Abstract
Background
Infectious diseases account for a significant global burden of disease and substantial investment in research and development. This paper presents a systematic assessment of research investments awarded to UK institutions and global health metrics assessing disease burden.
Methods
We systematically sourced research funding data awarded from public and philanthropic organisations between 1997 and 2013. We screened awards for relevance to infection and categorised data by type of science, disease area and specific pathogen. Investments were compared with mortality, disability-adjusted life years (DALYs) and years lived with disability (YLD) across three time points.
Findings
Between 1997–2013, there were 7398 awards with a total investment of £3.7 billion. An increase in research funding across 2011–2013 was observed for most disease areas, with notable exceptions being sexually transmitted infections and sepsis research where funding decreased. Most funding remains for pre-clinical research (£2.2 billion, 59.4%). Relative to global mortality, DALYs and YLDs, acute hepatitis C, leishmaniasis and African trypanosomiasis received comparatively high levels of funding. Pneumonia, shigellosis, pertussis, cholera and syphilis were poorly funded across all health metrics. Tuberculosis (TB) consistently attracts relatively less funding than HIV and malaria.
Interpretation
Most infections have received increases in research investment, alongside decreases in global burden of disease in 2013. The UK demonstrates research strengths in some neglected tropical diseases such as African trypanosomiasis and leishmaniasis, but syphilis, cholera, shigellosis and pneumonia remain poorly funded relative to their global burden. Acute hepatitis C appears well funded but the figures do not adequately take into account projected future chronic burdens for this condition. These findings can help to inform global policymakers on resource allocation for research investment.
Keywords: Infectious disease, Research investment, Disease burden, Global health, Funding
Highlights
-
•
We identified 7398 awards for infectious disease research awarded to UK institutions across 1997–2013, with total funding of £3.7 billion.
-
•
We compared research investment with global burden of disease. Acute hepatitis C, trypanosomiasis and leishmaniasis appear relatively well funded shigellosis, pertussis, cholera and syphilis consistently rank lowest for research funding relative to their disease burden.
-
•
Tuberculosis typically ranked lower than HIV or malaria, and pneumonia receives low levels of investment when considering global burdens.
-
•
By demonstrating potential inequities in allocation of investment, results can help to inform policymakers, funding agencies and researchers.
1. Introduction
Despite major advances in vaccines, diagnostics, therapeutics and infection control measures, the “unfinished agenda” of infectious diseases remains a global threat. The Global Burden of Disease (GBD) Study 2013 reports that lower respiratory tract infections, diarrhoeal disease, HIV, and malaria were four of the top ten causes of disease burden globally, as measured by disability-adjusted life years (DALYs) (Murray et al., 2015). These four disease areas, plus tuberculosis (TB), comprised five of the top eleven causes of death worldwide in 2013 (GBD 2013 Mortality and Causes of Death Collaborators, 2014). Infectious diseases generate a large economic burden (Fonkwo, 2008) with antimicrobial resistance (AMR), the subject of a World Health Organization (WHO) action plan (World Health Organization, 2014) and a priority for the United Kingdom (UK) (Anon.,), European Commission (2011), and the US Centers for Disease Control and Prevention (CDC) (Anon.,), projected to cost an estimated $100 trillion by the year 2050 if unadressed (Anon., 2014).
Research is essential to improve the evidence base for policy and clinical practice. The UK research funding landscape has several national and international awarding bodies that invest in pre-clinical (laboratory) science, observational studies, clinical trials, and translational research, with significant commitments to infectious disease research. Earlier research by the Research Investments in Global Health study (ResIn, www.researchinvestments.org) has systematically analysed public and philanthropic awards totalling £2.6 billion to UK institutions for infectious disease from 1997 to 2010, for funding awarded by infectious disease, microbiology and type of science (Head et al., 2013), including for respiratory infectious disease research (Head et al., 2014a) and pneumonia (Head et al., 2015a), sepsis (Fitchett et al., 2014a), AMR (Head et al., 2014b), and sexually-transmitted infections (Head et al., 2015b). Tracking research and development (R&D) investments provides information and evidence to inform policy on funding decisions. We present an update to the systematic analysis of infectious disease research awarded by public and philanthropic funders to UK institutions from 2011 to 2013, and assess funding from 1997–2013 against global measures of mortality, DALYs and years lived with disability (YLD) across three time points.
2. Methods
Our methods for the 1997–2010 analysis are described in detail elsewhere (Head et al., 2014a, Head et al., 2014b, Head et al., 2014c, Head et al., 2014d, Head et al., 2014e, Head et al., 2014f, Head et al., 2015a, Head et al., 2015b, Head et al., 2015c, Fitchett et al., 2013, Fitchett et al., 2014b) and adapted in subsequent peer-reviewed publications (www.researchinvestments.org/publications).
The methods for the updated analysis are broadly similar, in that we systematically examined award data from 585 public and philanthropic funding bodies by either searching the databases and information on their publically available websites, requesting data directly or searching other funding databases. From the information gathered, we manually screened each study individually for relevance to infectious disease research. We excluded studies not immediately relevant to infectious disease, symposium grants, studies related to purely veterinary or plant infectious disease (but included animal health research incorporating a clear zoonotic component), research where a viral vector was used in relation to non-communicable disease, and awards that were led by a non-UK institution, but had UK collaborators. Of the studies included in the final dataset, all had a title or brief descriptor and 65.1% had either an abstract attached or further information sourced from the internet (e.g. institutional webpages, clinical trials databases). Private sector data were not available to analyse in the same detail and therefore excluded.
Where awards were received from an international funder, currencies were converted to UK pounds using the mean exchange rate in the year of the award. All grant funding amounts were adjusted for inflation and reported in 2013 UK pounds. Awards from 1997–2010 had been previously adjusted for inflation to 2010 levels, but have here also been adjusted and reported in 2013 UK pounds to allow for updated comparison between years. Ongoing data management and revision to the overall dataset has resulted in 5 less studies (0.001% change) than previously reported. Unfunded studies were excluded.
Each study in the dataset was reviewed by MGH and assigned to as many disease categories as appropriate. Authors VN and NK provided support for this categorisation process. Studies were also allocated to one of five categories along the R&D pipeline: pre-clinical; phase I, II, or III; product development; public health; and cross-disciplinary research. The introduction of the cross-disciplinary category was a refinement of the previous methodology prompted by an increasing number of these types of awards (defined as an award containing significant components of a study that covers two areas along the R&D pipeline). We did not retrospectively apply this new category to the 1997–2010 dataset owing to resource constraints. The public health research category was previously entitled ‘implementation and operational research’. The change in name better reflects the content of this category, and has not involved any change in which studies are categorised here.
Provisional datasets were circulated to all authors for review and comment. Further checks involved authors JRF, VN and NK cross-checking 20% sections of randomly-selected rows of data with any disagreements settled by consensus, and also providing an opinion on any studies where initial categorisation proved difficult. Authors MGH and JRF further considered difficulties in categorisation between phase I–III studies, and the product development category that includes phase IV research. Final datasets were then again circulated for further review by all authors. Two fixed marginal κ scores were calculated and showed 0.95 for level of agreement on the ‘type of science’ categorisation, and 0.91 for application of the disease categories, highlighting high levels of independent agreement in the categorisation process.
As per the earlier analysis (Head et al., 2013), the category of antimicrobial resistance includes antibacterial, antiviral, and antifungal resistance. Reference to diagnostics includes screening programmes. Reference to sexually transmitted infections excludes HIV which is defined in its own category, and neglected tropical diseases were categorised based on the infections focused on by WHO (http://www.who.int/neglected_diseases/diseases/en/). Awards were defined as global health if they i) were considered to pursue a clear non-UK focus (e.g. ‘tuberculosis in Kenya’), or ii) focused on diseases not endemic in the UK (e.g. malaria).
