Abstract.
The serum levels of calcium (Ca) and phosphate are maintained higher in the fetus than in the pregnant mother, especially in late gestation, to meet the demands of fetal bone development. In order to maintain this fetal stage-specific mineral homeostasis, the placenta plays a critical role through active transcellular mineral transport. Although the molecular mechanism of transplacental Ca transport has been well studied, little is known about the transport mechanism of phosphate and magnesium. Maternal mineral homeostasis is also altered during pregnancy to supply minerals to the fetus. In the lactating mother, osteocytic osteolysis is suggested to be involved in the supply of minerals to the baby. The levels of some calcitropic and phosphotropic (Ca- and phosphate-regulating, respectively) hormones in the fetus are also different from those in the adult. The PTH level in the fetus is lower than that in the mother and nonpregnant adult. It is suggested, however, that low fetal PTH plays an important role in fetal mineral metabolism. The concentration of PTHrP in the fetus is much higher than that of PTH and plays a critical role in perinatal Ca homeostasis. Uncovering the molecular mechanisms for fetal stage-specific mineral metabolism will lead to better management of perinatal patients with mineral abnormalities.
Keywords: calcium, phosphate, placenta, fetus
Introduction
Perinatal mineral homeostasis differs from mineral homeostasis in adults, and fetal serum levels of calcium (Ca), inorganic phosphate (Pi), and magnesium (Mg) are maintained higher than the maternal levels during late gestation to meet the requirements of the developing fetus. The placenta plays a critical role in maintaining this fetal stage-specific mineral metabolism. The placental active transport of Ca, Pi, and Mg in the materno-fetal direction occurs mainly in late gestation, resulting in feto-maternal gradients in the plasma levels of these minerals. The calcitropic and phosphotropic (1) hormones also play distinct roles during the perinatal period from those in postnatal life. Although many fundamental studies have been performed in previous decades, some advances in the understanding of this field have been made in recent years. In this review article, we summarize the current understanding regarding the molecular basis for perinatal mineral metabolism.
Fetal Mineral Metabolism
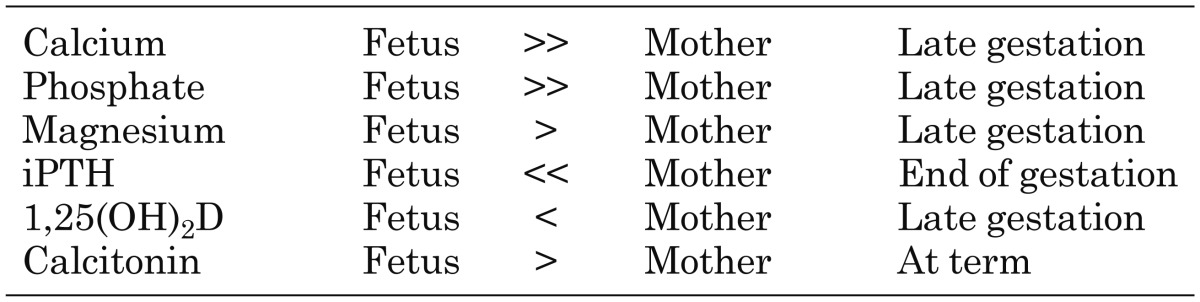
In late gestation, fetal serum levels of Ca and Pi are maintained above the maternal levels by ~1.2–2.0 mg/dl and 1.5 mg/dl, respectively (2). Although the fetal level of Mg is also increased compared with the maternal value, the gradient is less (~0.12 mg/dl) (2) than those of Ca and Pi. It is suggested that these high mineral levels in the fetus are needed for normal bone formation and calcification (3). In animal models, despite maternal hypocalcemia induced by a Ca-restricted diet (4), vitamin D deficiency (5), parathyroidectomy (6), or vitamin D receptor deletion model (7), fetuses were reported to be normocalcemic. The Ca concentration in the fetus is independent of the maternal Ca level. In the case of Pi, we reported that the fetal Pi level was maintained within the normal range despite maternal hypophosphatemia in the Hyp mouse, a murine model of X-linked hypophosphatemic rickets/osteomalacia (XLH), which carries an inactivating deletion of the Phex (phosphate-regulating gene with homologies to endopeptidases on the X-chromosome) gene (8). Similar data were also reported by other groups in both mouse (9) and rat (10) studies.
