Abstract
Background
Data on the long‐term impact of hydrolyzed formulas on allergies are scarce.
Objective
To assess the association between early intervention with hydrolyzed formulas in high‐risk children and allergic outcomes in adolescence.
Methods
GINI trial participants (n = 2252) received one of four formulas in the first four months of life as breastmilk substitute if necessary: partial or extensive whey hydrolyzate (pHF‐W, eHF‐W), extensive casein hydrolyzate (eHF‐C) or standard cow′s milk formula (CMF) as reference. Associations between these formulas and the cumulative incidence and prevalence of parent‐reported physician‐diagnosed asthma, allergic rhinitis (AR) and eczema, as well as spirometric indices and sensitization, were examined using generalized linear models.
Results
Between 11 and 15 years, the prevalence of asthma was reduced in the eHF‐C group compared to CMF (odds ratio (OR) 0.49, 95% confidence interval (CI) 0.26–0.89), which is consistent with the spirometric results. The cumulative incidence of AR was lower in eHF‐C (risk ratio (RR) 0.77, 95% CI 0.59–0.99]) and the AR prevalence in pHF‐W (OR 0.67, 95% CI 0.47–0.95) and eHF‐C (OR 0.59, 95% CI 0.41–0.84). The cumulative incidence of eczema was reduced in pHF‐W (RR 0.75, 95% CI 0.59–0.96) and eHF‐C (RR 0.60, 95% CI 0.46–0.77), as was the eczema prevalence between 11 and 15 years in eHF‐C (OR 0.42, 95% CI 0.23–0.79). No significant effects were found in the eHF‐W group on any manifestation,nor was there an effect on sensitization with any formula.
Conclusion
In high‐risk children, early intervention using different hydrolyzed formulas has variable preventative effects on asthma, allergic rhinitis and eczema up to adolescence.
Keywords: 15‐year follow‐up from birth, allergy prevention, hydrolyzed infant formulas, nutritional intervention, double‐blind, randomized trial
Abbreviations
- aOR
adjusted odds ratio
- AR
hay fever/allergic rhinitis
- aRR
adjusted relative risk
- CI
confidence interval
- CMF
standard cow´s milk formula
- eHF‐C
extensively hydrolyzed casein formula
- eHF‐W
extensively hydrolyzed whey formula
- GINI
German infant nutritional intervention
- IgE
immunoglobulin E
- ITT
intention‐to‐treat
- n.s.
statistically not significant at 5%
- OR
odds ratio
- pHF‐W
partially hydrolyzed whey formula
- PP
per protocol
- RR
relative risk
Early nutritional intervention with certain cow′s milk protein hydrolyzate infant formulas, one of several proposed approaches for allergy prevention in high‐risk infants, has been shown to be successful and is recommended by international scientific societies 1, 2, 3, 4, 5.
However, despite the existing evidence supporting their use, the benefits of hydrolyzed formulas with respect to allergy prevention have recently been questioned 6. The lack of a well‐understood underlying biologic mechanism is one concern, as is the reported lack of efficacy of a partial hydrolyzate 7. It is possible that beneficial effects may arise because of the absence of certain allergy‐inducing epitopes or alternatively because of the presence of certain peptide profiles as the result of the hydrolyzing process 8. Whether both or only one of these mechanisms is at work, or even whether other mechanisms may also play a role, such as those involving skin contact, remains to be discovered 9, 10.
Most 11, 12, 13, 14, 15, 16, 17, 18, 19 but not all 7 randomized, controlled trials that have compared different partially and/or extensively hydrolyzed formulas with standard cow′s milk formula (CMF) have confirmed some degree of preventative effect on allergies in the first 2–3 years of life. Results appear especially consistent for eczema 20 within the first year of life. Randomized, controlled trials of nutritional interventions with hydrolyzed formulas on allergic outcomes in adolescence, including asthma, hay fever/allergic rhinitis (AR) and eczema, are scarce 21 or have used a multifaceted approach that combined nutritional and aeroallergen avoidance measures 22, 23, 24. Data on the long‐term effectiveness of these interventions are important because asthma and especially AR risk increase during adolescence. The German Infant Nutritional Intervention (GINI) study allows a longitudinal evaluation of the long‐term impact of hydrolyzed formulas on allergic outcomes in 2252 children with a positive family history for allergic diseases, which were randomized at birth to three different hydrolyzed formulas or standard cow′s milk formula 25, 26, 27, 28.
The aim of the present study was to assess the long‐term relation between early use of certain formulas and the occurrence of asthma, AR and of eczema, including lung‐function and sensitization up to adolescence.
Materials and methods
Study design and population
The present study reports on the 15‐year follow‐up of the GINI trial, which was initiated in 1995 to investigate the potential preventive effects of three different hydrolyzates (pHF‐W1 , eHF‐W2 and eHF‐C3 ), compared to regular cow′s milk formula (CMF4 ), on allergy development in children at high risk of allergy. The design of the study, details of recruitment, randomization, blinding and allocation of the formulas, outcome definitions, and results from the one‐, three‐, six‐, and ten‐year follow‐ups have been published previously 21, 25, 26, 27, 28. In brief, between September 1995 and July 1998, a total of 2252 healthy term newborns at high risk of allergy were recruited in two regions in Germany (Wesel, Munich) and randomly allocated at birth to one of four blinded study formulas using a computer‐generated list. High risk was defined as having at least one parent or biologic sibling with a history of allergic disease. These formulas were used during the first four months of life as a milk substitute only if exclusive breastfeeding was not possible 25.
The 15‐year follow‐up examination was approved by the local ethic committees. The parents and adolescents gave their written informed consent.
15‐year follow‐up
All of the original 2252 trial participants, who had not actively declined further participation in the study, were invited to the 15‐year follow‐up examination (2011–2013). Parents received information about the aims and procedures of the follow‐up examination, a form for written informed consent, the main questionnaire, and a list of modules to which they could individually consent.
The parental questionnaire included questions on their child′s health, allergic symptoms, physician diagnoses of allergic diseases, and several covariates. The questions pertaining to the primary outcomes (details outlined in the online repository Data S1) were identical to those posed during the one to ten year follow‐ups 21. Participants were invited to the study center for a physical examination that included skin inspection, anthropometric data assessments, blood sampling for specific immunoglobulin E (IgE), and spirometric lung‐function measurements. All procedures were conducted according to the standardized protocols, and extensive quality control was ensured by monitoring the physical examinations.
