Abstract
DNA sequence analysis of dtxR has shown that the M(r) 25,316 regulatory protein contains a single cysteine residue at position 102. DtxR readily forms inactive disulfide-linked dimers. We have used saturation site-directed mutagenesis of the cysteine codon (TGC) at position 102 in order to determine the role of this residue in metal ion binding. We show that the insertion of amino acids other than cysteine or aspartic acid into this position abolishes DtxR function both in vitro and in recombinant Escherichia coli DH5 alpha:lambda RS45toxPO/lacZ. Only those mutant alleles in which the TGC codon for Cys-102 was replaced by either TGT (Cys) or GCA (Asp) were found to direct the expression of active forms of DtxR that regulate the expression of beta-galactosidase from the toxPO/lacZ transcriptional fusion.
Full text
PDF

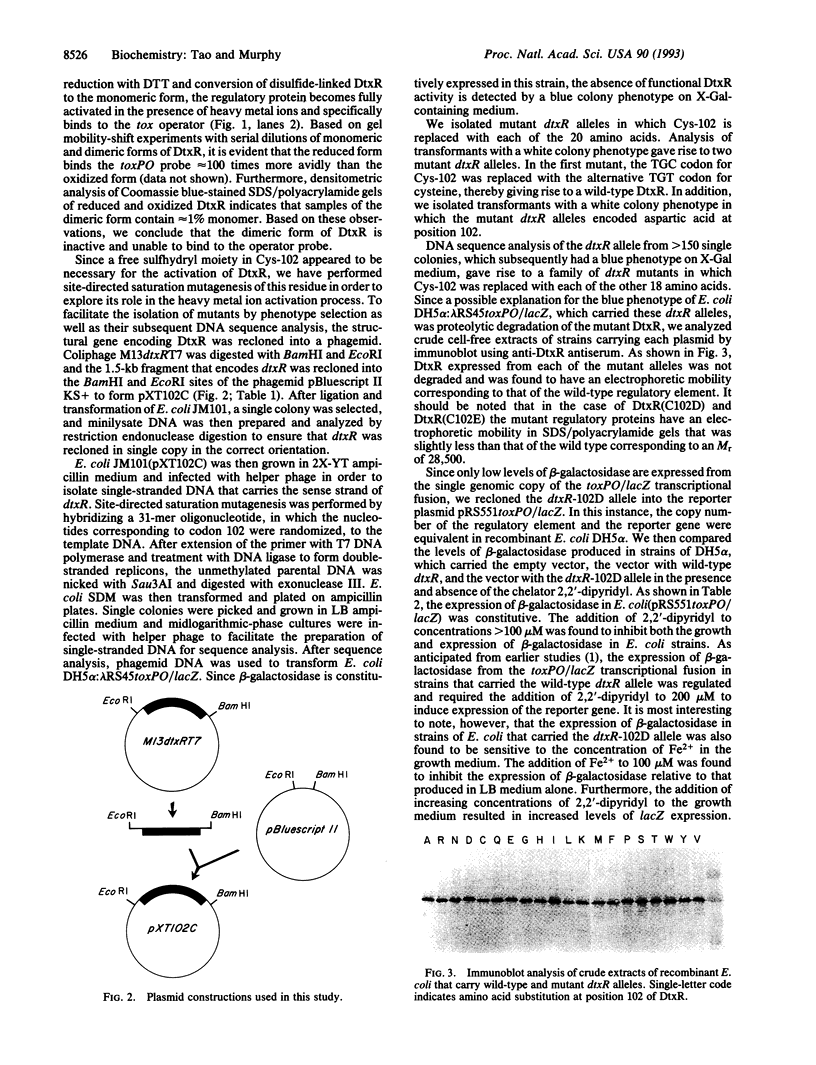
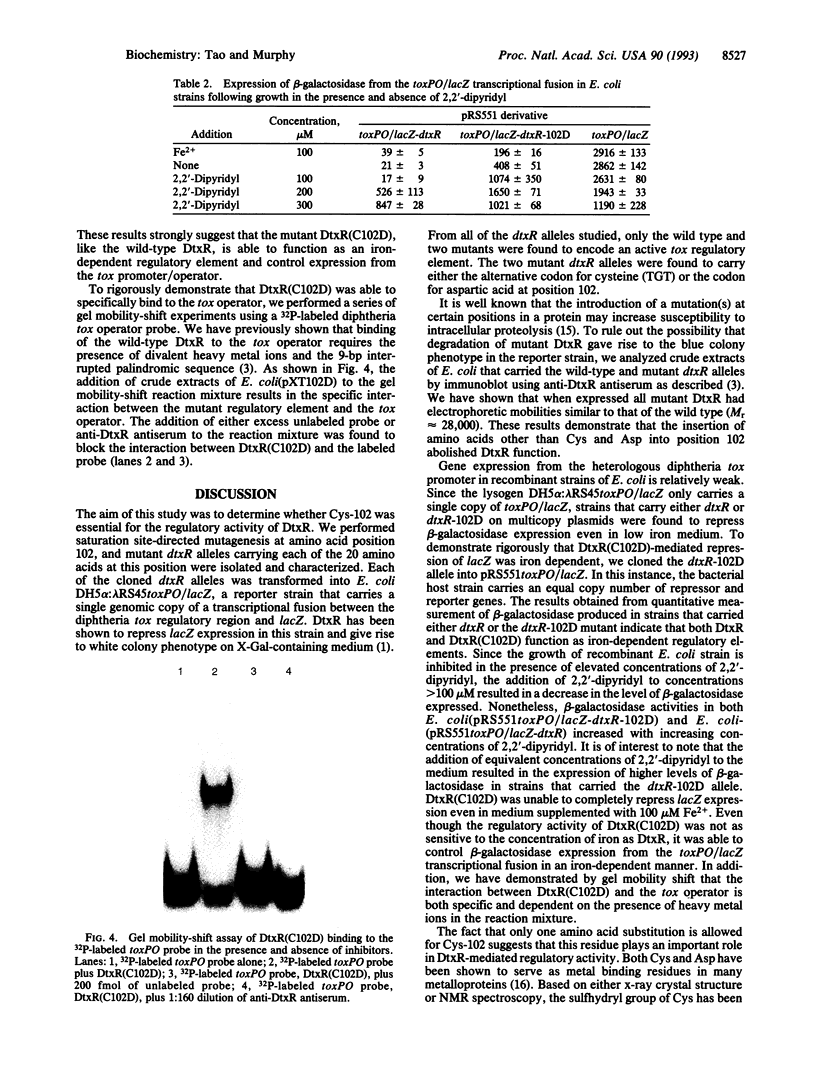

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J. M., Hall K. C., Murphy J. R. DNA sequences and characterization of dtxR alleles from Corynebacterium diphtheriae PW8(-), 1030(-), and C7hm723(-). J Bacteriol. 1992 Feb;174(4):1268–1272. doi: 10.1128/jb.174.4.1268-1272.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J., Oza M. N., Murphy J. R. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy M., Neilands J. B. Structural dynamics and functional domains of the fur protein. Biochemistry. 1991 Aug 20;30(33):8201–8210. doi: 10.1021/bi00247a016. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Kim E. E., Wyckoff H. W. Reaction mechanism of alkaline phosphatase based on crystal structures. Two-metal ion catalysis. J Mol Biol. 1991 Mar 20;218(2):449–464. doi: 10.1016/0022-2836(91)90724-k. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Gippert G. P., Soman K. V., Case D. A., Wright P. E. Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science. 1989 Aug 11;245(4918):635–637. doi: 10.1126/science.2503871. [DOI] [PubMed] [Google Scholar]