Mineral Transport Across the Placenta
In late gestation, the placenta transports large amounts of Ca and Pi to allow rapid fetal skeletal mineralization. The materno-fetal transfer of Ca and Pi uses an active transcellular transport mechanism that overcomes the concentration gradient across the placenta. This active transcellular transport mechanism occurs through the syncytiotrophoblast that covers the surface of villi and faces the intervillous space filled with maternal blood.
In the case of Ca, the mechanism of active placental transcellular transport is similar to the mechanism that occurs in the intestine. Transient receptor potential cation channel, subfamily V, member 6 (TRPV6), which is a Ca channel, opens in the maternal-facing basement membrane of the syncytiotrophoblast to allow Ca entry into the cells. Then Ca binds to calbindin-D9k, a Ca binding protein, and is transported to the opposite basement membrane. Finally, Ca is actively transported through the fetal-facing basement membranes toward the fetal circulation by the Ca2+-ATPase (11, 12). The expression of TRPV6 increases 14-fold during the last 4 d of gestation in wild-type mice, and Trpv6 null fetuses have severe hypocalcemia with a 40% reduction of transplacental Ca transport (11). The calbindin-D9k expression also increases considerably during the last week of gestation in wild-type mice (11, 13). The expression of Ca2+-ATPase doubles in the last 7 d of gestation (14, 15), and inhibition of its function by a neutralized antibody leads to a reduction of transplacental Ca transport in the perfused rat placenta (12, 16). These data suggest that these molecules are important in placental active Ca transport during late gestation.
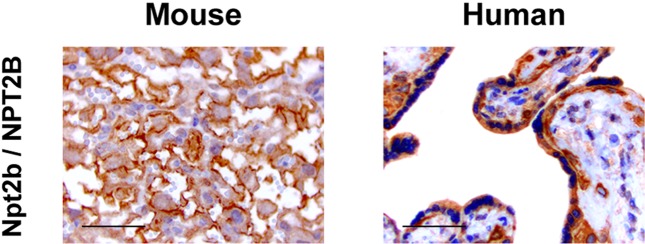
Compared with the Ca transport system, the mechanism for transplacental transport of Pi is largely unknown. It is suggested that the placental transfer of Pi in the materno-fetal direction also occurs in a transcellular manner and depends on an Na+- and pH-dependent active transport mechanism (17, 18). Moreover, Brunette et al. reported that the pH sensitivity of the placental Pi transport system is similar not to that of the renal system but to that of the intestinal system (19). In mammals, 3 types of Na+/Pi cotransporters have been identified (20). Type II Na+/Pi cotransporters have been considered to be involved in the regulation of plasma Pi concentrations (21). Type IIa (Npt2a) and IIc (Npt2c) are predominantly expressed in renal proximal tubules and contribute to reabsorbing Pi (22, 23), while type IIb (Npt2b) is predominantly expressed in the intestine and absorbs Pi (24).We have demonstrated that Slc34a2/SLC34A2, the gene for Npt2b/NPT2B, is expressed in the placenta also, and its expression increases during the development of the placenta (8). In addition, immunohistochemical staining revealed the localization of Npt2b/NPT2B in the feto-maternal interface of both mouse and human term placentas (Fig. 1), suggesting its critical role in the materno-fetal transport of Pi. Shibasaki et al. demonstrated that Slc34a2 is expressed in the labyrinth zone of the mouse placenta from E12.5 and that conventional knockout led to embryonic lethality just after implantation, indicating that Npt2b is essential for early embryonic development (25). Since the expression of genes for type III Na+/Pi cotransporters (Slc20a1 and Slc20a2) was also increased in the mouse placenta toward the end of gestation (8), we cannot exclude the possibility that they too have some role in prenatal Pi homeostasis. As for Npt2a and Npt2c, the placental expression of their genes (Slc34a1 and Slc34a3) was increased in late gestation, but it was still very low (8) and unlikely to have significant roles.
Fig. 1.
