Abstract
The kappa opioid receptor (KOR) is involved in mediating pruritus; agonists targeting this receptor have been used to treat chronic intractable itch. Conversely, antagonists induce an inch response at the site of injection. As a G protein-coupled receptor (GPCR), the KOR has potential for signaling via G proteins and βarrestins, however, it is not clear which of these pathways are involved in the KOR modulation of itch. In this study asked whether the actions of KOR in pruritus involve βarrestins by using βarrestin2 knockout (βarr2-KO) mice as well as a recently described biased KOR agonist that biases receptor signaling toward G protein pathways over βarrestin2 recruitment. We find that the KOR antagonists nor-binaltorphimine (NorBNI) and 5′-guanidinonaltrindole (5′GNTI) induce acute pruritus in C57BL/6J mice, with reduced effects in KOR-KO mice. βarr2-KO mice display less of a response to KOR antagonist-induced itch compared to wild types, however no genotype differences are observed from chloroquine phosphate (CP)-induced itch, suggesting that the antagonists may utilize a KOR-βarrestin2 dependent mechanism. The KOR agonist U50,488H was equally effective in both WT and βarr2-KO mice in suppressing CP-induced itch. Furthermore, the G protein biased agonist, Isoquinolinone 2.1 was as effective as U50,488H in suppressing the itch response induced by KOR antagonist NorBNI or CP in C57BL/6J mice. Together these data suggest that the antipruritic effects of KOR agonists may not require βarrestins.
Keywords: pruritus, kappa opioid receptor, biased ligand, mouse models of itch, U50, 488H, KOR antagonist
1. Introduction
Pruritus, commonly referred to as itch, is defined as “an unpleasant cutaneous sensation that provokes the desire to scratch” (Rothman, 1941). Pruritus secondary to disease can become pathological, resulting in an intense, chronic itch that can have a significantly negative impact on quality of life. Unfortunately, there are few therapeutic options for those that suffer from severe pathological itch. Recent advances in the understanding of the biology of pruritus implicate dynorphin and the kappa opioid receptor (KOR) as a potential therapeutic target (Kardon et al., 2014; Ross et al., 2010; Sardella et al., 2011) leading to new therapeutic options utilizing KOR agonists.
The KOR is a seven transmembrane G protein coupled receptor (GPCR) that is expressed throughout the nervous system. Elegant studies from the Ross laboratory demonstrate a functional role for KOR on dorsal root ganglion neurons for modulating itch responses (Kardon et al., 2014). Activation of KOR results in signaling through the pertussis toxin-sensitive Gαi/o proteins to inhibit adenylyl cyclase (Sharma et al., 1977), activate inward-rectifying potassium channels (North et al., 1987), inhibit voltage-dependent calcium channels (Hescheler et al., 1987; Surprenant et al., 1990) and increase intracellular calcium levels (Jin et al., 1992). However, it is becoming increasingly evident that GPCRs can signal via multiple downstream effectors, including other Gα protein subunits and βarrestins. βarrestins are multifunctional scaffolding proteins that can serve to scaffold to receptors thereby facilitating certain protein-protein interactions while disrupting others. In this role, βarrestins can serve to dampen or facilitate receptor signaling to downstream effectors. It has been suggested that KOR can also signal via βarrestins and shown that U69,593-mediated KOR activation of ERK1/2 is impaired in mice lacking βarrestin2 (βarr2-KO mice) (Schmid et al., 2013).
Receptors can conceptually interact with different G proteins and/or βarrestins to propagate downstream signaling, and certain agonists can facilitate signaling to one cascade over another. Therefore an opportunity arises to divert receptor signaling towards one cascade or another in an effort to harness receptor signaling to induce desired physiologies and avoid unwanted effects. The KOR has proven to be capable of signaling via G proteins and βarrestins, and different ligands have proven to be better at generating signals through one of these cascades over the others. Such ligands, referred to as “biased agonists,” may prove to be therapeutically beneficial when the desired effects are known to be mediated by one pathway while the deleterious effects are mediated by another pathway.
There is emerging information regarding the potential for “ligand-directed signaling” at the KOR. Activation of KOR is associated with dysphoria, depression and anxiety, and effects related to drug relapse and pro-addictive behaviors and has been proposed to be dependent upon GPCR kinase 3 (GRK3)-dependent βarrestin interactions with the receptor (Bruchas and Chavkin, 2010; Bruchas et al., 2006). KOR activation has also been proposed as a means to provide pain relief and activation of KOR in this manner appears to be due to G protein mediated mechanisms (Bruchas et al., 2007; White et al., 2014). Recently, agonists that bias KOR signaling toward G protein signaling and away from βarrestin2 recruitment have been identified (Rives et al., 2012; Schmid et al., 2013; White et al., 2015; White et al., 2014; Zhou et al., 2013). These tools, as well as βarr2-KO mice, are serving to define which KOR signaling pathways can be attributed to G protein or βarrestin2 signaling mechanisms. Thus far, it is apparent that KOR signaling via G proteins is sufficient to provide antinociception (White et al., 2014; Zhou et al., 2013). However, the question of how KOR modulates itch responses remains.
The KOR agonist nalfurafine (Remitch®) is used in treatment of the pruritus associated with chronic renal failure in humans, with intravenous (Ueno et al., 2013; Wikstrom et al., 2005) and oral (Kumagai et al., 2012; Kumagai et al., 2010) efficacy. In hemodialysis patients, nalfurafine can produce long-term suppression of pruritus without significant safety issues (Kumagai et al., 2012) or abuse liability (Ueno et al., 2013). In mice, KOR agonists are also effective. For example, the KOR agonist U50,488H reduces scratching induced by compound 48/80 (Kamei and Nagase, 2001). Additionally, nalfurafine (also known as TRK-820) inhibits the development of pruritus induced by substance P (Togashi et al., 2002; Umeuchi et al., 2003), histamine (Togashi et al., 2002), chloroquine phosphate (Inan and Cowan, 2004), as well as compound 48/80 (Inan et al., 2009b; Wang et al., 2005). Nalfurafine is a potent partial agonist with weak efficacy and no studies to date have determined whether it possesses biased signaling properties.
Conversely, KOR antagonists such as nor-binaltorphimine (NorBNI) and 5′guanadinonaltrindole (5′GNTI) induce acute pruritus (Inan t al., 2009a, b; Kamei and Nagase, 2001). Since the antagonists can induce itch it is attractive to speculate that the underlying tone of the endogenous KOR agonists, dynorphins, naturally suppress the itch response. However, the mechanisms by which KOR signaling modulates this response remains unclear, although recent reports have begun to uncover such mechanisms and confirm the importance of the KOR system (Kardon et al., 2014).
In this study, we ask whether βarrestins are involved in itch produced by KOR antagonists and whether the antipruritic effects of KOR agonists require βarrestin2. We utilize both βarr2-KO mice as well as a G protein biased KOR agonist, “Isoquinolinone 2.1” (Iso2.1), (Zhou et al., 2013). Iso2.1 produces more [35S]GTPγS binding than βarrestin2 recruitment (DiscoveRx PathHunter assay) with a calculated bias factor of 31. It has in vivo efficacy as it induces antinociception in the warm water tail immersion test (Zhou et al., 2013). Herein we test its function in a mouse model of pruritus.
2. Methods
2.1 Animals
Experiments were carried out with age matched (10-16 week old) male mice weighing between 25 and 35 g. C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME); KOR-KO mice were purchased from Jackson Laboratory and generated from homozygous breeding; βarr2-WT and βarr2-KO mice were derived from heterozygous breeding as previously described (Bohn et al., 1999). Mice were group housed (3-5 mice per cage) and maintained on a 12-hour light/dark cycle in a temperature-controlled room. All behavioral tests were performed during the light cycle between 8am-6pm. All mice were cared for in accordance to the guidelines set forth by the National Institutes of Health regarding the proper treatment and use of laboratory animals and with approval of The Scripps Research Institute Animal Care and Use Committee.