Primary outcome assessment
The primary outcomes of the present analysis are cumulative incidence up to 15 years of age and period prevalence between 11 and 15 years of age of asthma, AR, and eczema. A positive reply indicating a ‘yes response’ between 11 and 15 years and/or treatment in the last 12 months was used to determine the period prevalence. Any such positive reply during the lifetime of the child was used to determine the cumulative incidence. Both cumulative incidence and period prevalence were individually calculated for asthma, AR, and eczema using parental reports of physician diagnoses, asked separately for each year of life.
Secondary outcome assessment
Sensitization was assessed using specific IgE measurements as in previous examinations 21. The level of specific IgE to the most common food (FX5) and aeroallergens (SX1) were measured with the CAP System (Pharmacia, Freiburg, Germany) at the age of 15 years. If the screening test was positive, single allergens were subsequently tested (FX5: children's food, containing hen's egg, milk protein, codfish, soybean, peanut, and wheat; and inhalation mix SX1, containing dermatophagoides pteronyssinus, rye, timothy grass, mugwort, birch pollen, Cladosporium species, and cat and dog dander). Additionally, ragweed was tested as a single allergen. Sensitization was defined as positive if at least one specific IgE level was 0.35 kU/l or greater (i.e. CAP class 1 or higher). Sensitization to any food, aero‐ and pollen allergens was reported.
Spirometry measurements were performed in line with the ATS/ERS recommendations 29, before (baseline) and after bronchodilation with salbutamol. A pneumotachograph‐type spirometer (EasyOne Worldspiro meter, ndd, Zürich, Switzerland) was used to obtain flow‐volume curves. The procedures used are outlined in the online repository. GLI reference equations 30 were applied to calculate z‐scores for the spirometric indices using the software provided at http://www.ers-education.org/guidelines/global-lung-function-initiative/tools.aspx.
Statistics
Intention‐to‐treat (ITT) and per protocol (PP) analyses were performed. The ITT population consists of all primarily randomized children (n = 2252). The PP population consists of all children who received study formula within the first four months of life and who complied with the study protocol (n = 988) 31.
Logistic regression analyses were performed and odds ratios (OR) are reported for the associations between the study formulas with the period prevalence of the primary outcomes between 11 and 15 years of age, and with sensitization at 15 years.
Cumulative incidence was estimated by the life table method 32 and analyzed by generalized estimation equations 33 using PROC GENMOD with complementary log–log link and independent correlation structure. To assess whether the treatment effect remained constant over time, an interaction term between time and formula group was included in the models. The results are presented as relative risks (RR).
When analyzing the PP populations, all models were adjusted for a fixed set of known risk factors: family history of the modeled outcome (AR, asthma or eczema, respectively), heredity of family allergy, sex, study region, parental education, and older siblings. Results of the adjusted models are given as adjusted OR or RR (aOR, aRR). Interactions with sex and study region were assessed, but no indication of statistical significance was apparent.
In a sensitivity analysis, the effect of attrition was evaluated by repeating the analyses including only children who participated in all follow‐ups.
To investigate a potential effect of the early nutritional intervention on lung function, analyses of covariance were performed on the z‐scores and indices of spirometry (continuous variables). Age, height, and sex were included as covariates in the ITT and PP analyses (in PP in addition to the fix set of covariates listed above), and differences in least‐square means were tested. When the assumption of normality was not met, the outcomes were transformed using a suitable function. In a sensitivity analysis, physician‐diagnosed asthma was included as a covariate.
For analyses examining participation, multiple logistic regression models were performed and odds ratios (OR) are given. P values <0.05 were considered statistically significant, and estimates of OR and RR are given with 95% confidence intervals (95% CI). Statistical analyses were performed using the statistical software SAS for Windows, Release 9.2/9.3 (SAS Institute, Cary, NC, USA).
Results
Study population and participation
Questionnaire participation from the time of randomization up to 15 years of age for the ITT and PP populations is shown in Fig. 1. The mean age of the participants was 15.1 years. Response rates for the 15‐year questionnaire were 61.1% in the ITT population (1377/2252) and 66.0% in the PP population (652/988), and did not differ significantly across the formula groups (Table S1). Of the 1377 children in the ITT population and 652 children in the PP population who responded to the 15‐year questionnaire, 1056 (76.7%) and 492 (75%), respectively, also answered all prior questionnaires at one, two, three, four, six, and ten years (n.s. across the formula groups).
Figure 1.
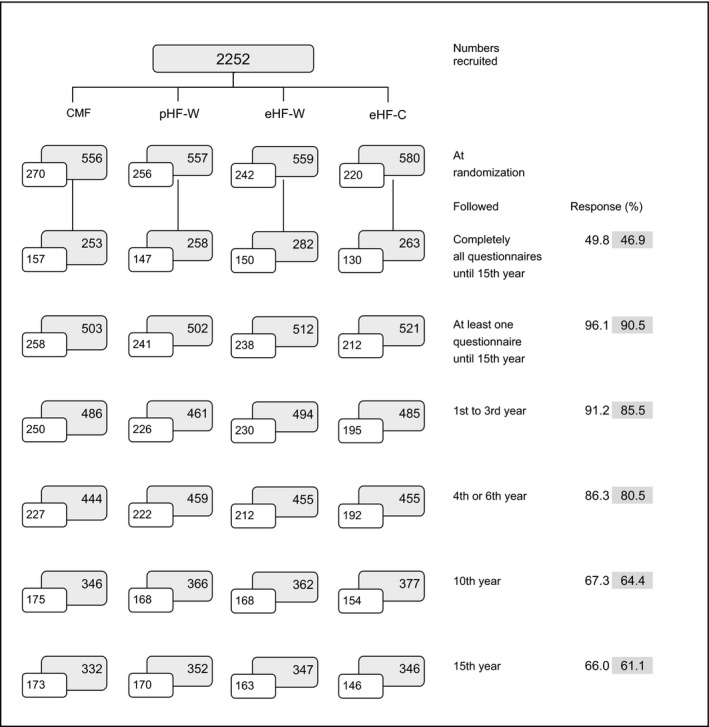
GINI study profile from birth to 15 years. Number of children followed and participation (%) for the ITT (shaded boxes) and PP (open boxes) population are shown.