The feto-maternal interface of the placenta expressed Npt2b/NPT2B in both mice and humans. Immunohistochemical staining using antibodies against the type IIb Na+/Pi cotransporter (Npt2b/NPT2B) in ICR mouse placentas at E18.5 and normal human placentas at 38 wk of gestation. The signals for these proteins were detected in the syncytiotrophoblasts of mouse and human placentas. Sections were counterstained with hematoxylin. Scale bars, 50 μm.
In adults, evidence has been accumulated for a central role of fibroblast growth factor 23 (FGF23) in regulating Pi homeostasis (26). To exert its effects, FGF23 requires an FGF receptor (FGFR) and α-Klotho as a co-receptor (27). Although we reported that the placenta expressed both of these molecules (8, 28), it has been unclear whether FGF23 can regulate Pi transplacental transport or not.
Little is known of the mechanism of placental Mg transport. Bidirectional Mg flux has been demonstrated in sheep, with materno-fetal flux (0.042 mg/kg fetal weight/hour) exceeding feto-maternal flux (0.012 mg/kg/h) (29). There is no direct information on the regulation of placental Mg transport.
Maternal Mineral Metabolism during Pregnancy and Lactation
High Ca demand during pregnancy and lactation alter Ca homeostasis in the mother. In pregnancy, 2–3% of maternal Ca is transferred to the fetus in late gestation (30). During lactation, 300–400 mg of Ca is transferred into breast milk per day (31). To meet this high Ca demand, the rate of intestinal absorption and bone turnover are increased during pregnancy (32). In the lactation period, renal excretion of Ca is reduced to maintain bone metabolism (33). It has been reported that, during pregnancy, the maternal lumbar spine and total bone mineral density (BMD) decrease by ~7.6% and ~3.9%, respectively (34). Other studies showed 4–6% bone loss during the first 6 months of lactation (35). However, there is no consensus about the long-term effects of pregnancy and lactation on bone.
The mechanism of bone loss during lactation is thought to be accelerated by suckling-induced, hypothalamic suppression of estrogen levels combined with increased levels of parathyroid hormone-related peptide (PTHrP) secreted from the lactating breast (36). However, experimentally decreasing estrogen levels and increasing PTHrP levels in mice do not reproduce the full effects of lactation on the skeleton (37). Therefore, it has been speculated that other mechanisms also contribute to the bone loss during lactation. Qing et al. showed that the size of osteocyte lacunae in both the tibia and vertebrae increased as a result of osteocytic osteolysis in lactating mice and that the change was reversed 7 days after weaning (38). They also reported that lactation is associated with the reversible activation of some genes related to osteoclastic osteolysis in the osteocyte: tartrate resistant acid phosphatase (TRAP), cathepsin K, carbonic anhydrase, matrix metallopeptidase 13 (MMP13), and several subunits of the H+-ATPase. Since conditional deletion of the type 1 PTH/PTHrP receptor (PTHR1) in osteocytes blocked the increase in lacunar size and the induction of TRAP activity observed during lactation, they concluded that lactation is associated with osteocytic osteolysis due to PTHR1 signaling (38).
PTH
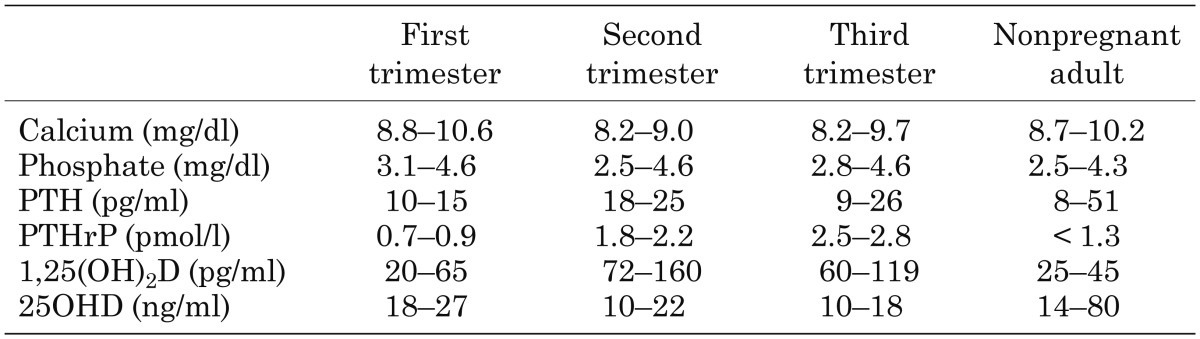
In the fetus, the concentrations of some calcitropic hormones are maintained at different levels than in adults. Fetal intact PTH levels are much lower than maternal or nonpregnant adult levels near the end of gestation (< 4.72 pg/ml) (Table 1) (39, 40). Since maternal PTH levels are suppressed during pregnancy compared with those in the nonpregnant adult (Table 2) (41), fetal PTH levels appear to be strongly suppressed. The fetal parathyroid glands produce PTH from 10 wk of gestation (42). However, it is unknown whether the human placenta produces PTH or not. Although the fetal PTH levels are very low, fetal PTH is thought to be important, since fetal mice lacking parathyroid glands, PTH or the PTH/PTHrP receptor are hypocalcemic and have undermineralized skeletons (3, 43,44,45).