2.2 Drugs
Nor-Binaltorphimine (NorBNI), 5′-guanidinonaltrindole (5′GNTI) and chloroquine phosphate (CP) were purchased from Sigma-Aldrich (St. Louis, MO) and U50,488H was purchased from Tocris Bioscience (Ellisville, MO). The synthesis of Iso2.1 has been previously described (Zhou et al., 2013). All drugs were prepared in a vehicle consisting of 1:1:8 dimethyl sulfoxide (DMSO), Tween80 and 0.9% sterile saline, with a pH of 6.0. Specifically, Iso2.1 was first dissolved in DMSO, then Tween80 and brought to volume with sterile saline; CP was first dissolved in 0.9% sterile saline and brought up to volume with DMSO and Tween80 to result in a 1:1:8 solution; pH 6.0. All drugs used to promote itch were freshly prepared and injected subcutaneously in the skin at the base of the neck (indicated by s.c.neck) at a volume of 5 μl/g body weight; KOR agonists used to block itch were injected subcutaneously (s.c.) in the flank area (5 μl/g body weight). When used as a pre-treatment, NorBNI was injected intraperitoneally (i.p.) at a volume of 10 μl/g body weight; dosing routes are indicated in the text. In order to conserve animals, all animals were pretreated with systemic injection of vehicle prior to treatments (10 minutes) so that separate groups would not be needed to compare to those animals receiving either agonist or antagonist pretreatments.
2.3 Evaluation of Pruritus-Induced Behavior
Mice were habituated to clear acrylic testing boxes [10 × 10 cm2] for one hour prior to the start of the experiment. All mice received a pretreatment of either vehicle (1:1:8 DMSO, Tween80, 0.9% sterile saline) or KOR agonists U50,488H or lso2.1 (5 μ/g, s.c). Ten minutes after pretreatment, mice receive a pruritogen injection (5 μ/g, s.c.neck) of either NorBNI, 5′GNTI or CP to induce acute pruritus. Immediately after the pruritogen injection the number of scratching bouts are recorded every 5 minutes for 1 hour by an investigator blinded to the treatment groups. During the experiments, mice were videotaped and for some studies, another investigator repeated scoring to validate the method. A “scratching bout” is defined as one or more rapid paw movements directed at the injection site, with the hindpaw being placed back on the floor as previously described (Holmes et al., 2012).
2.4 Spontaneous locomotor activity
C57BL/6J male mice were monitored in an open field activity monitor (Versamax [20 × 20 cm2] by Accuscan Instruments) immediately following injection (10 μ/g, s.c.) with vehicle, U50,488H or lso2.1 without habituation. In the twenty-four hour NorBNI pretreatment group, mice were injected with NorBNI (10 mg/kg, i.p.) and returned to the home cage until testing the following day. Spontaneous activity was measured over 60 minutes, with the number of horizontal beam breaks collected in 5-minute bins.
2.5 Statistical Analysis
All statistical comparisons were made using GraphPad Prism 6.01 software (GraphPad, LaJolla, CA) and are expressed as mean ± SEM. Differences between means were analyzed by oneway ANOVA for comparisons within the same genotype and by two-way ANOVA for comparisons between genotypes and time-course evaluation. All ANOVAs were followed with Bonferroni post hoc analysis, and in all analyses significance is set at p < 0.05.
3. Results
3.1 KOR antagonists NorBNI and 5′GNTI induce acute pruritus in C57BL/6J mice, with a reduced effect in KOR-KO mice
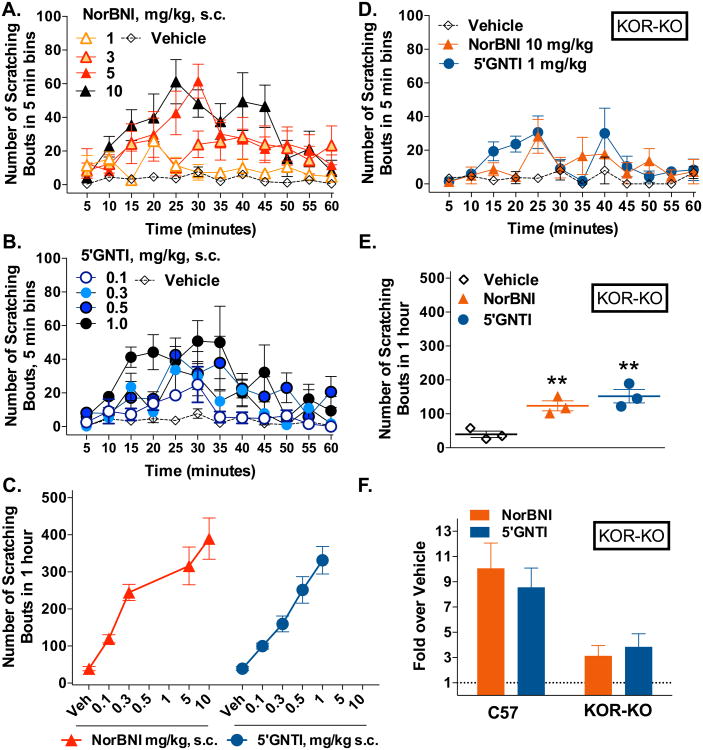
KOR antagonists have been characterized as acute pruritogen-inducing agents in ICR mice treated with NorBNI (Kamei and Nagase, 2001) and in Swiss Webster mice treated with 5′GNTI (Inan et al., 2009b). Here we show that these antagonists also induce itch in the C57BL/6J mouse line. This strain was chosen as it serves as the parental strain for the KOR-KO mice and is one of the parental strains of the βarr2-KO mice used later in this study. As seen in other strains of mice, these antagonists elicit a robust, dose-dependent increase in scratching behavior over the 60 minute observation period in adult male C57BL/6J mice compared to vehicle (Fig.1A-C) (two-way ANOVA interaction of dose and time: F(48,338) = 13.40, p < 0.0001, for NorBNI Fig 1A and F(48,325) = 14.75, p < 0.0001, for 5′GNTI, Fig 1B). To determine if these compounds were exerting their effects through KOR, we evaluated the itch response in KOR-KO mice. Interestingly, the scratching behavior is still present in KOR-KO mice treated with NorBNI (10 mg/kg) or 5′GNTI (1 mg/kg) compared to vehicle (Figure D: two-way ANOVA for interaction of treatment and time for NorBNI: F(12, 52) = 7.251, p < 0.0001 and 5′GNTI: F(12, 52) = 10.37, p < 0.0001). When the sum of the scratching bouts is compared to vehicle (Figure 1E, t-test comparing treatment to vehicle: **p < 0.01), it becomes evident that they induce an itch response that is ∼3-4 fold over that which is induced by vehicle (shown as fold over vehicle for comparison to WT responses in Figure 1F). These findings suggest that a maximal itch response is reached with both NorBNI and 5′GNTI and that deletion of KOR significantly ameliorates the response, however these agents still promote some itch independent of KOR expression.
Figure 1.