As observed in the six‐ and ten‐year follow‐ups 21, 27, participation at 15‐years was influenced by study region, birth order, and parental education, but not by formula group, family history of eczema, family history of asthma, and double heredity of familial allergy (Table S1).
Clinical outcomes
Results on cumulative incidence, prevalence, and effect sizes relative to CMF are given in Fig. 2a–c and in Table 1.
Figure 2.
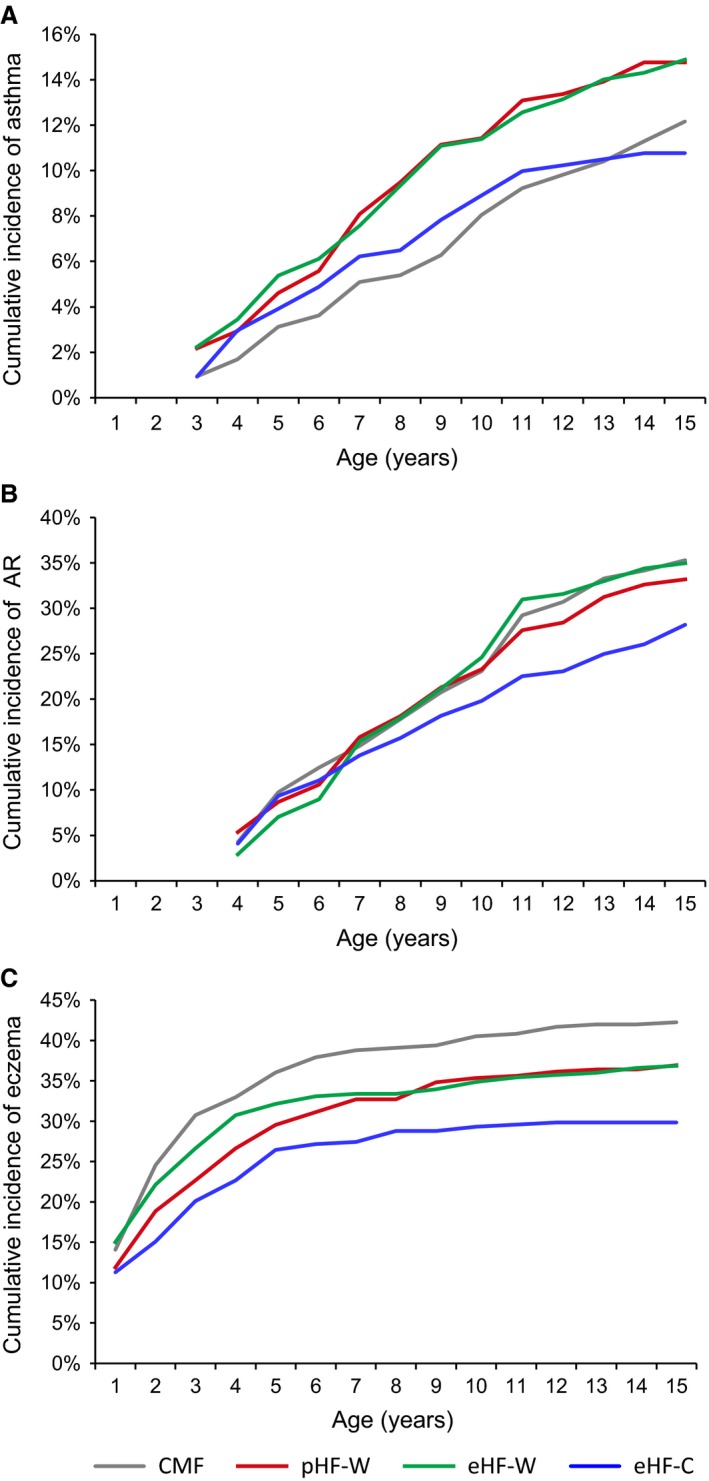
Cumulative incidence curves of the allergic manifestations asthma, hay fewer/allergic rhinitis and eczema until 15 years of age for the three different hydrolyzate formula groups and the cow's milk formula group (ITT population).
Table 1.
Cumulative incidence from birth to 15 years and period prevalence between 11 and 15 years
| CMF | pHF‐W | eHF‐W | eHF‐C | ||
|---|---|---|---|---|---|
| ITT, number of followed children (n = 2252) | n = 556 | n = 557 | n = 559 | n = 580 | |
| Asthma Cumulative incidence, 3 to 15 years | % | 12.2 | 14.8 | 14.9 | 10.8 |
| RR (95% CI) | 1 | 1.26 (0.85–1.88) | 1.29 (0.87–1.93) | 0.91 (0.60–1.40) | |
| Prevalence, 11 to 15 years, n = 1338 | % | 9.9 | 8.8 | 11.1 | 5.0 |
| OR (95% CI) | 1 | 0.88 (0.52–1.49) | 1.15 (0.69–1.89) | 0.49 (0.26–0.89) | |
| AR b Cumulative incidence, 4 to 15 years | % | 35.3 | 33.2 | 35.0 | 28.2 |
| RR (95% CI) | 1 | 0.90 (0.70–1.16) | 0.95 (0.74–1.22) | 0.77 (0.59–0.99) | |
| Prevalence, 11 to 15 years, n = 1331 | % | 30.7 | 22.8 | 27.2 | 20.6 |
| OR (95% CI) | 1 | 0.67 (0.47–0.95) | 0.84 (0.60–1.18) | 0.59 (0.41–0.84) | |
| Eczema Cumulative incidence, birth to 15 years | % | 42.3 | 36.9 | 36.9 | 29.8 |
| RR (95% CI) | 1 | 0.75 (0.59–0.96) | 0.84 (0.66–1.07) | 0.60 (0.46–0.77) | |
| Prevalence, 11 to 15 years, n = 1336 | % | 10.6 | 6.5 | 11.3 | 4.7 |
| OR (95% CI) | 1 | 0.58 (0.33–1.02) | 1.08 (0.66–1.77) | 0.42 (0.23–0.79) | |
| PP, number of followed children (n = 988) | n = 270 | n = 256 | n = 242 | n = 220 | |
| Asthma Cumulative incidence, 3 to 15 years | % | 13.5 | 16.8 | 13.4 | 12.3 |
| RR (95% CI) | 1 | 1.29 (0.75–2.21) | 1.01 (0.57–1.79) | 0.94 (0.52–1.71) | |
| aRRa (95%CI) | 1 | 1.22 (0.70–2.12) | 1.05 (0.59–1.87) | 0.94 (0.51–1.72) | |
| Prevalence, 11 to 15 years, n = 633 | % | 9.9 | 9.8 | 9.7 | 4.9 |
| OR (95% CI) | 1 | 0.99 (0.48–2.02) | 0.98 (0.47–2.03) | 0.47 (0.19–1.17) | |
| aORa (95% CI) | 1 | 0.95 (0.46–1.97) | 1.02 (0.48–2.15) | 0.47 (0.19–1.18) | |
| AR bCumulative incidence, 4 to 15 years | % | 32.8 | 34.7 | 32.9 | 31.4 |
| RR (95% CI) | 1 | 1.04 (0.72–1.51) | 0.95 (0.66–1.37) | 0.91 (0.62–1.33) | |
| aRRa (95%CI) | 1 | 0.99 (0.68–1.44) | 0.90 (0.62–1.30) | 0.88 (0.60–1.30) | |
| Prevalence, 11 to 15 years, n = 632 | % | 26.6 | 22.2 | 26.1 | 25.0 |
| OR (95% CI) | 1 | 0.79 (0.48–1.30) | 0.97 (0.60–1.60) | 0.92 (0.55–1.53) | |
| aORa (95% CI) | 1 | 0.78 (0.47–1.32) | 0.94 (0.56–1.56) | 0.91 (0.54–1.54) | |
| Eczema Cumulative incidence, birth to 15 years | % | 42.0 | 32.6 | 35.1 | 25.7 |
| RR (95% CI) | 1 | 0.62 (0.44–0.89) | 0.77(0.54–1.09) | 0.47 (0.32–0.70) | |
| aRRa (95%CI) | 1 | 0.58 (0.40–0.84) | 0.71 (0.49–1.03) | 0.45 (0.30–0.67) | |
| Prevalence, 11 to 15 years, n = 626 | % | 10.2 | 4.9 | 10.9 | 5.0 |
| OR (95% CI) | 1 | 0.45 (0.19–1.08) | 1.07 (0.53–2.18) | 0.46 (0.18–1.14) | |
| aORa (95% CI) | 1 | 0.43 (0.18–1.04) | 1.02 (0.49–2.12) | 0.43 (0.17–1.08) |