- Putnam S. L., Koch A. L. Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of beta-galactosidase. Anal Biochem. 1975 Feb;63(2):350–360. doi: 10.1016/0003-2697(75)90357-7. [DOI] [PubMed] [Google Scholar]
- Saito T., Duly D., Williams R. J. The histidines of the iron-uptake regulation protein, Fur. Eur J Biochem. 1991 Apr 10;197(1):39–42. doi: 10.1111/j.1432-1033.1991.tb15879.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Holmes R. K. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect Immun. 1991 Jun;59(6):1899–1904. doi: 10.1128/iai.59.6.1899-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Twiddy E. M., Holmes R. K. Purification and characterization of the diphtheria toxin repressor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7576–7580. doi: 10.1073/pnas.89.16.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Boyd J., Murphy J. R. Specific binding of the diphtheria tox regulatory element DtxR to the tox operator requires divalent heavy metal ions and a 9-base-pair interrupted palindromic sequence. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5897–5901. doi: 10.1073/pnas.89.13.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Murphy J. R. Binding of the metalloregulatory protein DtxR to the diphtheria tox operator requires a divalent heavy metal ion and protects the palindromic sequence from DNase I digestion. J Biol Chem. 1992 Oct 25;267(30):21761–21764. [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Active-site zinc ligands and activated H2O of zinc enzymes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):220–224. doi: 10.1073/pnas.87.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeyar M. A., Weiner M. P., Hutton C. J., Batt C. A. A simple and rapid method for the selection of oligodeoxynucleotide-directed mutants. Gene. 1988 May 15;65(1):129–133. doi: 10.1016/0378-1119(88)90425-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]