Table 1. Comparison of serum mineral values between the fetus and mother.
Table 2. Pregnant maternal serum mineral concentration (41).
Vitamin D
After birth, the metabolism of vitamin D to its major circulating form (25-hydroxy vitamin D: 25OHD) and functional active form [1,25-dihydroxyvitamin D: 1,25(OH)2D] takes place in the liver and kidney. In the kidney, 1α-hydroxylase converts 25OHD to 1,25(OH)2D, while 24-hydroxylase catabolizes 25OHD and 1,25(OH)2D to 24,25(OH)2D and 1,24,25(OH)3D, respectively. FGF23 decreases the production of 1,25(OH)2D by suppressing the expression of 1α-hydroxylase and inducing that of 24-hydroxylase (46).
During pregnancy, maternal 25OHD crosses the placenta, so the fetal level of 25OHD reaches 75–100% of the maternal level at term (47). On the other hand, the fetal 1,25(OH)2D level is lower than that in the mother (< 50%) (Table 1) (48). Both the fetal kidneys and the placenta express 1α-hydroxylase (8). Since the level of 1,25(OH)2D in the umbilical artery is higher than that in the umbilical vein (49), the fetal kidneys can contribute to produce 1,25(OH)2D in the fetus. However, it is thought that the synthesis of 1,25(OH)2D in the fetus is suppressed by the high levels of Ca and Pi and low levels of PTH. The maternal level of 1,25(OH)2D of during pregnancy is 2- to 3-fold higher than that in the nonpregnant adult (Table 2). It is suggested that the maternal kidneys contribute to the abundance of 1,25(OH)2D in the pregnant mother (50).
As for FGF23, we have reported that in cases of pathological conditions with increased levels of circulating FGF23, such as in the Hyp mouse, the high maternal levels of FGF23 can act on the placenta directly and increase the activity of placental 24-hydroxylase. Consequently, fetal 25OHD levels are decreased by the excessive conversion of 25OHD to 24,25(OH)2D (8).
Since the fetal Ca values and skeletal mineral content were within the normal ranges in vitamin D deficiency model mice and in vitamin D receptor null mice, vitamin D has been considered unimportant for fetal mineral homeostasis. However, vitamin D deficiency in mothers was associated with a lower bone mineral density in their children at 9 yr old (51). Based on this report, it is possible that vitamin D metabolism during pregnancy could affect childhood bone metabolism.
PTHrP
In cord blood, the concentration of PTHrP is 15-fold higher than that of PTH at term. In the fetus, PTHrP is produced in many tissues, including the placenta. The PTHrP level in the umbilical vein is higher than that in the umbilical artery in pigs (52), which suggests that the placenta may be a critical source of fetal PTHrP. PTHrP plays important roles in the fetus. It has been reported that abnormal endochondral bone development, hypocalcemia, hyperphosphatemia (even further above the normally high fetal Pi level), and decreased placental Ca transport were observed in fetuses of PTHrP-deficient mice (53, 54). On the other hand, the serum Mg level remained within the normal range. Although serum PTH levels increase 3-fold in PTHrP-deficient fetuses in a form of a secondary hyperparathyroidism (43), the fetuses still have hypocalcemia. This suggests that PTH cannot compensate for the lack of PTHrP completely. Fetuses lacking either parathyroid glands or PTH also have hypocalcemia and have no compensatory increase in PTHrP levels (3, 44). These data suggest that PTHrP also does not compensate for the absence of PTH.