Relative risks (RR), adjusted RR (aRR*), odds ratios (OR), and adjusted OR (aOR*) for the three different hydrolyzate formula groups, when compared to the cow's milk formula group (ITT and PP population).
Adjusted for family history of disease, heredity of family allergy, sex, study region, siblings, and parental education.
Hay fever/allergic rhinitis. Bold values and bold CI were used for significant effects, bold values for strong effects with loss of significance.
Asthma
Constant (not significant) effects in cumulative incidence over time for pHF‐W and eHF‐W were observed, that is proportional curves in comparison with CMF. However, the effect for the eHF‐C group changed after 10 years of age, and the increase between 11 and 15 years was reduced (Fig. 2a). The interaction term between time and eHF‐C compared to the time‐trend for CMF was significant (P = 0.036). The prevalence of asthma in the CMF group was 9.9%, and the prevalence was reduced in the eHF‐C group in the ITT (OR 0.49, 95% CI 0.26–0.89) and PP (OR 0.47, 95% C, 0.19–1.17) populations, although the latter was not significant.
Allergic rhinitis
A significant reduction in AR cumulative incidence was observed for eHF‐C in the ITT population (OR 0.77, 95% CI 0.59–0.99), but this effect was weaker and not significant in the PP population. The prevalence between 11 and 15 years was significantly reduced for pHF‐W (OR 0.67, 95% CI 0.47–0.95) and eHF‐C (OR 0.59, 95% CI 0.41–0.84) in the ITT population. However, in the PP population the effect for pHF‐W was weaker and no effect was observed for eHF‐C. The eHF‐W group did not differ from the CMF group.
Eczema
The cumulative incidence was significantly reduced in the eHF‐C (OR 0.60, 95% CI 0.46–0.77) and pHF‐W (OR 0.75, 95% CI 0.59–0.96) groups in the ITT population. Stronger effects were observed in the PP population. The numbers needed to treat to prevent one case of eczema up to 15 years of age for the pHF‐W, eHF‐W, and eHF‐C formulas were 7, 11, and 6, respectively, in the PP population, and 14, 30, and 8 in the ITT population.
The prevalence between 11 and 15 years in the eHF‐C (OR 0.42, 95% CI 0.23–0.79) and pHF‐W (OR 0.58, 95% C, 0.33–1.02) groups was reduced in the ITT population, although the latter failed to reach statistical significance. In the PP population, the ORs for the prevalence estimates in the eHF‐C and pHF‐W groups were lower than 0.5 but not statistically significant. No significant effect was found in the eHF‐W group.
Spirometry
Spirometry measurements were performed in 66% of the participants at the 15‐year follow‐up examination. Participation did not differ across formula groups. The spirometry results are given in Table 2. Lung volume indices (FVC, FEV1) did not significantly differ by formula group, but all flow indices were consistently highest in the eHF‐C group. Particularly, indices of small airway function (FEF50, FEF25, and FEF25/75) were significantly higher in the eHF‐C group compared to the CMF group. After bronchodilation, all indices increased in all formula groups, and the differences between formula groups weakened, suggesting partly reversible effects in the small airways (not shown). Adjusting for asthma did not change the results. The analyses based on z‐scores yielded comparable findings (not shown).
Table 2.