NorBNI and 5′GNTI induce scratching behaviors in C57BL/6J mice with some response still present in KOR-KO mice. (A) NorBNI (1.0-10 mg/kg, s.c.neck) and (B) 5′GNTI (0.1-1.0 mg/kg, s.c.neck) induce scratching behaviors over a 1 hour period compared to vehicle (two-way ANOVA for interaction of dose and time (p < 0.0001) for both; Vehicle n = 10; all other groups n = 5-6). (C) The sum of the total number of scratching bouts over the hour session is presented for each dose. (D) KOR-KO mice display scratching behaviors in response to NorBNI (10 mg/kg, s.c.neck) and 5′GNTI (0.1 mg/kg, s.c.neck) compared to vehicle (two-way ANOVA for interaction of treatment and time (p < 0.0001) for both; n = 3 all groups). (E) The sum of the scratching bouts over the hour session for KOR-KO reveals a significant effect of the KOR antagonists in the absence of KOR (t-test comparing treatment to vehicle, **p < 0.01; n = 3 all groups). (F) For visualization comparison, the fold stimulation over vehicle comparison of the antagonist effects presented in C and F are shown. Data are presented as mean ± SEM.
3.2 KOR antagonist-induced pruritus is reduced in βarr2-KO mice compared to βarr2-WT mice
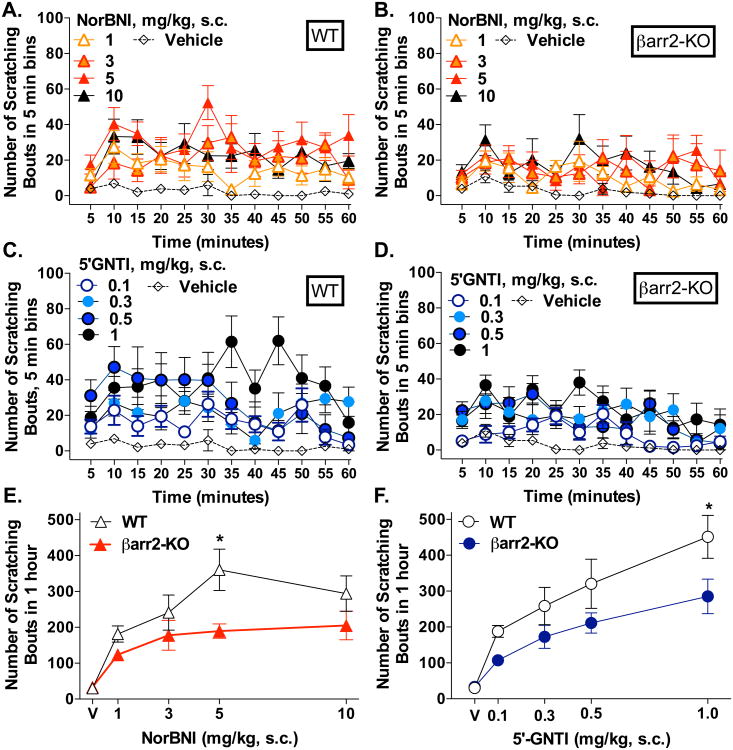
To examine the role of βarrestin2 in KOR-modulation of pruritus, we examined scratching behaviors induced by the KOR antagonists NorBNI and 5′GNTI in βarr2-WT and βarr2-KO mice. The KOR antagonists induced itch in both genotypes compared to vehicle (two-way ANOVA for dose effect of NorBNI in WT: F(4,360) = 24.43, p < 0.0001, Figure 2A; NorBNI in βarr2-KO: F(4,336) = 9.925, p < 0.0001, Figure 2B; 5′GNTI in WT: F(4,372) = 27.72, p < 0.0001, Figure 2C; 5′GNTI in βarr2-KO: F(4,420) = 23.40, p < 0.0001, Figure 2D). However, the response observed in the βarr2-KO mice is not as robust as that observed in the WT littermates for either KOR antagonist (two-way ANOVA for genotype: for NorBNI: F(4, 58) = 11.10, p = 0.0015; Bonferroni post-hoc analysis, *p < 0.05, Figure 2E; and 5′GNTI: F(1,63) = 13.02, p = 0.0006; Bonferroni post-hoc analysis, *p < 0.05, Figure 2F). These studies suggest that in the absence of βarrestin2, mice either display less of a scratching response or experience less of an induction of itch upon treatment with two different KOR antagonists in a dose dependent manner.
Figure 2.
βarr2-KO mice are less responsive to NorBNI and 5′GNTI. (A) WT and (B) βarr2-KO mice display a dose-dependent scratching response to NorBNI (s.c.neck) compared to vehicle (WT: two-way ANOVA for dose, p < 0.0001; n = 6-8; βarr2-KO: two-way ANOVA for dose, p < 0.0001; n = 6-8) as well as to 5′GNTI (C) WT and (D) barr2-KO mice display a dose-dependent response to 5′GNTI (s.c.neck) compared to vehicle (WT: two-way ANOVA for dose, p < 0.0001; n = 7-8; βarr2-KO: two-way ANOVA for dose, p < 0.0001; n = 7-8). (E) Comparison of the sum of dose effects over the hour test period, WT mice display a significantly greater response to NorBNI than barr2-KO mice (two-way ANOVA for genotype (p = 0.0015) and dose (p < 0.0001), *p<0.05 Bonferroni post hoc analysis; n = 6-8). (F) Comparison of the sum of dose effects over the hour test period, WT mice display a significantly greater response to 5′GNTI than βarr2-KO mice (two-way ANOVA for genotype (p = 0.0006) and dose (p < 0.0001), *p < 0.05 Bonferroni post hoc analysis; n = 7-8). Data presented as mean ± SEM.
3.3 Chloroquine phosphate-induced pruritus results in no genotype differences in βarr2-WT and βarr2-KO mice
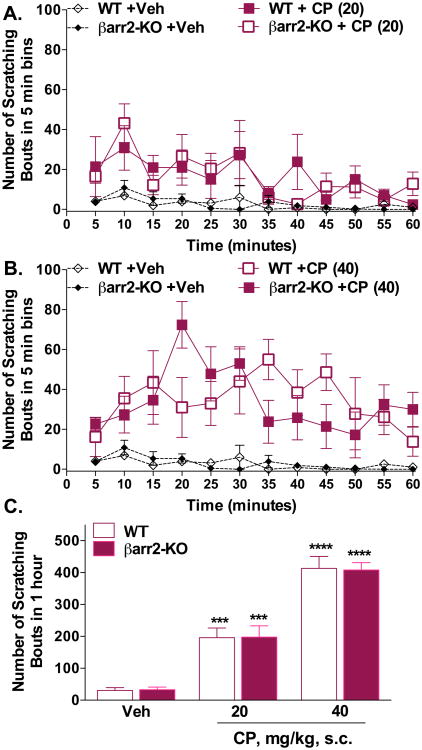
To test whether the WT and βarr2-KO mice are equally capable of expressing an itch response, we tested a general pruritic agent that is not known to be an antagonist at the KOR. Chloroquine phosphate (CP) is an antimalarial medication that, upon injection subcutaneously, promotes a robust itch response thought to be primarily due to triggering mast cell degranulation and a subsequent elevation of inflammatory cytokines as well as other itch-producing mediators (Aghahowa et al., 2010); it is often used to induce a model of pruritus in mice (Akiyama et al., 2014; Inan and Cowan, 2004). Figure 3 shows that CP-induced scratching was evident in both genotypes compared to vehicle (two-way ANOVA for treatment: WT: F(2,168) = 53.07, p < 0.0001; βarr2-KO: two-way ANOVA: F(2,180) = 63.28, p < 0.0001, (Figure 3A,B). Two-way ANOVA comparison between genotypes, within each dose, did not reveal a genotype effect when analyzed with the 5 minute binning data (p > 0.05) or when comparing the sum of the scratching response over the hour testing session at each dose (two-way ANOVA for genotype: F(1,28) = 0.00007, p = 0.9931; dose: F(2,28) = 132.8, p < 0.0001, Figure 3C) suggesting that the decrease in KOR antagonist-induced itch responses may be due to the actions of the antagonists at the KOR wherein βarrestin2 may play a role.
Figure 3.