In lactating mothers, as described above, PTHrP produced by the lactating breast plays an important role in meeting the Ca demands of lactation. Many studies have shown elevated serum PTHrP levels during lactation (36, 55, 56).
Calcitonin
Thyroid C-cells begin to differentiate at around the 12th wk of gestation (57), and calcitonin is detectable at around the 15th wk of gestation in the C-cells (58). Fetal circulating calcitonin levels are ~2-fold higher than those in the mother (Table 1) (2, 49, 59). The trophoblasts of the placenta also produce calcitonin and supply it to the fetus (60). Since the calcitonin does not cross the placenta in the mouse or rat (61, 62), fetal circulating calcitonin is derived from fetal sources. However, the major source of fetal circulating calcitonin has not been determined.
In a mouse model, fetuses with ablation of the Ctcgrp gene, which encodes both calcitonin and calcitonin gene-related peptide-α, exhibited normal Ca and Pi levels (61). However, since the serum Mg level of the fetuses decreased significantly, calcitonin may contribute to fetal Mg homeostasis (61).
Conclusion
To meet the demands of developing fetuses, perinatal mineral homeostasis differs from mineral homeostasis in adults. However, little is known about how mineral metabolism is regulated in the fetus compared with that in the adult because of the difficulty in studying human fetuses. It is expected that further investigations will uncover the molecular mechanism of fetal mineral homeostasis and may lead to the development of new therapeutic strategies for perinatal patients with mineral abnormalities.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (to T.M.) and the Ministry of Health, Labour and Welfare, Japan (to T.M.).
References
- 1.Fukumoto S, Shimizu Y. Fibroblast growth factor 23 as a phosphotropic hormone and beyond. J Bone Miner Metab 2011;29: 507–14. doi: 10.1007/s00774-011-0298-0 [DOI] [PubMed] [Google Scholar]
- 2.Pitkin RM, Cruikshank DP, Schauberger CW, Reynolds WA, Williams GA, Hargis GK. Fetal calcitropic hormones and neonatal calcium homeostasis. Pediatrics 1980;66: 77–82. [PubMed] [Google Scholar]
- 3.Simmonds CS, Karsenty G, Karaplis AC, Kovacs CS. Parathyroid hormone regulates fetal-placental mineral homeostasis. J Bone Miner Res 2010;25: 594–605. doi: 10.1359/jbmr.090825 [DOI] [PubMed] [Google Scholar]
- 4.Lima MS, Kallfelz F, Krook L, Nathanielsz PW. Humeral skeletal development and plasma constituent changes in fetuses of ewes maintained on a low calcium diet from 60 days of gestation. Calcif Tissue Int 1993;52: 283–90. doi: 10.1007/BF00296653 [DOI] [PubMed] [Google Scholar]
- 5.Halloran BP, De Luca HF. Effect of vitamin D deficiency on skeletal development during early growth in the rat. Arch Biochem Biophys 1981;209: 7–14. doi: 10.1016/0003-9861(81)90251-4 [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim MM, Thomas ML, Forte LR. Maternal-fetal relationships in the parathyroidectomized rat. Intestinal calcium transport, serum calcium, immunoreactive parathyroid hormone and calcitonin. Biol Neonate 1984;46: 89–97. doi: 10.1159/000242038 [DOI] [PubMed] [Google Scholar]
- 7.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 1997;94: 9831–5. doi: 10.1073/pnas.94.18.9831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohata Y, Yamazaki M, Kawai M, Tsugawa N, Tachikawa K, Koinuma T, et al. Elevated fibroblast growth factor 23 exerts its effects on placenta and regulates vitamin D metabolism in pregnancy of Hyp mice. J Bone Miner Res 2014;29: 1627–38. doi: 10.1002/jbmr.2186 [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Samaraweera M, Cooke-Hubley S, Kirby BJ, Karaplis AC, Lanske B, et al. Neither absence nor excess of FGF23 disturbs murine fetal-placental phosphorus homeostasis or prenatal skeletal development and mineralization. Endocrinology 2014;155: 1596–605. doi: 10.1210/en.2013-2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garel JM, Gilbert M. Dietary calcium and phosphorus manipulations in thyroparathyroidectomized pregnant rats and fetal liver glycogen stores. Reprod Nutr Dev 1981;21(6A): 969–77. doi: 10.1051/rnd:19810709 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Kovacs CS, Takanaga H, Peng JB, Landowski CP, Hediger MA. Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J Bone Miner Res 2008;23: 1249–56. doi: 10.1359/jbmr.080314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Care AD. The placental transfer of calcium. J Dev Physiol 1991;15: 253–7. [PubMed] [Google Scholar]
- 13.Delorme AC, Danan JL, Ripoche MA, Mathieu H. Biochemical characterization of mouse vitamin D-dependent calcium-binding protein. Evidence for its presence in embryonic life. Biochem J 1982;205: 49–57. doi: 10.1042/bj2050049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glazier JD, Atkinson DE, Thornburg KL, Sharpe PT, Edwards D, Boyd RD, et al. Gestational changes in Ca2+ transport across rat placenta and mRNA for calbindin9K and Ca(2+)-ATPase. Am J Physiol 1992;263: R930–5. [DOI] [PubMed] [Google Scholar]
- 15.Tuan RS, Bigioni N. Ca(2+)-activated ATPase of the mouse chorioallantoic placenta: developmental expression, characterization and cytohistochemical localization. Development 1990;110: 505–13. [DOI] [PubMed] [Google Scholar]
- 16.Borke JL, Caride A, Verma AK, Kelley LK, Smith CH, Penniston JT, et al. Calcium pump epitopes in placental trophoblast basal plasma membranes. Am J Physiol 1989;257: c341–6. [DOI] [PubMed] [Google Scholar]
- 17.Stulc J, Stulcová B. Transport of inorganic phosphate by the fetal border of the guinea-pig placenta perfused in situ. Placenta 1984;5: 9–19. doi: 10.1016/S0143-4004(84)80045-4 [DOI] [PubMed] [Google Scholar]
- 18.Stulc J, Stulcová B. Placental transfer of phosphate in anaesthetized rats. Placenta 1996;17: 487–93. doi: 10.1016/S0143-4004(96)90031-4 [DOI] [PubMed] [Google Scholar]
- 19.Brunette MG, Allard S. Phosphate uptake by syncytial brush border membranes of human placenta. Pediatr Res 1985;19: 1179–82. doi: 10.1203/00006450-198511000-00013 [DOI] [PubMed] [Google Scholar]
- 20.Virkki LV, Biber J, Murer H, Forster IC. Phosphate transporters: a tale of two solute carrier families. Am J Physiol Renal Physiol 2007;293: F643–54. doi: 10.1152/ajprenal.00228.2007 [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto K, Haito-Sugino S, Kuwahara S, Ohi A, Nomura K, Ito M, et al. Sodium-dependent phosphate cotransporters: lessons from gene knockout and mutation studies. J Pharm Sci 2011;100: 3719–30. doi: 10.1002/jps.22614 [DOI] [PubMed] [Google Scholar]
- 22.Murer H, Hernando N, Forster I, Biber J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev 2000;80: 1373–409. [DOI] [PubMed] [Google Scholar]
- 23.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, et al. Growth-related renal type II Na/Pi cotransporter. J Biol Chem 2002;277: 19665–72. doi: 10.1074/jbc.M200943200 [DOI] [PubMed] [Google Scholar]
- 24.Sabbagh Y, O’Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, et al. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol 2009;20: 2348–58. doi: 10.1681/ASN.2009050559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibasaki Y, Etoh N, Hayasaka M, Takahashi MO, Kakitani M, Yamashita T, et al. Targeted deletion of the tybe IIb Na(+)-dependent Pi-co-transporter, NaPi-IIb, results in early embryonic lethality. Biochem Biophys Res Commun 2009;381: 482–6. doi: 10.1016/j.bbrc.2009.02.067 [DOI] [PubMed] [Google Scholar]