Spirometry parameters. Means of measured values from linear models for the three different hydrolyzate formula groups and the cow's milk formula group (ITT and PP population)
| CMF | pHF‐W | eHF‐W | eHF‐C | |||||
|---|---|---|---|---|---|---|---|---|
| Meana | P b | Meana | P b | Meana | P b | Meana | P b | |
| ITT | n = 220 | n = 234 | n = 233 | n = 231 | ||||
| FEV1/FVC | 0.8643 | Ref | 0.8541 | 0.139 | 0.8642 | 0.935 | 0.8743 | 0.073 |
| PEF [l/s] | 7.0394 | Ref | 7.0083 | 0.477 | 7.1212 | 0.513 | 7.1412 | 0.299 |
| FEF75 [l/s] | 2.1783 | Ref | 2.1662 | 0.671 | 2.1902 | 0.854 | 2.2530 | 0.233 |
| FEF50 [l/s] | 4.3511 | Ref | 4.2954 | 0.457 | 4.3862 | 0.710 | 4.5636 | 0.020 |
| FEF25 [l/s] | 6.1179 | Ref | 6.0519 | 0.346 | 6.1748 | 0.639 | 6.3002 | 0.066 |
| FEF25/75 [l/s] | 3.8449 | Ref | 3.8025 | 0.487 | 3.8681 | 0.800 | 3.9943 | 0.068 |
| PP | n = 112 | n = 114 | n = 110 | n = 95 | ||||
| FEV1/FVC | 0.8600 | Ref | 0.8562 | 0.605 | 0.8673 | 0.374 | 0.8773 | 0.044 |
| PEF [l/s] | 6.9690 | Ref | 7.0774 | 0.593 | 7.2916 | 0.030 | 7.2039 | 0.085 |
| FEF75 [l/s] | 2.1064 | Ref | 2.1961 | 0.403 | 2.2261 | 0.220 | 2.2647 | 0.080 |
| FEF50 [l/s] | 4.2864 | Ref | 4.3092 | 0.922 | 4.5068 | 0.092 | 4.5960 | 0.018 |
| FEF25 [l/s] | 6.0488 | Ref | 6.0664 | 0.995 | 6.3504 | 0.031 | 6.3663 | 0.022 |
| FEF25/75 [l/s] | 3.7721 | Ref | 3.8353 | 0.646 | 3.9573 | 0.118 | 4.0214 | 0.035 |
Means for original values adjusted for age, sex, height, and weight, in PP additionally for family history of asthma, heredity of family allergy, study region, siblings, and parental education
Tests for the comparison with cow's milk formula (CMF) on original values or after normalizing using suitable function. FEV1 = forced expiratory volume in one second. FVC = forced vital capacity. PEF = peak expiratory flow. FEF25, FEF50, FEF75 = forced expiratory flow rates 25, 50 and 75% of exhaled FVC. FEF25/75 = mean flow rate between 25% and 75% of FVC.
Sensitization at 15 years
Sensitization data at 15 years were available for 963 and 449 children in the ITT and PP populations, respectively. Participation did not differ across formula groups.
Allergic sensitization, defined as at least one positive specific IgE value to any of the tested allergens, was 51.5% in the ITT population and 51.9% in the PP population. Most children were sensitized to aeroallergens (50.4% in the ITT population), specifically to pollen (40.7%). Only 12% of children were sensitized to food allergens. Similar proportions were observed across the analysis populations (ITT, PP) and formula groups (Table 3).
Table 3.
Sensitizationb. Point prevalence at 15‐year follow‐up. Odds ratio or adjusted odds ratio (aORa) from logistic regression for the three different hydrolyzate formulas in comparison with cow's milk formula (ITT and PP population)
| CMF | pHF‐W | eHF‐W | eHF‐C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ITT | n = 227 | n = 239 | n = 250 | n = 247 | |||||
| % | OR | % | OR (95%CI) | % | OR (95%CI) | % | OR (95%CI) | ||
| Sensitization | 51.5 | 1 | 48.5 | 0.89 (0.62–1.28) | 51.6 | 1.00 (0.70–1.44) | 54.3 | 1.12 (0.78–1.60) | |
| Food | 11.0 | 1 | 11.7 | 1.07 (0.61–1.90) | 12.0 | 1.10 (0.63–1.94) | 13.0 | 1.20 (0.69–2.10) | |
| Aero | 51.1 | 1 | 46.4 | 0.83 (0.58–1.19) | 51.2 | 1.00 (0.70–1.44) | 52.6 | 1.06 (0.74–1.53) | |
| Pollen | 39.6 | 1 | 39.3 | 0.99 (0.68–1.43) | 43.2 | 1.16 (0.72–1.50) | 40.5 | 1.04 (0.72–1.50) | |
| PP | n = 113 | n = 118 | n = 119 | n = 99 | |||||
| % | aORa | % | aORa (95%CI) | % | aORa (95%CI) | % | aORa (95%CI) | ||
| Sensitization | 48.7 | 1 | 50.8 | 1.11 (0.65–1.90) | 52.1 | 1.11 (0.65–1.89) | 56.6 | 1.41 (0.80–2.47) | |
| Food | 11.5 | 1 | 14.4 | 1.34 (0.61–2.93) | 11.8 | 0.98 (0.43–2.20) | 13.1 | 1.15 (0.50–2.64) | |
| Aero | 48.7 | 1 | 49.2 | 1.05 (0.61–1.78) | 51.3 | 1.08 (0.63–1.84) | 54.5 | 1.29 (0.74–2.27) | |
| Pollen | 38.9 | 1 | 41.5 | 1.16 (0.67–2.01) | 43.7 | 1.18 (0.68–2.03) | 36.4 | 0.87 (0.49–1.56) | |
Adjusted for heredity of family allergy, sex, study region, siblings, and parental education.
At least 1 specific IgE level ≥ 0.35 kU/l (i.e. CAP class ≥ 1).
Sensitivity analyses
Sensitivity analyses, restricted to children who completed all questionnaires (at one, two, three, four, six, ten and 15 years of age), yielded comparable point estimates to those observed in the original analyses (not shown).
Discussion
The 15‐year follow‐up study of the GINI cohort provides evidence that the interventional use of certain hydrolyzate formulas as breastmilk substitutes in the first four months of life in high‐risk children is associated with less eczema from birth until the age of 15 years. Furthermore, for the first time during this longitudinal follow‐up, some beneficial associations between the use of certain formulas and respiratory allergies emerged.
Of the three hydrolyzates used in this study, only the eHF‐C and pHF‐W were associated with a lower occurrence of an allergic disease from early childhood up to adolescence. There is hardly any effect on any allergic outcomes in the eHF‐W formula group, which is consistent with previous reports from the GINI cohort 21, 25.
Although the size of the preventative effect is always slightly higher for eHF‐C, the most striking and consistent effect for both the eHF‐C and pHF‐W formulas is the reduced development of eczema from birth onwards. Even though eczema peaks in the first 3 years of life and thus the preventive effect on eczema has an early ceiling, as shown in the previous publications of the GINI study 21, 25, the documentation of this effect as being well sustained and without any rebound up to 15 years is an important finding for the children, their families, and the health economy 34. For eHF‐C the prevalence of eczema between 11 and 15 years in the ITT population is significantly reduced, while for pHF‐W the reduction of >40% just fails to reach statistical significance. The fact that the ORs for the prevalence estimates (ORs <0.5) in the PP analysis are not significant could be explained by reduced power due to the small numbers in the PP analysis.