Available on the ResIn website (http://researchinvestments.org/about-the-study/study-methodology/) is the list of included funders (and excluded funders with reason for exclusions), list of keywords used to search funder's website, and examples of (and comment on) the definitions and categorisations. Stata (V13) software was used for data analysis.
Global mortality and DALY data were available at time points 2004, 2010 and 2013. Time points for global YLDs were 2005, 2010 and 2013. All burden data were sourced from the findings of the Global Burden of Disease study, for 2013 (Murray et al., 2015, GBD 2013 Mortality and Causes of Death Collaborators, 2014, Anon., 2015) and for 2010 (Anon., 2015, Murray et al., 2012, Lozano et al., 2012). Burden data from 2004/05 were obtained directly from colleagues at the Institute for Health Metrics and Evaluation, Washington. As defined by the GBD study, YLDs per person from a sequela are equal to the prevalence of the sequela multiplied by the disability weight for the health state associated with that sequela. YLDs for a disease or injury are the sum of the YLDs for each sequela associated with the disease or injury (Vos et al., 2012). DALYS are the product of adding YLLs and YLDs for each age–sex–country group (Murray et al., 2015).
In order to allow direct comparison of relative investment with global health metrics across disease areas and between different time periods, metrics were developed to show ‘investment per mortality/DALY/YLD observed’, and these were created using the following equation — (cumulative research investment up to the year before the time point / number of deaths, DALYs or YLD at time point) / number of years of investment included.
For example, for assessment of HIV mortality at the 2004 time point, we took the sum of HIV research investment 1997–2003 (£238,900,938) and divided that by number of deaths reported in 2004 (2,040,000), and divided the result by 7 (the number of years of investment included) to get an ‘investment per mortality observed’ metric of £16.73.
The use of cumulative investment and the division by number of years included aimed to reduce the impact of the volatility of annual research funding and short periods between time points.
Ranking scores of the investment metrics were developed for each infection and across each time point. Infections were ranked in order of relative investment against burden from high to low and assigned a score (from 1 to 25). The mean ranking scores across time points and across mortality, DALY and YLD were used to illustrate relative levels of investment.
In 2004 mortality, 2005 YLD and all 2013 datasets, only aggregated data was available for diarrhoea and enteric infectious disease and lower respiratory tract infections. For Salmonella, Escherichia coli, Shigellosis, Vibrio cholerae, Campylobacter, influenza and pneumonia, proportional estimates were compiled using disaggregated data from the 2010 dataset.
3. Results
The analysis for 1997–2013 analysis included funding of £3.7 billion across 7398 awards (Table 1). Mean funding for all infectious diseases was £219.1 million per year (435 awards annually). Mean funding per award was £503,524 (SD £1,412,776) with median funding per award of £192,143 (IQR £63,189–418,015).
Table 1.
Total funding, number of studies and mean and median award size of research investment by infection 1997–2013. SD, standard deviation. IQR, inter-quartile range.
| Investment 1997–2013 |
Investment 2011–2013 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Disease | Number of awards | Total investment (£) | Percentage of total | Mean award, (SD) | Median award, (IQR) | Number of awards 2011–2013 | Percentage of total | Total funding (£) | Percentage of 2011–2013 total |
| Overall | 7398 | 3,725,073,507 | n/a | 503,524 (1,412,776) | 192,143 (63,189–418,015) | 1232 | n/a | 916,960,747 | n/a |
| Disease areas and products | |||||||||
| Antimicrobial resistance | 413 | 164,419,467 | 4.4% | 398,110 (901,417) | 155,794 (44,065–347,091) | 76 | 6.17% | 53,195,899 | 5.80% |
| Global health | 1712 | 1,348,277,988 | 36.2% | 787,545 (2,468,415) | 253,262 (77,735–589,359) | 306 | 24.84% | 343,532,187 | 37.46% |
| Gastroenterology | 903 | 355,186,553 | 9.5% | 393,340 (687,605) | 218,799 (82,364–394,549) | 114 | 9.25% | 90,313,344 | 9.85% |
| Healthcare-associated infections | 348 | 105,957,588 | 2.8% | 304,475 (750,448) | 71,490 (10,656–252,931) | 51 | 4.14% | 43,165,873 | 4.71% |
| Hepatology | 366 | 125,058,113 | 3.4% | 341,688 (794,679) | 128,340 (43,420–290,954) | 44 | 3.57% | 46,794,839 | 5.10% |
| HIV | 919 | 651,351,095 | 17.5% | 708,760 (2,350,940) | 181,628 (41,468–481,721) | 155 | 12.58% | 135,569,246 | 14.78% |
| Neglected tropical diseases | 490 | 323,791,367 | 8.7% | 660,798 (2,191,508) | 276,730 (107,065–527,303) | 83 | 6.74% | 75,444,667 | 8.23% |
| Neurology | 399 | 151,371,666 | 4.1% | 379,377 (939,438) | 169,212 (70,749–390,007) | 60 | 4.87% | 4,226,646 | 0.46% |
| Respiratory | 1230 | 556,045,105 | 14.9% | 452,069 (838,744) | 207,736 (67,471–445,418) | 219 | 17.78% | 145,182,110 | 15.83% |
| Sepsis | 86 | 24,762,825 | 0.7% | 287,939 (577,197) | 151,855 (54,291–272,451) | 7 | 0.57% | 2,210,944 | 0.24% |
| Sexually transmitted infections | 402 | 166,144,022 | 4.5% | 413,293 (1,035,635) | 112,263 (19,251–269,686) | 24 | 1.95% | 17,014,003 | 1.86% |
| Diagnostics | 484 | 202,271,238 | 5.4% | 417,915 (1,055,385) | 106,001 (19,758–296,732) | 77 | 6.25% | 93,692,388 | 10.22% |
| Therapeutics | 788 | 662,160,655 | 17.8% | 840,305 (2,683,243) | 231,142 (62,947–639,642) | 262 | 21.27% | 217,159,123 | 23.68% |
| Vaccinology | 490 | 374,959,878 | 10.1% | 765,224 (1,556,168) | 266,315 (104,809–730,657) | 122 | 9.90% | 119,905,881 | 13.08% |
| Specific infection or disease | |||||||||
| African Trypanosomiasis | 170 | 98,621,900 | 2.6% | 580,128 (1,070,225) | 288,393 (156,960–505,168) | 35 | 2.84% | 29,561,488 | 3.22% |
| Aspergillus | 32 | 9,381,561 | 0.3% | 293,173 (680,033) | 68,304 (23,920–231,642) | 6 | 0.49% | 4,254,846 | 3.14% |
| Campylobacter | 113 | 35,796,296 | 1.0% | 316,781 (497,139) | 240,419 (95,468–346,045) | 26 | 2.11% | 9,775,861 | 1.07% |
