Abstract
Objectives: Ayurvedic oil-dripping treatment (Shirodhara) is often used for treating sleep problems. However, few properly designed studies have been conducted, and the quantitative effect of Shirodhara is unclear. This study sought to quantitatively evaluate the effect of sesame oil Shirodhara (SOS) against warm water Shirodhara (WWS) on improving sleep quality and quality of life (QOL) among persons reporting sleep problems.
Methods: This randomized, single-blinded, crossover study recruited 20 participants. Each participant received seven 30-minute sessions within 2 weeks with either liquid. The washout period was at least 2 months. The Shirodhara procedure was conducted by a robotic oil-drip system. The outcomes were assessed by the Pittsburgh Sleep Quality Index (PSQI) for sleep quality, Epworth Sleepiness Scale (ESS) for daytime sleepiness, World Health Organization Quality of Life 26 (WHO-QOL26) for QOL, and a sleep monitor instrument for objective sleep measures. Changes between baseline and follow-up periods were compared between the two types of Shirodhara. Analysis was performed with generalized estimating equations.
Results: Of 20 participants, 15 completed the study. SOS improved sleep quality, as measured by PSQI. The SOS score was 1.83 points lower (95% confidence interval [CI], −3.37 to −0.30) at 2-week follow-up and 1.73 points lower (95% CI, −3.84 to 0.38) than WWS at 6-week follow-up. Although marginally significant, SOS also improved QOL by 0.22 points at 2-week follow-up and 0.19 points at 6-week follow-up compared with WWS. After SOS, no beneficial effects were observed on daytime sleepiness or objective sleep measures.
Conclusions: This pilot study demonstrated that SOS may be a safe potential treatment to improve sleep quality and QOL in persons with sleep problems.
Introduction
The importance of adequate sleep is globally recognized. However, inadequate sleep and sleep disturbance are widespread problems that aggravate the risk of various lifestyle-related diseases and interfere with working capacity and concentration.1 It is estimated that 4% of American and 5% of Japanese adults take prescription sleep medications, and this number is consistently increasing.2,3 Patients are concerned about addiction, abuse, and adverse effects of sleep medications.3 Therefore, expectations for traditional medicine (TM) and complementary and alternative medicines (CAM) for treating sleep problems are rising. They are considered to be safe and could be substituted for conventional sleep medicines.4
The popularity of TM and CAM has increased in recent years, especially in developed countries.5–7 Recent studies indicate that a substantial number of people use CAM therapies in their lifetime with the expectation of maintaining and promoting health and curing diseases.8–10 Ayurveda is an ancient medicine that originated in the Indian subcontinent.11 It is recognized as a TM by the World Health Organization and is gaining global acceptance.12
In Ayurveda, sleep is considered one of the three supportive pillars to maintain life, along with diet and celibacy (i.e., regulated sexual conduct).13 Classic textbooks of Ayurveda describe the importance of sleep and various treatments to improve sleep.14,15 A treatment for insomnia in Ayurveda is Shirodhara,16 an oil-dripping treatment. In Shirodhara, a prescribed liquid (e.g., medicated oil or decoction) is continuously poured over the forehead at a specific temperature and speed for a certain period.
Several studies have evaluated the effect of Shirodhara on sleep.17–25 However, properly randomized studies that use standard indices for evaluation of sleep outcomes are limited. Therefore, the quantitative effect of Shirodhara is still unclear.
The current pilot intervention study was conducted in Okayama, Japan, to quantitatively evaluate the effect of sesame oil Shirodhara (SOS) against warm water Shirodhara (WWS) on improvements in sleep quality and quality of life (QOL) among those who report sleep problems.
Materials and Methods
Study design and participants
A randomized, single-blinded, crossover design was used to examine the effect of Shirodhara on sleep. Twenty-two adults who reported subjective poor sleep quality in the past month (e.g., difficulty in falling asleep or interrupted sleep) were recruited. Recruitment was done by distributing a pamphlet to each department at Okayama University and by placing an announcement on the homepage for the authors' office. Persons who were taking any treatment for sleep or had any underlying disease, such as diabetes or hypertension, at the time of recruitment were excluded.