Using a longitudinal design, this study is the first to show associations between hydrolyzed formulas with asthma and allergic rhinitis, diseases which typically occur later in childhood than eczema. Children who belonged to the eHF‐C formula group had a lower likelihood of being asthmatic between the age of 11 and 15 years, an effect that was confirmed by spirometry. Measures for distal airway function seemed to be especially positively affected by the eHF‐C formula. This is important to note because small‐airways dysfunction can already be present in patients with mild asthma 35. The associations between eHF‐C and pHF‐W with AR found in the ITT population were not apparent in the PP population. This is surprising, because one would expect stronger effects in the PP population. On the other hand, the number of subjects in each formula group is smaller in the PP population and this could affect instability in the estimation of effects. Nevertheless, although the association between early formula feeding and the emergence of a late‐onset preventative effect on respiratory allergies is interesting, it should be interpreted with caution until further results confirm these findings.
One of the critical arguments against the use of hydrolyzed formulas for allergy prevention is a lack of understanding of the mechanisms driving the beneficial effects. As 80–90% of respiratory allergic diseases in children and young adolescents are associated with sensitization to aeroallergens (mainly pollen and house dust mites), it is reasonable to hypothesize that IgE might be involved. However, we did not observe any association between specific IgE measured in serum and hydrolyzates at 15 years, which is consistent with our findings at six and ten years 21, 27 and with reports from the literature 17, 18, 19. Therefore, IgE does not appear to be involved in the observed findings in this study. Although we cannot exclude the possibility that, due to selective participation in blood sampling, IgE may still play a role, other (immune‐) mechanisms should be considered. It can be speculated that the observed differential effects of the individual formulas may be driven by the absence of allergy‐inducing epitopes, the presence of certain peptides that promote the development of tolerance via activation of T regulatory cells 36, or differential changes on the mucosal level in the gut, skin, or respiratory tract.
The differential effects of the formulas imply that all hydrolyzed formulas are not equal. Instead, each formula has to be considered as its own individual intervention. This is an important message that needs to be considered when evaluating which formula(s) should be provided to a child. Our results suggest that, apart from characteristics such as molecular weight profile and basic protein (whey or casein), other factors seem to be relevant to the biologic functionality of hydrolyzed formulas. Recent studies indicate that the peptide profiles of different commercially available cow′s milk protein hydrolyzates provide a descriptive and distinct signature as a result of the hydrolyzing process, which changes the peptide sequences 8. Through the use of new technologies, our previously reported hypothesis 21, that is the individual hydrolyzing process for each formula changes its biologic functionality, is now confirmed. This new knowledge may help explain the currently poorly understood differential effects of the three hydrolyzed formulas used in the GINI trial.
As reported data on the preventive potential of hydrolyzed formulas are inconsistent, an updated systematic review, which includes the study by Lowe et al. 7, should be used to guide feeding practices for high‐risk children who need breastmilk supplementation. In the study by Lowe, although the same pHF‐W formula was used, no preventive effect on allergy was observed up to 6–7 years of age. We appreciate the publication of null‐findings to avoid publication bias and to learn from the differences between studies, which may help explain why inconsistent results have been observed. However, based on the results reported in this study, we currently feel that – at least for eczema – there is sufficient evidence to recommend feeding certain hydrolyzed formulas to children at high risk who need breastmilk supplementation 37.
The main strengths of this study are the original large sample size of 2252 children, randomization and allocation concealment, and a fair participation at 15 years of age (ITT 61.1%, PP 66.0%), which are the formula groups. Identical questions were posed for the primary outcomes in all follow‐ups, from one up to 15 years of age 21. The current study nevertheless also has important limitations, such as the unblinding of the formulas when the youngest child turned three years old, as extensively discussed in our previous publication 21. Another potential source of bias is that the assessment of the formula effect is based on a parental report of a physician's diagnosis, as is the case for most epidemiological studies, and not on a clinical examination in the study centers. The inclusion of objective health assessments (skin examination, spirometry, and atopic sensitization) is a further strength, but the data should be interpreted with caution because the consent to lung‐function testing and blood sampling was self‐selective with response rates of 67% and 70%.
Conclusion
The results of the 15‐year follow‐up of the GINI study confirm that the previously reported preventative effect of the eHF‐C and pHF‐W formulas on eczema are sustained until adolescence without the existence of a rebound phenomenon. During the last years of follow‐up, fewer emergences of AR and asthma were related to the use of certain hydrolyzates, mainly eHF‐C. The effect in the eHF‐C formula group on asthma is consistent with the spirometric results. The findings with respect to the respiratory allergies should be interpreted with caution until confirmed in future studies. None of the formulas had an influence on IgE sensitization.
Author contributions
AvB contributed to the conception and design of the cohort study, was coordinator of the study and responsible for study execution in Wesel, interpreted the data, and drafted the manuscript. BFP performed statistical analysis, contributed to interpretation, and drafted parts of the manuscript. HS contributed to design of 15‐year follow‐up, spirometry measurement, interpretation of spirometry, and drafting the manuscript. UH contributed to data collection and clinical examination in Munich. EL contributed to data management since 6‐year follow‐up. MSu contributed to data management since 10‐year follow‐up. MSch contributed to study execution and data collection since 10‐year follow‐up. IB contributed to the design of 10‐ and 15‐year follow‐up. MSt contributed to the design of 15‐year follow‐up and data management. UK contributed to the design since 6‐year follow‐up and interpretation of the data. BH contributed to the design of 15‐year follow‐up, interpretation, and drafting the manuscript. JH contributed to the conception and design of the cohort study, interpretation, and drafting the manuscript. CPB contributed to the conception and design of the cohort study, interpretation, and drafting the manuscript. SK contributed to the conception and design of the cohort study, interpretation, and drafting the manuscript. DB is principal investigator of the GINI study, contributed to the conception and design of the cohort study, interpretation and drafting the manuscript, and agreed to be accountable for all aspects of the work. All authors without contribution to drafting revised the manuscript critically for important intellectual content. All authors approved the final manuscript for publishing.