| Candida | 87 | 31,410,745 | 0.8% | 361,043 (461,554) | 282,390 (92,281–416,521) | 11 | 0.89% | 6,676,377 | 0.73% |
| Chagas disease | 18 | 5,284,555 | 0.1% | 293,586 (222,290) | 233,625 (175,747–372,486) | 0 | 0.00% | 0 | 0.00% |
| Chlamydia | 119 | 25,899,326 | 0.7% | 217,641 (593,623) | 60,833 (12,590–196,419) | 7 | 0.57% | 2,783,510 | 0.30% |
| Cholera | 7 | 1,154,507 | 0.0% | 164,929 (123,277) | 89,667 (51,193–287,951) | 0 | 0.00% | 0 | 0.00% |
| Clostridium | 97 | 56,061,419 | 1.5% | 577,952 (1,089,221) | 226,732 (49,926–475,684) | 19 | 1.54% | 17,009,293 | 1.85% |
| Cytomegalovirus | 79 | 35,695,572 | 1.0% | 451,842 (673,587) | 220,703 (118,302–531,000) | 11 | 0.89% | 5,287,629 | 0.58% |
| Dengue | 38 | 54,430,748 | 1.5% | 1,432,388 (5,674,662) | 309,695 (124,361–693,323) | 9 | 0.73% | 6,236,268 | 0.68% |
| Diphtheria | 2 | 149,094 | 0.0% | n/a | n/a | 0 | 0.00% | 0 | 0.00% |
| Ebola | 0.0% | 0 | 0.00% | 0 | 0.00% | ||||
| Escherichia coli | 130 | 38,984,636 | 1.0% | 299,881 (290,578) | 234,560 (119,663–380,995) | 23 | 1.87% | 10,782,819 | 1.18% |
| Epstein-Barr Virus | 155 | 51,901,868 | 1.4% | 334,850 (479,035) | 164,142 (52,255–389,593) | 9 | 0.73% | 4,333,912 | 0.47% |
| Gonorrhoea | 20 | 1,448,016 | 0.0% | 72,400 (99,604) | 14,485 (3963–146,098) | 2 | 0.16% | 440,305 | 0.05% |
| Helicobacter | 104 | 18,233,832 | 0.5% | 175,325 (280,233) | 95,172 (12,217–203,294) | 3 | 0.24% | 2,282,283 | 0.25% |
| Helminths | 158 | 80,232,248 | 2.2% | 507,799 (1,221,547) | 251,698 (93,055–459,074) | 8 | 0.65% | 6,852,922 | 0.75% |
| Hepatitis B | 82 | 26,834,637 | 0.7% | 327,251 (708,712) | 87,810 (24,062–238,425) | 13 | 1.06% | 10,996,796 | 1.20% |
| Hepatitis C | 272 | 110,257,438 | 3.0% | 405,358 (926,977) | 141,135 (50,250–301,331) | 36 | 2.92% | 43,153,957 | 4.71% |
| HIV | 919 | 651,351,095 | 17.5% | 708,760 (2,350,940) | 181,628 (41,468–481,721) | 155 | 12.58% | 135,569,246 | 14.78% |
| Human papillomavirus | 164 | 62,219,508 | 1.7% | 379,387 (872,744) | 119,037 (39,642–260,832) | 17 | 1.38% | 5,994,339 | 0.65% |
| Herpes simplex virus | 55 | 26,750,879 | 0.7% | 486,379 (733,513) | 225,701 (60,655–458,073) | 7 | 0.57% | 3,163,889 | 0.35% |
| Influenza | 194 | 126,643,152 | 3.4% | 652,799 (1,124,119) | 308,455 (164,025–736,503) | 53 | 4.30% | 39,139,703 | 4.27% |
| Leishmaniasis | 89 | 52,894,292 | 1.4% | 594,317 (868,448) | 309,522 (121,761–582,645) | 11 | 0.89% | 7,257,334 | 0.79% |
| Leprosy | 2 | 633,855 | 0.0% | n/a | n/a | 0 | 0.00% | 0 | 0.00% |
| Listeria | 13 | 6,566,639 | 0.2% | 505,126 (452,060) | 263,445 (139,604–730,472) | 0 | 0.00% | 0 | 0.00% |
| Lymphatic filariasis | 10 | 57,693,197 | 1.5% | 5,769,320 (11,200,387) | 806,101 (208,913–3,247,287) | 3 | 0.24% | 5,986,094 | 0.65% |
| Malaria | 621 | 518,734,860 | 13.9% | 835,321 (2,413,049) | 256,064 (75,813–641,805) | 117 | 9.50% | 137,212,998 | 14.96% |
| Measles | 13 | 6,556,866 | 0.2% | 504,374 (463,858) | 331,040 (77,576–777,779) | 1 | 0.08% | 1,113,633 | 0.12% |
| Meningitis | 264 | 90,609,407 | 2.4% | 343,217 (1,060,577) | 155,610 (73,893–266,103) | 42 | 3.41% | 32,968,225 | 3.60% |
| Norovirus | 23 | 13,181,682 | 0.4% | 573,116 (937,560) | 218,767 (61,583–533,797) | 11 | 0.89% | 7,580,760 | 0.83% |
| Onchocerciasis | 8 | 7,755,554 | 0.2% | 969,444 (1,315,292) | 217,587 (30,798–2,005,428) | 3 | 0.24% | 5,986,094 | 0.65% |
| Pertussis | 13 | 4,108,262 | 0.1% | 316,020 (250,671) | 341,788 (46,284–539,766) | 4 | 0.32% | 1,527,779 | 0.17% |
| Pneumonia | 137 | 59,051,642 | 1.6% | 431,033 (820,052) | 206,399 (61,652–401,771) | 35 | 2.84% | 28,849,125 | 3.15% |
| Polio | 13 | 6,786,968 | 0.2% | 522,074 (596,514) | 419,773 (67,163–580,209) | 9 | 0.73% | 5,978,072 | 0.65% |
| Pseudomonas | 59 | 11,577,984 | 0.3% | 196,237 (239,174) | 153,990 (29,367–263,384) | 16 | 1.30% | 4,553,921 | 0.50% |
| Rotavirus | 22 | 7,752,326 | 0.2% | 352,378 (423,329) | 179,221 (148,949–352,758) | 3 | 0.24% | 1,097,942 | 0.12% |
| Respiratory syncytial virus | 56 | 20,984,547 | 0.6% | 374,724 (482,391) | 197,172 (51,855–527,461) | 11 | 0.89% | 2,756,796 | 0.30% |
| Salmonella | 168 | 81,422,224 | 2.2% | 484,656 (595,724) | 291,574 (172,554–518,393) | 23 | 1.87% | 21,291,316 | 2.32% |
| Schistosomiasis | 50 | 45,767,421 | 1.2% | 915,348 (3,963,046) | 215,294 (65,146–469,526) | 3 | 0.24% | 2,069,845 | 0.23% |
| Shigellosis | 12 | 7,390,226 | 0.2% | 615,852 (534,875) | 527,553 (148,386–923,817) | 3 | 0.24% | 3,815,542 | 0.42% |
| Syphilis | 5 | 1,112,066 | 0.0% | 222,413 (152,855) | 221,474 (117,055–253,533) | 0 | n/a | 0 | n/a |
| Tetanus | 5 | 5,727,398 | 0.1% | 1,145,480 (2,083,733) | 244,849 (201,515–401,104) | 0 | n/a | 0 | n/a |
| Trachoma | 6 | 7,883,360 | 0.2% | 1,313,893 (1,171,657) | 977,242 (342,867–1,928,703) | 4 | 0.32% | 3,877,578 | 0.42% |
| Tuberculosis | 413 | 239,232,401 | 6.4% | 579,255 (1,097,612) | 226,748 (96,244–542,191) | 83 | 6.74% | 71,110,945 | 7.76% |
| Varicella zoster virus | 21 | 4,721,860 | 0.0% | 224,850 (281,832) | 152,770 (50,883–243,513) | 1 | 0.08% | 177,901 | 0.02% |
Across 2011–2013, there were additional 1232 awards with total new funding of £917.0 million. Mean annual funding was greater in 2011–2013, amounting to £305.7 million across 411 awards each year. Mean funding per award was £744,286 (SD £1,360,777), with median funding per award at £315,918 (IQR £156,283–779,794).
Table 1 shows comparisons of funding for specific infection and disease area in 1997–2013 and specifically the addition of 2011–2013 data. Total and proportional funding for antimicrobial resistance (5.8% of all infection research), healthcare-associated infections (4.71%), and viral hepatology (5.1%) increased in 2011–13 compared with 1997–2010. Relative funding for sepsis (0.24% of all infection research), sexually-transmitted (1.86%) and neurological infections (0.46%) declined in 2011–2013 compared with 1997–2010. An observed 36.2% of the total investment was directly related to global health. There was no public or philanthropic research investment for Ebola or other haemorrhagic fevers in 2011–2013. There were large increases in funding for new products, specifically diagnostics, therapeutics and vaccines.