WT and βarr2-KO mice display equally robust scratching behaviors in response to administration of the general pruritic agent, chloroquine phosphate (CP). (A, B) CP (20, 40 mg/kg, s.cneck) induces itch in WT and βarr2-KO mice (two-way ANOVA for dose, p < 0.0001 for both WT and βarr2-KO genotypes; WT, Vehicle n = 7, CP n = 5 for both doses; βarr2-KO, Veh n = 8, CP n = 5 for both doses). Two-way ANOVA comparison between genotypes, within each dose including vehicle, did not reveal a genotype effect when analyzed with the 5 minute binning data (p > 0.05). (C) Comparison of the sum of the response over the hour testing session at each dose revealed a significant effect of dose with no difference between the genotypes (two-way ANOVA for genotype and dose (p < 0.0001), ***p < 0.001, ****p < 0.0001 Bonferroni post hoc analysis). Data are presented as mean ± SEM.
3.4 KOR agonists suppress antagonist- induced pruritus
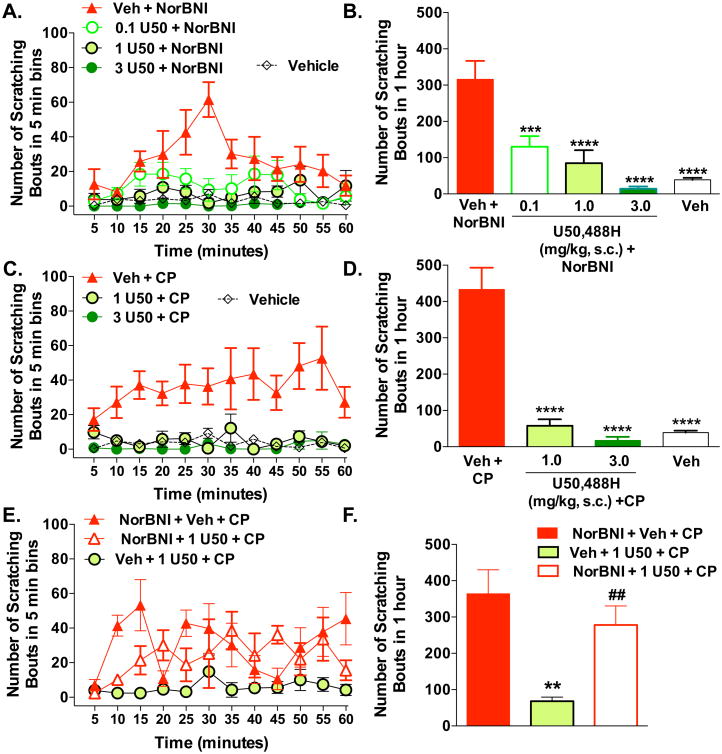
KOR agonist pretreatment can prevent KOR antagonist-induced acute pruritus as previously demonstrated in ICR mice by use of nalfurafine administration prior to 5′GNTI and U50,488H administration before NorBNI (Inan et al., 2011; Kamei and Nagase, 2001). Here we show that systemic pretreatment with U50,488H dose-dependently blocks the development of NorBNI-induced itch in C57BL/6J mice (two-way ANOVA for dose: F(4,360) = 49.23, p < 0.0001 Figure 4A; one-way ANOVA vs. Veh + NorBNI: F(4,29) = 17.35, p < 0.0001, Figure 4B). Suppression of CP-induced pruritus has also been demonstrated using KOR agonist nalfurafine in ICR mice (Inan and Cowan, 2004). Here we show systemic pretreatment with U50,488H also dose-dependently blocks the development of the CP-induced itch response in C57BL/6J mice (two-way ANOVA for dose: F(3, 276) = 93.30, p < 0.0001, Fig 4C; one-way ANOVA vs. Veh + CP: F(4,29) = 17.35, p < 0.0001, Figure 4D). The suppression of CP-induced scratching by U50,488H is be prevented by a 24 hour systemic pretreatment of the long-lasting KOR antagonist NorBNI (10 mg/kg, i.p.) (two-way ANOVA comparing Veh + U50,488H + CP and NorBNI + U50,488H + CP: F(1,96) = 30.42, p < 0.0001, Figure 4E). This is also evident in the comparison of the sum of the scratching bouts occurring during the 1 hour test session (NorBNI + Veh + CP vs. Veh + U50,488H + CP: **p < 0.01; Veh + U50,488H + CP and NorBNI + U50,488H + CP: ##p > 0.05, student's t-test) (Figure 4F).
Figure 4.
A balanced kappa agonist suppresses scratching behaviors induced by NorBNI and chloroquine phosphate in C57BL/6J mice. (A, B) U50,488H (0.1 - 3.0 mg/kg, s.c.) administered 10 minutes prior to NorBNI (5 mg/kg, s.c.neck) suppresses the antagonist-induced scratching response. For the vehicle response, vehicle (s.c.) was administered 10 min prior to a challenge with vehicle (s.c.neck). (A) Dose effect over the time course compared to vehicle: two-way ANOVA for dose (p < 0.0001); Vehicle, n = 11; all other groups, n = 5-7. (B) Sum of the response over the hour test session: one-way ANOVA vs. Veh + NorBNI (p < 0.0001), ***p < 0.001, ****p < 0.0001 Bonferroni post hoc analysis. (C, D) U50,488H (1.0 and 3.0 mg/kg, s.c.) administered 10 minutes prior to CP (40 mg/kg, s.c.neck) suppresses the scratching response. For the vehicle response, vehicle (s.c.) was administered 10 min prior to a challenge with vehicle (s.c.neck). (C) Dose effect over the time course compared to vehicle: two-way ANOVA for dose (p < 0.0001); Vehicle, n = 11; all other groups, n = 6-7. (D) Sum of the response over the hour test session: one-way ANOVA vs. Veh + CP (p < 0.0001), ****p < 0.0001 Bonferroni post hoc analysis. (E, F) Twenty-four hour pretreatment with kappa antagonist NorBNI blocks the antipruritic effects of U50,488H on CP-induced itch. (E) Effect of Veh (i.p.) + U50,488H (1 mg/kg, s.c.) + CP (40 mg/kg, s.c.neck) vs. NorBNI (10 mg/kg, i.p.) + U50,488H (1 mg/kg, s.c.) + CP (40 mg/kg, s.c.neck): two-way ANOVA (p < 0.0001). There were no differences detected between vehicle pretreatment and NorBNI pretreatment groups (p > 0.05). (F) The comparison of the sum of the responses over the hour test session reveals suppression of CP-induced itch by systemic U50,488H injection and reversal of U50,488H effects by the 24 hour pretreatment with NorBNI: one-way ANOVA, ****p < 0.0001 Bonferroni post hoc analysis compared to vehicle pretreatment. Data are presented as the mean ± SEM.