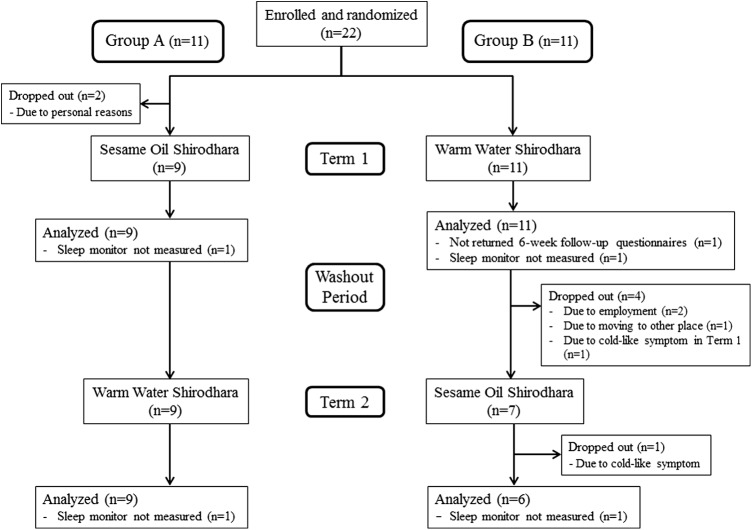
Participants were randomly assigned by block randomization with two participants per block26 using a random number table.27 Participants were allocated randomly to group A or B. Because this was a crossover study, each participant received two different procedures in the first and second terms with a washout period in between of at least 2 months. Group A received SOS in term 1 and WWS in term 2, and group B received WWS in term 1 and SOS in term 2 (Fig. 1).
FIG. 1.
Flowchart of the 22 participants throughout the trial.
Procedures were conducted from April to August 2013 in term 1 and from September to December 2013 in term 2 at a study office in the Department of Human Ecology, Okayama University Graduate School of Environmental and Life Science.
The Institutional Review Board of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences approved this study (no. 652). The participants were informed and fully prepared for the study procedure at an explanatory session before intervention. All participants gave written informed consent.
Demographic questionnaire
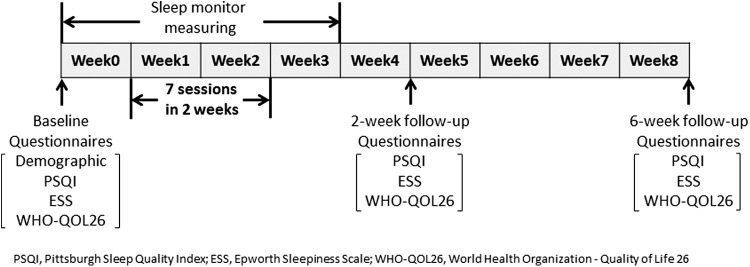
Each participant completed a general demographic questionnaire at baseline (Fig. 2). The questionnaire included birth date; sex; occupation; marital, smoking, alcohol, and exercise status; and medical history.
FIG. 2.
Schedule of outcome measures in each term. Questionnaires include general demographic questionnaire, Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and World Health Organization Quality of Life-26 (WHO-QOL26). The general demographic questionnaire was asked only at baseline in term 1. Sleep quality was measured with a Sleepscan SL-503.
Shirodhara procedures
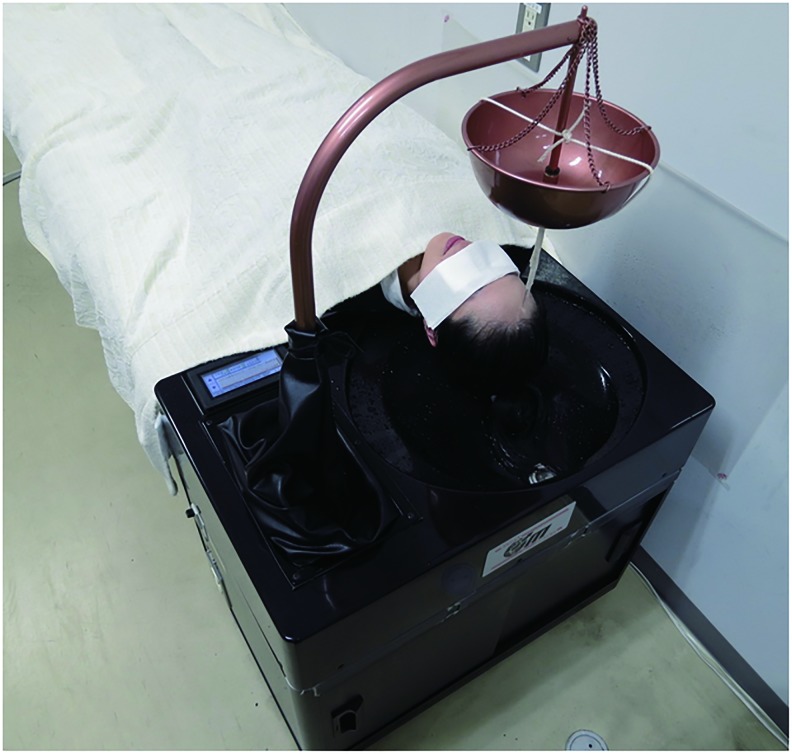
Seven sessions of Shirodhara per participant were conducted within 2 weeks during each term. All procedures were conducted by a therapist who was an Ayurvedic doctor certified by the Indian government and were performed with a robotic oil-drip system called Shirodhara Robot (EM-Techno Co., Ltd., Toyama, Japan). This system automatically pours the liquid over the forehead of the subject with a specified temperature and flow rate (Fig. 3).
