doi: 10.1002/mds.20792. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Memory Scale, Third Edition. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Weingartner H, Burns S, Diebel R, LeWitt PA. Cognitive impairments in Parkinson's disease: distinguishing between effort-demanding and automatic cognitive processes. Psychiatry Research. 1984;11(3):223–235. doi: 10.1016/0165-1781(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Wicklund AH, Johnson N, Rademaker A, Weitner BB, Weintraub S. Word list versus story memory in Alzheimer disease and frontotemporal dementia. Alzheimer Disease and Associated Disorders. 2006;20(2):86–92. doi: 10.1097/01.wad.0000213811.97305.49. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Lewis SJG, Barker RA. Cognitive deficits and psychosis in Parkinson’s disease: a review of pathophysiology and therapeutic options. CNS Drugs. 2006;20(6):477–505. doi: 10.2165/00023210-200620060-00004. [DOI] [PubMed] [Google Scholar]
- Williams EJ. The comparison of regression variables. Journal of the Royal Statistical Society, Series B (Methodology) 1959;21:396–399. [Google Scholar]
- Zahodne LB, Bowers D, Price CC, Bauer RM, Nisenzon A, Foote KD, Okun MS. The case for testing memory with both stories and word lists prior to DBS surgery for Parkinson's Disease. The Clinical Neuropsychologist. 2011;25(3):348–358. doi: 10.1080/13854046.2011.562869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zesiewicz TA, Shaw JD, Allison KG, Staffetti JS, Okun MS, Sullivan KL. Update on treatment of essential tremor. Current Treatment Options in Neurology. 2013;15(4):410–423. doi: 10.1007/s11940-013-0239-4. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology. 2003;16(4):193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Sian J, Gerlach M, Youdim MB, Riederer P. Parkinson's disease: a major hypokinetic basal ganglia disorder. Journal of Neural Transmission. 1999;106(5-6):443–476. doi: 10.1007/s007020050171. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Bain P, Brin M, Ad Hoc Scientific Committee Consensus statement of the Movement Disorder Society on Tremor. Movement Disorders. 1998;3:2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Golden SM. Epidemiology of movement disorders. Current Opinion in Neurology. 1994;7(4):340–345. doi: 10.1097/00019052-199408000-00011. [DOI] [PubMed] [Google Scholar]