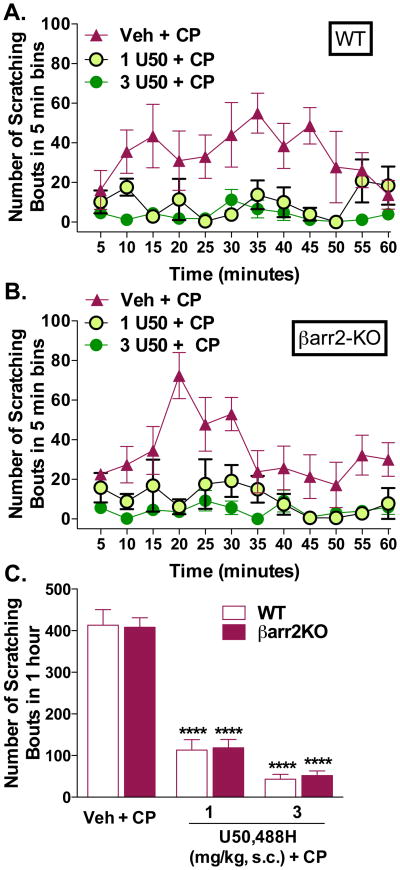
Since our studies in the βarr2-KO mice suggest that antagonism of KOR to induce itch may involve βarrestin2, we asked whether βarrestin2 is involved in the agonist-induced suppression of the itch response. WT and βarr2-KO mice were treated with U50,488H (1, 3 mg/kg s.c.) prior to challenge with CP (40 mg/kg, s.c.neck), a dose that produces an equivalent scratching response between the genotypes. Compared to vehicle, U50,488H suppresses the scratching behaviors in WT (two-way ANOVA for dose effect: F(2,144) = 47.14, p < 0.0001, Figure 5A) and βarr2-KO mice (two-way ANOVA for dose effect: F(2,144) = 52.96, p < 0.0001, Figure 5B). Two-way ANOVA comparison between genotypes, within each dose, did not reveal a genotype effect when analyzed with the 5 minute binning data (p > 0.05) or when comparing the sum of the scratching response over the hour testing session at each dose (two-way ANOVA for genotype: F(1,24) = 0.02729, p = 0.8702; dose: F(2,24) = 138.0, p < 0.0001, Figure 5C) suggesting that βarrestin2 may not be required for inducing the antipruritic effects of KOR agonists.
Figure 5.
The KOR agonist U50,488H suppresses scratching behaviors induced by chloroquine phosphate independent of the presence of βarr-2. (A) U50,488H (1.0 and 3.0 mg/kg, s.c.) administered 10 minutes prior to CP (40 mg/kg, s.c.neck) suppresses the scratching response in both genotypes. For the vehicle response, vehicle (s.c.) was administered 10 min prior to a challenge with vehicle (s.c.neck). Compared to vehicle: (A) WT: two-way ANOVA for dose (p < 0.0001); (B) βarr2-KO: two-way ANOVA for dose p < 0.0001); all groups, both genotypes n = 5. Two-way ANOVA comparison between genotypes, within each dose, did not reveal a genotype effect when analyzed with the 5 minute binning data (p > 0.05). (C) Comparison of the sum of the response over the hour testing session at each dose revealed a significant effect of dose, but no difference between the genotypes (two-way ANOVA for genotype (p > 0.05), and dose (p < 0.0001), ****p < 0.0001 Bonferroni post hoc analysis; all groups, both genotypes n = 5). Data are presented as mean ± SEM.
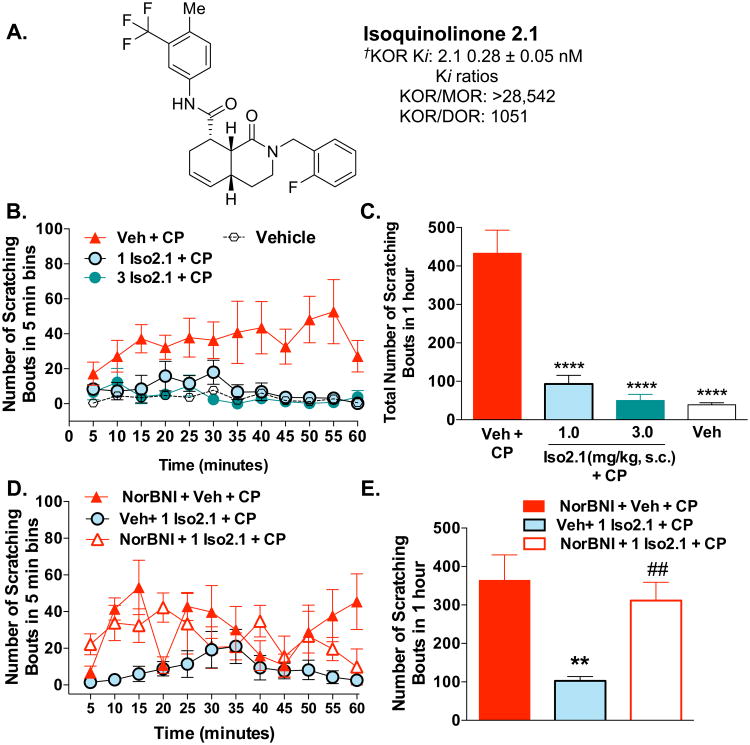
3.5 A KOR agonist that induces bias towards G protein signaling, Isoquinolinone 2.1, dose-dependently prevents the development of chloroquine phosphate-induced pruritus yet does not induce sedation
Recently, we described the development of KOR agonists that displays bias for G protein signaling over βarrestin2 recruitment, a “G protein signaling biased agonist” (Figure 6A) (Zhou et al., 2013). Isoquinolinone 2.1 (Iso2.1) dose dependently suppresses the itch response induced by CP (40 mg/kg, s.c.neck) (two-way ANOVA for dose: F(2,156) = 49.77, p < 0.0001, Figure 6B; one-way ANOVA for 1 hour sums: F(3,22) = 38.98, p < 0.0001, Figure 6C). The effects of Iso2.1 could be fully blocked by a pretreatment of systemic NorBNI (10 mg/kg, i.p.) 24 hours prior to Iso2.1 treatment, supporting that Iso2.1 is acting at KOR (two-way ANOVA comparing Veh + Iso2.1 + CP and NorBNI + Iso2.1 + CP: F(1,96) = 25.21, p < 0.0001, Figure 6D). A comparison of the sum effects over the one hour test period also reveals significant reversal of Iso2.1 effects by NorBNI + Veh + CP vs. Veh + Iso2.1 + CP: **p < 0.01; Veh + Iso2.1 + CP and NorBNI + Iso2.1 + CP: ##p > 0.05, student's t-test) (Figure 6E).
Figure 6.
A KOR agonist that is biased towards G protein signaling over βarrestin2 recruitment suppresses chloroquine phosphate-induced itch. (A). Isoquinolinone 2.1 (Iso2.1) is a highly selective KOR agonist; †affinity measures for radioligand binding are from Zhou et al., 2013. (B) Iso2.1 (1.0 and 3.0 mg/kg, s.c.) administered 10 minutes prior to CP (40 mg/kg, s.c.neck) suppresses the scratching response compared to vehicle: two-way ANOVA for dose (p < 0.0001); n = 5 - 6. (C) Significance is also revealed by the comparison of the sum of the response over the hour test session: one-way ANOVA, ***p < 0.001, ****p < 0.0001 Bonferroni post hoc analysis; n = 5-6). (D, E), Twenty-four hour pretreatment with KOR antagonist NorBNI (10 mg/kg, i.p.) blocks the antipruritic effects of Iso2.1 (1 mg/kg, i.p.) on CP-induced itch. (D) Effect of Veh + Iso2.1 + CP vs. NorBNI + Iso2.1 + CP: two-way ANOVA (p < 0.0001). There were no differences detected between vehicle pretreatment and NorBNI pretreatment groups (p > 0.05). (E) The comparison of the sum of the responses over the hour test session reveals suppression of CP-induced itch by systemic Iso2.1 injection and reversal of Iso2.1 effects by the 24 hour pretreatment with systemic NorBNI: one-way ANOVA, NorBNI + Veh + CP vs. Veh + Iso2.1 + CP, **p < 0.01; NorBNI + Iso2.1 + CP vs. Veh + Iso2.1 + CP, #p < 0.05, student's t-test. Data are presented as the mean ± SEM.