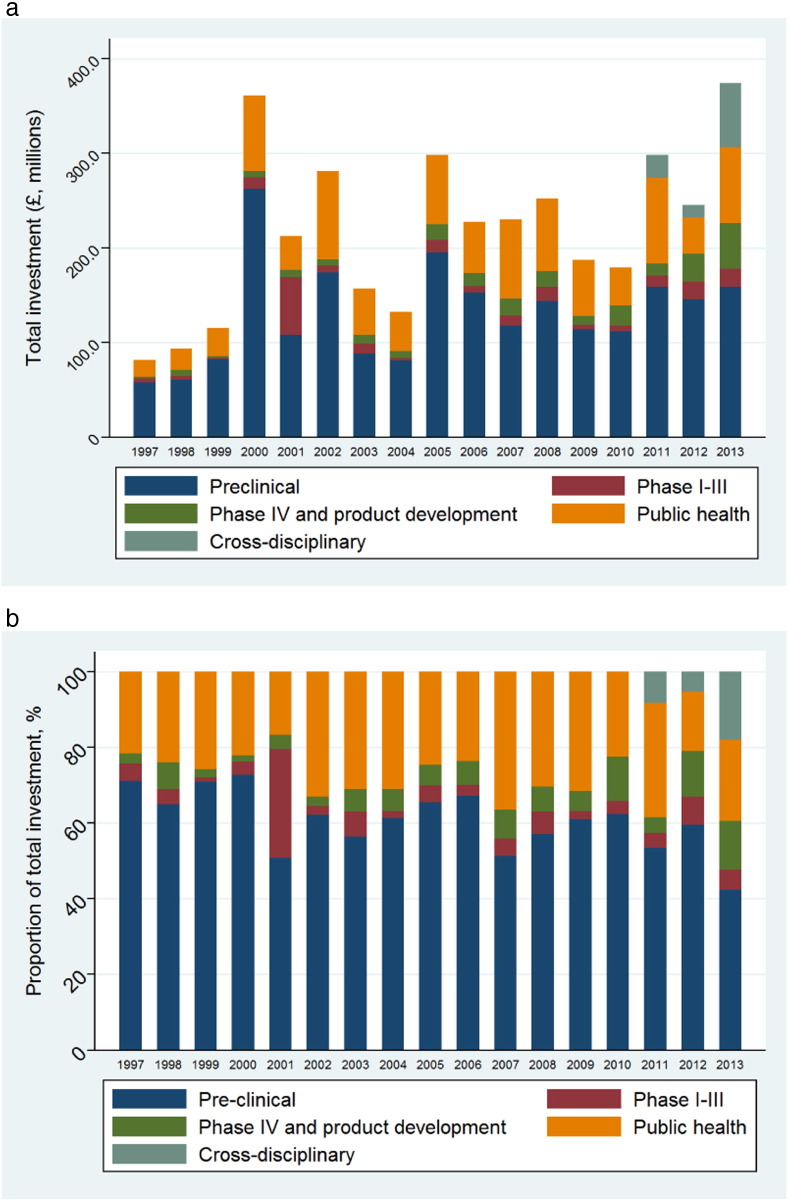
There is volatility in annual research funding though an overall increase between 1997 and 2013 (Fig. 1a). The Wellcome Trust is the largest investor in research, having funded 2285 studies (30.9%) totalling £935.0 million (25.1%), followed by the Medical Research Council (£924.9 million, 24.8%). The European Commission funded 12.9% of total investment across 1997–2013, and this increased to 21.6% specifically across 2011–2013, ahead of the Wellcome Trust (20.9%) and slightly behind the MRC (21.9%) (Supplementary 1). Alongside these three funders, the National Institute for Health Research (NIHR, main funding stream of the UK Department of Health), Biotechnology and Biological Sciences Research Council (BBSRC) and the Bill & Melinda Gates Foundation combined contributed 84.1% of the overall investment. The remainder was provided by institutions such as the US National Institutes for Health (NIH), UK government departments, UK research councils, other research charities and professional bodies and societies.
Fig. 1.
a) Aggregate and b) proportionate funding from 1997–2013 by type of science along the research and development pipeline (1997–10 data published previously (Head et al., 2013)).
The proportion of funding awarded by the type of science (supplementary 2, Fig. 1b) show a gradual increase in investment for public health research with slight decline for pre-clinical science. Pre-clinical science overall receives the greatest investment of £2.2 billion (59.4%), followed by public health research (£954.6 million; 25.9%), with relatively little public or philanthropic investment for phase I–III trials (£207.3 million; 5.6%) or product development studies (£234.7 million, 6.3%). Cross-disciplinary research accounted for a small number of awards (0.7% overall, 4.0% in 2011–2013), but a greater proportion of the funding (£105.4 million; 2.8% overall, 11.5% in 2011–2013) and these studies were often consortia-led or programme grants.
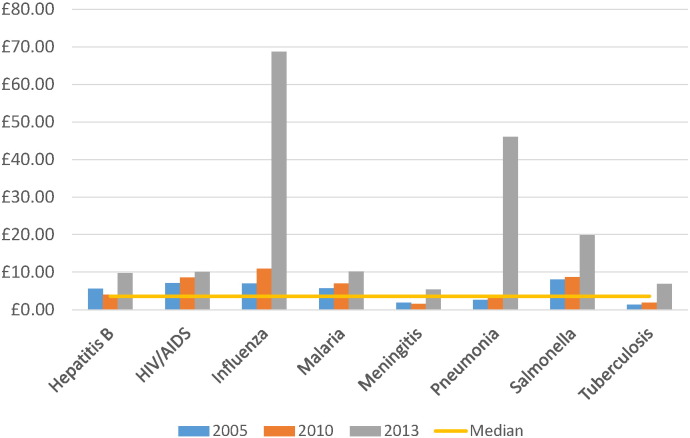
Across three time points (2005, 2010, 2013), YLDs for infectious diseases broadly declined (Table 2, Fig. 2). The median ‘investment by YLD observed’ metric was highest in 2013 (£6.01 compared with £3.56 overall). Acute hepatitis C and trypanosomiasis consistently received the greatest relative investment respectively (Table 2). Investment by YLD observed for influenza greatly increased in 2013 (£68.69, compared with £10.93 in 2010, after the 2009 pandemic). Infections such as shigellosis, cholera, syphilis and gonorrhoea were typically amongst those receiving the lowest relative investment (less than £1.00 per YLD observed).
Table 2.
Years lived with disability (YLD) and ‘investment by YLD observed’ at three time points.