FIG. 3.
Shirodhara robotic oil-drip system. The liquid was warmed and circulated inside the system and poured on the forehead of the subject through a bundle of cotton strings attached under the nozzle. The ends of the strings were set 8 cm above the forehead. Temperature, pattern, and flow speed can be specified. Color images available online at www.liebertpub.com/acm
The liquid used for SOS was unroasted plain sesame oil (Kadoya Sesame Mills Inc., Tokyo, Japan) and that for WWS was plain tap water. Although medicated oils are commonly used for Shirodhara, plain sesame oil was selected in this study because it is considered effective and safe and is also often used for Shirodhara in Japan and India. In previous studies, prescribed liquids that were meant for subsiding Vata (one of the biological energies in Ayurveda) were used as Shirodhara liquids. In Ayurveda, the vitiation of Vata is considered a cause of sleep disturbance.28,29 According to classic textbooks, sesame oil also has properties to subside vitiated Vata,30–32 and applying sesame oil on the head produces sound sleep and happiness.33 A prior patch test of sesame oil was performed on all participants to assess allergic reactions.
Each procedure was conducted for 30 minutes. The liquid temperature was set at 38.0 ± 0.5°C (April–October) or 39.0 ± 0.5°C (November–December) and the flow rate was fixed at 1.5 L/min. The nozzle from which the liquid came out was set to move side-to-side over the forehead at 10.0 mm/s. The procedure room was maintained at a temperature of 25 ± 1°C with quiet and dim surroundings.
Participants received Shirodhara in the supine position, with the eyes covered with cotton pads and gauze. The body below the neck was covered with a towel. Participants with long hair had their hair bound at the top of the head with a hair band. After the procedure, the therapist toweled the participant's hair gently; then the participant was asked to rise.
Sleep quality and QOL indices
Three questionnaires were used as primary outcomes to assess treatment effects on sleep quality and QOL. Participants answered these questionnaires three times in each term: 1 week before the first session and at 2-week and 6-week follow-up after the intervention period (Fig. 2).
Pittsburgh Sleep Quality Index (PSQI)
PSQI is a self-rating scale to measure sleep quality and disturbances over a 1-month interval. Higher scores indicate worse sleep quality.34,35
Epworth Sleepiness Scale (ESS)
ESS is a self-report instrument to measure perception of sleepiness. Higher scores indicate stronger subjective daytime sleepiness.36
World Health Organization-Quality of Life-26 (WHO-QOL26)
WHO-QOL26 is a self-reported measure to assess QOL. Higher scores indicate higher QOL.37
Sleep monitor measures
A mat sleep monitor was used to provide an objective measure for sleep status (Sleepscan SL-503; TANITA Corp., Tokyo, Japan). The monitor was placed under the mattress while the participant was sleeping. The monitor measures sleep status by respiration, pulse, and body motion and provides information on time in bed (TIB, starting from the moment of intention to fall asleep and concluding with the final arising), total sleep time (actual time slept), sleep onset latency (how many minutes it takes to fall asleep, starting from the moment of intention to fall asleep), sleep efficiency (percentage of time in bed spent asleep, calculated as total sleep time/time in bed × 100), and wake after sleep onset (total amount of time awake during the night).34 This measurement was performed by participants at home every night starting 1 week before the first session until 1 week after the 2-week intervention period (i.e., a total of 28 days). The daily averaged values of the sleep monitor during week 0, weeks 1 and 2, and week 3 were calculated as values of preintervention, 2-week intervention, and postintervention, respectively, and these values were used for analysis (Fig. 2). When the participants failed to monitor and the data were missing, the average values were calculated with the data from remaining days.