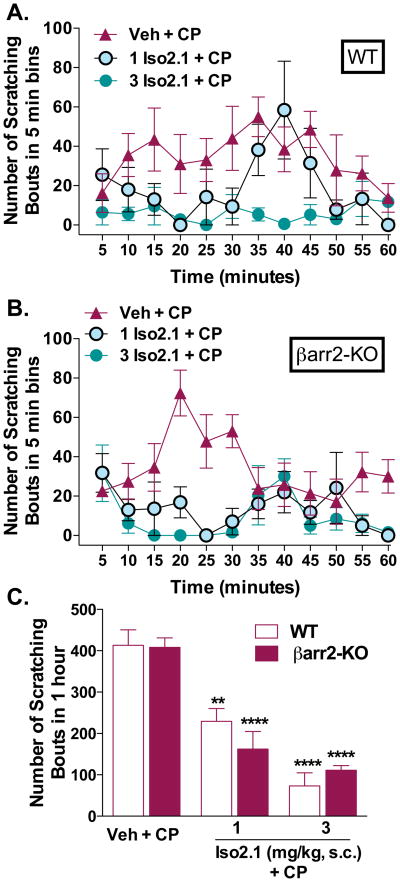
We then asked whether a G protein-biased KOR agonist would remain potent in the absence of βarrestin2. Both WT (two-way ANOVA for dose,: F(2,144) = 20.60, p < 0.0001, Figure 7A) and βarr2-KO mice (two-way ANOVA for dose: F(2,144) = 26.29, p < 0.0001, Figure 7B) respond to pretreatment with G protein biased KOR agonist Iso2.1 to reduce the development of the CP-induced scratching behavior compared to vehicle treated mice. Two-way ANOVA comparison between genotypes, within each dose, did not reveal a genotype effect when analyzed with the 5 minute binning data (p > 0.05) or when analyzing the sum of the response over the hour testing session (two-way ANOVA for genotype: F(1,23) = 0.2042, p = 0.6556; dose: F(2,23) = 56.80, p < 0.0001) (Figure 7C).
Figure 7.
A G protein biased KOR agonist remains potent in the absence of βarr-2. (A) Iso2.1 (1 and 3 mg/kg, s.c.) administered 10 minutes prior to CP (40 mg/kg, s.c.neck) suppresses the scratching response in both genotypes. Compared to vehicle: (B) WT: two-way ANOVA for dose (p < 0.0001); βarr2-KO: (p < 0.0001); all groups, both genotypes n = 5. Two-way ANOVA comparison between genotypes, within each dose, did not reveal a genotype effect when analyzed with the 5 minute binning data (p > 0.05). (C) Comparison of the sum of the response over the hour testing session at each dose revealed a significant effect of dose, but no difference between the genotypes: two-way ANOVA for genotype (p > 0.05), and dose (p < 0.0001), ****p < 0.0001 Bonferroni post hoc analysis; all groups, both genotypes n = 5. Data are presented as mean ± SEM.
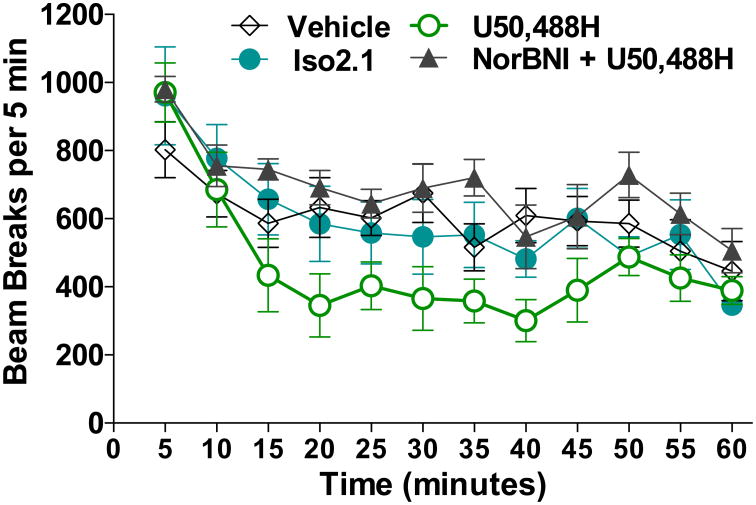
Since KOR agonists are known to suppress locomotor activity, we asked whether Iso2.1 decreased locomotor activity to a greater degree than U50,488H at the highest dose used to suppress the scratching response, with the idea that if Iso2.1 had sedative effects, it may result in less CP-induced scratching behavior that would be independent of its direct actions at KOR relating to the sensory perception of itch. Spontaneous locomotor activity was assessed in the open field following a 3 mg/kg, i.p. injection of U50,488 or Iso2.1 (Figure 8). While U50,488H significantly suppresses activity (two-way ANOVA vs. vehicle: F(1,132) = 13.81, p = 0.0003); Iso2.1 has no significant effect on spontaneous activity (two-way ANOVA vs. vehicle: p > 0.05). The effects of U50,488H on locomotor activity are prevented with a twenty-four hour pretreatment of NorBNI (10 mg/kg, i.p.) (two-way ANOVA vs 3 mg/kg U50,488H: F(1, 132) = 52.94, p < 0.0001), showing that the effects of U50,488H on locomotor activity can be blocked by a KOR antagonist.
Figure 8.
A G protein biased KOR agonist does suppress spontaneous locomotor activity. While U50,488H (3 mg/kg, s.c.) significantly suppressed activity (two way ANOVA vs. vehicle, p = 0.0003), Iso2.1 (3 mg/kg, s.c.) had no significant effect on spontaneous activity when compared to vehicle (two-way ANOVA vs. vehicle, p>0.05). Twenty-four hour pretreatment with NorBNI (10 mg/kg, i.p.) prevented the decreased locomotor activity associated with U50,488H (two-way ANOVA vs. 3 mg/kg U50488H, p < 0.0001). Vehicle, n = 9; Iso2.1, n = 6; U50,488H, n = 7; NorBNI + U50,488H, n = 6. Data presented as mean ± SEM.
4. Discussion
In this study we show that the actions of KOR antagonists, NorBNI and 5′GNTI, dose dependently promote a scratching response in C57BL/6J mice when injected s.c. at the nape of the neck, confirming observations made in other mouse strains (Figure 1A-C). We also show the effects of these antagonists are less severe in the KOR-KO mice, however, the itch response that is produced is still significant suggesting that the antagonist effects cannot be completely attributed to actions at the KOR (Figure 1D-E). The effects of the KOR antagonists were slightly but significantly decreased in the βarr2-KO mice compared to WT littermate controls (Figure 2), while the effects of chloroquine phosphate were equivalent in both genotypes (Figure 3). A systemic, 24 hour pretreatment with NorBNI reverses the effects of U50,488H implicating a KOR-mediated mechanism in the antipruritic effects of U50,488H (Figure 4). The ability of NorBNI to reverse the effects of U50,488H in the chloroquine phosphate model of pruritus was maintained in the βarr2-KO mice (Figure 5). A KOR agonist biased for G protein signaling over βarrestin2 recruitment in cell culture assay, Isoquinolinone 2.1, was effective in preventing CP-induced itch (Figure 6); this effect was blocked by NorBNI implicating KOR mechanisms are preserved in βarrestin2-KO mice as one might anticipate for an agonist that has very low functional affinity for recruiting βarrestin2 in cell-based assays (Figure 7). Finally, the suppression of itch induced by Iso2.1 could not be attributed to increased sedative properties of the biased agonist (Figure 8).
Together these data suggest that 5′GNTI and NorBNI may induce itch via a KOR-dependent mechanism, however, they may also exert their effects by nonspecific effects as they maintained significant efficacy in the KOR-KO mice. There have also been speculations of non-opioid targets (Munro et al., 2013, Zhou et al., 2015, Zhou et al., in press). For example, screening against a panel of non-opioid receptors and ion channels reveal 5′GNTI to be a weak M1 muscarinic receptor antagonist (Inan et al., 2011; Munro et al., 2013). Interestingly, intrathecal treatment with M1 agonist McN-A-343 inhibits 5′GNTI-induced pruritus (Inan et al., 2009b). This supports the idea that the residual pruritus produced in KOR-KO mice by 5′GNTI could be attributed to antagonism at the M1 muscarinic receptor, however, it does not rule out other potential mechanisms of action.