| Disease | YLD |
Investment per YLD observed |
||||
|---|---|---|---|---|---|---|
| 2005 | 2010 | 2013 | 2005 | 2010 | 2013 | |
| Campylobacter | 746,000 | 746,000 | 637,440 | £2.10 | £2.51 | £3.51 |
| Chagas disease | 308,932 | 303,000 | 97,500 | £1.61 | £1.24 | £3.39 |
| Chlamydia | 632,136 | 669,000 | 646,500 | £3.64 | £2.53 | £2.50 |
| Cholera | 80,000 | 80,000 | 95,958 | £0.21 | £1.11 | £0.75 |
| Dengue | 9938 | 12,000 | 565,900 | £59.79 | £308.03 | £6.01 |
| Diphtheria | 130 | 110 | 100 | £143.36 | £104.26 | £93.18 |
| E. coli | 1,910,000 | 1,910,000 | 1,624,445 | £1.08 | £1.08 | £1.50 |
| Gonorrhoea | 233,757 | 249,000 | 225,400 | £0.43 | £0.26 | £0.40 |
| Hepatitis A | 182,394 | 185,000 | 198,000 | £0.64 | £0.39 | £0.29 |
| Hepatitis B | 233,179 | 248,000 | 172,600 | £5.58 | £3.90 | £9.72 |
| Hepatitis C | 36,450 | 39,000 | 16,900 | £136.61 | £119.70 | £407.76 |
| Hepatitis E | 66,215 | 69,000 | 56,600 | £0.00 | £0.00 | £0.00 |
| HIV/AIDS | 4,707,104 | 4,342,000 | 4,063,700 | £7.07 | £8.57 | £10.02 |
| Influenza | 552,922 | 583,000 | 115,225 | £6.98 | £10.93 | £68.69 |
| Leishmaniasis | 128,339 | 124,000 | 49,700 | £16.71 | £25.52 | £66.52 |
| Malaria | 3,887,171 | 4,070,000 | 3,170,500 | £5.63 | £6.95 | £10.23 |
| Measles | 59,444 | 31,000 | 17,300 | £7.06 | £13.51 | £43.35 |
| Meningitis | 2,528,495 | 2,628,000 | 1,679,100 | £1.84 | £1.55 | £5.35 |
| Pertussis | 149,225 | 122,000 | 125,500 | £1.42 | £1.63 | £3.33 |
| Pneumonia | 572,838 | 604,000 | 119,373 | £2.56 | £3.68 | £46.03 |
| Salmonella | 513,000 | 513,000 | 438,668 | £7.96 | £8.66 | £19.83 |
| Schistosomiasis | 2,639,036 | 2,986,000 | 2,861,700 | £1.77 | £1.12 | £1.95 |
| Shigellosis | 744,000 | 744,000 | 596,315 | £0.34 | £0.37 | £1.15 |
| Syphilis | 89,379 | 91,000 | 584,000 | £1.11 | £0.94 | £0.24 |
| Tetanus | 30,245 | 21,000 | 13,200 | £3.56 | £20.98 | £54.24 |
| Trypanosomiasis | 10,742 | 80,000 | 54,000 | £386.71 | £63.41 | £190.48 |
| Tuberculosis | 6,797,443 | 6,774,000 | 3,669,700 | £1.37 | £1.83 | £6.81 |
| Varicella | 185,361 | 202,000 | 197,200 | £1.37 | £1.73 | £1.50 |
| Overall | 28,033,875 | 28,425,110 | 22,092,524 | £28.37 | £54.02 | £87.58 |
Fig. 2.
Investment by years lived with disability (YLD) observed for selected infections and across three time points.
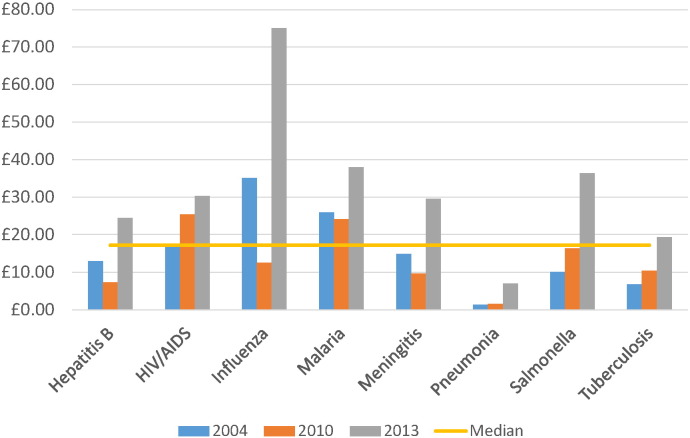
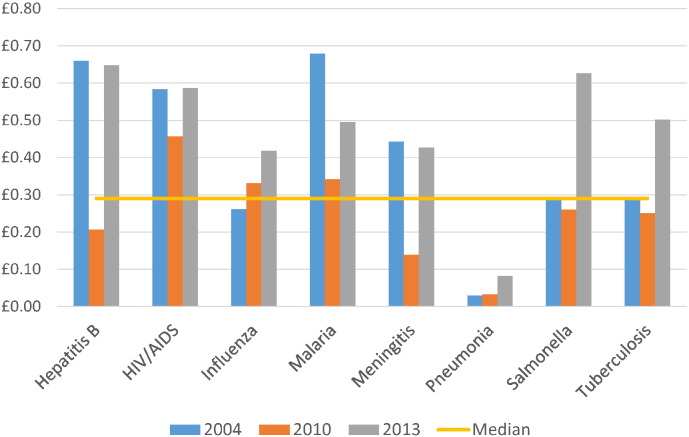
The relative level of investment per death, DALY or YLD for all infections combined consistently increased over time (Table 2, Table 3, Table 4). When considering time points 2004, 2010 and 2013, the median investment by mortality observed was £17.20, but greatest in 2013 (£31.16). HIV and malaria investments were above the median level at each time point; tuberculosis was below the median level (Table 3, Fig. 3). Compared to mortality, little investment was directed towards cholera, syphilis and pneumonia. Vaccine-preventable diseases such as diphtheria, measles and pertussis were mostly ranked as poorly-invested, though their respective investment by mortality observed noticeably increased in 2013 as global burdens declined. The ‘investment per DALY observed’ metric (Table 4, Fig. 4) demonstrated similar findings to the mortality metric, with pneumonia appearing poorly-invested compared to other particularly high-burden infections such as malaria, and cholera, syphilis and pertussis also receiving little funding. Acute hepatitis C, African trypanosomiasis and chlamydia demonstrated the highest relative investment when compared to DALYs.
Table 3.
Mortality and investment by mortality observed at three time points.
| Number of deaths |
Investment per mortality observed |
|||||
|---|---|---|---|---|---|---|
| 2004 | 2010 | 2013 | 2004 | 2010 | 2013 | |
| Campylobacter | 163,092 | 109,000 | 14,100 | £10.50 | £17.20 | £158.67 |
| Chagas disease | 11,000 | 10,300 | 10,600 | £48.61 | £36.44 | £31.16 |
| Chlamydia | 9000 | 1200 | 1100 | £280.96 | £1412.06 | £1471.55 |
| Cholera | 86,932 | 58,100 | 69,900 | £0.22 | £1.53 | £1.03 |
| Clostridium difficile | 41,500 | £84.43 | ||||
| Dengue | 18,000 | 14,700 | 9100 | £23.01 | £251.46 | £373.84 |
| Diphtheria | 5000 | 2900 | 3300 | £3.61 | £3.95 | £2.82 |
| E. coli | 313,465 | 209,500 | 61,000 | £7.31 | £9.84 | £39.94 |
| Gonorrhoea | 1000 | 900 | 2300 | £78.48 | £72.03 | £39.35 |
| Hepatitis A | 102,800 | 14,900 | £0.00 | £0.00 | ||
| Hepatitis B | 105,000 | 132,200 | 68,600 | £12.99 | £7.32 | £24.45 |
| Hepatitis C | 54,000 | 16,000 | 3500 | £98.51 | £291.77 | £1968.88 |
| Hepatitis E | 56,600 | 49,700 | £0.00 | £0.00 | ||
| HIV/AIDS | 2,040,000 | 1,465,400 | 1,341,000 | £16.73 | £25.39 | £30.36 |
| Influenza | 117,632 | 507,900 | 105,400 | £35.03 | £12.55 | £75.10 |
| Leishmaniasis | 47,000 | 51,600 | 62,500 | £46.21 | £61.33 | £52.89 |
| Malaria | 889,000 | 1,169,500 | 854,600 | £25.94 | £24.19 | £37.94 |
| Measles | 424,000 | 125,400 | 95,600 | £0.65 | £3.34 | £7.85 |
| Meningitis | 340,000 | 422,900 | 303,500 | £14.87 | £9.65 | £29.58 |
| Norovirus | 1800 | £457.70 | ||||
| Pertussis | 254,000 | 81,400 | 60,600 | £0.96 | £2.44 | £6.90 |
| Pneumonia | 1,235,381 | 1,460,700 | 784,600 | £1.33 | £1.52 | £7.00 |
| Salmonella | 406,233 | 271,500 | 239,300 | £10.04 | £16.37 | £36.35 |
| Schistosomiasis | 47,000 | 11,700 | 5500 | £109.01 | £284.76 | £1012.27 |
| Shigellosis | 182,543 | 122,000 | 73,900 | £1.58 | £2.25 | £9.27 |
| Syphilis | 99,000 | 113,300 | 136,800 | £1.15 | £0.76 | £1.02 |
| Tetanus | 163,000 | 61,300 | 58,900 | £0.76 | £7.19 | £12.15 |
| Trypanosomiasis | 52,000 | 9100 | 6900 | £78.36 | £557.48 | £1490.69 |
| Tuberculosis | 1,464,000 | 1,196,000 | 1,290,300 | £6.78 | £10.35 | £19.38 |
| Varicella | 6800 | 7000 | ||||
| Overall | 8,527,278 | 7,790,700 | 5,734,500 | £85.28 | £197.11 | £337.39 |
Table 4.