- 26.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 2008;118: 3820–8. doi: 10.1172/JCI36479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006;444: 770–4. doi: 10.1038/nature05315 [DOI] [PubMed] [Google Scholar]
- 28.Ohata Y, Arahori H, Namba N, Kitaoka T, Hirai H, Wada K, et al. Circulating levels of soluble alpha-Klotho are markedly elevated in human umbilical cord blood. J Clin Endocrinol Metab 2011;96: E943–7. doi: 10.1210/jc.2010-2357 [DOI] [PubMed] [Google Scholar]
- 29.Care AD, Pickard DW, Weatherley A, Appleby D. The measurement of transplacental magnesium fluxes in the sheep. Res Vet Sci 1979;27: 121–2. [PubMed] [Google Scholar]
- 30.Pitkin RM. Calcium metabolism in pregnancy and the perinatal period: a review. Am J Obstet Gynecol 1985;151: 99–109. doi: 10.1016/0002-9378(85)90434-X [DOI] [PubMed] [Google Scholar]
- 31.Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrinol Metab 2001;86: 2344–8. [DOI] [PubMed] [Google Scholar]
- 32.Krebs NF, Reidinger CJ, Robertson AD, Brenner M. Bone mineral density changes during lactation: maternal, dietary, and biochemical correlates. Am J Clin Nutr 1997;65: 1738–46. [DOI] [PubMed] [Google Scholar]
- 33.Kalkwarf HJ. Hormonal and dietary regulation of changes in bone density during lactation and after weaning in women. J Mammary Gland Biol Neoplasia 1999;4: 319–29. doi: 10.1023/A:1018780425600 [DOI] [PubMed] [Google Scholar]
- 34.Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int 2001;12: 828–34. doi: 10.1007/s001980170033 [DOI] [PubMed] [Google Scholar]
- 35.Hopkinson JM, Butte NF, Ellis K, Smith EO. Lactation delays postpartum bone mineral accretion and temporarily alters its regional distribution in women. J Nutr 2000;130: 777–83. [DOI] [PubMed] [Google Scholar]
- 36.VanHouten JN, Wysolmerski JJ. Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology 2003;144: 5521–9. doi: 10.1210/en.2003-0892 [DOI] [PubMed] [Google Scholar]
- 37.Ardeshirpour L, Brian S, Dann P, VanHouten J, Wysolmerski J. Increased PTHrP and decreased estrogens alter bone turnover but do not reproduce the full effects of lactation on the skeleton. Endocrinology 2010;151: 5591–601. doi: 10.1210/en.2010-0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jähn K, Kato S, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res 2012;27: 1018–29. doi: 10.1002/jbmr.1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.David L, Anast CS. Calcium metabolism in newborn infants. The interrelationship of parathyroid function and calcium, magnesium, and phosphorus metabolism in normal, “sick,” and hypocalcemic newborns. J Clin Invest 1974;54: 287–96. doi: 10.1172/JCI107764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillman LS, Slatopolsky E, Haddad JG. Perinatal vitamin D metabolism. IV. Maternal and cord serum 24,25-dihydroxyvitamin D concentrations. J Clin Endocrinol Metab 1978;47: 1073–7. doi: 10.1210/jcem-47-5-1073 [DOI] [PubMed] [Google Scholar]
- 41.Ardawi MS, Nasrat HA, BA’Aqueel HS. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol 1997;137: 402–9. doi: 10.1530/eje.0.1370402 [DOI] [PubMed] [Google Scholar]
- 42.Leroyer-Alizon E, David L, Anast CS, Dubois PM. Immunocytological evidence for parathyroid hormone in human fetal parathyroid glands. J Clin Endocrinol Metab 1981;52: 513–6. doi: 10.1210/jcem-52-3-513 [DOI] [PubMed] [Google Scholar]
- 43.Kovacs CS, Chafe LL, Fudge NJ, Friel JK, Manley NR. PTH regulates fetal blood calcium and skeletal mineralization independently of PTHrP. Endocrinology 2001;142: 4983–93. doi: 10.1210/endo.142.11.8509 [DOI] [PubMed] [Google Scholar]