Funding
The GINI Intervention study was funded for 3 years by grants from the Federal Ministry for Education, Science, Research and Technology (Grant no. 01 EE 9401‐4). The companies Milupa, Nestlé, Mead Johnson, and Nutricia provided the blinded study formulas for the participating children for the first 4–6 months. The 3–6‐ and 10‐year‐follow‐up examinations of the GINI study were covered from the respective budgets of the initial 4 study centers (Wesel, LMU Munich, TU Munich, and Helmholtz Center Munich (former GSF)). From 6 years onwards, GINI was additionally partly supported by the Federal Ministry for the Environment (IUF, FKZ 20462296) and by the budget of the IUF‐ Leibniz Research Institute for Environmental Medicine at the University of Düsseldorf. The 15‐year follow‐up of the GINI study was supported by the companies Mead Johnson and Nestlé and in cooperation with European Studies (e.g. MeDALL, ESCAPE). Some projects not directly related to the intervention effect of the hydrolyzates (e.g. effect of cesarean section, effect of solid food introduction) were partly supported by Nestlé, Mead Johnson, Numico, Pharmacia and Stiftung Kindergesundheit, and in cooperation with European studies (e.g. MeDALL). None of the funding bodies had any influence on the study design, data analysis, or manuscript preparation.
Conflicts of interest
AvB has received speakers' fees from Nestlé Nutrition Institute. The Research Institute at the Marien‐Hospital has received grant from Nestlé Vevey, Switzerland. SK has received research support from Mead Johnson; has board memberships with MSD, Nestlé, Danone, Merk, Nutricia; is a consultant for Boehringer Ingelheim, MSA, Merck, Abbvie, Danone; has received speakers' fees by Centocor, MSD, Danone, Merck, Vifor, Nestlé Nutrition Institute, Euroimmun, Thermo‐Fischer (Phadia), Abbvie, Schär, Hipp, Falk and travel support by MSD; the institution has received grants from Nestlé Nutrition, Mead Johnson, Thermosphere, Euroimmun, INOVA, R‐Biopharm, Schär. The rest of the authors declare that they have no conflicts of interest.
Supporting information
Data S1 Supplementary information on study methods, primary outcomes and procedure of lung‐function assessment
Table S1 Association between possible factors and participation (%) at 15 years expressed as adjusted OR (aOR*)
Acknowledgments
We thank the children and families in the GINI study for their continuous participation, as well as the GINIplus study team for excellent work.
Appendix A.
GINIplus 15 Study Group: Helmholtz Zentrum München, German Research Centre for Environmental Health, Institute of Epidemiology I, Neuherberg (J. Heinrich, I. Brüske, H. Schulz, M. Standl, M. Schnappinger, M. Sußmann, E. Thiering, C. Tiesler, C. Flexeder, C. Zeller); Marien‐Hospital Wesel, Department of Pediatrics, Research Institute, Wesel (D. Berdel, A. von Berg, B. Filipiak‐Pittroff); Ludwig‐Maximilians‐University of Munich, Dr von Hauner Children's Hospital (S. Koletzko, K. Werkstetter); Technical University Munich, Department of Pediatrics, and Deutsche Rentenversicherung Bayern (C.P. Bauer, U. Hoffmann); and IUF‐Leibniz Institute for Environmental Research, Düsseldorf (B. Hoffmann, E. Link, C. Klümper, U. Krämer, D. Sugiri).
von Berg A, Filipiak‐Pittroff B, Schulz H, Hoffmann U, Link E, Sußmann M, Schnappinger M, Brüske I, Standl M, Krämer U, Hoffmann B, Heinrich J, Bauer C‐P, Koletzko S, Berdel D, for the GINIplus study group . Allergic manifestation 15 years after early intervention with hydrolyzed formulas – the GINI Study. Allergy 2016; 71: 210–219.
Edited by: Thomas Bieber
GINIplus 15 Study Group details are given in Appendix
Notes
Partial whey hydrolyzate (pHF‐W) Beba‐ HA
extensive whey hydrolyzate (eHF‐W) HIPP‐HA (at that time identical to Nutrilon Pepti)
extensive casein hydrolyzate (eHF‐C) Nutramigen
standard cow´s milk formula (CMF) Nutrilon Premium
References
- 1. American Academy of Pediatrics Committee on Nutrition . Hypoallergenic infant formulas. Pediatrics 2000;106:236–347. [PubMed] [Google Scholar]
- 2. Muraro A, Dreborg S, Halken S, Høst A, Niggemann B, Aalberse R et al. Dietary prevention of allergic diseases in infants and small children. Part III: critical review of published peer‐reviewed observational and interventional studies and final recommendations. Pediatr Allergy Immunol 2004;15:291–307. [DOI] [PubMed] [Google Scholar]
- 3. Greer FR, Sicherer SH, Burks W, the Committee on Nutrition and Section on Allergy and Immunology . Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. 2008;121:183–191. [DOI] [PubMed] [Google Scholar]
- 4. Fleischer DM, Spergel JM, Assa'ad AH, Pongracic JA. Primary prevention of allergic disease through nutritional intervention. J Allergy Clin Immunol Pract 2013;1:29–36. [DOI] [PubMed] [Google Scholar]
- 5. Prescott S, Tang M, Björksten B. Primary allergy prevention in children: updated summary of a position statement of the Australasian Society of Clinical Immunology and Allergy In: Kemp A, Mullin R, Weiner J, editors. MJA Practice Essentials Allergy. Sydney: Australasian Medical Publishing Company; 2007: 54–58. [Google Scholar]
- 6. Brandt PL, Vlieg‐Boerstra BJ, Dubois AE. Dietary prevention of allergic diseases in children: are current recommendations really based on good evidence? Pediatr Allergy Immunol 2007;18:475–479. [DOI] [PubMed] [Google Scholar]
- 7. Lowe AJ, Hosking CS, Bennett CM, Allen KJ, Axelrad C, Carlin JB et al. Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high‐risk children: a randomized controlled trial. J Allergy Clin Immunol 2011;128:360–365. [DOI] [PubMed] [Google Scholar]
- 8. Lambers TT, Gloerich J, van Hoffen E, Alkema W, Hondmann DH, van Tol EA. Clustering analyses in peptidomics revealed that peptide profiles of infant formulae are descriptive. Food Science nutrition 2015;3:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allen KJ, Lowe AJ, Dharmage SC. The role of hydrolysates for allergy prevention – con. Pediatr Allergy Immunol 2013;24:724–726. [DOI] [PubMed] [Google Scholar]
- 10. Arshad SH. Primary prevention of allergy by using protein hydrolysate: an achievable objective? J Allergy Clin Immunol 2013;131:1574–1575. [DOI] [PubMed] [Google Scholar]
- 11. Hays T, Wood RA. A systematic review of the role of hydrolyzed infant formulas on allergy prevention. Arch Pediatr Adolesc Med 2005;159:810–816. [DOI] [PubMed] [Google Scholar]
- 12. Osborne DA, Sinn J Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev 2006;4:CD003664. [DOI] [PubMed] [Google Scholar]
- 13. Szajewska H, Horvath A. A meta‐analysis of the evidence for a partially hydrolyzed 100% whey formula for the prevention of allergic diseases. Curr Med Res Opin 2010;6:423–427. [DOI] [PubMed] [Google Scholar]
- 14. Alexander DD, Cabana MD. Partially hydrolyzed 100% whey protein infant formula and reduced risk of atopic dermatitis: a meta‐analysis. JPGN 2010;50:356–358. [DOI] [PubMed] [Google Scholar]
- 15. Food and Drug administration . 100% whey‐protein partially hydrolyzed infant formula and reduced risk of atopic dermatitis. 2011. Available at http://www.fda.gov/Food/LabelingNutrition/LabelClaims/QualifiedHealthClaims/ucm256731.htm .