Disability-adjusted life years (DALYs) and ‘investment by DALY observed’ at three time points.
| DALYs |
Cumulative investment per DALY observed |
|||||
|---|---|---|---|---|---|---|
| 2004 | 2010 | 2013 | 2004 | 2010 | 2013 | |
| Campylobacter | 6,131,039 | 7,541,000 | 6,132,681 | £0.28 | £0.25 | £0.36 |
| Chagas disease | 429,872 | 546,000 | 338,000 | £1.24 | £0.69 | £0.98 |
| Chlamydia | 3,748,198 | 714,000 | 692,000 | £0.67 | £2.37 | £2.34 |
| Cholera | 3,628,541 | 4,463,000 | 3,629,512 | £0.01 | £0.02 | £0.02 |
| Clostridium difficile | ||||||
| Dengue | 669,647 | 825,000 | 1,142,000 | £0.62 | £4.48 | £2.98 |
| Diphtheria | 173,575 | 236,000 | 253,000 | £0.10 | £0.05 | £0.04 |
| E. coli | 11,736,863 | 14,436,000 | 11,740,005 | £0.20 | £0.14 | £0.21 |
| Gonorrhoea | 3,549,975 | 282,000 | 313,000 | £0.02 | £0.23 | £0.29 |
| Hepatitis A | 1,214,000 | £0.05 | ||||
| Hepatitis B | 2,067,533 | 4,674,000 | 2,587,000 | £0.66 | £0.21 | £0.65 |
| Hepatitis C | 954,622 | 518,000 | 138,000 | £5.57 | £9.01 | £49.94 |
| Hepatitis E | 2,616,000 | £0.00 | ||||
| HIV/AIDS | 58,512,843 | 81,547,000 | 69,363,000 | £0.58 | £0.46 | £0.59 |
| Influenza | 15,784,216 | 19,244,000 | 18,932,694 | £0.26 | £0.33 | £0.42 |
| Leishmaniasis | 1,974,465 | 3,317,000 | 4,283,000 | £1.10 | £0.95 | £0.77 |
| Malaria | 33,976,025 | 82,685,000 | 65,493,000 | £0.68 | £0.34 | £0.50 |
| Measles | 14,852,775 | 10,420,000 | 8,051,000 | £0.02 | £0.04 | £0.09 |
| Meningitis | 11,426,376 | 29,399,000 | 21,014,000 | £0.44 | £0.14 | £0.43 |
| Norovirus | ||||||
| Pertussis | 9,881,887 | 7,018,000 | 5,250,000 | £0.02 | £0.03 | £0.08 |
| Pneumonia | 56,343,025 | 68,693,000 | 67,581,770 | £0.03 | £0.03 | £0.08 |
| Salmonella | 13,891,385 | 17,086,000 | 13,895,104 | £0.29 | £0.26 | £0.63 |
| Schistosomiasis | 1,707,143 | 3,309,000 | 3,062,000 | £3.00 | £1.01 | £1.82 |
| Shigellosis | 5,733,469 | 7,052,000 | 5,735,004 | £0.05 | £0.04 | £0.12 |
| Syphilis | 2,846,113 | 9,578,000 | 11,324,000 | £0.04 | £0.01 | £0.01 |
| Tetanus | 5,283,485 | 4,663,000 | 3,654,000 | £0.02 | £0.09 | £0.20 |
| Trypanosomiasis | 1,672,728 | 560,000 | 390,000 | £2.44 | £9.06 | £26.37 |
| Tuberculosis | 34,216,000 | 49,396,000 | 49,816,000 | £0.29 | £0.25 | £0.50 |
| Varicella | 487,000 | £0.61 | ||||
| Overall | 301,191,800 | 428,202,000 | 379,126,770 | £2.41 | £3.59 | £5.10 |
Fig. 3.
Investment by mortality observed for selected infections and across three time points.
Fig. 4.
Investment by DALYs observed for selected infections and across three time points.
Ranking infections by investment and disease burden in 2013, Table 5 describes how HIV (9th) and malaria (10th) were mid-ranking diseases, with tuberculosis (14th) and pneumonia (20th) ranked lower. Acute hepatitis C, African trypanosomiasis and leishmaniasis were the top three infections, and pertussis, cholera and syphilis the bottom three. Diphtheria was ranked in the lowest three when compared against mortality and DALYs, whilst gonorrhoea was ranked in the lowest three when compared against YLD.
Table 5.
Rankings of research investment for 25 infectious diseases compared with 2013 YLD, mortality and DALYs.
| Disease | Research investment (UK pound) by burden observed, 2013 |
|||
|---|---|---|---|---|
| Mean ranking across all burden metrics | Mortality | Years lived with disability | Disability-adjusted life years | |
| Hepatitis C | 1.00 | 1 | 1 | 1 |
| Trypanosomiasis | 2.00 | 2 | 2 | 2 |
| Leishmaniasis | 6.67 | 8 | 5 | 7 |
| Dengue | 7.33 | 5 | 14 | 3 |
| Influenza | 8.33 | 7 | 4 | 14 |
| Chlamydia | 8.67 | 3 | 19 | 4 |
| Schistosomiasis | 9.67 | 4 | 20 | 5 |
| Salmonella | 10.00 | 12 | 9 | 9 |
| Malaria | 11.00 | 11 | 10 | 12 |
| HIV/AIDS | 11.67 | 14 | 11 | 10 |
| Chagas Disease | 12.00 | 13 | 17 | 6 |
| Hepatitis B | 12.00 | 16 | 12 | 8 |
| Campylobacter | 12.33 | 6 | 16 | 15 |
| Tuberculosis | 13.67 | 17 | 13 | 11 |
| Tetanus | 14.00 | 18 | 6 | 18 |
| Meningitis | 14.33 | 15 | 15 | 13 |
| E. coli | 15.67 | 9 | 21 | 17 |
| Measles | 16.00 | 20 | 8 | 20 |
| Diphtheria | 16.33 | 23 | 3 | 23 |
| Pneumonia | 16.33 | 21 | 7 | 21 |
| Gonorrhoea | 16.67 | 10 | 24 | 16 |
| Shigellosis | 20.00 | 19 | 22 | 19 |
| Pertussis | 20.67 | 22 | 18 | 22 |
| Cholera | 23.67 | 24 | 23 | 24 |
| Syphilis | 25.00 | 25 | 25 | 25 |
4. Discussion
We identified 7398 awards for infectious disease research awarded to UK institutions across the 17 year time period of 1997–2013, with total funding of £3.7 billion. Relative to measures of investment compared to global mortality, DALYs and YLD, acute hepatitis C, trypanosomiasis and leishmaniasis rank highly whilst Shigellosis, pertussis, cholera and syphilis consistently rank lowest; tuberculosis typically ranked lower than HIV or malaria, and pneumonia appears to receive particularly low levels of investment compared to mortality and DALYs. The overall level of investment and the median investment by mortality, DALY and YLD observed increased in 2013 compared with previous time points, owing to both increases in research investment and decreases in the global burden of infectious disease.