While the data suggest that the βarr2-KO mice may be less sensitive to the antagonists for promoting the itch response via KOR, it is difficult to support this conclusion as the differences between the WT and βarr2-KO mice may be due to differences in the, pruritic effects that persist in the absence of KOR in response to these antagonists. As such, the βarr2-KO mice could simply be less responsive to this unidentified effect. Moreover, the βarr2-KO mice may be impaired in their ability to display a scratching response compared to the WT mice. In an attempt to address these issues, chloroquine phosphate (CP) was used as a general pruritic agent. In those studies, CP induced an equivalent itch response in both genotypes, arguing against a disruption in the ability to display the response. The general mechanism of action of CP, an antimalarial medication, has been attributed to inducing a mast cell degranulation resulting in the localized release of inflammatory factors (Aghahowa et al., 2010). NorBNI and 5′GNTI could also be producing nonspecific effects that may or may not include the same mechanisms induced by CP. Therefore, it remains difficult to attribute the differences seen between the WT and βarr2-KO mice solely to differential regulation of KOR.
Aside from investigating the role of βarrestin2, our results contribute to and expand upon studies that report KOR antagonists NorBNI and 5′GNTI are pruritogens in mice. NorBNI was first described as a pruritogen in ICR mice (Kamei and Nagase, 2001), whereas a majority of 5′GNTI-induced pruritus has been characterized in Swiss-Webster mice (Inan et al., 2009a, b, 2011). Different strains of mice can inherently have different phenotypes that present when challenged with a pruritogen. For example, ICR mice show an increased release of histamine from mast cells, which is thought to facilitate scratching behavior, whereas the histamine release from mast cells in C57BL/6 mice is relatively low (Toda et al., 1989). Our study demonstrates that C57BL/6J adult male mice significantly respond to KOR antagonists and CP for the induction of a pruritic response; this will be useful information as many genetic mouse models have been developed on the C57BL/6J background.
A growing number of studies report that KOR is important in the processing of pruritus (Kardon et al., 2014; Ross et al., 2010; Sardella et al., 2011). Additionally, the development and characterization of nalfurafine (TRK-820) in the clinic to treat hemodialysis-associated pruritus (Kumagai et al., 2012; Kumagai et al., 2010; Ueno et al., 2013; Wikstrom et al., 2005) supports the therapeutic potential of KOR agonists in treating pruritus. Indeed, there is significant evidence supporting activation of KOR as an avenue for the treatment of itch arising from multiple pruritic effectors in both rodents (Gmerek and Cowan, 1988; Kamei and Nagase, 2001) and non-human primates (Ko and Husbands, 2009; Ko et al., 2003). For example, nalfurafine, a 4,5-epoxymorphinan derivative full agonist for KOR and partial agonist for the mu opioid receptor (MOR) (Nagase et al., 1998; Seki et al., 1999), has potent antipruritic-activity in both antihistamine-effective and -ineffective animal models of pruritus. These include pruritus induced by substance P, histamine (Togashi et al., 2002), compound 48/80 (Wang et al., 2005), chloroquine (Inan and Cowan, 2004), scratching secondary to cholestasis (Inan and Cowan, 2006) and KOR antagonist-induced itch (Inan et al., 2009b) all in rodents and intravenously administered morphine in monkeys (Wakasa et al., 2004). Nalfurafine (Remitch®) is also clinically utilized in Japan for the treatment of patients suffering from uremic pruritus (Kumagai et al., 2012; Kumagai et al., 2010; Ueno et al., 2013; Wikstrom et al., 2005). Like nalfurafine, the novel KOR agonist Iso2.1 also prevents the development of itch from multiple pruritogens, specifically KOR antagonist-induced (Figure 3A-B) and chloroquine phosphate-induced pruritus (Figure 3C-D) with a similar efficacy as U50,488H.
Iso2.1 possesses high selectivity and affinity for KOR and induces receptor signaling biased toward G protein coupling over βarrestin2 recruitment (Zhou et al., 2013). Recently, another G protein signaling biased KOR agonist, RB-64, derivative of salvinorin A, was found to produce antinociception and conditioned place aversion (CPA) and not motor incoordination (White et al., 2014). These results suggest KOR-mediated G protein signaling leads to antinociception and CPA, whereas KOR-mediated βarrestin2 signaling may contribute to the motor deficits. Interestingly, Iso2.1 maintained antinociceptive efficacy in a mouse warm water tail immersion test (Zhou et al., 2013) while maintaining potency in suppressing CP-induced pruritus, independent of βarrestin2 (Figures 6 & 7). Whether this biased agonist suppresses locomotor coordination like U50,488H does, remains to be fully evaluated; however, our studies suggest that it does not suppress spontaneous locomotor activity to a greater extent than U50,488H (Figure 8).
It is attractive to speculate that the use of a compound that possesses bias in cell-based assays (shows a mathematically derived preference for G protein signaling in one assay over βarrestin2 recruitment in another assay as compared to the performance of a reference agonist, U69,593 in both assays) can be used to define a preferred signaling pathway in vivo. However, this may not necessarily be the case. Cell-based signaling assays used to assess whether a compound is biased only provide an opportunity to compare the compound to the performance of a reference compound in the context of the assay. The context of the assay is the immediate cellular environment, which includes the signaling effectors that are available for interaction as well as the thermodynamic exchanges favored by membrane compositions and interacting partners. As the cellular environment is assuredly different between cell-based assay systems and endogenous sensory neurons, one cannot simply infer that the bias observed in cell culture will directly translate to the exact recapitulation of bias in the endogenous setting (Zhou and Bohn, 2014). However, the cell-based assay systems serve as very useful models in determining how compounds differ from the way a conventional agonist or endogenous hormone functions in vivo. Future studies, and likely, the development of better assay tools, are needed to determine the actual signal that propagates from KOR in its modulation of pruritus. However, as compounds with diverse chemotypes that share classifications among the cell-based systems venture into the endogenous setting, correlations will become apparent and inform as to the pharmacological properties that may translate from in vitro characterizations to behavioral modulations.
Supplementary Material
Highlights.
KOR agonists are clinically used for pruritis yet most KOR agonists induce sedation.
G protein/βarrestin2 biased KOR agonists are antipruritic yet not sedating in mice.
Biased KOR agonists may have therapeutic utility in treating pruritis.
Acknowledgments
We thank Stephen Slauson of KU for his chemical synthesis efforts. This work was funded by NIDA grant R01DA031927 to L. Bohn and J. Aubé.