- 44.Kovacs CS, Manley NR, Moseley JM, Martin TJ, Kronenberg HM. Fetal parathyroids are not required to maintain placental calcium transport. J Clin Invest 2001;107: 1007–15. doi: 10.1172/JCI11321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest 2002;109: 1173–82. doi: 10.1172/JCI0214817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 2004;113: 561–8. doi: 10.1172/JCI200419081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hillman LS, Haddad JG. Human perinatal vitamin D metabolism. I. 25-Hydroxyvitamin D in maternal and cord blood. J Pediatr 1974;84: 742–9. doi: 10.1016/S0022-3476(74)80024-7 [DOI] [PubMed] [Google Scholar]
- 48.Steichen JJ, Tsang RC, Gratton TL, Hamstra A, DeLuca HF. Vitamin D homeostasis in the perinatal period: 1,25-dihydroxyvitamin D in maternal, cord, and neonatal blood. N Engl J Med 1980;302: 315–9. doi: 10.1056/NEJM198002073020603 [DOI] [PubMed] [Google Scholar]
- 49.Wieland P, Fischer JA, Trechsel U, Roth HR, Vetter K, Schneider H, et al. Perinatal parathyroid hormone, vitamin D metabolites, and calcitonin in man. Am J Physiol 1980;239: E385–90. [DOI] [PubMed] [Google Scholar]
- 50.Turner M, Barré PE, Benjamin A, Goltzman D, Gascon-Barré M. Does the maternal kidney contribute to the increased circulating 1,25-dihydroxyvitamin D concentrations during pregnancy? Miner Electrolyte Metab 1988;14: 246–52. [PubMed] [Google Scholar]
- 51.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Princess Anne Hospital Study GroupMaternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 2006;367: 36–43. doi: 10.1016/S0140-6736(06)67922-1 [DOI] [PubMed] [Google Scholar]
- 52.Abbas SK, Ratcliffe WA, Moniz C, Dixit M, Caple IW, Silver M, et al. The role of parathyroid hormone-related protein in calcium homeostasis in the fetal pig. Exp Physiol 1994;79: 527–36. doi: 10.1113/expphysiol.1994.sp003785 [DOI] [PubMed] [Google Scholar]
- 53.Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev 1994;8: 277–89. doi: 10.1101/gad.8.3.277 [DOI] [PubMed] [Google Scholar]
- 54.Kovacs CS, Lanske B, Hunzelman JL, Guo J, Karaplis AC, Kronenberg HM. Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci USA 1996;93: 15233–8. doi: 10.1073/pnas.93.26.15233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 1997;18: 832–72. [DOI] [PubMed] [Google Scholar]
- 56.VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, et al. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest 2003;112: 1429–36. doi: 10.1172/JCI200319504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan AS, Conen PE. Ultrastructural observations on cytodifferentiation of parafollicular cells in the human fetal thyroid. Lab Invest 1971;25: 249–59. [PubMed] [Google Scholar]
- 58.Leroyer-Alizon E, David L, Dubois PM. Evidence for calcitonin in the thyroid gland of normal and anencephalic human fetuses: immunocytological localization, radioimmunoassay, and gel filtration of thyroid extracts. J Clin Endocrinol Metab 1980;50: 316–21. doi: 10.1210/jcem-50-2-316 [DOI] [PubMed] [Google Scholar]
- 59.Woloszczuk W, Kovarik J, Pavelka P. Calcitonin in pregnant women and in cord blood. Gynecol Obstet Invest 1981;12: 272–6. doi: 10.1159/000299613 [DOI] [PubMed] [Google Scholar]
- 60.Balabanova S, Kruse B, Wolf AS. Calcitonin secretion by human placental tissue. Acta Obstet Gynecol Scand 1987;66: 323–6. doi: 10.3109/00016348709103646 [DOI] [PubMed] [Google Scholar]
- 61.McDonald KR, Fudge NJ, Woodrow JP, Friel JK, Hoff AO, Gagel RF, et al. Ablation of calcitonin/calcitonin gene-related peptide-alpha impairs fetal magnesium but not calcium homeostasis. Am J Physiol Endocrinol Metab 2004;287: E218–26. doi: 10.1152/ajpendo.00023.2004 [DOI] [PubMed] [Google Scholar]
- 62.Garel JM, Milhaud G, Sizonenko P. Thyrocalcitonin and the placental barrier in rats. C R Acad Sci Hebd Seances Acad Sci D 1969;269: 1785–7. [PubMed] [Google Scholar]