The comparison with global burden of disease and the development of associated metrics demonstrates the need for consideration of more than one measure of burden. Pneumonia, a disease with relatively high mortality, receives low levels of research investment when considering just mortality or DALYs, but ranks higher when considering lifelong measures of burden such as YLDs. Chlamydia, a disease of low mortality, ranks highly when comparing investment with mortality but the YLD ranking is lower. Chronic infections are also difficult to account for, as demonstrated by the high ranking of acute hepatitis C, which does not fully take into account the chronic and undiagnosed burden or the projected future burden.
Several of the infections studied are vaccine-preventable, such as measles, pertussis and diphtheria, and policymakers need to decide whether to fund, for example, operational research to identify improvements in the delivery process of vaccine, or to invest more heavily in implementation measures known to be effective. Expanding on this work to incorporate further measures of burden across different time points and to take into account proportions of funding for each type of science would strengthen these models. Replication of the study using data from other countries and in non-communicable disease area would also provide a more complete picture of diseases where there have been adequate or inadequate investment. Further work to decipher the impact of predominantly UK-focused funders, such as the NIHR, and the global funding remit of the organisations such as the Wellcome Trust would also be useful.
There are other published analyses that have similar aims in terms of considering investment and burden. Consideration of NIH funding in 2006 suggested a modest correlation with USA disease burden from 2004, (Gillum et al., 2011) with comparable findings in analyses from Australia (Mitchell et al., 2009) and Norway (Kinge et al., 2014), showing variable correlation depending upon the chosen burden metric and whether that metric considered national or global burden. Thus, policymakers have a decision to make about the extent to which there is targeting of investment on health burdens within their own country, and how much funding targets international priorities. These decisions will be driven to an extent by the remits of existing funding agencies, plus also the existing research expertise in the UK. Disease areas such as the NTDs appear well-funded here, relative to their global disease burden, and this is may be in part due to previous and ongoing excellent performance by individuals and institutions in addressing these areas. In terms of the location of research investment activity, the UK demonstrably provides greater relative international investment in nations where there are colonial ties (Fitchett et al., 2014c). It may be that other nations also favour countries in which they have historic connections (and there are good reasons for doing so, such as a common language or infrastructure requirements); however, this may also mean that some countries are neglected in terms of receiving research investment from which they could greatly benefit.
Funding for infections with pandemic potential remains inconsistent and it is difficult to project accurate future burdens of new and emerging infectious diseases. The UK influenza research portfolio is now arguably relatively strong, combined with a Department of Health focus to identify research gaps and priorities in this area (Infectious Disease Research Network, 2014). The emergence of the large Ebola outbreak in West Africa over 2014 and 2015 has highlighted how little research there has been by UK institutions prior to 2014 on filoviruses; an unpublished analysis using ResIn data demonstrates that the US NIH is the only funder with a track record of sustained funding in this area. Reactive efforts across 2014 and beyond will greatly increase that total research investment; however, the examples of Ebola and novel coronaviruses with pandemic potential (Head et al., 2014a) show that there is a need for a proactive approach in terms of commissioning, capacity-strengthening and carrying out research in diseases that have potential to impact on global health security (though this area of commissioning faces significant challenges). Decisions around resource allocation for disease eradication may reflect the interests of specific donors and high-profile individuals, such as former US President Jimmy Carter and his efforts towards eradicating dracunculiasis (Guinea worm) (Hopkins et al., 2014), though most investment here would be considered implementation rather than research. In 2015, the UK government announced a high-profile implementation and research investment in collaboration with the Bill & Melinda Gates Foundation to advance knowledge against infectious diseases, particularly malaria (HM Treasury D for ID, 2015). Clearly, there are other factors beyond current and projected disease burdens that should influence decisions around research investment priorities, such as the effectiveness of available interventions, what constitutes the ‘best buy’ for a particular health service and consideration of health inequity in neglected populations (Wiseman and Mooney, 1998). There should also be reflections on what research is feasible and the likelihood of advances in knowledge if investment is targeted in specific disease or geographical areas. A checklist for health research priority setting, with the aim of achieving maximum public health benefit, has been suggested previously (Viergever et al., 2010), and the data presented here showing the relative scale of investment compared to disease burden can usefully inform the commissioning process for research that assesses how to alleviate the impact of disease on public health.
There have been demonstrable shifts in the proportions of the type of science allocated to some disease areas, and this will affect the likely temporal impacts of R&D on public health and patient care and thus the burden of disease. For example, over 80% of gastroenterology research was pre-clinical in 1997–2010; across 2011–2013, less than half was pre-clinical with relatively equal sums of money for the other four phases of research. This may be the result of the previous pre-clinical research being translated into tools, products or knowledge in other areas of the research pipeline, and may have contributed to the reduction in global burden of disease observed in enteric infections in 2013. The level of investment by the private sector in new tools such as vaccines, therapeutics and diagnostics for infectious diseases is important but uncertain given the low market attractiveness for these conditions which predominantly affect lower income and middle income countries, though it is plausible that investment decisions in the public, philanthropic and private sector are made as a result of some level of interaction between the sectors. There are examples of public–private product development partnerships for infections such as malaria and TB, while that for maternal and child health, where there is also low market attractiveness, has been lacking (Fisk and Atun, 2008). Although there has been progress made with transparency of reporting in the pharmaceutical and biotechnology industries (Brown, 2013), commercial sensitivities mean that it is difficult to obtain investment data to analyse in similar detail to the awards included here.
The low levels of research investment for syphilis and gonorrhoea remain (Head et al., 2013, Head et al., 2015b), both in terms of total funding but also when compared to disease burden. Given the increasing levels of antibiotic resistance in strains of Neisseria gonorrhoeae, and the continuing high burden of both these otherwise-treatable infections, they seem an area worthy of greater focus by UK research institutions and funders. Sepsis remains hugely problematic in many healthcare settings, and the research portfolio needs to be increased to reflect this.
The temporal trends in funding for infectious disease research has been noted as being volatile (Fitchett et al., 2014b), for example the peak in year 2000 (Fig. 1a) being partly due to markedly increased contributions from the Bill & Melinda Gates Foundation in that year (£40.1 million, compared with zero UK research investment in 1999 or 2001). This will result in fluctuations in levels of funding for several infections; for example here across 1997–2010 and 2011–2013, investments for Candida proportionately increased and investments for HPV decreased The rationale for fluctuations in funding are complex, and will reflect various issues such as funder priorities, numbers of applications received by those funders, disbursement of research funds over the study period, research capacity in each topic area, and potentially the quality of burden data available.
This study has several limitations, some of which have been described previously (Head et al., 2013). An additional limitation is that our analyses will exclude much infrastructure funding that some funders invest in quite heavily and this may underestimate total investment in infectious disease research as well as the contribution of specific funders. As described above, the lack of private sector investment information leaves a data gap. Analyses have not taken into account proportions of awards allocated to indirect or estate costs. Any distribution of funding from the lead centre to collaborating partners is also not documented. Categorisation is subjective, but checks by at least one other author reduce the impact of any observer error. This analysis is also vulnerable to the effects of any changes in methodology of the GBD study, and there will also be uncertainty in the disease estimates calculated in the GBD analyses.
Public and philanthropic research funding for infectious diseases has increased in the time period 2011–2013 as compared with previous years, with greater mean and median award amounts, indicating a shift towards funding award types such as consortia or programme grants. Measuring these investments against the global burden of disease highlight areas such as influenza and trypanosomiasis where the UK has relatively strong investment and probable research strengths, but areas such as sepsis, pneumonia, syphilis and gonorrhoea remain areas of relative underinvestment, and should be considered by funding organisations and policymakers. Co-ordinated efforts across the global health community must consider research investment in emerging infectious diseases and pathogens of pandemic potential. By demonstrating potential inequities in allocation of investment and disease burden, analyses by the ResIn study can contribute to priority setting by funders and inform strategy of policymakers, government departments and research institutions.