Statistical analysis
All outcome measures (i.e., PSQI, ESS, WHO-QOL26, and sleep monitor measures) were treated as continuous variables. Before assessing the effects of SOS, the pretest to check the assumption of negligible carryover effects38 was done for outcomes, as is routine for crossover studies. To assess the effects of SOS, linear generalized estimating equations (GEE) were used,39 with consideration of within-individual correlation in the crossover study design. In the analysis, the effects of SOS on all outcomes during the follow-up periods were estimated compared with WWS, considering the baseline differences between the two groups. Within-individual exchangeable correlation was assumed, and covariates were not adjusted for given the design (i.e., randomized crossover design).
For primary outcomes (PSQI, ESS, and WHO-QOL26), the effects of SOS were first estimated from baseline to 2-week follow-up (model 1) and then from baseline to 6-week follow-up (model 2) (Fig. 2). For sleep monitor measures, the effects of SOS on changes in average values were first estimated between week 0 (preintervention) and weeks 1 and 2 (2-week intervention) (model 1) and then between week 0 and week 3 (postintervention) (model 2).
All confidence intervals (CIs) were calculated at the 95% level. All analyses were performed using Stata statistical software (Stata SE, version 12.1, Stata Corp. LP, College Station, TX).
Results
Figure 1 shows the participant flow chart. The 22 participants were randomly allocated equally to each group. Two participants allocated to group A dropped out for personal reasons before the intervention began. Therefore, in term 1, 9 and 11 participants received SOS and WWS, respectively. All participants completed term 1, but 1 participant in each group declined to measure the sleep monitor and 1 participant in group B did not return the 6-week follow-up questionnaires. Four participants withdrew from group B during the study before term 2, and 1 participant from group B dropped out during term 2. Therefore, 20 participants (group A: n = 9; group B: n = 11) in term 1 and 15 participants (group A: n = 9; group B: n = 6) in term 2 were included in the analysis.
Baseline characteristics of the participants are shown in Table 1. The proportion of female participants was 75.0% (n = 15) and the mean age of all participants (± standard deviation) was 42.0 ± 7.2 years. No substantial baseline differences existed between the two groups, which were well matched for possible known confounders (e.g., age, sex, smoking status, alcohol consumption, and exercise).
Table 1.
Participant Characteristics
| Characteristic | Group A (n = 9) | Group B (n = 11) | Total |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 7 (77.8) | 8 (72.7) | 15 (75.0) |
| Male | 2 (22.2) | 3 (27.3) | 5 (25.0) |
| Age (y) | 40.6 ± 7.4 | 43.1 ± 7.2 | 42.0 ± 7.2 |
| Smoking behavior, n (%) | |||
| Never smoked | 6 (66.7) | 9 (81.8) | 15 (75.0) |
| Current smoker | 2 (22.2) | 1 (9.1) | 3 (15.0) |
| Ex-smoker | 1 (11.1) | 1 (9.1) | 2 (10.0) |
| Alcohol consumption, n (%) | |||
| No/rarely | 5 (55.6) | 7 (63.6) | 12 (60.0) |
| ≥1 time per week | 4 (44.4) | 4 (36.4) | 8 (40.0) |
| Exercise, n (%) | |||
| No | 3 (33.3) | 4 (36.4) | 7 (35.0) |
| ≥1 time per week | 6 (66.7) | 7 (63.6) | 13 (65.0) |
| Baseline PSQI score | 7.1 ± 2.3 | 5.8 ± 1.9 | 6.4 ± 2.1 |
| Baseline ESS score | 12.3 ± 3.4 | 10.0 ± 2.9 | 11.1 ± 3.3 |
| Baseline WHO-QOL26 score | 3.1 ± 0.3 | 3.3 ± 0.4 | 3.2 ± 0.4 |
Values expressed with a plus/minus sign are the mean ± standard deviation.
PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale; WHO-QOL26, World Health Organization Quality of Life 26.
Table 2 shows the results from GEE analysis on the effect of SOS compared with WWS on primary outcomes. SOS reduced the PSQI score by 1.83 (95% CI, −3.37 to −0.30) and 1.73 (95% CI, −3.84 to 0.38) compared with WWS (i.e., improved sleep quality) from baseline to 2-week follow-up and to 6-week follow-up, respectively. Although marginally significant, SOS also improved QOL by 0.22 points at 2-week follow-up and 0.19 points at 6-week follow-up compared with WWS. However, no beneficial effect of SOS was observed for ESS.
Table 2.