- 16. Vandenplas Y, Hauser B, Van den Borre C, Clybouw C, Mahler T, Hachimi‐Idrissi S et al. The long‐term effect of a partial whey hydrolyzate formula on the prophylaxis for atopic disease. Eur J Pediatr 1995;154:488–494. [DOI] [PubMed] [Google Scholar]
- 17. Oldaeus G, Anjou K, Bjørksten B, Moran JR, Kjellman NI. Extensively and partially hydrolysed infant formulas for allergy prophylaxis. Arch Dis Child 1997;77:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatr Allergy Immunol 2000;11:149–161. [DOI] [PubMed] [Google Scholar]
- 19. Zeiger RS. Food allergen avoidance in the prevention of food allergy in infants and children. Pediatrics 2003;111:1662–1671. [PubMed] [Google Scholar]
- 20. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the world allergy organization, October 2003. J Allergy Clin Immunol 2004;113:832–836. [DOI] [PubMed] [Google Scholar]
- 21. von Berg A, Filipiak‐Pittroff B, Krämer U, Hoffmann B, Link E, Beckmann C et al. Allergies in high‐ risk schoolchildren after early intervention with cow′s milk protein hydrolysates: 10‐year results from the German Infant Nutritional intervention (GINI) study. J Allergy Clin Immunol 2013;131:1565–1573. [DOI] [PubMed] [Google Scholar]
- 22. Chan‐Yeung M, Ferguson A, Watson W, Dimich‐Ward H, Rousseau R, Lilley M et al. The Canadian childhood asthma primary prevention study: outcomes at 7 years of age. J Allergy Clin Immunol 2005;116:49–55. [DOI] [PubMed] [Google Scholar]
- 23. Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic diseases during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol 2007;119:307–313. [DOI] [PubMed] [Google Scholar]
- 24. Scott M, Roberts G, Kurukulaaratchy RJ, Matthews S, Nove A, Arshad SH. Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax 2012;67:1046–1051. [DOI] [PubMed] [Google Scholar]
- 25. von Berg A, Koletzko S, Grübl A, Filipiak‐Pittroff B, Wichmann HE, Bauer CP et al. The effect of hydrolyzed cow's milk formula for allergy prevention in the first year of life: the German Infant Nutritional Intervention Study, a randomized double‐blind trial. J Allergy Clin Immunol 2003;111:533–540. [DOI] [PubMed] [Google Scholar]
- 26. von Berg A, Koletzko S, Filipiak‐Pittroff B, Laubereau B, Grübl A, Wichmann HE et al. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: three‐year results of the German Infant Nutritional Intervention Study. J Allergy Clin Immunol 2007;119:718–725. [DOI] [PubMed] [Google Scholar]
- 27. von Berg A, Filipiak‐Pittroff B, Krämer U, Link E, Bollrath C, Brockow I et al. Preventive effect of hydrolyzed infant formulas persists until age 6 years: long‐term results from the German Infant Nutritional Intervention Study (GINI). J Allergy Clin Immunol 2008;121:1442–1447. [DOI] [PubMed] [Google Scholar]
- 28. Berg Av, Krämer U, Link E, Bollrath C, Heinrich J, Brockow I et al. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course ‐ the GINIplus study up to the age of 6 years. Clin Exp Allergy 2010;40:627–36. [DOI] [PubMed] [Google Scholar]
- 29. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A et al. Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 30. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH et al. Multi‐ethnic reference values for spirometry for the 3–95‐year age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schoetzau A, Gehring U, Franke K, Grübl A, Koletzko S, von Berg A et al. Maternal compliance with nutritional recommendations in an allergy preventive programme. Arch Dis Child 2002;86:180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research. Principles and quantitative methods: Belmont Lifetime learning publications, 1982. [Google Scholar]
- 33. Diggle P, Haegerty P, Liang K, Zeger S. Analysis of Longitudinal Data. Oxford University Press, 2nd edition, 2002. [Google Scholar]
- 34. Mertens J, Stock S, Lüngen M, von Berg A, Krämer U, Filipiak‐Pittroff B et al. Is prevention of atopic eczema with hydrolyzed formulas cost‐effective? A health economic evaluation from Germany. Pediatr Allergy Immunol 2012;23:597–604. [DOI] [PubMed] [Google Scholar]
- 35. Van der Wiel E, ten Hacken NH, Postma DS, van den Berge M. Small‐airways dysfunction associates with respiratory symptoms and clinical features of asthma: a systematic review. J Allergy Clin Immunol 2013;131:646–657. [DOI] [PubMed] [Google Scholar]
- 36. Pabst O, Mowat AM. Oral tolerance to food protein. Muscosal Immunol 2012;5:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koletzko S, Filipiak‐Pittroff B, Koletzko B, vonBerg A , Krämer U, Berdel D et al. No reason to change the current guidelines on allergy prevention. J Allergy Clin Immunol 2012;129:262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supplementary information on study methods, primary outcomes and procedure of lung‐function assessment
Table S1 Association between possible factors and participation (%) at 15 years expressed as adjusted OR (aOR*)