Abbreviations
- βarr2-KO
βarrestin2 knockout
- CP
chloroquine phosphate
- CPA
conditioned place aversion
- DMSO
dimethyl sulfoxide
- 5′-GNTI
5′-guanidinonaltrindole
- GPCR
G protein coupled receptor
- GRK3
GPCR kinase 3
- [35S]GTPγS
guanosine 5′-O-(3-[35S]thio)triphosphate
- KOR
kappa opioid receptor
- KOR-KO
kappa opioid receptor knockout
- MOR
mu opioid receptor
- NorBNI
nor-binaltorphimine
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghahowa SE, Obianwu HO, Isah AO, Arhewoh IM. Chloroquine-induced Pruritus. Indian J Pharm Sci. 2010;72:283–289. doi: 10.4103/0250-474X.70471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. Role of spinal bombesin-responsive neurons in nonhistaminergic itch. J Neurophysiol. 2014;112:2283–2289. doi: 10.1152/jn.00409.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A. Role of opioid receptors in bombesin-induced grooming. Ann N Y Acad Sci. 1988;525:291–300. doi: 10.1111/j.1749-6632.1988.tb38614.x. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Rosenthal W, Trautwein W, Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. Nature. 1987;325:445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Holmes FE, Vanderplank P, Wynick D. Galanin-expression and galanin-dependent sensory neurons are not required for itch. Mol Pain. 2012;8:87. doi: 10.1186/1744-8069-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S, Cowan A. Kappa opioid agonists suppress chloroquine-induced scratching in mice. Eur J Pharmacol. 2004;502:233–237. doi: 10.1016/j.ejphar.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Inan S, Cowan A. Nalfurafine, a kappa opioid receptor agonist, inhibits scratching behavior secondary to cholestasis induced by chronic ethynylestradiol injections in rats. Pharmacol Biochem Behav. 2006;85:39–43. doi: 10.1016/j.pbb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A. Inhibitory effect of lidocaine on pain and itch using formalin-induced nociception and 5′-guanidinonaltrindole-induced scratching models in mice: behavioral and neuroanatomical evidence. Eur J Pharmacol. 2009a;616:141–146. doi: 10.1016/j.ejphar.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A. Nalfurafine prevents 5′-guanidinonaltrindole- and compound 48/80-induced spinal c-fos expression and attenuates 5′-guanidinonaltrindole-elicited scratching behavior in mice. Neuroscience. 2009b;163:23–33. doi: 10.1016/j.neuroscience.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A. Investigation of gastrin-releasing peptide as a mediator for 5′-guanidinonaltrindole-induced compulsive scratching in mice. Peptides. 2011;32:286–292. doi: 10.1016/j.peptides.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Lee NM, Loh HH, Thayer SA. Dual excitatory and inhibitory effects of opioids on intracellular calcium in neuroblastoma x glioma hybrid NG108-15 cells. Mol Pharmacol. 1992;42:1083–1089. [PubMed] [Google Scholar]
- Kamei J, Nagase H. Norbinaltorphimine, a selective kappa-opioid receptor antagonist, induces an itch-associated response in mice. Eur J Pharmacol. 2001;418:141–145. doi: 10.1016/s0014-2999(01)00941-4. [DOI] [PubMed] [Google Scholar]
- Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82:573–586. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Husbands SM. Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. J Pharmacol Exp Ther. 2009;328:193–200. doi: 10.1124/jpet.108.143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH, Naughton NN. Activation of kappa-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther. 2003;305:173–179. doi: 10.1124/jpet.102.044909. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, Kurihara M, Yanagita T, Suzuki H. Efficacy and safety of a novel k-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol. 2012;36:175–183. doi: 10.1159/000341268. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25:1251–1257. doi: 10.1093/ndt/gfp588. [DOI] [PubMed] [Google Scholar]
- Munro TA, Huang XP, Inglese C, Perrone MG, Van't Veer A, Carroll FI, Beguin C, Carlezon WA, Jr, Colabufo NA, Cohen BM, Roth BL. Selective kappa opioid antagonists nor-BNI, GNTI and JDTic have low affinities for non-opioid receptors and transporters. PLoS One. 2013;8:e70701. doi: 10.1371/journal.pone.0070701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Hayakawa J, Kawamura K, Kawai K, Takezawa Y, Matsuura H, Tajima C, Endo T. Discovery of a structurally novel opioid kappa-agonist derived from 4,5-epoxymorphinan. Chem Pharm Bull (Tokyo) 1998;46:366–369. doi: 10.1248/cpb.46.366. [DOI] [PubMed] [Google Scholar]
- North RA, Williams JT, Surprenant A, Christie MJ. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987;84:5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives ML, Rossillo M, Liu-Chen LY, Javitch JA. 6′-Guanidinonaltrindole (6′-GNTI) is a G protein-biased kappa-opioid receptor agonist that inhibits arrestin recruitment. J Biol Chem. 2012;287:27050–27054. doi: 10.1074/jbc.C112.387332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman S. Physiology of itching. Physiological Reviews. 1941;21:357–381. [Google Scholar]
- Sardella TC, Polgar E, Garzillo F, Furuta T, Kaneko T, Watanabe M, Todd AJ. Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol Pain. 2011;7:76. doi: 10.1186/1744-8069-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Groer CE, Munro TA, Zhou L, Bohn LM. Functional selectivity of 6′-guanidinonaltrindole (6′-GNTI) at kappa-opioid receptors in striatal neurons. J Biol Chem. 2013;288:22387–22398. doi: 10.1074/jbc.M113.476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Awamura S, Kimura C, Ide S, Sakano K, Minami M, Nagase H, Satoh M. Pharmacological properties of TRK-820 on cloned mu-, delta- and kappa-opioid receptors and nociceptin receptor. Eur J Pharmacol. 1999;376:159–167. doi: 10.1016/s0014-2999(99)00369-6. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci U S A. 1977;74:3365–3369. doi: 10.1073/pnas.74.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Shen KZ, North RA, Tatsumi H. Inhibition of calcium currents by noradrenaline, somatostatin and opioids in guinea-pig submucosal neurones. J Physiol. 1990;431:585–608. doi: 10.1113/jphysiol.1990.sp018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Kimura M, Tohya K. Strain differences in histamine release from mouse peritoneal mast cells induced by compound 48/80 or A23187. Jikken Dobutsu. 1989;38:135–137. doi: 10.1538/expanim1978.38.2_135. [DOI] [PubMed] [Google Scholar]
- Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–264. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Mori A, Yanagita T. One year long-term study on abuse liability of nalfurafine in hemodialysis patients. Int J Clin Pharmacol Ther. 2013;51:823–831. doi: 10.5414/CP201852. [DOI] [PubMed] [Google Scholar]
- Umeuchi H, Togashi Y, Honda T, Nakao K, Okano K, Tanaka T, Nagase H. Involvement of central mu-opioid system in the scratching behavior in mice, and the suppression of it by the activation of kappa-opioid system. Eur J Pharmacol. 2003;477:29–35. doi: 10.1016/j.ejphar.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Wakasa Y, Fujiwara A, Umeuchi H, Endoh T, Okano K, Tanaka T, Nagase H. Inhibitory effects of TRK-820 on systemic skin scratching induced by morphine in rhesus monkeys. Life Sci. 2004;75:2947–2957. doi: 10.1016/j.lfs.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL. The G protein-biased kappa-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352:98–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Scopton AP, Rives ML, Bikbulatov RV, Polepally PR, Brown PJ, Kenakin T, Javitch JA, Zjawiony JK, Roth BL. Identification of novel functionally selective kappa-opioid receptor scaffolds. Mol Pharmacol. 2014;85:83–90. doi: 10.1124/mol.113.089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, Ueno Y. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16:3742–3747. doi: 10.1681/ASN.2005020152. [DOI] [PubMed] [Google Scholar]
- Zhou L, Bohn LM. Functional selectivity of GPCR signaling in animals. Curr Opin Cell Biol. 2014;27:102–108. doi: 10.1016/j.ceb.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, Cameron MD, Prisinzano TE, Aube J, Bohn LM. Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem. 2013;288:36703–36716. doi: 10.1074/jbc.M113.504381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Stahl EL, Lovell KM, Frankowski KJ, Prisinzano TE, Aube J, Bohn LM. Characterization of kappa opioid receptor mediated, dynorphin-stimulated [35S]GTPγS binding in mouse striatum for the evaluation of selective KOR ligands in an endogenous setting. Neurophamracology. 2015 doi: 10.1016/j.neuropharm.2015.07.001. in Press (minor revisions submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.