Generalized Estimating Equation Analysis of Effects of Sesame Oil Shirodhara Versus Warm Water Shirodhara on Sleep Quality and Quality of Life Between Baseline and Two Follow-Up Periods
| Effect of SOS (95% CI) | ||
|---|---|---|
| Outcome | Model 1 (between baseline and 2-wk follow-up) | Model 2 (between baseline and 6-wk follow-up) |
| PSQI | −1.83 (−3.37 to −0.30)a | −1.73 (−3.84 to 0.38) |
| ESS | −0.75 (−3.38 to 1.88) | 1.31 (−0.75 to 3.36) |
| QOL | 0.22 (−0.02 to 0.46) | 0.19 (−0.07 to 0.45) |
p < 0.05.
SOS, sesame oil Shirodhara; CI, confidence interval.
The results on sleep monitor measures are shown in Table 3. SOS had no beneficial effects on changes in average values between preintervention and 2-week intervention and postintervention.
Table 3.
Generalized Estimating Equation Analysis of Effects of SOS Versus Warm Water Shirodhara as Measured by the Sleep Monitor on Changes in Average Values Before Intervention, at 2 Weeks of Intervention and After Intervention
| Effect of SOS (95% CI) | ||
|---|---|---|
| Outcome | Model 1 (between preintervention and 2-wk intervention) | Model 2 (between preintervention and postintervention) |
| Total time in bed (min) | 8.4 (−27.2 to 44.0) | −22.4 (−61.4 to 16.6) |
| Total sleep time (min) | 8.8 (−27.6 to 45.2) | −25.9 (−60.5 to 8.7) |
| Sleep onset latency (min) | 3.6 (−4.6 to 11.8) | 4.9 (−5.3 to 15.2) |
| Sleep efficiency (%) | 1.8 (−1.6 to 5.2) | 0.6 (−2.6 to 3.8) |
| Wake after sleep onset (min) | −4.4 (−13.1 to 4.3) | −1.9 (−11.0 to 7.3) |
Although no serious adverse events occurred during procedure periods, four participants reported five minor events. One participant reported slight pain in the right knee and cold-like symptoms in term 1 while taking WWS. Although this participant completed only four of seven sessions in term 1, this participant was included in the analysis. Another three participants reported cold-like symptoms during term 2, one of whom was taking WWS and the others taking SOS. One of those taking SOS discontinued participation after 1 session and was excluded from the analysis of term 2, as shown in Figure 1. The other two participants rescheduled and restarted the sessions after recovery.
Discussion
This pilot study evaluated the effect of SOS on sleep quality and QOL compared with WWS for those reporting sleep problems. SOS improved sleep quality and QOL, but not subjective daytime sleepiness or sleep monitor measures.
SOS improved subjective sleep quality measured by PSQI, in particular from baseline to 2-week follow-up. This result is consistent with the findings of previous studies that demonstrated the effect of Shirodhara on improvement of subjective sleep quality.17–25 However, those studies assessed sleep quality with different indices, most of which are neither standardized nor validated and are difficult to compare with this study. Furthermore, they showed the results merely by p-value, which did not provide any quantitative information. In contrast, this study used standardized indices for assessing sleep quality and showed quantitative point estimates with interval estimates considering baseline differences by using GEE analyses. In that respect, the current study provides the additional evidence. In addition, the current results indicate that the effect of SOS on sleep quality trended in a positive direction at 6 weeks after intervention. SOS may have long-term effects on sleep quality.
Because the minimal clinically important difference for PSQI is sometimes set at an improved score of 3,40 the difference of 1.83 (in the short term) or 1.73 (in the long term) in total (Table 2) may not be large enough to generate clinical significance. Although the result did not reach the statistical significance at the 5% level because of the small number of cases, 27% of the SOS group achieved the improved score of 3 for PSQI from baseline to 2-week follow-up compared with 5% of the WWS group (data not shown), which may show some potentials for clinical significance of the intervention.
For the ESS, no beneficial effect of SOS was observed. Most previous studies18–25 did not use this scale to assess daytime sleepiness (one study17 did use this scale). Instead, they used subjective experiences, such as yawning, drowsiness, fatigue, and lack of concentration, and they just compared subjective symptoms from before to after treatment. There was a trend of subjective improvement on those symptoms. One study17 mentioned the use of ESS in its Materials and Methods section, but the result was not described in the Results section. Therefore, it is difficult to compare the results of this study with those of previous studies. One possible reason that SOS did not show beneficial effects in the present study is that seven sessions may not have been enough to produce significant changes in daytime sleepiness. Most other studies set the number of interventions to more than seven sessions.