Funding
There was no funding source for this study.
Ethical approval
An ethics statement was not required for this work.
Contributors
MGH and JRF designed the study. MGH wrote the submission with edits by all authors. MGH, JRF, NK and VN collected and categorised the data. AH and RA provided commentary on the dataset and interpretation of the results. All authors gave comments on draft and final versions of this manuscript.
Competing interests
The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to acknowledge the input of the Infectious Disease Research Network (www.idrn.org) and the funding agencies who contributed data to these analyses.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.12.016.
Appendix A. Supplementary data
Supplementary materials 1
Supplementary materials 2
References
- Anon. UK 5 Year Antimicrobial Resistance Strategy 2013 to 2018 - Publications — GOV.UK. (https://www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018).
- Anon. Threat report 2013 | Antimicrobial Resistance | CDC. (http://www.cdc.gov/drugresistance/threat-report-2013/ (accessed Oct 14, 2013).
- Anon. The review on antimicrobial resistance. Antimicrobial resistance: tackling a crisis for the future health and wealth of nations. London, 2014 http://amr-review.org/Publications (accessed Jan 8, 2015).
- Anon. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 doi: 10.1016/S0140-6736(15)60692-4. (published online June. http://www.thelancet.com/article/S0140673615606924/fulltext (accessed June 8, 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. It's time for alltrials registered and reported. Cochrane Database Syst. Rev. 2013;4:ED000057. doi: 10.1002/14651858.ED000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission Communication from the Commission to the European Parliament and the Council Action Plan Against the Rising Threats from Antimicrobial Resistance. 2011. http://ec.europa.eu/dgs/health_food-safety/docs/communication_amr_2011_748_en.pdf (accessed Sept 15, 2015)
- Fisk N.M., Atun R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008;5:e22. doi: 10.1371/journal.pmed.0050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitchett J.R., Head M.G., Atun R. Investing in sepsis research: systematic analysis of UK public and philanthropic funding 1997–2010. JRSM Open. 2014;5 doi: 10.1177/2054270414538954. (2054270414538954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitchett J.R., Head M.G., Atun R. Infectious disease research investments follow colonial ties: questionable ethics. Int. Health. 2014;6:74–76. doi: 10.1093/inthealth/iht036. [DOI] [PubMed] [Google Scholar]
- Fitchett J.R., Head M.G., Cooke M.K., Wurie F.B., Atun R. Funding infectious disease research: a systematic analysis of UK research investments by funders 1997–2010. PLoS One. 2014;9:e105722. doi: 10.1371/journal.pone.0105722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitchett J.R., Head M.G., Atun R. Infectious disease research investments: systematic analysis of immunology and vaccine research funding in the UK. Vaccine. 2013;31:5930–5933. doi: 10.1016/j.vaccine.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Fonkwo P.N. Pricing infectious disease. The economic and health implications of infectious diseases. EMBO Rep. 2008;9(Suppl. 1):S13–S17. doi: 10.1038/embor.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum L.A., Gouveia C., Dorsey E.R. NIH disease funding levels and burden of disease. PLoS One. 2011;6:e16837. doi: 10.1371/journal.pone.0016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Atun R. Systematic analysis of funding awarded for norovirus research to institutions in the United Kingdom, 1997–2010. J. R. Soc. Med. 2014;107:110–115. doi: 10.1177/0141076813511450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Atun R., May R.C. Systematic analysis of funding awarded for mycology research to institutions in the UK, 1997–2010. BMJ Open. 2014;4:e004129. doi: 10.1136/bmjopen-2013-004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Cooke M.K. Investments in respiratory infectious disease research 1997–2010: a systematic analysis of UK funding. BMJ Open. 2014;4:e004600. doi: 10.1136/bmjopen-2013-004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Cooke M.K. Systematic analysis of funding awarded for antimicrobial resistance research to institutions in the UK, 1997–2010. J. Antimicrob. Chemother. 2014;69:548–554. doi: 10.1093/jac/dkt349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Holmes A.H., Atun R. Funding healthcare-associated infection research: a systematic analysis of UK research investments, 1997–2010. J. Hosp. Infect. 2014;87:84–91. doi: 10.1016/j.jhin.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Moore D.A., Atun R. Systematic analysis of funding awarded to institutions in the United Kingdom for infectious disease research, 1997–2010. JRSM Open. 2015;6 doi: 10.1177/2054270415577056. (2054270415577056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Newell M.-L. Mapping pneumonia research: a systematic analysis of UK investments and published outputs 1997–2013. EBioMedicine. 2015;2(9):1993–1999. doi: 10.1016/j.ebiom.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Cassell J.A., Atun R. Investments in sexually transmitted infection research, 1997–2013: a systematic analysis of funding awarded to UK institutions. J. Glob. Health. 2015;5:020405. doi: 10.7189/jogh.05.020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Cooke G.S., Foster G.R., Atun R. Systematic analysis of funding awarded for viral hepatitis-related research to institutions in the United Kingdom, 1997–2010. J. Viral Hepat. 2014 doi: 10.1111/jvh.12300. (published online Aug 22. http://www.ncbi.nlm.nih.gov/pubmed/25146854, (accessed Nov 10, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M.G., Fitchett J.R., Cooke M.K., Wurie F.B., Hayward A.C., Atun R. UK investments in global infectious disease research 1997–2010: a case study. Lancet Infect. Dis. 2013;13:55–64. doi: 10.1016/S1473-3099(12)70261-X. [DOI] [PubMed] [Google Scholar]
- HM Treasury D for ID . News stories. GOV.UK; 2015. Chancellor George Osborne and Bill Gates to join forces to end malaria. ( https://www.gov.uk/government/news/chancellor-george-osborne-and-bill-gates-to-join-forces-to-end-malaria (accessed Dec 10, 2015) [Google Scholar]
- Hopkins D.R., Ruiz-Tiben E., Eberhard M.L., Roy S.L. Progress toward global eradication of dracunculiasis—January 2013–June 2014. MMWR Morb. Mortal. Wkly Rep. 2014;63:1050–1054. [PMC free article] [PubMed] [Google Scholar]
- Infectious Disease Research Network Planning for a pandemic — addressing research for influenza and other respiratory threats. London. 2014. http://www.idrn.org/pandemicplanning/ (accessed Jan 27, 2015)
- Kinge J.M., Roxrud I., Vollset S.E., Skirbekk V., Røttingen J.-A. Are the Norwegian health research investments in line with the disease burden? Health Res. Policy Syst. 2014;12:64. doi: 10.1186/1478-4505-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Barker-Collo S., Bartels D.H., Bell M.L., Benjamin E.J., Bennett D., Bhalla K., Bikbov B.,.M.Z. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R.J., McClure R.J., Olivier J., Watson W.L. Rational allocation of Australia's research dollars: does the distribution of NHMRC funding by National Health Priority Area reflect actual disease burden? Med. J. Aust. 2009;191:648–652. doi: 10.5694/j.1326-5377.2009.tb03365.x. [DOI] [PubMed] [Google Scholar]
- Murray C.J.L., Barber R.M., Foreman K.J. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015 doi: 10.1016/S0140-6736(15)61340-X. (published online Aug. http://www.thelancet.com/article/S014067361561340X/fulltext (accessed Aug 28, 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J.L., Vos T., Lozano R. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Viergever R.F., Olifson S., Ghaffar A., Terry R.F. A checklist for health research priority setting: nine common themes of good practice. Health Res. Policy Syst. 2010;8:36. doi: 10.1186/1478-4505-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Flaxman A.D., Naghavi M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman V., Mooney G. SOUNDING BOARD. Health Policy (N. Y.) 1998;43:243–251. doi: 10.1016/s0168-8510(98)00003-7. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2014. Antimicrobial Resistance: Global Report on Surveillance 2014. ( http://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed June 15, 2014) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials 1
Supplementary materials 2