Although the effect was marginally significant, the result of WHO-QOL26 indicated that SOS improved QOL during both periods. Improvement in sleep quality might be related to better QOL. No previous studies measured QOL changes, and the result cannot be compared.
No beneficial effects of SOS were observed from the results of the sleep monitor. This may be because the validity of the instrument has not been verified. Moreover, some participants might have difficulty following instructions (e.g., forgetting to turn on the instrument before sleep). Assessing objective sleep quality using valid objective instruments in future studies is necessary.
The PSQI result would be supported by the findings of the previous studies that demonstrate Shirodhara is associated with decreased sympathetic nervous system tone and increased skin temperature of the hand and foot.41,42 When the sympathetic nervous system relaxes, peripheral blood circulation and skin temperature increase.43 This situation might induce shorter sleep latency and better sleep quality.42,44–46 However, the long-term duration of this mechanism is unknown.
This appears to be the first study to compare the effect of SOS on sleep quality and QOL with that of WWS in a randomized and single-blinded design. The crossover design adopted in this study eliminates between-patient variation and requires smaller sample sizes than those in parallel-group trials to obtain the same number of observations.38,47,48 Moreover, a robotic Shirodhara system was used, which eliminates between- and within-therapist variability and maintains stable procedures with fixed flow and temperature of the liquid in each session.
This study had some limitations. Because this was a pilot and exploratory study, the number of participants was relatively small. Moreover, 25% of the total participants (n = 5) did not complete the study.
Second, again because this was a pilot study, the number of sessions was relatively small and might have been too few to generate beneficial changes in results. Most of the Shirodhara studies conducted in India usually set 21 sessions per participant, and Shirodhara treatment may need to be performed for longer periods to expect therapeutic effects.
Practically, in India various medicated oils, decoctions, or other liquids are used for Shirodhara depending on the purpose of treatment.16 On the other hand, plain sesame oil instead of medicated oil is usually used in Japan because of availability and pharmaceutical regulations. Thus, this study used plain sesame oil. If medicated oil had been used, the results would have likely been more favorable.
Ideally, the duration of the procedure is chosen depending on the nature of the illness and the condition of the client; it ranges from 30 to 90 minutes.16 In this study, the length of time was fixed at 30 minutes for all participants. Again, the result would have been different if we had customized the time length of procedure for each participant. The rate of the dripping and amount of liquid in this study were as per the specification of Shirodhara Robot, and they were similar to ordinal Shirodhara setting.
Third, in this study, the control intervention was set as WWS, so both the Shirodhara treatments share common conditions except the liquid properties. This weakens any of the differences in outcomes between these two methods.
Fourth, participant blinding may not have been perfectly maintained. According to the poststudy questionnaire, some participants correctly guessed which liquid was used for each treatment. If they noticed that SOS was the targeted treatment of this study and WWS was the control, it might have influenced their questionnaire scores that strengthened the results.
Fifth, some participants took sessions during hot and cold seasons (i.e., July–August and December, respectively). These climatic alterations probably affected sleep, which could be reflected in their questionnaire scores.
Finally, the inclusion criteria were developed according to subjective complaints of sleep problems and were not assessed or diagnosed by standard measures. In four participants, the PSQI scores were less than 5 at baseline in term 1, which suggested that these participants did not have sleep problems. If the inclusion criteria were restricted to persons who actually had sleep problems at the time of recruitment, then the effects would have been more apparent.
In conclusion, this pilot study demonstrated that SOS may be a safe potential treatment to improve sleep quality and QOL for those who have sleep problems.
Acknowledgments
The authors thank the participants for their cooperation and Sethukumar Kamath, Kazuo Uebaba, Morimichi Ogata, and Michiyo Yamakawa for their valuable advice and support during this study.
This study was supported by the Foundation for Total Health Promotion (FY2012).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Your Guide to Healthy Sleep. NIH Publication No. 11-5271. Bethesda, MD: National Institutes of Health, 2005, revised in 2011 [Google Scholar]
- 2.Chong Y, Fryer CD, Gu Q. Prescription sleep aid use among adults: United States, 2005–2010. NCHS Data Brief 2013;127:1–8 [PubMed] [Google Scholar]
- 3.Mishima K. Clinical practice guidelines for proper use and withdrawal of sleep medication. Jpn J Sleep Med 2013;7:514–520 [Google Scholar]
- 4.U.S. Department of Health and Human Services, National Institutes of Health, National Center for Complementary and Alternative Medicine. Sleep Disorders and Complementary Health Approaches: What You Need to Know. NIH Publication No. D437. Bethesda, MD: National Institutes of Health, 2009, updated in 2014 [Google Scholar]
- 5.Adams J, Sibbritt D, Broom A, et al. A comparison of complementary and alternative medicine users and use across geographical areas: a national survey of 1,427 women. BMC Complement Altern Med 2011;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manya K, Champion B, Dunning T. The use of complementary and alternative medicine among people living with diabetes in Sydney. BMC Complement Altern Med 2012;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagawa H, Terao J, Takeda E, et al. Consultation clinics for complementary and alternative medicine at Japanese university hospitals: an analysis at Tokushima University Hospital. Exp Ther Med 2010;1:481–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 2008;12:1–23 [PubMed] [Google Scholar]
- 9.Bishop FL, Lewith GT. Who uses CAM? A narrative review of demographic characteristics and health factors associated with CAM use. Evid Based Complement Alternat Med 2010;7:11–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis MA, West AN, Weeks WB, et al. Health behaviors and utilization among users of complementary and alternative medicine for treatment versus health promotion. Health Serv Res 2011;46:1402–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Benchmarks for training in traditional/complementary and alternative medicine: benchmarks for training in Ayurveda. Geneva, Switzerland: World Health Organization, 2010 [Google Scholar]
- 12.Chaudhary A, Singh N. Contribution of world health organization in the global acceptance of Ayurveda. J Ayurveda Integr Med 2011;2:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agnivesha. Charaka samhita. In: Sharma RK, Dash B, eds. Vol 1. Varanasi: Chowkhamba Sanskrit Series Office, 2001. (Ca.Su.11/35). [Google Scholar]
- 14.Agnivesha. Charaka samhita. In: Sharma RK, Dash B, eds. Vol 1. Varanasi: Chowkhamba Sanskrit Series Office, 2001. (Ca.Su.21/25-59). [Google Scholar]
- 15.Vagbhata. Ashtanga hrdayam. In: Shrikantha Murty KR, ed. Vol 1. Varanasi: Krishnadas Academy, 1999. (A.H, Su.7/53-68). [Google Scholar]
- 16.Acharya GS. Panchakarma: Illustrated. Delhi: Chaukhamba Sanskrit Pratishthan, 2006 [Google Scholar]
- 17.Bhaduri T, Chowdhury K, Biswas S, et al. Clinical evaluation of sirodhara and yoga therapy in management of chronic insomnia. IOSR J Pharm Biol Sci 2013;4:78–80 [Google Scholar]
- 18.Bharti , Makhija R, Kumar A, et al. Shirodhara - pilot observations in anidra (insomnia). J Ayurveda 2008;2:60–63 [Google Scholar]
- 19.Gotmare A, Tawalare K, Nanote K, Dehankara M. Godugdha shirodhara: a non-pharmacological treatment of nidranash (insomnia). Int J Res Ayurveda Pharmacy 2013;4:541–44 [Google Scholar]
- 20.Pokharel S, Sharma A. Evaluation of Insomrid Tablet and shirodhara in the management of anidra (insomnia). Ayu 2010;31:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahu A, Sharma A. A clinical study on anidra and its management with shirodhara and mansyadi kwatha. J Ayurveda 2009;3:4–15 [Google Scholar]
- 22.Sarkar TK, Gupta N. A comparative study on Guduchi and rose water shirodhara in stress induced insomnia w.s.r. to the principle - brinhanani yatca tat sarvam prasastham vataroginam (Ch.Chi.28/106). J Ayurveda 2012;6:82–90 [Google Scholar]
- 23.Seetha M, Sharma O, Sharma R. Dashmoola siddha ksheer shirodhara in the management of anidra w.s.r. to insomnia. J Ayurveda 2007;1(3):21–25 [Google Scholar]
- 24.Singh AK, Chandola HM, Ravishankar B. Clinical study on psychic traits in stress induced chronic insomnia and its management with Mamsyadi ghrita & Dashamula kwatha Shirodhara. Ayu 2006;29:9–18 [Google Scholar]
- 25.Vanish B, Chandola HM. Clinical study on psychic traits in stress included insomnia (anidra) and its management with tagaradi kwatha & mahishi dugdha shirodhara. Ayu 2008;29:133–139 [Google Scholar]
- 26.Sato T. Human Nutrition Lecture Series: introduction to randomized clinical trials. Part 4: methods of randomization. Jpn J Nutr Diet 2007;65:255–260 [Google Scholar]
- 27.Matsubara N, Nawata K, Nakai N. Introduction to Statistics. Tokyo: University of Tokyo Press, 1991. [in Japanese, Toukeigaku Nyumon]. [Google Scholar]
- 28.Agnivesha. Charaka samhita. In: Sharma RK, Dash B, eds. Vol 1 Varanasi: Chowkhamba Sanskrit Series Office, 2001. (Ca.Su.21/57). [Google Scholar]
- 29.Agnivesha. Charaka samhita. In: Sharma RK, Dash B, eds. Vol 1 Varanasi: Chowkhamba Sanskrit Series Office, 2001. (Ca.Su.20/11). [Google Scholar]
- 30.Agnivesha. Charaka samhita. In: Sharma RK, Dash B, eds. Vol 1 Varanasi: Chowkhamba Sanskrit Series Office, 2001. (Ca.Su.27/286-287). [Google Scholar]
- 31.Agnivesha. Charaka samhita. In: Sharma RK, Dash B, eds. Vol 5 Varanasi: Chowkhamba Sanskrit Series Office, 2001. (Ca.Chi.28/181). [Google Scholar]
- 32.Vagbhata. Ashtanga hrdayam. In: Shrikantha Murty KR, ed. Vol 1 Varanasi: Krishnadas Academy, 1999. (A.H.Su.1/25). [Google Scholar]
- 33.Agnivesha. Charaka samhita. In: Sharma RK, Dash B, eds. Vol 1 Varanasi: Chowkhamba Sanskrit Series Office, 2001. (Ca.Su.5/83). [Google Scholar]
- 34.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep 2006;29:1155–1173 [DOI] [PubMed] [Google Scholar]
- 35.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 36.Takegami M, Suzukamo Y, Wakita T, et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med 2009;10:556–565 [DOI] [PubMed] [Google Scholar]
- 37.Tazaki M, Nakane Y. WHO QOL26 Handbook. Revised ed. Tokyo: Kaneko Shobo, 2007. [Google Scholar]
- 38.Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2012;109(15):276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz MH. Multivariable Analysis: A Practical Guide for Clinicians and Public Health Researchers. 3rd ed. Cambridge: Cambridge University Press, 2011 [Google Scholar]
- 40.Hughes CM, McCullough CA, Bradbury I, et al. Acupuncture and reflexology for insomnia: a feasibility study. Acupunct Med 2009;27:163–168 [DOI] [PubMed] [Google Scholar]
- 41.Uebaba K, Xu FH, Tagawa M, et al. Using a healing robot for the scientific study of shirodhara: altered states of consciousness and decreased anxiety through Indian dripping oil treatments. IEEE Eng Med Biol Mag 2005;24:69–78 [DOI] [PubMed] [Google Scholar]
- 42.Xu F, Uebaba K, Ogawa H, et al. Pharmaco-physio-psychologic effect of Ayurvedic oil-dripping treatment using an essential oil from Lavendula angustifolia. J Altern Complement Med 2008;14:947–956 [DOI] [PubMed] [Google Scholar]
- 43.Tortora GJ, Grabowski SR, Roesch B, et al. Principles of Anatomy and Physiology. 9th ed. New York: Wiley, 2000. [Google Scholar]
- 44.Krauchi K, Cajochen C, Werth E, et al. Warm feet promote the rapid onset of sleep. Nature 1999;401:36–37 [DOI] [PubMed] [Google Scholar]
- 45.Krauchi K, Cajochen C, Werth E, et al. Functional link between distal vasodilation and sleep-onset latency? Am J Physiol Regul Integr Comp Physiol 2000;278:R741–748 [DOI] [PubMed] [Google Scholar]
- 46.Liao WC, Wang L, Kuo CP, et al. Effect of a warm footbath before bedtime on body temperature and sleep in older adults with good and poor sleep: an experimental crossover trial. Int J Nurs Stud 2013;50:1607–1616 [DOI] [PubMed] [Google Scholar]
- 47.Mehrotra DV. A recommended analysis for 2 × 2 crossover trials with baseline measurements. Pharm Stat 2014;13:376–387 [DOI] [PubMed] [Google Scholar]
- 48.Senn SS. Cross-over Trials in Clinical Research. 2nd ed. New York: Wiley, 2003 [Google Scholar]